SUMMARY
The prevalence and consequences of nasal obstruction in untreated obstructive sleep apnoea patients are not known. The study objectives were to investigate the frequency of subjective and objective nasal obstruction in untreated sleep apnoea patients, and the associations with sleep and quality of life. Patients in the Icelandic Sleep Apnea Cohort were subjected to a type 3 sleep study, answered questionnaires, and had their nasal dimensions measured by acoustic rhinometry. In total, 810 patients participated (including 153 females), aged 54.5 ± 10.6 years (mean ± SD) with an apnoea/hypopnea index 44.7 ± 20.7/hour. Nocturnal nasal obstruction (≥ 3 times/week) was reported by 35% of the patients. These patients had smaller nasal dimensions measured by the minimum cross-sectional area within the smaller nasal valve (0.42 ± 0.17 cm2 vs. 0.45 ± 0.16 cm2, p = 0.013), reported more daytime sleepiness (Epworth sleepiness scale score 12.5 ± 4.9 vs. 10.8 ± 5.0; p < 0.001) and slightly lower mental quality of life than patients without nocturnal nasal obstruction. Nocturnal nasal obstruction is reported in one-third of the sleep apnoea patients and they are more likely to suffer from daytime sleepiness and slightly reduced quality of life than other sleep apnoea patients.
Keywords: Nose, breathing, nasal anatomy, sleep apnoea, questionnaire, quality of life
INTRODUCTION
Healthy people normally breathe through the nose during sleep with only 0–4% of the sleeping time reported as oral breathing (Fitzpatrick et al., 2003). Nasal obstruction is a problem reported by approximately 15% of the general population (Eriksson et al., 2011) with decreased quality of life as consequence (Hellgren, 2007). Several structural problems may cause reduced nasal patency including septal deviation, enlarged turbinates, and nasal valve collapse. Moreover, inflammatory diseases of the nasal mucosa, such as allergic and non-allergic rhinitis as well as chronic rhinosinutis with and without nasal polyposis, can cause nasal obstruction (Georgalas, 2011). We have reported recently that patients with nasal obstruction due to chronic rhinosinusitis with nasal polyps had impaired sleep quality that improved with surgery, and that the OSA risk was also decreased (Värendh et al., 2017).
OSA is a common disease affecting 25–50% of middle-aged people in the general population (Heinzer et al., 2015). Hoffstein et al. (1992) asked patients by questionnaires for side effects during continuous positive airway pressure (CPAP) treatment and reported that nasal obstruction was a common issue. However, the degree of nasal symptoms before CPAP treatment was not reported and the patients had been on CPAP for varying lengths of time. Krakow et al. (2016) retrospectively studied non-allergic nasal obstruction in patients referred to a sleep investigation, but they did not specify differences in nasal obstruction between patients with and without OSA. Furthermore, they found more daytime sleepiness in patients with non-allergic nasal obstruction. No Randomized controlled study has shown effect of nasal surgery on the AHI (Koutsourelakis et al., 2008), but one meta-analysis showed a minor effect (Wu et al., 2017). Two small meta-analyses by Ishii et al. (2015) and Li et al. (2011) concluded that nasal surgery in OSA patients with nasal obstruction leads to a decline in daytime sleepiness.
Several papers state that many OSA patients have nasal obstruction, but no well-defined, large studies have addressed the prevalence of subjective and objective nasal obstruction in these patients before initiating treatment. The pathophysiological role of the nose and the consequences of nasal obstruction for health related quality of life in OSA, are therefore not fully understood. Accordingly, the objectives of this study were to investigate the frequency of subjective and objective nasal obstruction in OSA patients while untreated, and to assess if nasal obstruction was associated with sleep-related symptoms and quality of life.
Our hypothesis was that subjective nocturnal nasal obstruction is common in OSA patients and is associated with objective narrowing of one nasal passage. Moreover we hypothesized that nasal obstruction would influence insomnia and some other aspects of sleep quality or Quality of life.
METHODS
Study design and study subjects
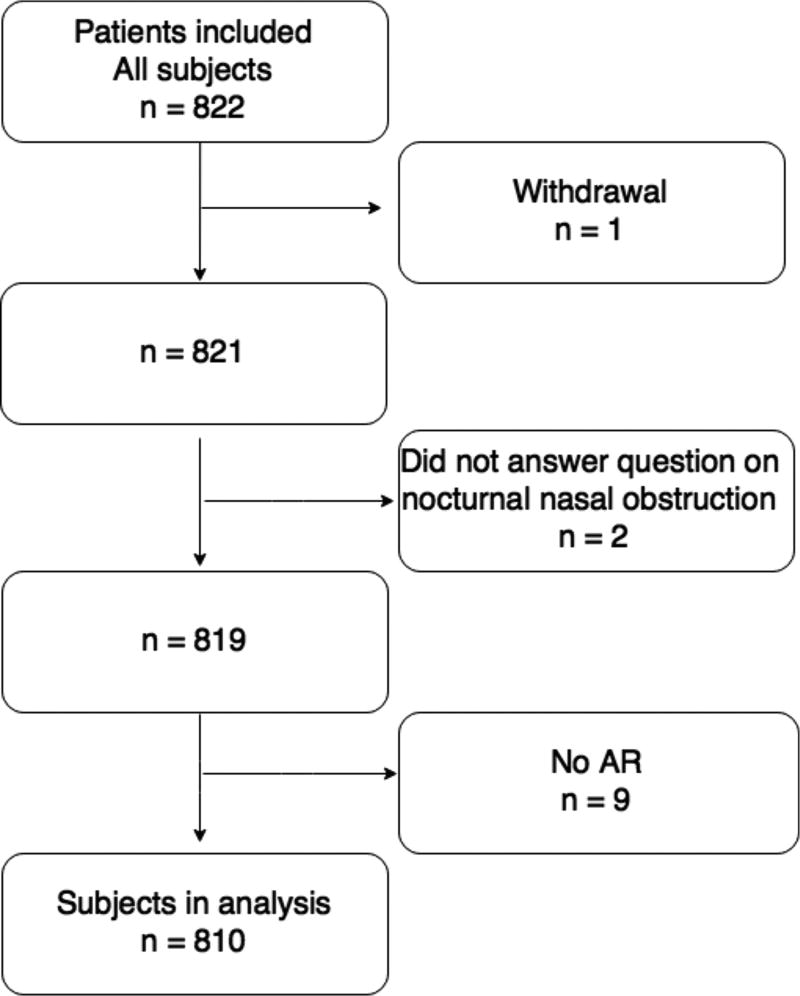
This is a cross-sectional study. The Icelandic Sleep Apnea Cohort (ISAC) is a project with the overall aim of studying the genetics of OSA. The project is performed in collaboration between the University of Iceland Reykjavik, Iceland and the University of Pennsylvania, US. The major project is divided into many smaller studies, investigating different aspects of the OSA disease. Patients diagnosed with OSA who were referred to the Department of Respiratory Medicine and Sleep, Landspitali – The National University Hospital (LSH) of Iceland, for treatment with positive airway pressure (PAP) from September 2005 to December 2009, were invited to participate in (ISAC) study. Over 90% of eligible and approached subjects (n = 822) agreed to participate and started PAP treatment following baseline assessment. Nine patients were excluded due to missing acoustic rhinometry (AR) data and one withdrew from the study (Fig. 1).
Figure 1.
Outline of the patient sample.
Furthermore, two patients were excluded since they did not answer the question about nocturnal nasal obstruction. No other exclusion or inclusion criteria were used (Arnardottir et al., 2013). The National Bioethics Committee of Iceland, the Data Protection Authority of Iceland and the Institutional Review Board of the University of Pennsylvania approved the ISAC study. All patients signed a written informed consent.
Measurements and Questionnaires
While untreated, the patients answered standardized questionnaires about their health and sleep. Nasal obstruction was evaluated with the question: “Is your nose congested at night”. The response categories were a frequency scale from 1 to 5: 1 = never or very seldom, 2 = less than once a week, 3 = once to twice a week, 4 = 3–5 times a week, and 5 = every night or almost every night of the week. A score of 4 or 5 was defined as nocturnal nasal obstruction. Patients filled out the questionnaires the same day, or for some within the days before, they were examined with Acoustic Rhinometry.
The Basic Nordic Sleep Questionnaire was used to evaluate sleep symptoms including insomnia symptoms (Partinen and Gislason, 1995). The following questions were asked: “I have difficulties falling asleep at night“ (Initial insomnia), “I wake up often during the night” (Middle insomnia), and “I wake up early and find it difficult to fall back asleep”(Late insomnia). Symptoms of insomnia were considered present if reported three times per week or more often. All questions were based on the past month´s experience. Nocturnal sweating was also considered present if reported three times per week or more often. Nocturnal gastroesophageal reflux was considered present if reported more than one time per week (Gislason et al., 2002; Emilsson et al., 2012).
Daytime sleepiness was evaluated with the Epworth Sleepiness Scale (ESS), and an ESS score of ≥ 10 was considered excessive daytime sleepiness (Johns, 1991).
Health related quality of life was examined with the Short Form Health Survey (SF-12) questionnaire (Ware et al., 1996). Scores are divided into either physical or mental health scores. Physical health is exemplified as moving a table or climbing several flights of stairs and if physical activities were limited due to compromised physical health. Concerning mental health, patients were asked if emotional issues like feeling depressed or anxious have limited their daily activities. The scores range from 0–100 (score of 100 indicate the best health related quality of life).
Patients were also asked if they were on nasal cortisone medication (yes/no).
Acoustic rhinometry (AR)
The AR technique works through an acoustic pulse sent into the nostrils. A single-impulse rhinometer (RhinoScan™ SRE2000, Rhinometrics, Assens, Denmark) was used. The method gives an anatomical description of the measurements of the nasal cavity. It compares the amplitude (representing the area) of sound waves that are reflected by the structures in the nasal cavity of an incident sound wave, this as a function of time (representative for the distance to the nasal cavity) (Clement and Gordts, 2005). Patients were examined sitting in an upright position.
The variables examined were before nasal spray: total minimal cross-sectional area in both nasal valves added together (TMCA, cm2), minimal cross-sectional area within the smaller nasal valve (either left or right) (MCA-min, cm2), total volume of left and right nasal cavity added together (TVOL, cm3), and the difference between MCA before and after nasal decongestive spray (MCA-diff, cm2). The decongestive spray, oxymethazoline (0,5 mg/ml) was given with, two puffs in each nostril after the first AR. All AR measurements were re-evaluated 2–6th of Nov 2015 by MV. Three measurements were not of sufficient quality and were not used in calculations.
Sleep study
A type 3 sleep study was conducted with an Embletta portable monitor, an Embla 12 channel system (EMBLA™; Flaga Inc., Reykjavik, Iceland) or a T3 device (Nox Medical, Reykjavik, Iceland). All systems recorded the same channels. The sleep study included nasal airflow, oxygen desaturation, pulse, chest and abdominal movements by respiratory inductive plethysmography as well as body position and activity by accelerometer.
All sleep studies were re-read by a centralized scoring laboratory at the University of Pennsylvania using the Somnologica Studio (Embla™) software and used for the analysis. More than 4 hours of a scorable oxygen saturation (SaO2) signal was needed for a sleep study to be scored. The apnoea- hypopnea index (AHI) was defined as the mean number of apnoea and hypopnea per hour of recording (upright time excluded). A hypopnea was classified as ≥ 30% decrease in the flow with ≥ 4% oxygen desaturation or ≥ 50% decrease in flow for ≥ 10 sec with a sudden increase in flow at the end of the event. The oxygen desaturation index (ODI) was defined as the number of transient drops in oxygen saturation ≥ 4% per hour of recording. OSA severity was defined as: severe OSA (AHI ≥ 30), moderate OSA (AHI 15 – 29.9), and mild OSA (AHI 5 –14.9). See previous publications for further details (Arnardottir et al., 2012).
Nasal surgery
Information on prior nasal surgery was derived from patient files including septoplasty, turbinectomy, and endoscopic surgery sometimes with polypectomy.
Statistical analysis
Nominal data were presented as frequencies and percentages without decimals. In comparisons between nominal data in independent groups, the chi-squared test was used. Fisher’s exact test was used when the expected values were insufficient for a chi-squared test. Ordinal data as well as quantitative data were presented by mean and standard deviation (± SD). Independent group differences were calculated with the Mann-Whitney U Test for 2 groups and Kruskal-Wallis test for > 2 group comparison. Post hoc tests were calculated with Mann-Whitney U Test between two groups when Kruskal-Wallis test showed a significance of < 0.05 for > 2 group comparisons. Multiple regression analyses were calculated with Enter method. SPSS 22.0 was used in all analyses. A two-sided p-value of < 0.05 was considered significant in all calculations. All p-values, significant or not, are presented in the comparisons.
RESULTS
Study sample
The characteristics of the patients are shown in Table 1, (153 females and 657 males). The mean ± SD BMI was 33.5 ± 5.7 kg/m2. A large proportion of the patients (57%) was diagnosed with hypertension, 21% were current smokers and 27% former smokers. Hypertension was more frequent in females (p < 0.05). Daytime sleepiness was common and the overall mean score for ESS was 11.7 ± 5.0 (mean ± SD). Also, the SF-12 survey demonstrated a low mental and physical health related quality of life. A larger proportion of the women reported nocturnal sweating, nocturnal gastric reflux and insomnia (both initial, middle, and late) (p < 0.05). Women also scored lower on mental and physical quality of life compared to men (p < 0.05).
Table 1.
Women had smaller nasal dimensions, more insomnia and a lower Quality of life.
| Baseline Characteristics, Nasal Dimensions, and Sleep Quality (n=810) | ||||
|---|---|---|---|---|
| All n= 810 |
Female n (%) 153 (19) |
Male n (%) 657 (81) |
p-value for sex comparison |
|
| Age (year) | 54.5 ± 10.6 | 58.6 ± 9.0 | 53.6 ± 10.8 | <0.001 |
| Current smoker | 21% | 19% | 22% | 0.58 |
| Body mass index (kg/m2) | 33.5 ± 5.7 | 34.1 ± 6.3 | 33.3 ± 5.5 | 0.19 |
| Weight (kg) | 104.3 ± 19.2 | 93.0 ± 17.2 | 106.9 ± 18.7 | <0.001 |
| Hypertension | 57 % | 67 % | 55 % | 0.03 |
| Diabetes | 11 % | 12 % | 11 % | 0.70 |
| Coronary heart disease including coronary heart occlusion, heart failure, or/and stroke | 18 % | 10 % | 20 % | 0.006 |
| Apnoea-hypopnea index | 44.8 ± 20.7 | 42.2 ± 20.0 | 45.4 ± 20.8 | 0.058 |
| Oxygen desaturation index, (4%) | 35.5 ± 20.3 | 32.6 ± 20.5 | 36.2 ± 20.2 | 0.008 |
| Nocturnal nasal obstruction ≥ 3× week | 35% | 37% | 35% | 0.68 |
| TMCA, (cm2) | 1.06 ± 0.31 | 0.94 ± 0.28 | 1.08 ± 0.31 | <0.001 |
| MCA-min, (cm2) | 0.43 ± 0.16 | 0.40 ± 0.15 | 0.44 ± 0.17 | 0.02 |
| TVOL, (cm3) | 4.10 ± 0.81 | 3.48 ± 0.65 | 4.25 ± 0.77 | <0.001 |
| Diff TMCA, (cm2) | 0.19 ± 0.21 | 0.16 ± 0.20 | 0.20 ± 0.22 | 0.02 |
| Diff MCA-min, (cm2) | 0.10 ± 0.12 | 0.08 ± 0.11 | 0.11 ± 0.12 | 0.03 |
| Diff TVOL, (cm3) | 0.21 ± 0.34 | 0.22 ± 0.31 | 0.21 ± 0.35 | 0.30 |
| Nocturnal gastroesophageal reflux ≥ 1× week | 14 % | 18 % | 13 % | 0.006 |
| Initial insomnia, ≥ 3× week | 16 % | 27 % | 13 % | <0.001 |
| Middle insomnia, ≥ 3× week | 58 % | 62 % | 57 % | <0.001 |
| Late insomnia, ≥ 3× week | 28 % | 33 % | 27 % | <0.001 |
| Nocturnal sweating ≥ 3× week | 31 % | 33 % | 31 % | <0.001 |
| Daytime sleepiness (ESS) | 11.7 ± 5.0 | 11.2 ± 5.2 | 11.8 ± 5.0 | 0.23 |
| Mental quality of life (SF-12) | 48.3 ±10.9 | 46.8 ± 11.1 | 48.6 ± 10.8 | 0.048 |
| Physical quality of life (SF-12) | 40.2 ± 10.9 | 35.5 ±10.9 | 41.3 ±10.6 | <0.001 |
ESS: Epworth sleepiness scale
Diff MCA-min: Difference between MCA-min before and after nasal decongestant spray
Diff TMCA: Difference between TMCA before and after nasal decongestant spray
Diff TVOL: Difference between before and after nasal decongestant spray
MCA-min: Minimal cross-sectional area within the smallest nostril of either left or right before decongestant spray
TMCA: Total minimal cross-section area in the nose, left and right nostril combined before nasal decongestant spray
TVOL: Total volume of left and right nasal volume combined before nasal decongestant spray
SF-12: The 12-Item Short Form Health Survey (SF-12) a smaller version of the SF-36v2 Health Survey
Significance in bold.
Numbers given as mean ± SD if not specified and p-values when comparing mean values was calculated with Mann-Whitney U test.
The chi-squared test was used comparisons between nominal data in independent groups (here show in %).
A majority of the patients (73%) had severe OSA, 23% had moderate OSA, and 3% had mild OSA.
Prevalence of subjective and objective nasal obstruction in OSA
Overall, 65% reported nasal obstruction during the night once per week or more often and 35% ≥ 3 times per week. No differences were seen in OSA severity, as measured by the AHI, between the three groups (p = 0.57) (Table 2).
Table 2.
Patients with frequent nocturnal nasal obstruction were slightly more likely to have one smaller nasal valve. No other differences were found between the groups.
| Nocturnal Nasal Obstruction (n = 810) | ||||
|---|---|---|---|---|
| Never n = 285 |
1–2 × week n = 240 |
≥ 3 × week n = 285 |
p-value | |
| Age (year) | 55.4 ± 10.4 | 53.7 ± 10.3 | 54.1 ± 11.0 | 0.16 |
| Current smoker | 21% | 27 % | 20 % | 0.85 |
| Body mass index, (kg/m2) | 33.7 ± 5.8 | 33.1 ± 5.6 | 33.6 ± 5.6 | 0.30 |
| Apnoea-hypopnea index | 43.5 ± 10.0 | 45.8 ± 20.5 | 45.2 ± 21.5 | 0.57 |
| Oxygen desaturation index | 34.3 ± 19.8 | 36.1 ± 20.0 | 36.3 ± 20.9 | 0.54 |
| TMCA, (cm2) | 1.11 ± 0.30 | 1.07 ± 0.30 | 1.03 ± 0.32 | 0.19 |
| MCA-min, (cm2) | 0.45 ± 0.16 | 0.44 ± 0.17 | 0.42 ± 0.17 | 0.04** |
| TVOL, (cm3) | 4.10 ± 0.83 | 4.13 ± 0.78 | 4.08 ± 0.81 | 0.78 |
| Diff TMCA, (cm2) | 0.17 ± 0.21 | 0.21 ± 0.19 | 0.20 ± 0.23 | 0.11 |
Diff MCA-min: Difference between MCA-min before and after nasal decongestant spray
Diff TMCA: Difference between TMCA before and after nasal decongestant spray
Diff TVOL: Difference between before and after nasal decongestant spray
MCA-min: Minimal cross-sectional area within the smallest nostril of either left or right before decongestant spray
TMCA: Total minimal cross-section area in the nose, left and right nostril combined before nasal deobstruction spray
TVOL: Total volume of left and right nasal volume combined before nasal decongestant spray
Significance in bold.
Numbers given as mean ± SD if not specified.
Independent group differences were calculated with Kruskal-Wallis test for >2 group comparison of mean values.
The chi-squared test was used comparisons between nominal data in independent groups (here show in %).
The p-value for post hoc test: 0.013 comparing the groups “Never” and “≥ 3 × week”.
Nasal cavity dimensions assessed by AR showed mean values of TMCA 1.06 ± 0.31, MCA-min 0.43 ± 0.16 and TVOL 4.10 ± 0.81. TMCA and TVOL were significantly smaller in female patients than in males (p<0.05) but no sex differences were found in subjective nocturnal nasal obstruction see Table 1.
Sleep related symptom and nocturnal nasal obstruction
We divided the patients into three groups, depending on their subjective nocturnal nasal obstruction symptoms (Table 2). Women and men were equally distributed between the three groups (p = 0.45). There was a difference between the groups in MCA-min, assessed by AR, with the smallest mean value of 0.42 ± 0.17 cm2 in the nocturnal nasal obstruction group compared to 0.45 ± 0.16 cm2 in the group without any nocturnal nasal obstruction (post hoc analysis between “never nasal obstruction” and “> 3 × week” p = 0.013) (Table 2).
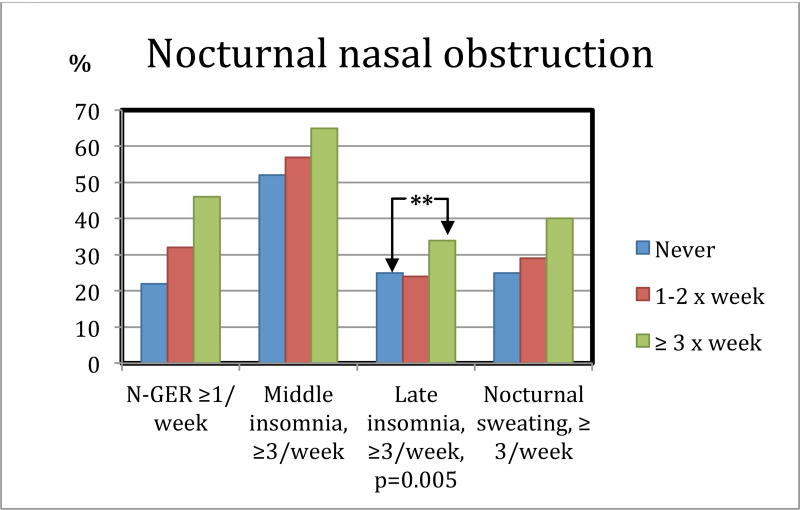
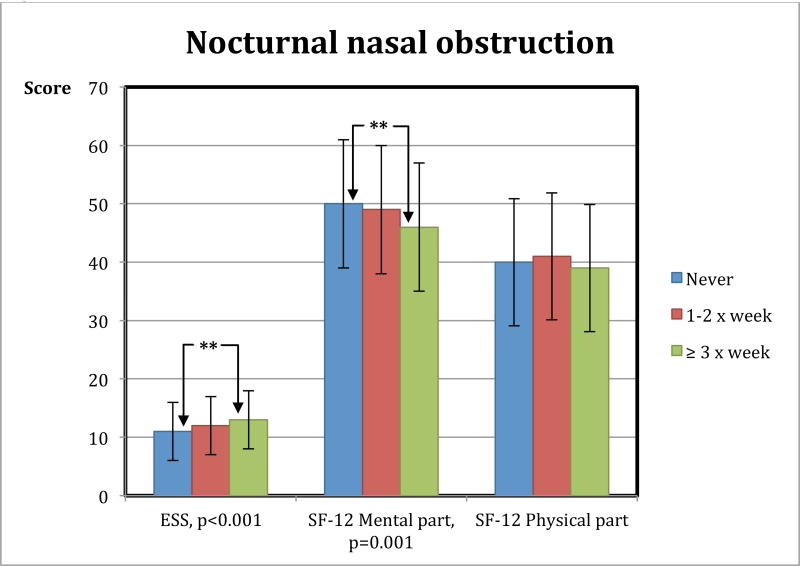
Late insomnia was reported by a larger proportion of the patients with nocturnal nasal obstruction > 3×week compared to the group without (post hoc: p = 0.013) (Fig. 2, p-value 0.005 is calculated between all three groups). 65% of patients with nocturnal nasal obstruction ≥ 3× week have middle insomnia. Patients with nocturnal nasal obstruction also had more daytime sleepiness compared to patients without any nocturnal nasal obstruction (ESS 12.5 ± 4.9 vs. 10.8 ± 5.0, post hoc comparison p < 0.001) (Fig. 3). Mental quality of life was reported lower in the group with nocturnal nasal obstruction compared to those without obstruction (46.4 ± 11.4 vs. 49.8 ± 10.5, p < 0.001) (Fig. 3).
Figure 2.
Patients with nocturnal nasal obstruction are more likely to have late insomnia and 65% of patients with nocturnal nasal obstruction ≥ 3× week have middle insomnia. **=significance between the group of patients never nocturnal nasal obstruction and the group with nocturnal nasal obstruction ≥ 3× week. N-GER: Nocturnal Gastroesophageal reflux.
Figure 3.
Patients with more nocturnal nasal obstruction have more daytime sleepiness and lower score on Quality of life, mental part. Figure describing nocturnal nasal obstruction and daytime sleepiness (ESS: Epworth sleepiness scale) and Quality of life measured by SF-12. ** = significance difference between the groups never nocturnal nasal obstruction and the group with nocturnal nasal obstruction ≥ 3× week.
A multiple regression analysis was performed to predict subjective nocturnal nasal obstruction. The differences found in subjective nocturnal nasal obstruction remains significant after adjusting for sex, BMI, nocturnal gastroesophageal reflux and smoking.
Nasal surgery
A total of 86 patients had nasal surgery prior to PAP treatment and prior to being included in the study. Some patients underwent more than one kind of surgery and 18 patients underwent nasal surgery on two occasions. The different surgeries were: septal deviation surgery (61), turbinoplasty (37), endoscopic sinus surgery and polypectomy (11). As a group, these patients reported significantly more frequent nasal obstruction compared to the others, despite the surgery (47% vs. 34%, respectively, p = 0.02), but no differences were found in OSA severity or measured nasal dimensions (Table 4).
Medication
Concerning medication with possible impact on nasal obstruction the following results were found: 37 patients used nasal steroids, 14 patients systemic steroids, and 6 patients oral antihistamines. A total of 55 patients had one or more of these medications. There were, however, no differences between the users of these drugs and non-users in terms of AHI (p=0.8), TMCA (p=0.34), MCA-min (p=0.77) or TVOL (p=0.66).
DISCUSSION
Summary
The present study demonstrates that the prevalence of reported nocturnal nasal obstruction was 35% in untreated OSA patients. Patients with nocturnal nasal obstruction were more likely to have one small nasal valve area (MCA-min). Moreover, OSA patients with nasal obstruction slightly more often reported symptoms of late insomnia, daytime sleepiness, and had generally a lower mental quality of life compared to OSA patients without nasal obstruction.
Prevalence of nocturnal nasal obstruction
The present study revealed a nocturnal nasal obstruction prevalence of almost 65% once per week or more often and 35% ≥ 3 times in treatment naïve OSA patients. The prevalence of nasal obstruction in OSA has, to our knowledge, not previously been described. A previous, retrospective study reported a prevalence of non-allergic nasal obstruction of 45% in unselected sleepy patients (Krakow et al., 2016) The Wisconsin Sleep Cohort reported nasal obstruction to be a risk factor for apnoeas, hypopnoeas and habitual snoring (Young et al., 1997). However, they did not report a prevalence of nasal obstruction in the patients with OSA.
Acoustic rhinometry
The minimal cross section area within the smallest nasal valve of either left or right side, MCA-min, was the only parameter that was found to differ between OSA patients with and without nasal obstruction. In contrast to our results Vidigal et al. (2013) used AR to study the nasal geometry in a small sample of OSA patients and a control group. They found more nasal symptoms in OSA patients compared to controls, but no difference in AR values.
However they did not investigate the smallest nasal valve compared to subjective obstruction. There are at least two parts of nasal obstruction. The first part is the structural part consisting of skeletal bone and cartilage and the second part is the swollen mucosa causing congestion. The later is varying with the nasal cycle, the normal ‘corporo-nasal’ reflex, and possibly a separate airflow cycle within each nasal valve (Kahana-Zweig et al. 2016). These normal events could be an explanation to the influence of MCA-min on subjective nasal obstruction in the current study. If one side of the nose is structurally obstructed, subjective nasal obstruction will increase if subjects lay on the other side. Then the more open (lower) half of the nose becomes congested, and the more resistant (upper) half of the nose will not be patent (Pevernagie D. et al. 2005).
OSA severity between the groups
No differences were observed in OSA severity between the patients with and without nocturnal nasal obstruction. No other large study has, to our knowledge, investigated the relation between AHI and nocturnal nasal obstruction. There are conflicting results concerning OSA severity and impact of nasal surgery. Two previously mentioned meta-analyses by Ishii et al. (2015) and Li et al. (2011) including small studies, and only randomized and controlled. These studies showed no improvement on OSA severity with nasal surgery. One small meta analysis of Wu et al. (2017) showed an improvement of OSA severity with surgery.
Insomnia
Late insomnia was reported more often by patients with nocturnal obstruction compared to OSA patients without nasal obstruction, (p = 0.01) despite similar OSA severity. This finding is in line with a previous study that reported more insomnia problems in patients with undifferentiated sleep problems and nasal obstruction than in patients without these problems. However, it was a retrospective questionnaire study and the patients were not diagnosed with OSA (Krakow et al., 2016). It is possible that nocturnal nasal obstruction has an influence on late insomnia in OSA patients.
Daytime sleepiness
Daytime sleepiness was found to be more slightly more pronounced in OSA patients with nocturnal nasal obstruction compared to patients without obstruction (p < 0.001). With a mean value of 12.5 ± 4.9, the sleepiness will most likely have an impact on everyday life. Our results are therefore in agreement with previous studies showing that nasal obstruction has an impact on daytime sleepiness (Värendh et al., 2017; Ishii et al., 2015; Li et al., 2011).
Quality of life
Mental quality of life in patients with nasal obstruction was found to be slightly lower than in other OSA patients (p < 0.001) and lower compared to normal reference values for healthy adults (Hilberg, 2002). This matter has, to our knowledge, not been studied before. A possible explanation for the decreased quality of life is that the patients are influenced by their nasal obstruction, which is associated with more insomnia complaints and daytime sleepiness. Nocturnal nasal obstruction might increase the problems of insomnia and daytime sleepiness, which influences quality of life.
Medication
Using oral antihistamines, nasal or systemic corticosteroids did not have an impact on nasal dimensions.
Strengths and limitations of the study
A major strength of this study is the large, well defined clinical cohort of OSA patients in ISAC and that the nose is examined both subjectively and objectively.
AR is a valid technique so long as the limitations are understood (Clement and Gordts, 2005; Arnardottir et al., 2016). The method describes anatomical structures, but does not give extensive information about nasal function. AR is conducted in an upright position during the daytime and therefore it is difficult to draw conclusions about nasal dimensions during sleep. However, an anatomical description of OSA patients prior to treatment is lacking in the literature and is of interest and importance.
Sleep was recorded with a type 3 sleep study without EEG and therefore it was not possible to study arousals. However, a type 3 sleep study is clinically acceptable to diagnose OSA (Berry et al., 2015; Mols et al., 2009).
A limitation to the objective evaluation of insomnia in this study is that polysomnography was not used.
The nasal questions used were not validated, and additional validated questionnaires like SNOT-22, would probably have provided a better evaluation of the patients symptoms. Questionnaires have limitations, but subjective symptoms of patients are very valuable and important. It is difficult to obtain objective measurements in some issues in real life circumstances and the patient complaint indicates what is affecting her/his quality of life.
A control group of healthy individuals would have been of major interest but to gather such a large group of non-sleep-apnoea patients of comparable age, sex, and weight remains a future task.
Clinical implications
The findings in this study show that it is of great importance to increase the awareness of clinicians of the high incidence of nasal obstruction is OSA patients and how much it influences their daily life.
CONCLUSION
Nocturnal nasal obstruction was found in over one-third of the OSA patients. Subjects with nocturnal nasal obstruction had on average one nasal valve with a smaller minimum cross section area. Furthermore, measures of late insomnia, daytime sleepiness, and mental quality of life were slightly worse compared to patients without nasal obstruction.
Table 3.
Former nasal surgery had no impact on AHI or nasal dimensions. A larger proportion of patients with previous nasal surgery were reporting nocturnal nasal obstruction.
| Former Nasal Surgery | |||
|---|---|---|---|
| Nasal surgery n (%) 86 (11) |
No nasal surgery n (%) 724 (89) |
p-value | |
| Apnoea-hypopnea index | 40.7 ± 16.2 | 45.3 ± 21.1 | 0.12 |
| TMCA, (cm2) | 1.05 ± 0.30 | 1.05 ± 0.31 | 0.99 |
| MCA-min, (cm2) | 0.43 ± 0.16 | 0.43 ± 0.17 | 0.95 |
| TVOL, (cm3) | 4.06 ± 0.75 | 4.11 ± 0.81 | 0.84 |
| Nocturnal nose obstruction | 47 % | 34 % | 0.02 |
MCA-min: Minimal cross-sectional area within the smallest nostril of either left or right before decongestant spray
TMCA: Total minimal cross-section area in the nose, left and right nostril combined before nasal decongestant spray
TVOL: Total volume of left and right nasal volume combined before nasal decongestant spray
Significance in bold.
Numbers given as mean ± SD if not specified and p-values when comparing mean values was calculated with Mann-Whitney U test.
The chi-squared test was used comparisons between nominal data in independent groups (here show in %).
Acknowledgments
We would like to thank Sigrun Gudmundsdottir and Lovisa Gudmundsdottir for data collection and Eyjolfur Sigurdsson for statistical preparations. A special thank you to prof. Robin L. Anderson, Peter MacCallum Cancer Centre and the University of Melburne for English proofreading and valuable review on the manuscript. The authors are also thankful to Allan I. Pack, Greg Maislin, Bethany Staley, Brendan Keenan and the other staff at the Centre for Sleep and Circadian Neurobiology at the University of Pennsylvania who helped analyse the sleep studies and other data of the cohort.
FUNDING
NIH supported project, grant number: R01HL072067.
ABBREVIATIONS
- AHI
Apnoea-hypopnea index
- AR
+Acoustic rhinometry
- BMI
Body mass index
- CPAP
Continuous positive airway pressure
- CT
Computerized tomography
- ESS
Epworth sleepiness scale
- Diff MCA-min
Difference between non-decongested and congested Minimal cross-sectional area within one nasal valve
- Diff TMCA
Difference between non-decongested and congested Total minimal cross-section area in the nose, left and right nasal valve combined
- Diff TVOL
Difference between non-decongested and congested Total volume of left and right nasal volume combined
- ISAC
The Icelandic Sleep Apnea Cohort
- LSH
Landspitali – The National University Hospital in Iceland
- MCA
Minimal cross-sectional area within one nasal valve, before nasal decongestant spray
- MCA-min
Minimal cross-sectional area within the smaller nasal valve (either left or right), before nasal decongestant spray
- MRI
Magnetic resonance imaging
- NAR
Nasal airflow resistance
- ODI
Oxygen desaturation index
- OSA
Obstructive sleep apnoea
- PAP
Positive airway pressure
- RCT
Randomized Controlled Trial
- RDI
Respiratory disturbance index
- SDB
Sleep disordered breathing
- SF-12
The 12-Item Short Form Health Survey (SF-12) a smaller version of the SF-36v2 Health Survey.
- TMCA
Total minimal cross-section area in the nose, left and right nasal valve combined, before nasal decongestant spray
- TVOL
Total volume of left and right nasal volume combined before nasal decongestant spray
- UPPP
Uvulopalatopharyngoplasty
Footnotes
Conflict of interests:
Maria Värendh: No conflict of interest.
Morgan Andersson: No conflict of interest.
Erla Bjørnsdottir: No conflict of interest.
Harald Hrubos-Strøm: Received payments for lectures from the companies NOX, TAKEDA and RESmed outside the submitted work.
Arne Johannisson: No conflict of interest.
Erna Sif Arnardottir: Part-time consultant for Nox Medical, Reykjavik, Iceland unrelated to manuscript, outside the submitted work.
Thorarinn Gislason: No conflict of interest.
Sigurdur Juliusson: No conflict of interest.
Author contributorship:
Maria Värendh: Was part of the designing of the calculations, did statistical calculations, took part in evaluating the results, and drafted the paper. Participated in all revisions of the paper with the co-authors.
Morgan Andersson: Contributed to the designing the calculations, took part in evaluating the results, and reviewed the manuscript. Participated in all revisions of the paper with the co-authors.
Erla Bjørnsdottir: Contributed to the designing the calculations, took part in evaluating the results, and reviewed the manuscript. Participated in all revisions of the paper with the co-authors.
Harald Hrubos-Strøm: Contributed to the designing the calculations, took part in evaluating the results, and reviewed the manuscript. Participated in all revisions of the paper with the co-authors.
Arne Johannisson: Contributed to the design of statistical analysis. Did statistical calculations and analysis.
Erna Sif Arnardottir: Designed the study. Contributed to the designing the calculations, took part in evaluating the results, and reviewed the manuscript. Participated in all revisions of the paper with the co-authors.
Thorarinn Gislason: Designed the study. Contributed to the in designing the calculations, took part in evaluating the results, and reviewed the manuscript. Participated in all revisions of the paper with the co-authors.
Sigurdur Juliusson: Designed the study. Paticipated in datacollection. Contributed to the designing the calculations, took part in evaluating the results, and reviewed the manuscript. Participated in all revisions of the paper with the co-authors.
References
- Arnardottir ES, Janson C, Bjørnsdottir E, et al. Nocturnal sweating - a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013;3:e002795. doi: 10.1136/bmjopen-2013-002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnardottir ES, Maislin G, Schwab RJ, et al. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the Icelandic Sleep Apnea Cohort. Sleep. 2012;35:921–32. doi: 10.5665/sleep.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnardottir ES, Verbraecken J, Gonçalves M, et al. Variability in recording and scoring of respiratory events during sleep in Europe: a need for uniform standards. J Sleep Res. 2016;25:144–57. doi: 10.1111/jsr.12353. [DOI] [PubMed] [Google Scholar]
- Berry RB, Gamaldo CE, Harding SM, et al. AASM Scoring Manual Version 2.2 Updates: New Chapters for Scoring Infant Sleep Staging and Home Sleep Apnea Testing. J Clin Sleep Med. 2015;11:1253–4. doi: 10.5664/jcsm.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement PA, Gordts F. Consensus report on acoustic rhinometry and rhinomanometry. Rhinology. 2005;43:169–79. [PubMed] [Google Scholar]
- Emilsson OI, Janson C, Benediktsdóttir B, Júlíusson S, Gíslason T. Nocturnal gastroesophageal reflux, lung function and symptoms of obstructive sleep apnea: Results from an epidemiological survey. Respir Med. 2012;106:459–66. doi: 10.1016/j.rmed.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Eriksson J, Ekerljung L, Pullerits T, et al. Prevalence of Chronic Nasal Symptoms in West Sweden: Risk Factors and Relation to Self-Reported Allergic Rhinitis and Lower Respiratory Symptoms. Int Arch Allergy Immunol. 2011;154:155–63. doi: 10.1159/000320230. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick M, Driver H, Chatha N, Voduc N, Girard A. Partitioning of inhaled ventilation between the nasal and oral routes during sleep in normal subjects. J Appl Physiol (1985) 2003;94:883–90. doi: 10.1152/japplphysiol.00658.2002. [DOI] [PubMed] [Google Scholar]
- Georgalas C. The role of the nose in snoring and obstructive sleep apnoea: an update. Eur Arch Otorhinolaryngol. 2011;268:1365–73. doi: 10.1007/s00405-010-1469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gislason T, Janson C, Vermeire P, et al. Respiratory symptoms and nocturnal gastroesophageal reflux: a population-based study of young adults in three European countries. Chest. 2002;121:158–63. doi: 10.1378/chest.121.1.158. [DOI] [PubMed] [Google Scholar]
- Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–8. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellgren J. Quality of life in nonallergic rhinitis. Clin Allergy Immunol. 2007;19:383–7. [PubMed] [Google Scholar]
- Hilberg O. Objective measurement of nasal airway dimensions using acoustic rhinometry: methodological and clinical aspects. Allergy. 2002;57(Suppl 70):5–39. doi: 10.1046/j.0908-665x.2001.all.doc.x. [DOI] [PubMed] [Google Scholar]
- Hoffstein V, Viner S, Mateika S, Conway J. Treatment of obstructive sleep apnea with nasal continuous positive airway pressure. Patient compliance, perception of benefits, and side effects. Am Rev Respir Dis. 1992;145:841–5. doi: 10.1164/ajrccm/145.4_Pt_1.841. [DOI] [PubMed] [Google Scholar]
- Ishii L, Roxbury C, Godoy A, Ishman S, Ishii M. Does Nasal Surgery Improve OSA in Patients with Nasal Obstruction and OSA? A Meta-analysis. Otolaryngol Head Neck Surg. 2015;153:326–33. doi: 10.1177/0194599815594374. [DOI] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Kahana-Zweig R, Geva-Sagiv M, Weissbrod A, Secundo L, Soroker N, Sobel N. Measuring and Characterizing the Human Nasal Cycle. PLoS One. 2016;11:e0162918. doi: 10.1371/journal.pone.0162918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsourelakis I, Georgoulopoulos G, Perraki E, Vagiakis E, Roussos C, Zakynthinos SG. Randomised trial of nasal surgery for fixed nasal obstruction in obstructive sleep apnoea. Eur Respir J. 2008;31:110–7. doi: 10.1183/09031936.00087607. [DOI] [PubMed] [Google Scholar]
- Krakow B, Foley-Shea M, Ulibarri VA, McIver ND, Honsinger R. Prevalence of potential nonallergic rhinitis at a community-based sleep medical center. Sleep Breath. 2016;20:987–93. doi: 10.1007/s11325-016-1322-3. [DOI] [PubMed] [Google Scholar]
- Li HY, Wang PC, Chen YP, Lee LA, Fang TJ, Lin HC. Critical appraisal and meta-analysis of nasal surgery for obstructive sleep apnea. Am J Rhinol Allergy. 2011;25:45–9. doi: 10.2500/ajra.2011.25.3558. [DOI] [PubMed] [Google Scholar]
- Mols M, Pelle AB, Kupper N. Normative data of the SF-12 health survey with validation using postmyocardial infarction patients in the Dutch population. Qual Life Res. 2009;18:403–414. doi: 10.1007/s11136-009-9455-5. [DOI] [PubMed] [Google Scholar]
- Partinen M, Gislason T. Basic Nordic Sleep Questionnaire (BNSQ): a quantitated measure of subjective sleep complaints. J Sleep Res. 1995;4:150–5. doi: 10.1111/j.1365-2869.1995.tb00205.x. [DOI] [PubMed] [Google Scholar]
- Pevernagie DA, De Meyer MM, Claeys S. Sleep, breathing and the nose. Sleep Med Rev. 2005;9:437–51. doi: 10.1016/j.smrv.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhao G, Li Y, et al. Apnea-hypopnea index decreased significantly after nasal surgery for obstructive sleep apnea: A meta-analysis. Medicine (Baltimore) 2017;96:e6008. doi: 10.1097/MD.0000000000006008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T, Finn L, Kim H. Nasal obstruction as a risk factor for sleep-disordered breathing. The University of Wisconsin Sleep and Respiratory. Research Group. J Allergy Clin Immunol. 1997;99:S757–62. doi: 10.1016/s0091-6749(97)70124-6. [DOI] [PubMed] [Google Scholar]
- Vidigal TA, Haddad FLM, Gregorio LC, Poyares D, Tufik S, Bittencourt LRA. Subjective, anatomical, and functional nasal evaluation of patients with obstructive sleep apnea syndrome. Sleep Breath. 2013;17:427–433. doi: 10.1007/s11325-012-0667-5. [DOI] [PubMed] [Google Scholar]
- Värendh M, Johannisson A, Hrubros-Strøm H, Andersson M. Sleep quality improves with endoscopic sinus surgery in patients with chronic rhinosinusitis and nasal polyposis. Rhinology. 2017;55:45–52. doi: 10.4193/Rhin16.065. [DOI] [PubMed] [Google Scholar]