Introduction
Asthma is a chronic, heterogeneous disease that ranges from mild, intermittent asthma to severe asthma. In 2015, asthma affected approximately 18 million adults and 6 million children in the United States.1 Asthma is characterized by coughing, wheezing, shortness of breath and/or chest tightness driven by increased airway reactivity, inflammation, and/or mucus production. The majority of patients with asthma have allergic airway inflammation characterized by type 2-mediated airway inflammation.2 However, some patients with asthma have low (or no) type 2-mediated airway inflammation but have increased neutrophils driven by type 1 or IL17-mediated airway inflammation.2, 3
As depicted in Figure 1, increased airway inflammation, mucus production, and airway hyperresponsiveness (AHR) are caused by multiple pathways. Initiation of type 2 allergic airway inflammation occurs with exposures to allergens, including house dust mite, pollen, mammalian antigens, cockroach antigens, and/or fungal antigens, and results in increased production of inflammatory cytokines, including thymic stromal-derived lymphopoietin (TSLP), IL-33, IL-25, and/or IL-4. Increased production of these stimulatory cytokines resulted in increased expression of type-2 cytokines, IL-4, IL-5, IL-13, and IL-9 produced from CD4+ T cell helper (Th)2 cells, group-2 innate lymphoid cells (ILCs), eosinophils, basophils, mast cells, macrophages and other cells. The release of type-2 cytokines results in increased IgE (immunoglobulin E)-triggered hypersensitivity to allergens, activation of airway epithelial cells, increased infiltration and activation of eosinophils, and increased airway remodeling.2
Figure 1. How sex hormones regulate different airway inflammatory pathways in asthma.
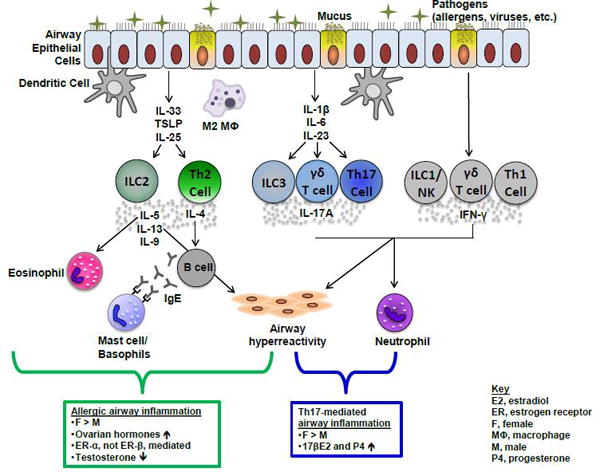
Schematic of type 2, IL-17A-mediated, and IFN-y-mediated pathways associated with different phenotypes of asthma. Summary of gender differences and the role of sex hormone signaling in type 2 and IL-17A-mediated airway inflammation are shown in boxes below pathways.
In patients with more severe, type 1 or IL17-mediated phenotypes of asthma, neutrophils are increased in the sputum and bronchoalveolar lavage (BAL) fluid. Typically, these patients do not respond well to corticosteroid treatments and have increased morbidity and asthma-related health care costs when compared to patients with milder phenotypes of asthma.4 Increased neutrophils in the airway are driven by increased IL-17A-secreting and/or IFN (interferon)-γ-secreting cells, including Th17, γδ T cells, Th1, and NK (natural killer) cells.5,6 In this review, we will summarize the clinical, epidemiological, and animal studies that show a gender disparity in asthma and the mechanisms by which sex hormones regulate asthma pathogenesis.
Gender disparity in asthma
A gender disparity exists in asthma and changes with age. As children, boys have an increased prevalence of asthma compared to girls with increased atopy, wheeze, serum IgE levels, and use of asthma medications.7, 8 Several longitudinal studies, including The Prevention and Incidence of Asthma and Mite Allergy (PIAMA) study, have shown this gender disparity in asthma. PIAMA was unique because it recruited 4,146 pregnant women and assessed 3,308 of their children yearly for wheeze (ages 1-7) and asthma (age 8) by questionnaires. Starting at age 1 and maintained until age 8, boys had an increased cumulative incidence of parent-reported wheeze compared to girls. At age 8, 15.1% of the boys and 10.8% of the girls had asthma,7 suggesting the gender disparity in asthma begins early in childhood. Boys also had increased atopy, measured by specific IgE or skin prick testing to common allergens, compared to girls prior to adolescence.7–12 These findings suggest that atopy and allergen sensitization is greater in boys compared to girls as children, and may provide some rationale for increased the gender disparity in asthma prevalence in children.
Other differences in immune responses and anatomical differences may also play a role in explaining this observed gender difference in wheeze and/or asthma in boys and girls, when sex hormones are low. PHA (phytohemagglutinin)-induced mononuclear cells from boys, compared to girls, had significantly increased IFN-γ at 1 year of life and increased IFN-γ, IL-5, and IL-13 in children that wheezed at 3 years of life.13 Further, boys had increased rates of sensitization, total IgE levels, and blood eosinophil counts compared to girls.13 Dysanapsis, smaller airway diameters relative to lung volumes, is also detected more often in boys compared to girls.10 Therefore, a more robust immune response and decreased airway size likely contribute to increased wheezing in young boys compared to girls.
In contrast, as adults, women have an increased prevalence of asthma compared to men. This switch in asthma prevalence coincides with puberty, suggesting that sex hormones are important in asthma pathogenesis.14, 15 The Childhood Asthma Management Program (CAMP) study determined this gender switch at puberty by longitudinally tracking the average asthma symptom scores and the progression of puberty, using Tanner puberty stages, in children from ages 4 to 17.16 Tanner stage puberty scores characterize predictable changes in puberty using a 1-5 scale, with 1 being pre-pubertal and 5 being puberty completed, fully mature. Tanner stage puberty scores started to increase in girls at approximately 10 years old, and this coincided with increases in the asthma symptom scores in girls. For boys, Tanner stage puberty scores started to increase around age 10, but the average asthma symptom scores began to decrease at approximately age 14.16 Additional studies have also shown that early aged menarche (≤11 years old) in girls increased the incidence of asthma.17
After puberty, the gender difference in asthma in men and women may be explained by increased incidence in women. A longitudinal, questionnaire-based study that tracked children into adulthood, ages 10-20, with asthma (n=274) as well as healthy controls (n=1000) determined that at age 20, the prevalence of asthma persisted in 24.5% of asthmatic participants and remained male dominated in ratio.18 In the healthy controls, 4.8% of participants had developed asthma by age 20, and most of these patients were female. Atopy, measured by skin prick testing to inhaled allergens at age 10, was not associated with wheeze in the healthy individuals diagnosed with asthma at age 20. Therefore, these findings suggest that the gender switch in asthma prevalence that occurs during adolescence and early adulthood is driven by increased incidence in females over resolution in males with asthma.
Pediatric and adult gender disparities in asthma were also shown in a large, retrospective cohort study using the Kaiser-Permanente care computerized data from 60,694 patients. This study determined that asthma-related health care utilization and medication use was increased in boys compared to girls from ages 2-13, was not statistically different by gender from ages 14-22, and was increased in women compared to men after age 23.19 This large, health-care utilization cohort study highlights the gender differences in asthma health utilization throughout life.
All of these studies have provided insight on when the highest asthma prevalence begins to shift from boys to women, but additional studies need to be conducted to determine the hormones that are important for driving the observed switch in asthma prevalence. It is important to leverage information on which sex hormones are associated with asthma symptoms, control, and frequency of exacerbations as established pediatric asthma cohorts age through puberty and into adolescence and adulthood. This information will be imperative to further defining the mechanisms of asthma pathogenesis and potential hormonal-related causes to later onset asthma in females.
Gender disparity across phenotypes of asthma
The heterogeneity in patients’ asthma severity and the mechanisms that drive airway inflammation, mucus production, and/or airway reactivity led researchers to categorize asthma based on phenotypes and endotypes. Unsupervised clustering analyses to identify and categorize asthma phenotypes with similar characteristics using multiple variables have been ongoing in both pediatric and adult asthma populations. Pediatric population cluster analyses utilizing the CAMP, the Inner City Asthma Consortium, or The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) studies showed that boys had an increased percentage in clusters with more exacerbations and/or lower baseline pulmonary function compared to girls.20–22 The CAMP study determined 5 clusters based on atopic burden, degree of airway obstruction, and history of exacerbation. The clusters with the largest percentages of boys (clusters 3-5) also had high airway obstruction, medium to high atopy, and high exacerbation rates compared to clusters with milder phenotypes of asthma (clusters 1 and 2).20 The Inner-City Asthma Consortium Asthma Phenotypes in the Inner City (APIC) identified 5 clusters from 616 pediatric patients and found an increased percentage of boys compared to girls in all participants. Further, the more type 2-mediated allergic asthma clusters (clusters C, D, and E in the study) had more boys than girls.22 The TENOR study used hierarchical clustering algorithms and determined clusters in children based on gender, atopic status, smoke exposure, and race. Boys were the majority of children in all asthma clusters except one cluster that was defined by female gender.21
Clustering analysis in adults with asthma determined that women are more likely have more severe, less corticosteroid responsive phenotypes of asthma compared to men.23, 24 In particular, women had a higher prevalence than men in a cluster of later onset with less atopy and worse lung function and a cluster of obese, less atopic, and older asthmatics in one study.23 The U-BIOPRED cohort, which enrolled patients with asthma ranging from mild to severe, showed that women have an increased prevalence in a cluster composed of obese patients with uncontrolled severe asthma and increased exacerbations but normal lung function.25 Further, the European Network For Understanding Mechanisms Of Severe Asthma (UNFUMOSA) study reported that females were 4.4X more likely to be classified with severe asthma than males.26 Cluster analysis of asthma phenotypes in 611 adult patients utilizing primary care providers in the ACCURATE trial also showed a female predominance in clusters with patients that have later onset of asthma or more asthma exacerbations.27 Combined, cluster analyses in pediatric and adult asthma populations show that asthma is a heterogeneous disease and that the gender disparity is observed in the more severe or exacerbation prone clusters in children and adults.
Changes in asthma symptoms during the menstrual cycle
Changes in ovarian hormone concentrations each month as well as through the reproductive years of a women’s life have provided valuable insight on the role of sex hormones on asthma control and exacerbations. Early studies reported worsening of asthma symptoms, decreased peak flow rates, and increased use of rescue medications in 30-40% of women with asthma during the pre or perimenstrual phase of the cycle.28, 29,30–32 Sputum eosinophils and fractional exhaled nitric oxide (FeNO) were also increased during this pre-menstrual phase when compared to just after menses (7th day of cycle).33, 34 Further, increased oral corticosteroid bursts and increased emergency department visits were reported in the SARP study in women that had peri-menstrual worsening of asthma compared to women without peri-menstrual worsening of asthma.35 These studies suggest that pre-menstrual asthma impacts many women with asthma, but additional studies with mild to severe asthma patients reported no differences in the asthma-related emergency department visits in any phase of the menstrual cycle.36, 37 Further, Juniper et al. determined that asthma symptoms were increased during menstruation but that medication use and FEV1 in response to methacholine were similar in patients 1 week prior and 1 week after menstruation and that changes in serum progesterone had no effect.38 Therefore, the mechanisms driving these cyclic changes in some women are unclear.
Based on the cyclic changes in asthma symptoms in some women, asthma investigators became interested in determining if use of hormonal birth control medications affected asthma symptoms in women. A cross-sectional postal survey study reported that contraceptive pill use in pre-menopausal women was associated with self-reported increased risk for asthma and increased wheezing in normal and overweight women.39 However, in another study, use of oral contraceptives decreased serum progesterone levels, but had no effect on asthma medication use and changes in FEV1 in response to methacholine.38 Other studies, such as the Swiss cohort study on Air Pollution And Lung Disease in Adults (SAPALDIA) reported that women taking oral contraceptives had a decrease in methacholine-induced AHR compared to women not taking oral contraceptives. Additionally, the Scottish Health Surveys determined that women on oral contraceptives had a reduced risk of current, physician-diagnosed asthma and urgent care use of more than 3 times per year for asthma.40 These conflicting findings suggest that additional studies are needed to determine the therapeutic value of birth control medications on asthma.
Utilizing dehydroepiandrosterone (DHEA), a hormone secreted by the adrenal cortex that is converted into androgens and estrogen, has also been explored as a potential therapeutic for patients with asthma. Mice fed DHEA containing chow during dust mite-induced allergic airway inflammation had decreased airway eosinophils as well as decreased serum IL-4 and IL-5 levels.41 Further, DHEA suppressed methacholine-induced AHR and airway eosinophils in OVA sensitized and challenged mice as well as decreased Ca2+-induced airway smooth muscle contraction in tracheal rings from guinea pigs.42, 43 In humans, nebulized dehydroepiandrosterone-3-sulfate (DHEAS), but not DHEA, improved asthma control, as measured by the asthma control questionnaire, in moderate to severe asthmatics after a 6-week, randomized, double-blind, placebo-controlled study.44 Use of contraceptives or DHEAS as therapeutics for patients with asthma, particularly moderate to severe asthma, is plausible, but additional studies are needed.
Pregnancy and asthma
Changes in asthma severity and symptoms have also been reported by some women during pregnancy. Results from an initial study in which women kept daily asthma symptom diaries and had monthly spirometries conducted during pregnancy and 3 months post-partum suggested that asthma symptoms increased in approximately one-third of patients with asthma.45 This study also showed that 73% of women who experienced worsening of asthma symptoms had a decline in asthma symptoms (back to pre-pregnancy levels) by 3 months post-partum.45 Additional studies showed that women with severe asthma were at increased risk for asthma exacerbations during pregnancy compared to mild and moderate asthma patients,46 and that the main triggers for these asthma exacerbations during pregnancy were viral infections and non-adherence to inhaled corticosteroid therapies.47 In contrast, airway responsiveness and asthma severity, measured as a percentage of FEV1 to vital capacity as well as asthma medication use, was decreased in sixteen asthmatic women during pregnancy but returned toward pre-conception levels 1 month after delivery.48 In addition, Belanger et al. reported that pregnancy did not affect asthma severity when patients continued to use their prescribed medications and that the trimester of pregnancy was not associated with changes in asthma severity.49 Controlling asthma during pregnancy is vital to reducing perinatal risks that have been associated with asthma during pregnancy, including low birth rates and small sizes for gestational age, but the effects of pregnancy on asthma need further study.50
Menopause and asthma
If ovarian hormones were imperative for increasing asthma symptoms, then a decline in asthma symptoms and asthma prevalence would be expected in women after menopause. However, as discussed below, studies have reported variations in asthma symptoms, prevalence, and phenotypes in peri and post-menopausal women. The Respiratory Health in Northern Europe (RHINE) study reported that the risk of respiratory symptoms increased in early postmenopausal and late postmenopausal women, and that transitional times in reproductive aging were more prone to new-onset asthma and respiratory symptoms.51 Further, use of hormonal replacement therapy for perimenopausal women, particularly women with body mass indexes less than 25, in the RHINE study increased asthma prevalence and asthma symptoms as assessed by a questionnaire.52 Additional studies also showed that use of hormonal replacement therapy in perimenopausal women increased the rate of new, physician diagnosed asthma53 and asthma incidence.54 These results suggest that fluctuations in hormone levels during menopause or use of hormonal replacement therapy during menopause may increase asthma symptoms. However, further investigated is warranted to determine the effects of menopause and hormone replacement therapy on asthma symptoms and newly described asthma phenotypes in older patients.55
Animal Studies on asthma
Clinical and epidemiological studies in pediatric and adult patients with asthma have provided valuable insight into the gender disparity in asthma. However, mouse models of eosinophilic or neutrophilic-mediated airway inflammation have been essential in providing insight on the role of testosterone and ovarian hormones in asthma pathogenesis. Since most patents with asthma have allergic asthma, many of these studies have been focused on type 2-mediated airway inflammation, including the role of sex hormones on mediating CD4+ Th2 cell, M2 macrophage, dendritic cell (DC), mast cell and basophil responses. Results from these studies are summarized in Figure 1.
Estrogen signaling regulates allergic airway inflammation in mice
Since females have an increase in asthma prevalence and incidence starting around puberty, the role of female sex hormones have been explored in mouse models of allergic asthma. Female mice undergoing OVA-induced or house dust mite (HDM)-induced allergic airway inflammation had increased eosinophils in the BAL fluid, increased IL-5 and IL-13 production in whole lung homogenates, and/or increased serum IgE production compared to male mice.56–58 Ovarian hormones are important in inducing allergic airway inflammation because gonadectomized female mice have decreased OVA-induced eosinophils in the BAL fluid, IL-5 production, AHR, and total serum IgE compared to hormonally intact, sham-operated female mice.59
Estrogen is the primary ovarian hormone that has been studied in airway inflammation. Estrogen signals through the nuclear receptors ER-α and ER-β as well as the membrane bound G protein-couple estrogen receptor 1 (GPER1). Upon binding estrogen, estrogen receptors homodimerize and recruit essential cofactors to activate or repress transcription of genes with estrogen response elements. ER-α and ER-β are expressed on hematopoietic cells and stromal cells, but the expression levels of ER-α and ER-β vary in cell types. For example, CD4+ T cells and M2 macrophages express more ER-α while B cells and airway epithelial cells express more ER-β.60–62
Estrogen receptor knockout mice have been used to study the effects of estrogen signaling on asthma. Studies suggest that ER-α signaling increases allergic airway inflammation. For example, mice deficient in ER-α (esr1−/− mice) had decreased OVA-induced allergic airway inflammation and AHR compared to WT mice, but mice deficient in ER-β (esr2−/− mice) had similar airway inflammation and AHR as WT mice.63 An additional study also determined that ER-α signaling increased OVA-induced AHR, but no effect on airway inflammation was seen.63
Studies also suggest that estrogen signaling is important for increasing DC function in type 2-mediated airway inflammation. Antigen presentation by DCs is essential for inducing allergic airway inflammation, and a sexual dimorphism is seen with DC accumulation after allergen challenge. Myeloid DCs and plasmacytoid DCs from OVA sensitized and challenged female mice are increased in the mediastinal lymph nodes compared to male OVA sensitized and challenged mice.64 Additionally, DCs treated with 17β-E2 (20 μg/ml) had increased cytokine expressions of IL-6, IL-8, and MCP-1 compared to baseline control, and pre-treatment of DCs with 17β-E2 (20 μg/ml) increased lipopolysaccharide (LPS)-induced T cell proliferation compared to vehicle.65 CD11c+ CD11bint DC differentiation was also inhibited by ER antagonists and in esr1−/− mice.66 Combined, these data show that estrogen signaling is important for increasing DC function in type 2-mediated airway inflammation.
Estrogen signaling is also important for increasing allergen-mediated type 2 airway inflammation in M2 macrophages, mast cells, and basophils. M2-polarized macrophages are increased in the BAL fluid in patients with asthma compared to healthy controls,67 and in mice, intratracheal administration of alveolar macrophages from OVA-sensitized and challenged mice increased eosiniophilic airway inflammation.64 Further, M2, but not M1, alveolar macrophages are increased in female mice compared to male mice after OVA sensitization and challenge.60 Recent findings suggest that female mice may skew more readily to this M2 macrophage phenotype upon allergen exposure because of increased IL-4R-α expression as well as increased ER-α expression and production of YM1 and Arg1 when compared to macrophages from male mice.60 Serum IgE is also increased in OVA sensitized and challenged female mice compared to male mice,56–58 suggesting increased activation and degranulation of mast cells and basophils in females. Although these results show the importance of estrogen signaling in allergen-mediated type 2 airway inflammation, additional studies are needed to determine if estrogen and other ovarian hormones, including progesterone, synergistically enhance allergic airway inflammation.
Ovarian hormones regulate neutrophilic inflammation and mucus production in mice
Neutrophils and IL-17A are increased in the BAL fluid in patients with more severe asthma compared to milder phenotypes of asthma.68, 69 Studies conducted by our laboratory showed that women with severe asthma had increased IL-17A producing memory Th17 cells compared to men with severe asthma. Further, we showed in mice that both 17β-estradiol and progesterone were required for enhancing Th17 cell differentiation and IL-17A production as well as IL-17A-mediated airway inflammation.70 Additionally, estradiol treatment of γδ T cells, which produce IL-17A and augment the IL-17A-mediated airway response, decreased numbers of IL-17+ T cells in the draining lymph nodes, suggesting that estrogen mediates γδ T cell migration from the lymph nodes to various tissues.71 However, studies examining the role of sex hormones on IL-17A producing γδ T cells and ILC3s, which also produce IL-17A and augment the IL-17A-mediated airway response, are limited and an important area for future research.
Estrogen and progesterone are also important in mucus production and mucociliary clearance as airway epithelial cells express estrogen receptors, predominantly ER-β, and progesterone receptors. Administration of estradiol to human airway or nasal epithelial cells increased mucin proteins, Muc5AC and Muc5B, and mucus production through various mechanisms, including an ERK1/2-dependent mechanism, an ER-β signaling dependent mechanism that increased NFATc1, and post-transcriptionally modifying fucosylation of mucin proteins.72, 73 Progesterone alone also decreased cilia beat frequency from cultured primary human airway epithelial cells compared to vehicle. However, primary human airway epithelial cells co-administered 17β-E2 with progesterone had a cilia beat frequency that was similar to vehicle treated cells.74 In summary, estrogen and progesterone signaling are important in regulating airway inflammation, AHR, and mucus production.
Androgen signaling regulates asthma pathogenesis
The role of androgens, including testosterone, have also been explored in mouse models of allergic asthma. Gonadectomized male mice, which lack testosterone, had increased eosinophils, lymphocytes, and IL-13 protein expression compared to hormonally intact, sham-operated male mice. Further, as mentioned in an above section, administration of DHEA in the chow of mice undergoing the HDM protocol decreased allergic airway inflammation compared to mice on normal chow.41 Recent studies have also reported that androgen receptor signaling regulates ILC2-mediated airway inflammation. ILC2 production of IL-13 and IL-5 was increased in female mice compared to male mice stimulated with Alternaria alternata extract (Alt Ext), HDM, or OVA.75–77 Further, male mice with a mutation in the androgen receptor (AR) that prevents AR signaling had increased Alt Ext or HDM-induced airway inflammation compared to WT male mice.75, 77 Androgens were also important in the secretion of IL-33 and TSLP, cytokines that stimulate ILC2 production of IL-5 and IL-13, because gonadectomized male mice, which lack testosterone, had increased Alt Ext-induced IL-33 and TSLP secretion compared to hormonally intact, sham-operated male mice.77 These data show that AR signaling is important in regulating type 2-mediated airway inflammation.
Androgens also regulate smooth muscle contractility. At baseline, male mice from both BALB/c and C57BL/6 strains of mice have increased AHR as well as increased smooth muscle contractility compared to female mice.78, 79 This baseline difference is attributed to androgens increasing vagal nerve responses, since gonadectomized male mice had similar levels of vagal nerve responses as intact female mice and the administration of androgens to gonadectomized male mice increased vagal nerve responses to AHR.79 Combined, these studies suggest that androgen signaling is important in decreasing airway inflammation/AHR and provide insight on the decline in asthma prevalence and incidence in adolescent males compared to females.
Conclusions
A gender disparity exists in asthma, which changes at puberty from males having the highest prevalence to females having the highest prevalence. Further, fluctuations in hormones during menstruation, pregnancy, and menopause are associated with changes in asthma symptoms. These findings suggest that sex hormones are important in asthma pathogenesis, but the mechanisms by which estrogen and/or androgen signaling regulate airway inflammation, mucus production, and airway hyperreactivity are not fully elucidated. Animal studies using genetic deletions of estrogen receptors and a mutated androgen receptor have shown that estrogen signaling promotes and androgen signaling attenuates type 2-mediated airway inflammation. Further, animal studies have shown that ovarian hormones are important for IL-17A-mediated airway inflammation. However, additional studies need to be conducted to determine mechanisms by which sex hormones are regulating neutrophil infiltration into the airway as well as regulating alarmins that are important in initiating the inflammatory response. Elucidating these pathways will provide the foundational research necessary for the development of treatment strategies, including use of hormonal contraceptives or DHEAS, for women with asthma during the various reproductive phases of life.
Acknowledgments
Funding sources: National Institute of Health R01 HL122554 and R21 AI121420.
Abbreviations
- AHR
airway hyperresponsiveness
- Alt Ext
Alternaria alternata extract
- AR
androgen receptor
- BAL
bronchoalveolar lavage
- DC
dendritic cell
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone-3-sulfate
- GPER1
G protein-couple estrogen receptor 1
- HDM
house dust mite
- IFN
interferon
- IgE
immunoglobulin E
- ILCs
innate lymphoid cells
- LPS
lipopolysaccharide
- NK
natural killer
- PHA
phytohemagglutinin
- Th
T helper cell
- TSLP
thymic stromal-derived lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Center for Disease Control USDoHaHS. National Health Interview Survey. 2015 [Google Scholar]
- 2.Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray A, Raundhal M, Oriss TB, Ray P, Wenzel SE. Current concepts of severe asthma. J Clin Invest. 2016;126(7):2394–2403. doi: 10.1172/JCI84144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Lung Association Trends in Asthma Morbidity and Mortality. 2012 [Google Scholar]
- 5.Fuseini H, Newcomb DC. Mechanisms Driving Gender Differences in Asthma. Current Allergy and Asthma Reports. 2017;17(3):19. doi: 10.1007/s11882-017-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raundhal M, Morse C, Khare A, et al. High IFN-gamma and low SLPI mark severe asthma in mice and humans. J Clin Invest. 2015;125(8):3037–3050. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wijga A, Tabak C, Postma DS, et al. Sex differences in asthma during the first 8 years of life: the Prevention and Incidence of Asthma and Mite Allergy (PIAMA) birth cohort study. J Allergy Clin Immunol. 2011;127(1):275–277. doi: 10.1016/j.jaci.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Almqvist C, Worm M, Leynaert B, working group of GALENWPG Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63(1):47–57. doi: 10.1111/j.1398-9995.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 9.Sears MR, Burrows B, Flannery EM, Herbison GP, Holdaway MD. Atopy in childhood. I. Gender and allergen related risks for development of hay fever and asthma. Clin Exp Allergy. 1993;23(11):941–948. doi: 10.1111/j.1365-2222.1993.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson CC, Peterson EL, Ownby DR. Gender differences in total and allergen-specific immunoglobulin E (IgE) concentrations in a population-based cohort from birth to age four years. Am J Epidemiol. 1998;147(12):1145–1152. doi: 10.1093/oxfordjournals.aje.a009413. [DOI] [PubMed] [Google Scholar]
- 11.Kulig M, Tacke U, Forster J, et al. Serum IgE levels during the first 6 years of life. J Pediatr. 1999;134(4):453–458. doi: 10.1016/s0022-3476(99)70203-9. [DOI] [PubMed] [Google Scholar]
- 12.Mohammad HR, Belgrave D, Kopec Harding K, Murray CS, Simpson A, Custovic A. Age, sex and the association between skin test responses and IgE titres with asthma. Pediatr Allergy Immunol. 2016;27(3):313–319. doi: 10.1111/pai.12534. [DOI] [PubMed] [Google Scholar]
- 13.Uekert SJ, Akan G, Evans MD, et al. Sex-related differences in immune development and the expression of atopy in early childhood. J Allergy Clin Immunol. 2006;118(6):1375–1381. doi: 10.1016/j.jaci.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Vink NM, Postma DS, Schouten JP, Rosmalen JG, Boezen HM. Gender differences in asthma development and remission during transition through puberty: the TRacking Adolescents’ Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol. 2010;126(3):498–504. doi: 10.1016/j.jaci.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Kynyk JA, Mastronarde JG, McCallister JW. Asthma, the sex difference. Curr Opin Pulm Med. 2011;17(1):6–11. doi: 10.1097/MCP.0b013e3283410038. [DOI] [PubMed] [Google Scholar]
- 16.Fu L, Freishtat RJ, Gordish-Dressman H, et al. Natural progression of childhood asthma symptoms and strong influence of sex and puberty. Ann Am Thorac Soc. 2014;11(6):939–944. doi: 10.1513/AnnalsATS.201402-084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castro-Rodriguez JA. A new childhood asthma phenotype: obese with early menarche. Paediatric Respiratory Reviews. 2016;18:85–89. doi: 10.1016/j.prrv.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Nicolai T, Pereszlenyiova-Bliznakova L, Illi S, Reinhardt D, von Mutius E. Longitudinal follow-up of the changing gender ratio in asthma from childhood to adulthood: role of delayed manifestation in girls. Pediatr Allergy Immunol. 2003;14(4):280–283. doi: 10.1034/j.1399-3038.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 19.Schatz M, Camargo CA., Jr The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann Allergy Asthma Immunol. 2003;91(6):553–558. doi: 10.1016/S1081-1206(10)61533-5. [DOI] [PubMed] [Google Scholar]
- 20.Howrylak JA, Fuhlbrigge AL, Strunk RC, et al. Classification of childhood asthma phenotypes and long-term clinical responses to inhaled anti-inflammatory medications. J Allergy Clin Immunol. 2014;133(5):1289–1300. 1300 e1281–1212. doi: 10.1016/j.jaci.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schatz M, Hsu JW, Zeiger RS, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2014;133(6):1549–1556. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Zoratti EM, Krouse RZ, Babineau DC, et al. Asthma phenotypes in inner-city children. J Allergy Clin Immunol. 2016;138(4):1016–1029. doi: 10.1016/j.jaci.2016.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu W, Bleecker E, Moore W, et al. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J Allergy Clin Immunol. 2014;133(5):1280–1288. doi: 10.1016/j.jaci.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefaudeux D, De Meulder B, Loza MJ, et al. U-BIOPRED clinical adult asthma clusters linked to a subset of sputum omics. J Allergy Clin Immunol. 2017;139(6):1797–1807. doi: 10.1016/j.jaci.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 26.The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur Respir J. 2003;22(3):470–477. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 27.Khusial RJ, Sont JK, Loijmans RJB, et al. Longitudinal outcomes of different asthma phenotypes in primary care, an observational study. NPJ Prim Care Respir Med. 2017;27(1):55. doi: 10.1038/s41533-017-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eliasson O, Scherzer HH, DeGraff AC., Jr Morbidity in asthma in relation to the menstrual cycle. J Allergy Clin Immunol. 1986;77(1 Pt 1):87–94. doi: 10.1016/0091-6749(86)90328-3. [DOI] [PubMed] [Google Scholar]
- 29.Gibbs CJ, Coutts II, Lock R, Finnegan OC, White RJ. Premenstrual exacerbation of asthma. Thorax. 1984;39(11):833–836. doi: 10.1136/thx.39.11.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal AK, Shah A. Menstrual-linked asthma. J Asthma. 1997;34(6):539–545. doi: 10.3109/02770909709055398. [DOI] [PubMed] [Google Scholar]
- 31.Shames RS, Heilbron DC, Janson SL, Kishiyama JL, Au DS, Adelman DC. Clinical differences among women with and without self-reported perimenstrual asthma. Ann Allergy Asthma Immunol. 1998;81(1):65–72. doi: 10.1016/S1081-1206(10)63111-0. [DOI] [PubMed] [Google Scholar]
- 32.Pauli BD, Reid RL, Munt PW, Wigle RD, Forkert L. Influence of the menstrual cycle on airway function in asthmatic and normal subjects. Am Rev Respir Dis. 1989;140(2):358–362. doi: 10.1164/ajrccm/140.2.358. [DOI] [PubMed] [Google Scholar]
- 33.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oguzulgen IK, Turktas H, Erbas D. Airway inflammation in premenstrual asthma. J Asthma. 2002;39(6):517–522. doi: 10.1081/jas-120004921. [DOI] [PubMed] [Google Scholar]
- 35.Rao CK, Moore CG, Bleecker E, et al. Characteristics of perimenstrual asthma and its relation to asthma severity and control: data from the Severe Asthma Research Program. Chest. 2013;143(4):984–992. doi: 10.1378/chest.12-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brenner BE, Holmes TM, Mazal B, Camargo CA., Jr Relation between phase of the menstrual cycle and asthma presentations in the emergency department. Thorax. 2005;60(10):806–809. doi: 10.1136/thx.2004.033928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmerman JL, Woodruff PG, Clark S, Camargo CA. Relation between phase of menstrual cycle and emergency department visits for acute asthma. Am J Respir Crit Care Med. 2000;162(2 Pt 1):512–515. doi: 10.1164/ajrccm.162.2.9910105. [DOI] [PubMed] [Google Scholar]
- 38.Juniper EF, Kline PA, Roberts RS, Hargreave FE, Daniel EE. Airway responsiveness to methacholine during the natural menstrual cycle and the effect of oral contraceptives. Am Rev Respir Dis. 1987;135(5):1039–1042. doi: 10.1164/arrd.1987.135.5.1039. [DOI] [PubMed] [Google Scholar]
- 39.Macsali F, Real FG, Omenaas ER, et al. Oral contraception, body mass index, and asthma: a cross-sectional Nordic-Baltic population survey. J Allergy Clin Immunol. 2009;123(2):391–397. doi: 10.1016/j.jaci.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 40.Nwaru BI, Sheikh A. Hormonal contraceptives and asthma in women of reproductive age: analysis of data from serial national Scottish Health Surveys. J R Soc Med. 2015;108(9):358–371. doi: 10.1177/0141076815588320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu CK, Liu YH, Chen CL. Dehydroepiandrosterone attenuates allergic airway inflammation in Dermatophagoides farinae-sensitized mice. J Microbiol Immunol Infect. 2002;35(3):199–202. [PubMed] [Google Scholar]
- 42.Liou CJ, Huang WC. Dehydroepiandrosterone suppresses eosinophil infiltration and airway hyperresponsiveness via modulation of chemokines and Th2 cytokines in ovalbumin-sensitized mice. J Clin Immunol. 2011;31(4):656–665. doi: 10.1007/s10875-011-9529-3. [DOI] [PubMed] [Google Scholar]
- 43.Espinoza J, Montano LM, Perusquia M. Nongenomic bronchodilating action elicited by dehydroepiandrosterone (DHEA) in a guinea pig asthma model. J Steroid Biochem Mol Biol. 2013;138:174–182. doi: 10.1016/j.jsbmb.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Wenzel SE, Robinson CB, Leonard JM, Panettieri RA., Jr Nebulized dehydroepiandrosterone-3-sulfate improves asthma control in the moderate-to-severe asthma results of a 6-week, randomized, double-blind, placebo-controlled study. Allergy Asthma Proc. 2010;31(6):461–471. doi: 10.2500/aap.2010.31.3384. [DOI] [PubMed] [Google Scholar]
- 45.Schatz M, Harden K, Forsythe A, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol. 1988;81(3):509–517. [PubMed] [Google Scholar]
- 46.Schatz M, Dombrowski MP, Wise R, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol. 2003;112(2):283–288. doi: 10.1067/mai.2003.1516. [DOI] [PubMed] [Google Scholar]
- 47.Murphy VE, Gibson PG, Talbot PI, Kessell CG, Clifton VL. Asthma self-management skills and the use of asthma education during pregnancy. Eur Respir J. 2005;26(3):435–441. doi: 10.1183/09031936.05.00135604. [DOI] [PubMed] [Google Scholar]
- 48.Juniper EF, Daniel EE, Roberts RS, Kline PA, Hargreave FE, Newhouse MT. Improvement in airway responsiveness and asthma severity during pregnancy. A prospective study. Am Rev Respir Dis. 1989;140(4):924–931. doi: 10.1164/ajrccm/140.4.924. [DOI] [PubMed] [Google Scholar]
- 49.Belanger K, Hellenbrand ME, Holford TR, Bracken M. Effect of pregnancy on maternal asthma symptoms and medication use. Obstet Gynecol. 2010;115(3):559–567. doi: 10.1097/AOG.0b013e3181d06945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy VE, Schatz M. Asthma in pregnancy: a hit for two. Eur Respir Rev. 2014;23(131):64–68. doi: 10.1183/09059180.00008313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Triebner K, Johannessen A, Puggini L, et al. Menopause as a predictor of new-onset asthma: A longitudinal Northern European population study. J Allergy Clin Immunol. 2016;137(1):50–57 e56. doi: 10.1016/j.jaci.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 52.Gomez Real F, Svanes C, Bjornsson EH, et al. Hormone replacement therapy, body mass index and asthma in perimenopausal women: a cross sectional survey. Thorax. 2006;61(1):34–40. doi: 10.1136/thx.2005.040881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barr RG, Wentowski CC, Grodstein F, et al. Prospective study of postmenopausal hormone use and newly diagnosed asthma and chronic obstructive pulmonary disease. Arch Intern Med. 2004;164(4):379–386. doi: 10.1001/archinte.164.4.379. [DOI] [PubMed] [Google Scholar]
- 54.Troisi RJ, Speizer FE, Willett WC, Trichopoulos D, Rosner B. Menopause, postmenopausal estrogen preparations, and the risk of adult-onset asthma. A prospective cohort study. Am J Respir Crit Care Med. 1995;152(4 Pt 1):1183–1188. doi: 10.1164/ajrccm.152.4.7551368. [DOI] [PubMed] [Google Scholar]
- 55.Baptist AP, Hao W, Karamched KR, Kaur B, Carpenter L, Song PXK. Distinct Asthma Phenotypes among Older Adults with Asthma. J Allergy Clin Immunol Pract. 2017 doi: 10.1016/j.jaip.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blacquiere MJ, Hylkema MN, Postma DS, Geerlings M, Timens W, Melgert BN. Airway inflammation and remodeling in two mouse models of asthma: comparison of males and females. Int Arch Allergy Immunol. 2010;153(2):173–181. doi: 10.1159/000312635. [DOI] [PubMed] [Google Scholar]
- 57.Takeda M, Tanabe M, Ito W, et al. Gender difference in allergic airway remodelling and immunoglobulin production in mouse model of asthma. Respirology. 2013;18(5):797–806. doi: 10.1111/resp.12078. [DOI] [PubMed] [Google Scholar]
- 58.Hayashi T, Adachi Y, Hasegawa K, Morimoto M. Less sensitivity for late airway inflammation in males than females in BALB/c mice. Scand J Immunol. 2003;57(6):562–567. doi: 10.1046/j.1365-3083.2003.01269.x. [DOI] [PubMed] [Google Scholar]
- 59.Riffo-Vasquez Y, Ligeiro de Oliveira AP, Page CP, Spina D, Tavares-de-Lima W. Role of sex hormones in allergic inflammation in mice. Clin Exp Allergy. 2007;37(3):459–470. doi: 10.1111/j.1365-2222.2007.02670.x. [DOI] [PubMed] [Google Scholar]
- 60.Keselman A, Fang X, White PB, Heller NM. Estrogen Signaling Contributes to Sex Differences in Macrophage Polarization during Asthma. J Immunol. 2017;199(5):1573–1583. doi: 10.4049/jimmunol.1601975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97(1):107–113. doi: 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 62.Campbell L, Emmerson E, Williams H, et al. Estrogen receptor-alpha promotes alternative macrophage activation during cutaneous repair. J Invest Dermatol. 2014;134(9):2447–2457. doi: 10.1038/jid.2014.175. [DOI] [PubMed] [Google Scholar]
- 63.Carey MA, Card JW, Bradbury JA, et al. Spontaneous airway hyperresponsiveness in estrogen receptor-alpha-deficient mice. Am J Respir Crit Care Med. 2007;175(2):126–135. doi: 10.1164/rccm.200509-1493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melgert BN, Oriss TB, Qi Z, et al. Macrophages: regulators of sex differences in asthma? Am J Respir Cell Mol Biol. 2010;42(5):595–603. doi: 10.1165/rcmb.2009-0016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bengtsson AK, Ryan EJ, Giordano D, Magaletti DM, Clark EA. 17beta-estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood. 2004;104(5):1404–1410. doi: 10.1182/blood-2003-10-3380. [DOI] [PubMed] [Google Scholar]
- 66.Paharkova-Vatchkova V, Maldonado R, Kovats S. Estrogen preferentially promotes the differentiation of CD11c CD11b dendritic cells from bone marrow precursors. J Immunol. 2004;172(3):1426. doi: 10.4049/jimmunol.172.3.1426. [DOI] [PubMed] [Google Scholar]
- 67.Melgert BN, ten Hacken NH, Rutgers B, Timens W, Postma DS, Hylkema MN. More alternative activation of macrophages in lungs of asthmatic patients. J Allergy Clin Immunol. 2011;127(3):831–833. doi: 10.1016/j.jaci.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 68.Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc. 2009;6(3):256–259. doi: 10.1513/pats.200808-087RM. [DOI] [PubMed] [Google Scholar]
- 69.Newcomb DC, Peebles RS., Jr Th17-mediated inflammation in asthma. Curr Opin Immunol. 2013;25(6):755–760. doi: 10.1016/j.coi.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Newcomb DC, Cephus JY, Boswell MG, et al. Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. J Allergy Clin Immunol. 2015;136(4):1025–1034 e1011. doi: 10.1016/j.jaci.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andersson A, Grahnemo L, Engdahl C, et al. IL-17-producing γδT cells are regulated by estrogen during development of experimental arthritis. Clinical Immunology. 2015;161(2):324–332. doi: 10.1016/j.clim.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 72.Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. Estradiol increases mucus synthesis in bronchial epithelial cells. PLoS One. 2014;9(6):e100633. doi: 10.1371/journal.pone.0100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi HJ, Chung YS, Kim HJ, et al. Signal pathway of 17beta-estradiol-induced MUC5B expression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2009;40(2):168–178. doi: 10.1165/rcmb.2007-0377OC. [DOI] [PubMed] [Google Scholar]
- 74.Jain R, Ray JM, Pan JH, Brody SL. Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. Am J Respir Cell Mol Biol. 2012;46(4):446–453. doi: 10.1165/rcmb.2011-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laffont S, Blanquart E, Savignac M, et al. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med. 2017;214(6):1581–1592. doi: 10.1084/jem.20161807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Warren KJ, Sweeter JM, Pavlik JA, et al. Sex differences in activation of lung-related type 2 innate lymphoid cells in experimental asthma. Ann Allergy Asthma Immunol. 2017;118(2):233–234. doi: 10.1016/j.anai.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cephus JY, Stier MT, Fuseini H, et al. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep. 2017;21(9):2487–2499. doi: 10.1016/j.celrep.2017.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Card JW, Carey MA, Bradbury JA, et al. Gender Differences in Murine Airway Responsiveness and Lipopolysaccharide-Induced Inflammation. J Immunol. 2006;177(1):621–630. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Card JW, Voltz JW, Ferguson CD, et al. Male sex hormones promote vagally mediated reflex airway responsiveness to cholinergic stimulation. Am J Physiol Lung Cell Mol PHysiol. 2007;292(4):L908. doi: 10.1152/ajplung.00407.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


