Abstract
This article reviews the key biochemical mechanisms that govern O2 transport, NO scavenging, and oxidative degradation of acellular hemoglobin and how these ideas have been used to try to develop strategies to engineer safer and more effective hemoglobin-based oxygen carriers (HBOCs). Significant toxicities due to acellular hemoglobin (Hb) have been observed after the administration of HBOCs or after the lysis of red cells, and include: (a) rapid clearance and kidney damage due to dissociation into dimers, haptoglobin binding, and macrophage activation; (b) early O2 release leading to decreased tissue perfusion in capillary beds; (c) interference with endothelial and smooth muscle signaling due to nitric oxide (NO) scavenging; (d) autooxidization of heme iron followed by production of reactive oxygen species; and (e) iron overload symptoms due to hemin loss, globin denaturation, iron accumulation, and further inflammation. Protein engineering can be used to mitigate some of these side effects but requires an in-depth mechanistic understanding of the biochemical and biophysical features of hemoglobin that regulate quaternary structure, O2 affinity, NO dioxygenation, and resistance to oxidation, hemin loss, and unfolding.
Keywords: blood substitutes, hemoglobin-based oxygen carriers (HBOCs), hemoglobin toxicity, oxygen affinity, NO scavenging, autooxidation, hemin loss, globin unfolding
INTRODUCTION
The objective of this article is to review the biochemical strategies that have been used to try to engineer safer and more effective acellular hemoglobin-based oxygen carriers (HBOCs). In the preceding article on the toxicity of hemoglobin-based oxygen carriers (HBOCs), Alayash (1) reviewed the history, development, and side effects of some key commercial products, with an emphasis on oxidative reactions of the heme group. Our focus is on the fundamental biochemical mechanisms that govern clearance, O2 transport, NO scavenging, and denaturation of acellular hemoglobin, all of which determine the physiological activity and toxicities of HBOC products. These processes are described in molecular detail to allow the development of strategies to inhibit the reactions that lead to serious side effects.
The use of hemoglobin-based transfusion alternatives to donated blood has been explored since the 1930s (2–4). The development of these blood substitutes has matured along with a greater understanding of the chemistry and physiology of erythrocytes and hemoglobin. Initial tests focused on replacing whole blood in animal models with acellular hemoglobin-based oxygen carriers (HBOCs). Identification of the different physiological roles of whole blood informed the requirements for distinct transfusing agents. For example, saline solutions were identified early on as substitutes for the volume and isotonic properties of blood that are important for maintaining heart pumping. When the role of hemoglobin in oxygen transport was fully established, it became apparent that acellular hemoglobin could be used for efficient oxygen transport during transfusion (3).
The intrinsic value of acellular blood substitutes is that these products can potentially eliminate clinically significant problems associated with using donated blood (5, 6). First, acellular hemoglobin solutions do not contain antigens and require no blood typing. Second, production of blood substitutes can be done in vitro under sterile laboratory conditions, reducing or eliminating the risk of transmitting disease or infection. Third, the shelf life of most acellular HBOCs is dramatically longer than that of donated blood (in some cases years versus one month), which is crucial for production, storage, and transport to the scene of trauma. Fourth and perhaps most important, large supplies of HBOCs can be maintained and stockpiled, eliminating shortages and transfers between institutions during large-scale emergencies.
Unfortunately, there are significant problems associated with the use of acellular hemoglobin products (7–12). Initial studies with acellular hemoglobin began in the early 20th century. In these experiments, hemoglobin was purified from cell lysates and injected directly into patients (2, 4). Purified acellular hemoglobin was able to provide oxygen delivery without antigenic reactions, but not without significant side effects. Injection of unmodified acellular hemoglobin into animals and humans resulted in the rapid appearance of high levels of hemoglobin in urine, hypertension, and renal failure (2–4).
DISSOCIATION OF ACELLULAR HEMOGLOBIN INTO DIMERS
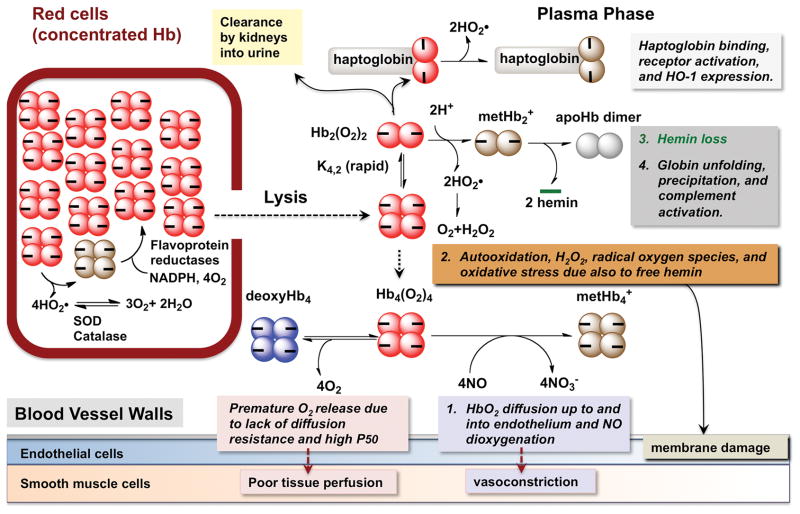
A summary of the causes of plasma hemoglobin toxicity is given in Fig. 1. A list of some of the acellular hemoglobin based products, most of which have undergone human trials, is given in Table 1 along with some of their properties. In general, it was difficult to obtain blood pressure effects, autooxidation rates, and hemin loss kinetic parameters under comparable conditions for all these products. As result care should be taken when making comparisons and the primary literature in the footnotes should be examined.
Fig. 1.
Possible causes of acellular hemoglobin toxicity
Table 1. Summary of O2 binding, blood pressure effects, autooxidation, and hemin loss properties of HBOC products that have been used in human trials.
Except for the P50 values, most of these parameters are under reported, particularly the rates of autooxidation and hemin loss. Thus, the values for the last four columns may be approximate. The companies should be consulted for exact values. ΔMAP represents the increase in mean arterial pressure in 10% topload or 50% isovolemic exchange experiments with rats or guinea pigs. The rate constants for autooxidation and hemin loss were normally measured at 37 °C, near physiological pH. NR indicates not reported.
| Hemoglobin Product Name (other names) | Modification | Company | Oxygen P50 mmHg | ΔMAP mmHg (Top load) | kautox initial h−1 | k-H (β) h−1 | k-H (α) h−1 |
|---|---|---|---|---|---|---|---|
| Stroma-free HbA Tetramer (Dimer) | None | 8a | ≥25b | ~0.001c (~0.03) | 1.5 (15)c | 0.3 (0.6)c | |
| HemAssist@ (DCLHb, ααHb) | Human HbA chemically crosslinked tetramer | Baxter International | 32d,e | ~30f | NR | NR | NR |
| Polyheme@ (SFH-P) | Glutaraldehyde crosslinked HbA with covalently attached pyridoxal-phosphate | Northfield Laboratories | 20 – 22d, 26–30e | 30f | 0.007g | 8.1g | 1.2g |
| Hemopure@ (HBOC-201) | Glutaraldehyde crosslinked bovine Hb | Biopure | 32–38d, 40e | 15h | NR | 4.3i | 0.4i |
| Hemospan@ (MP4) | Pegylated human HbA | Sangart | 10e | ≤5j | 0.021g | 40g | 1.3g |
| Optro1.1@ | Crosslinked Recombinant HbA with β N108K mutation | Somatogen | 33k | ≥20k | NR | 52g | 1.5g |
| Sanguinate@ | Pegylated bovine Hb | Prolong | 12l | ≤10m | NR | NR | NR |
| rHb2.0 (rHb3011) | Crosslinked Recombinant HbA with (βV67W-αH58Q/L28W) | Baxter Hemoglobin Therapeutics | 31–33n | ≤5n | 0.41o | 90o | 90o |
Maillet et al. 2008 (38)
McCarthy et al. 2001 (84)
The initial kautox value for native HbA was determined in this report. The values of k-hemin for tetrameric (and dimeric) HbA were taken from Hargrove et al.1997 (77)
Chen et al. (85)
Natanson et al. (86)
Gulati et al.1995 (87)
Vandegriff et al. 2006 (88)
Katz et al. 2010 (89)
Unpublished data from Olson, J.S. and Light, W. R., 2008.
Vandegriff et al.2003 (90)
Raat et al. 2005 (47)
Abuchowski 2017 (91)
Abuchowski 2010 (92)
Hermann et al. 2007 (93)
Varnado et al. 2013(5)
Bunn et al. (13, 14) identified the dissociation of tetrameric hemoglobin into dimers as the probable cause of renal toxicity and rapid clearance. Hemolysis due to red cell aging, chronic inflammation, and exposure to chemical oxidants shows similar toxicities (15, 16). When human adult hemoglobin is diluted into plasma, the tetramers dissociate into dimers, which can pass through the glomerular filters. Alternatively, the dimers can rapidly bind to haptoglobin to form complexes that are specifically taken up and degraded by macrophages through a pathway involving the CD163 receptor and stimulation of heme oxygenase activity. Schaer, Alayash, and their colleagues have also shown that CD163 can also take up hemoglobin alone (albeit at a much higher concentration), and that these interactions may reduce oxidative damage by acellular hemoglobin, although this effect has not been shown directly (17–19).
To prevent dissociation, the first generation of acellular hemoglobins products used chemical crosslinking methods to polymerize hemoglobin and prevent dimerization. Although some of these polymerized and crosslinked hemoglobins led to a reduction in side effects, there were still issues with hypertension and reduced cardiac output (see (20) or reviews (8–10, 12, 21) and Table 1). One of the initial roadblocks for optimization of the chemically crosslinked hemoglobins was the variability of unmodified, non-crosslinked hemoglobin tetramers in the preparations. This problem was solved by Biopure, Inc. (Hemopure@) and Northfield Laboratories (Polyheme@) in the late 1980s by reproducibly removing virtually all non-crosslinked Hb tetramers from their polymerized samples ((22, 23) and Table 1).
The second generation of recombinant hemoglobin oxygen carriers used more precise chemical reactions or genetic manipulation to produce cross-linked tetrameric HbA variants. The first simple hemoglobin tetramer product contained α chains crosslinked with bis-(3,5-dibromosalycil) fumarate between two α Lys99 side chains (HemAssist@, Baxter, Table 1) (24). Several years later, recombinant DNA technology was used to fuse two α globin genes with a glycine codon linker to create a di-α subunit that associated with β subunits to create a crosslinked recombinant hemoglobin tetramer, called rHb0.1 (25). However, tests with these tetrameric HBOCs still revealed significant vasoconstrictive effects and renal toxicity (Table 1 and (8, 21, 26)). As with the polymerized HBOCs, the circulation half-lives of these simple crosslinked tetramers were significantly increased, and no hemoglobin was excreted in urine. Marden, Ho, and their coworkers constructed and evaluated a recombinant hemoglobin that spontaneously becomes an octamer by formation of two inter-tetramer disulfide bonds between Cys side chains introduced at the β Gly83 and α Asn78 positions (27).
Genetic crosslinking of the hemoglobin genes and their expression in E. coli allowed the use of rational protein engineering approaches to determine the underlying causes of the side effects associated with acellular HBOCs (28, 29). Previously, Varnado et al. (5) have reviewed the methodology used to express and purify recombinant HBOCs in bacteria, the problems associated with this technology, and various attempts to mitigate the side effects of acellular Hb. In this paper, we review the mechanisms and strategies for mitigating the key processes that are associated with plasma hemoglobin toxicity (numbered 1–4 in Figure 1).
OXYGEN BINDING AND TRANSPORT
Optimization of ligand binding parameters is critical to mimic the oxygen transport properties of intact red blood cells. One of the first differences noted between extracellular and intracellular hemoglobin was the increased O2 affinity of the cell-free protein due to the loss of intracellular 2,3-bis-phosphoglycerate, which decreases oxygen affinity inside erythrocytes. Chemical and genetic crosslinking also often reduces P50 (the partial pressure of O2 when hemoglobin is half saturated). The efficiency of oxygen transport is governed primarily by the release of O2 from hemoglobin and diffusion into actively respiring tissues (i.e. heart muscle, brain tissue, etc.) The diffusion gradient at 50% unloading is proportional to the P50 of the HBOC, and as a result, the efficiency of transport increases for hemoglobins with lower O2 affinity, as long as the P50 is low enough for complete uptake in the lungs (29–31).
In the case of red cells flowing through capillaries, there is additional diffusion resistance due to both unstirred plasma layers around the red cell and cell free layers along the capillary surface. These resistances do not occur when the hemoglobin is present in the plasma phase, and as a result, the efficiency of O2 transport increases 2–3 fold for acellular HBOCs (29, 32–35). A consequence of this enhanced delivery is that the dosage of acellular hemoglobin can be 2–3 times lower than that for whole blood (i.e. ~ 4–7 g/dl of acellular Hb versus 10–14 g/dl Hb in whole blood). However, Winslow and colleagues have argued that this difference leads to premature O2 unloading in arterioles and less delivery to capillary beds (21, 36). To avoid this problem, he and his colleagues proposed that acellular HBOCs should have a higher oxygen affinity than what is needed for efficient delivery by red cells and argued that Hemospan@ or MP4 was a more efficient HBOC because of its lower P50 (Table 1). Regardless of whether this idea is correct, it is relatively straightforward to engineer the oxygen affinity of Hb-based blood substitutes.
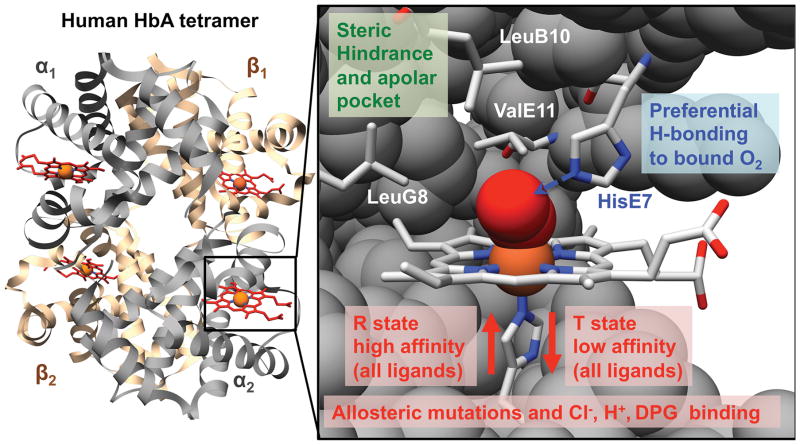
The basic biochemical mechanisms that govern O2 affinity in human hemoglobin are well understood as outlined in Fig. 2 and as described by Olson and coworkers (37–39). First, iron reactivity is governed by the ease of movement of the Fe(II)-proximal His(F8) complex into the plane of the porphyrin ring, which in turn is regulated by the R (high affinity) to T (low affinity) quaternary conformational transition. Allosteric effectors that bind and stabilize the T quaternary conformation can raise P50. This strategy was employed to raise the P50 of three initial products: Polyheme@ by covalently attaching pyridoxal phosphate (a 2,3-bisphoshoglycerate analogue) (Table 1 and (23)); Hemopure@ by using bovine Hb, which binds Cl− very tightly in the T state (22); and HemAssist@ by chemically crosslinking HbA in the deoxy or T state conformation (24). This approach has also been taken with genetically crosslinked rHb, using naturally occurring mutations that favor the low affinity T state (i.e. Hb Presbyterian (β N108K) in Optro1.1@ (25)). O2 affinity can also be adjusted by distal pocket mutations which alter the strength of hydrogen bonding to bound O2 from the polar E7 side chain (39) or enhance steric hindrance by the apolar B10, E11, or G8 amino acids that line the back of the distal pocket (40) (see Fig. 2, where the amino acid positions are given as residue number from the N-terminal position along one of the 8 helices labeled A through G). Many examples of these types of allosteric and active site substitutions occur in nature and serve to guide protein-engineering efforts (37–39). One of the more interesting studies involved examining substitutions in recombinant woolly mammoth hemoglobin that allowed the protein to maintain efficient oxygen transport during exposure to very low temperatures at the animal’s extremities (41, 42). Thus, it is relatively straightforward to chemically or genetically engineer HBOCs with altered O2 affinities and rate constants.
Fig. 2. Factors governing O2 binding in human adult hemoglobin (HbA).
The crystal structure of the oxygenated form of the tetramer and the active site of an α subunit in adult human oxyhemoglobin (PDB access code 2DN1).
NO SCAVENGING AND HYPERTENSION
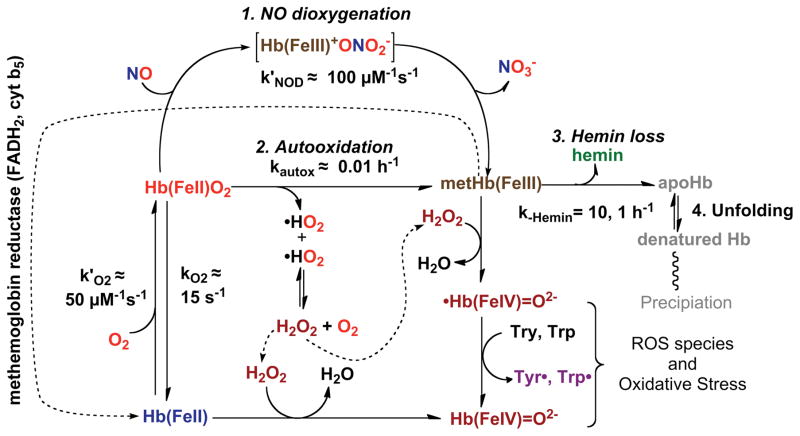
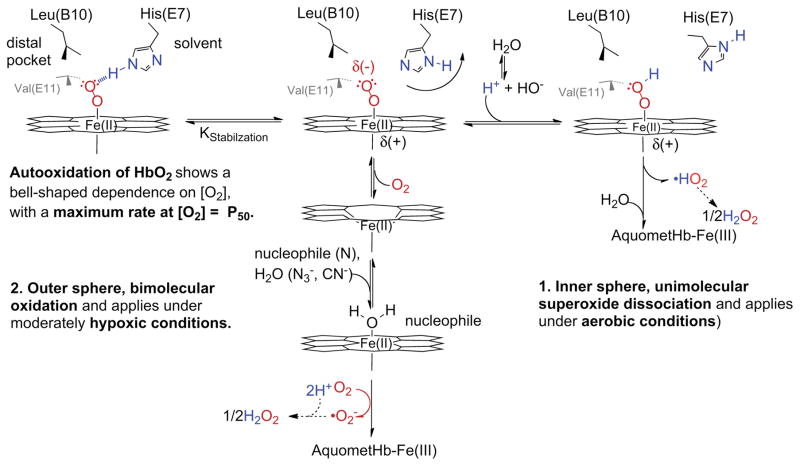
The most serious side effects of acellular hemoglobins involve oxidative processes that lead to methemoglobin formation and include autooxidation and the reaction of nitric oxide (NO) with oxyhemoglobin (HbO2). For example, increases in systemic blood pressure due to acellular hemoglobin (Hb) have been observed in hemolytic diseases, transfusions with old blood, and administration of extracellular HBOCs (Table 1, column 5 (8, 15, 16). In the early 1990s, there was considerable discussion of what role extracellular hemoglobin played in these hypertensive events. However, currently almost all workers in the field agree that scavenging of nitric oxide (NO) by hemoglobin inhibits vasorelaxation, which is mediated by the NO complex of soluble guanylyl cyclase (sGC) in blood vessel walls. The key reaction involves dioxygenation of NO by O2 bound to reduced hemoglobin and produces nitrate (NO3−) and methemoglobin (26, 43). As shown in Fig. 3, NO enters the active site of an oxyhemoglobin subunit (HbO2) and quickly reacts with the bound O2 to generate transient intermediates that rapidly dissociate into oxidized hemoglobin (metHb) and nitrate. The overall reaction is very fast (μs to ms), irreversible, and its speed is determined by the rate of NO diffusion into the active site of the protein. Some direct NO binding to deoxyhemoglobin does occur, but it is very minor because most of the plasma and red cell hemoglobin is oxygenated in arteriole blood. In addition, the NO that is bound reversibly to deoxyHb will slowly dissociate and then be dioxygenated by surrounding HbO2 molecules. Thus, NO scavenging is due exclusively to irreversible dioxygenation, producing NO3− and metHb.
Fig. 3. Four key reactions involved in NO scavenging and oxidative degradation of human hemoglobin.
The reactions of H2O2 are also shown, and these processes are described in detail by Alayash and coworkers (paper that is part of this series).
There are three strategies for inhibiting NO scavenging. The first strategy is to encapsulate the hemoglobin in a cell or vesicle, which prevents the HbO2 from getting up to and into endothelial cells (44, 45). To be consumed NO has to diffuse through cell free plasma layers adjacent to the blood vessel walls and through unstirred layers adjacent to the cell surface. In contrast acellular hemoglobin flows up to and into the blood vessel walls where it can directly interfere with NO signaling between endothelial and smooth muscle cells. A second strategy for reducing NO scavenging is to inhibit acellular Hb extravasation into the endothelium by increasing the size of the hemoglobin molecule or coating its surface with polyethylene glycol polymers (46). Various groups have shown that increasing the size of the HBOC reduces the extent of the hypertensive side effect (see Fig. 7 in Olson et al. (26)), and PEGylation of acellular Hb by itself also inhibits elevation of mean arterial blood pressure (36). These effects explain why the polymerized HBOCs listed in Table 1 (i.e. Polyheme@ and Hemopure@) have lower hypertensive side effects (increases in mean arterial blood pressure, ΔMAP, in Table 1) than the simple crosslinked tetramers (Hemassist (DCLHb or ααHb) and Optro(rHb1.1)) and why the pegylated HBOCs (Hemospan@ or MP4 –pegylated human Hb) and Sanguinate@ (pegylated bovine Hb) also have lower blood pressure effects.
The third strategy for decreasing NO scavenging is to reduce the bimolecular rate of NO dioxygenation by genetic engineering. In the late 1990s, the group at Somatogen (later Baxter Hemoglobin Therapeutics (28)) introduced large aromatic amino acids (Phe, Trp) at the B10, E11, and G8 helical positions in the α and β subunits of recombinant hemoglobin (rHb) to decrease the rate of NO entry by preventing its capture in the back of the distal portion of the heme pocket (see Fig. 2). They created a library of rHbs with bimolecular rates of NOD dioxygenation (k′NOD values) that decreased from ~ 80 μM−1s−1 to ~ 4 μM−1s−1, and observed corresponding 10 to 20-fold decreases in the mean arterial pressure (MAP) effect in experiments using an isovolemic exchange transfusion protocol in rats (28). These results were later extended to total peripheral resistance (TPR) measurements (26, 29). The observed increases in MAP or total peripheral resistance (TPR) only correlated with the rate of NO dioxygenation and not with the oxygen affinity of the genetically crosslinked HBOC variants (26, 29). These studies led to the development of a product called rHb2.0 by Baxter Hemoglobin Therapeutics, Inc., which contained α Leu(B10)Trp/His(E7)Gln and β Val(E11)Trp mutations. rHb2.0 had little or no hypertensive side effect in transfusion experiments with animal models (Table 1 and (47)). However, as described in Varnado et al. (5), this product oxidized and denatured relatively quickly and was not successful. This failure points out the need to consider all the key properties at the same time when developing engineering strategies, i.e. the rate of NO dioxygenation needs to be reduced without decreasing resistance to oxidative degradation. The rapid reaction of HbO2 with free NO also suggests that efforts to mitigate the hypertensive side effect of HBOCs by simply adding or attaching NO releasing agents are unlikely to be successful because the heme bound O2 will rapidly react with the free gas, oxidizing the hemoglobin and generating nitrate.
AUTOOXIDATION
Many workers in the HBOC field have argued that oxidative degradation of acellular hemoglobin is the major cause of the observed side effects, particularly those associated with endothelial damage and inflammation caused by oxidative stress from reactive oxygen species (8, 9). Similar pathological effects are associated with transfusions using old blood and with hemolytic diseases. Spontaneous autooxidation of oxyhemoglobin generates both methemoglobin and superoxide, which rapidly dismutates to hydrogen peroxide (H2O2), a very reactive oxygen species. As shown in Fig. 3, the resultant H2O2 can in turn react with either reduced or oxidized hemoglobin to produce even more reactive protein radicals that damage both hemoglobin itself and nearby proteins and membrane components (48).
In red blood cells, reductases quickly reverse the oxidation of intracellular hemoglobin, and superoxide dismutase and catalase efficiently remove O2•− and H2O2 (Fig. 1). However, no superoxide or catalase is present in plasma, and as a result, these reactive oxygen species lead to cyclic redox reactions that can produce free radicals, which cause significant damage to hemoglobin itself, plasma proteins, blood vessels, and surrounding tissues. H2O2 can react with either reduced deoxyHb (FeII) or oxidized metHb (FeIII) to produce highly reactive ferryl (FeIV) species and protein radicals. These ferryl forms can be reduced back to ferric forms by cyclic pseudoperoxidase activities, which sustain the generation of radicals and ROS (48, 49). These cyclic redox reactions appear to account in part for oxidative damage to the kidneys, where the acellular hemoglobin tends to accumulate.
In recent reviews, Alayash (1, 10, 50, 51) has described the reactions of acellular Hb with H2O2, and Cooper and his colleagues at the University of Essex have described various approaches to mitigate these processes including mutations in β chains to enhance reduction of radicals (52–55). In this paper, we have focused in much greater detail on the initial autooxidation reaction, which is the key rate-determining step in the oxidative degradation of hemoglobin. Ironically, most workers in the hemoglobin field avoid studying autooxidation because of the slowness of the reaction, the instability of the reaction products, and the complexity of the mechanism (missing numbers in Table 1). However, in our view inhibition of this reaction, even if only 2 to 3-fold, would generate a much more stable, effective, and commercially viable product. Thus, we have presented below a detailed molecular description of this process in the hope that the proposed mechanisms will lead to engineering strategies to reduce the rate of autooxidation of acellular hemoglobin products.
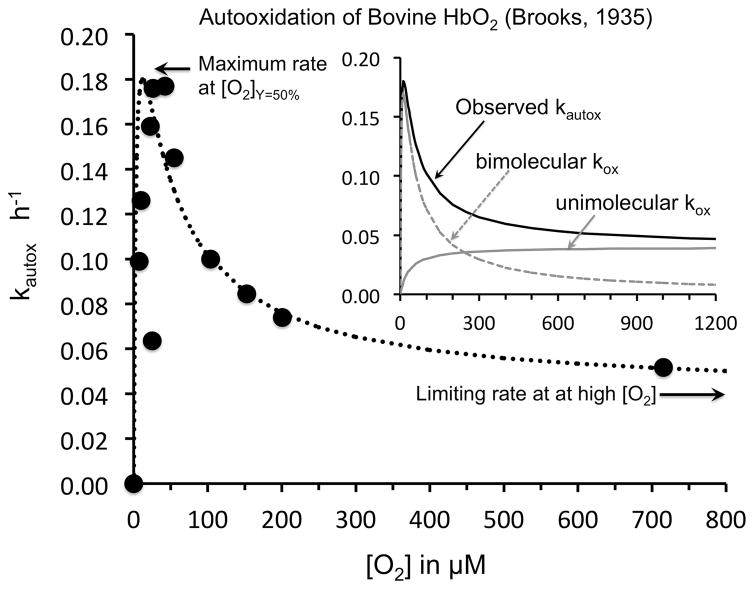
Many of the important phenomenological features of autooxidation of oxyhemoglobin and oxymyoglobin were established in the 1930s using van Slyke Apparatus manometric methods to control O2 pressure and measure O2 binding capacity (56–58). However, attempts at detailed biochemical interpretations were not made until many years later (59–61). Early studies by Brooks on hemoglobin (57) and George and Stratmann (62) on myoglobin showed that the rate of autooxidation, kautox, has a “bell” shaped dependence on the concentration of dioxygen, [O2], with a maximum kautox at the P50 of the globin sample (i.e. [O2]free at 50% saturation) and then a smaller, limiting value of kautox equal to 0.1 to 0.02 h−1 at very high [O2] (Fig. 4). They also showed that kautox dramatically increases with decreasing pH in the range from 9 to 5.
Fig. 4. Dependence of the rate of autooxidation of bovine oxyhemoglobin on [O2] in 0.6 M phosphate at pH 5.7, 30° C.
Data were taken from Brooks (57) and plotted versus [O2] in μM instead of PO2 in mmHg. The dashed line in the main figure is an empirical fit to the combined mechanism shown in Fig. 5, using the equations derived in Brantley et al. (61) for sperm whale myoglobin. The dependence of the bimolecular rate of oxidation and the unimolecular rate of oxidation on [O2] are shown in the inset as gray dashed and solid lines.
Roughly 60 years later, Brantley et al. (61) examined the factors that governed autooxidation of mammalian myoglobin and proposed the complex scheme shown in Fig. 5 based on work with a large library of recombinant mutants, previous studies by Caughey and coworkers (59, 63) on the effects of cyanide and azide on the rates of autooxidation of globins, and theoretical considerations of direct superoxide dissociation, originally discussed by Weiss in 1964 (64). The outer valence electron in ferrous iron can be partially transferred to bound dioxygen to produce a complex that resembles low spin ferric iron and bound superoxide (O2•−). If protonated, the bound O2•− can dissociate as the neutral hydrosuperoxide and then rapidly migrate out of the distal pocket at rates similar to those of the neutral diatomic gases (~ 107s−1 (65)). This unimolecular process is shown as Mechanism 1 on the right hand side of Fig. 5. Under physiological conditions, the rate of this process will be slow because the extent of protonation of bound O2 is very small and the probability of thermal dissociation of HO2• is also very low. This mechanism predicts a hyperbolic dependence of kautox on [O2], which is identical to that for simple reversible O2 binding, and a limiting value at high [O2] equal to the thermal rate of HO2• dissociation, i.e. a value of ~ 0.04 h−1 for the results obtained by Brooks (57) in Fig. 4.
Fig. 5. Mechanisms involved in the autooxidation of HbO2.
This scheme was taken from Brantley et al. (61) where the mechanism of autooxidation of myoglobin was examined quantitatively.
The mechanism in Fig. 5 also explains the strong inverse dependence of kautox on pH, with the rates increasing several-fold for each decrease in pH unit from ~ pH 8 to pH 6 (56, 57, 60, 61). The pKa for superoxide in solution is roughly 4.9 so that at neutral pH the extent of protonation is very small for the free superoxide anion and perhaps even lower when it is bound to iron (61). This scheme also explains the key role of the distal histidine in inhibiting autooxidation by forming a strong hydrogen bond with the Fe-O2 complex. Hydrogen bonding to His(E7) blocks protonation of the bound ligand by lowering the effective pKa even further. When His(E7) is replaced with apolar amino acids, the rates of autooxidation of mammalian Hbs and Mbs increase markedly, by factors of 10 to 1000, depending on the size of the amino acid side chain (61).
The unimolecular, HO2• dissociation mechanism cannot explain the marked 3 to 4-fold increase in kautox as [O2] decreases into the region of the hemoglobin’s P50 value (Fig. 4). In 1982, Wallace et al. (59) proposed a mechanism similar to the bimolecular process shown in the middle of Fig. 5. This second mechanism involves transient formation of a 6-coordinate ferrous complex in which an external ligand or nucleophile binds on the distal side of the heme group and pulls the iron atom into the plane of the porphyrin ring. Under these conditions, outer sphere electron transfer to an acceptor can occur without a large quantum mechanical barrier and forms a stable Fe(III) complex with the proximal His(F8) and the nucleophile (59). In the case of autooxidation, the acceptor is dissolved O2 which reacts with the edge of the porphyrin ring to produce O2•− directly. In this mechanism, no autooxidation can occur until some deoxyhemoglobin is formed to react with either an added nucleophile like azide or a solvent water molecule. For this mechanism, kautox is zero at high [O2] when no deoxyHb is present and at very low [O2] when no electron acceptor is present. The maximum rate will occur at ~ 50% saturation. This mechanism explains why the observed rate of autooxidation shows a “bell-shaped” dependence on [O2] and why the observed rate at low [O2] increases when strong ferric ligands such as azide, cyanide, and imidazole are added.
Hargrove and coworkers (66) have also pointed out that this mechanism explains why autooxidation of naturally occurring hexacoordinate plant and microbial hemoglobins is so fast. In these cases, the distal histidine is binding to the iron atom in the ferrous state, inhibiting reversible O2 binding and, at the same time, speeding up electron transfer to solvent O2 and other electron acceptors. In myoglobins and hemoglobins, the structure of the distal pocket evolved to keep the distal histidine away from the iron atom in the native tertiary structure to prevent rapid autooxidation by this bimolecular mechanism, but still close enough to form a strong hydrogen bond to prevent both simple dissociation of the bound O2 and protonation to facilitate the dissociative mechanism.
As shown in Fig. 4 and discussed in Brantley et al. (61) both mechanisms are occurring in hemoglobins and myoglobins. If the P50 of the hemoglobin is low, then autooxidation occurs primarily by the dissociative process in air or at higher [O2]. However, if the P50 is high, the rate of autooxidation will have a greater contribution from the bimolecular process. A detailed discussion of the effects of mutagenesis on these processes in Mb is given in Brantley et al. (61), and we are currently working on trying to apply those ideas to studies with recombinant Hbs.
Autooxidation of hemoglobin has three additional complications compared to monomeric myoglobin, all of which are physiologically relevant. First, the rate of autooxidation, kautox, of human adult oxyhemoglobin depends dramatically on total protein concentration. This observation was first made by Zhang and Rifkind (67) and then quantitated more thoroughly by Mollan et al. (68). An example of the dramatic increase in kautox at low protein concentration is shown in Fig. 6 using data that we have recently collected. The observed kautox value becomes 30 to 40-fold higher at very low concentrations where human oxyhemoglobin dissociates into dimers. Mollan et al. (68) analyzed this dependence quantitatively, using a tetramer to dimer dissociation constant K4,2, ≈ 1 μM, and obtained fitted limiting values of 0.01 h−1 for tetramers (upper gray line, Fig. 6) and 0.4 h−1 for dimers (lower gray line, Fig. 6).
Fig. 6. Effect of quaternary structure on autooxidation of human hemoglobin in 0.1 M Na phosphate, pH 7.0, 37° C.
rHb0.1 is genetically crosslinked human hemoglobin containing a glycine linker between the C terminus of one α chain and the N-terminus of the other α chain. The points represent triplicate measurements and the fraction of HbO2 remaining was obtained from deconvoluting the observed spectra into components representing HbO2, metHb, hemichromes, and light scattering due to precipitation (83) The upper and lower gray lines represent theoretical time courses for completely tetrameric and dimeric HbA, respectively, based on the analysis of Mollan et al. (68).
The second complication is that the autooxidation of Hb tetramers could potentially be cooperative, with oxidation of the first few subunits facilitating the oxidation of the remaining ones. In the absence of ligands, equilibrium redox curves for human HbA do show cooperativity (69), and the time course we observe for autooxidation of almost fully tetrameric human HbA at high protein concentrations shows some acceleration (Fig. 6). However, no one has yet attempted to analyze the extent of the increase in rate in terms of an allosteric model, and this acceleration is lost at lower protein concentrations (≤ 100 μM heme) where most experiments are done and significant levels of non-cooperative HbO2 dimers are present. However, the important observation is that the initial rate of HbA autooxidation is small and on the order of 0.001 h−1 for tetramers at 37 °C, pH 7 and underestimates the speed of the overall reaction (Table 1 and Fig. 6).
The third potential complication is differences between the autooxidation rates of the α and β subunits. Shikama and coworkers (70) have argued that the time course for HbA autooxidation is biphasic and that the α subunits autooxidize more rapidly. In unpublished work, we have looked for differences between the autooxidation rates of the subunits by reacting partially oxidized samples of HbA with azide and measuring the relative fractions of oxidized β versus α subunits. Because met-β subunits react ~ 5 times more rapidly with azide than met-α, the fraction of subunits that react rapidly with azide provides the ratio of met-β/met-α. This approach was used by MacQuarrie and Gibson (69) to show that β subunits have a more positive reduction potential than α subunits, implying that the α subunits might more readily oxidize. However, under the conditions shown in Fig. 6, the ratio met-β/met-α was unchanged throughout the autooxidation time course for HbO2 at 100 μM. In addition, if the subunits were significantly different, the overall time course should have been biphasic and not accelerating under these conditions. At lower protein concentrations, the overall time course does become exponential and sometimes biphasic, and we do see evidence that the α subunits autooxidize more rapidly (≤ 2.5-fold), but the differences are small.
The key issue for this review is whether or not the mechanisms and results shown in Figs. 4–6 can be used as a framework to engineer HBOCs with greater resistance to autooxidation. Most HBOC preparations contain crosslinked hemoglobin to prevent dissociation into dimers, which was done initially to prevent clearance by the kidneys. Thus, many of these stabilized tetramers or Hb polymers actually do show smaller kautox values than HbA at low protein concentrations because dissociation into rapidly oxidizing dimers is prevented (i.e. 10 μM rHb0.1 in Fig. 6 compared with 10 μM HbA and see values of kautox for the HBOCs in Table 1). However, at higher more physiological globin concentrations, all of these crosslinked hemoglobin preparations show larger rates of autooxidation than native HbA tetramers due to the effects of the mutations or chemical modifications used for crosslinking (i.e. compare 100 μM rHb0.1 with 100μM HbA, the upper gray curve in Fig. 6 and initial kautox values in Table 1).
Both mechanisms shown in Fig. 5 require loss of hydrogen bonding from the distal histidine to facilitate either O2 dissociation into deoxyHb or to allow protonation of bound O2 and dissociation of HO2•−. Autooxidation can be slowed dramatically by inserting more hydrogen bond donating amino acids in the active site as is seen in some invertebrate and bacterial hemoglobins. However, these proteins have such high O2 affinities that they are not capable of O2 transport (71, 72).
A better strategy is to try to limit solvent accessibility to the distal pocket because water is required, either to combine transiently with deoxyHb to facilitate the outer sphere bimolecular process or to act as a proton donor to the bound O2 in the unimolecular mechanism. We were successful in reducing the rate of autooxidation of Mb roughly 5-fold by inserting a Phe for Leu(B10), which limited water penetration into the active site (73). However, this same strategy has not worked for human HbA. So far no one has been able to construct a mutant or chemically modified Hb with a lower rate of autooxidation than native tetrameric human HbA (i.e. see (74, 75)).
In our view, hemoglobin autooxidation is governed by transient water penetration into the distal pocket, which is dampened when more rigid quaternary states occur. This idea would explain the dramatic ~ 100-fold decrease in kautox observed in going from isolated subunits (~ 1–2 h−1 at 37° C) to dimers (~ 0.4 h−1) to tetramers (≥ 0.01 h−1). Thus, mutations that dampen fluctuations in the tertiary structures of tetrameric subunits need to be considered but are not obvious from static crystal structures. Choices of regions to mutate or modify will require both computational analyses and screening of large libraries of variants with changes in regions at interfaces and turns between helical regions (i.e. CD, EF, and FG corners). Finally the results in Figs. 4 and 6 also indicate that the autooxidation characteristics of potential HBOCs require studies as a function of O2 concentration and pH. Maximum rates of autooxidation in vivo will occur in capillaries adjacent to actively respiring muscle and neuronal tissues where [O2] approaches the P50 of the product and the blood pH is lowered.
HEMIN DISSOCIATION
The third key process in the oxidative degradation of hemoglobin is hemin dissociation (Figs. 1 and 3). This process leads to irreversible precipitation of apoglobin, incorporation of the redox active hemin into nearby serum proteins and endothelial membranes, and self-aggregation of the hemin to form insoluble particulates. These three reactions prevent reformation of HbO2 from metHb even when reducing agents are added. MetHb can be reduced back to HbO2 or deoxyHb if the hemin is still bound by adding ascorbate, glutathione, or other naturally occurring reducing agents. In red cells, autooxidation is reversed by methemoglobin reductases using NADPH, and catalase and superoxide dismutase to prevent an accumulation of H2O2 (Fig. 1). Thus, another key engineering goal in the HBOC field is to reduce the rate of hemin dissociation.
As shown in Fig. 7B, dissociation of heme requires hydrolysis of the Fe-His(F8) bond and then movement of the Fe-protoporphyrin IX out of the protein. The strength of the Fe-His(F8) bond depends on the oxidation state of the iron atom and the presence or absence of bound ligands. Heme does not dissociate readily when the iron is reduced and is completely inhibited in the presence of bound ligands, particularly CO. The ferrous forms of myoglobin and hemoglobin have equilibrium heme dissociation constants ≤ 10−16 M (76). In contrast, the equilibrium constants for hemin dissociation from the aquo-ferric forms of these proteins are one hundred to a million-fold higher and on the order of 10−14 to 10−10 M (76, 77). In effect, autooxidation has to occur before the heme group can dissociate.
Fig. 7.
A. Time courses for hemin dissociation from various quaternary states of HbA and HbA dimers bound to haptoglobin (Hp) 1.1. The time courses for isolated α and β subunits, metHbA dimers, metHbA, and crosslinked met-rHb0.1were computed using the rate constants determined by Hargrove et al. (77). The hemin loss assay was performed in 0.1 M potassium phosphate, pH7.0, 37° C in 20% sucrose as an osmolyte to inhibit precipitation of apohemoglobin at the end of the reaction (77). The time course for hemin dissociation from the Hp:HbA dimer complex was computed from the rate constant reported by Mollan et al. (68). B. Active site of human 7agr; subunits showing how penetration of water can facilitate both autooxidation and hemin dissociation.
Twenty-five years ago Hargrove et al. (78) developed an assay to measure the rate of hemin dissociation from metMb and metHb. They constructed a double mutant of sperm whale myoglobin in which the distal histidine (His(E7)) was replaced with a Tyr (H64Y), which chelates to the ferric iron atom and gives the holo metMb a green color. The distal valine (Val(E11)) was replaced with a Phe (V68F) to enhance the stability of the apoprotein. When excess H64Y/V68F apoMb is mixed with metHb samples, the color of the solution turns from brown to green as the apoMb reagent scavenges hemin from the hemoglobin sample. The rate-limiting step is hemin dissociation, which occurs with rate constants, k-H, on the order of 40 to 0.3 h−1, and half-times of 1 to 2 minutes to 2 to 3 hours, depending on pH and protein concentration.
As with autooxidation, the rate of hemin dissociation from HbA depends strongly on oligomeric structure and thus globin concentration (77). Comparisons of time courses for hemin dissociation from isolated subunits, dimers, tetramers, and the dimer:haptoglobin complex are shown in Fig. 7A using data taken primarily from Mollan et al. (68). Monomeric subunits are highly unstable in the ferric form and lose hemin completely in 10 to 20 minutes; dimers lose all of their hemin to the scavenger in an hour; and tetramers are significantly more stable. The fast and slow phases for hemin loss from dimers and tetramers represent hemin dissociation from the β and α subunits respectively (77, 78). The most remarkable result in Fig. 7 is the dramatic stabilization of bound hemin by the binding of HbA dimers to haptoglobin (68). Virtually no loss of hemin was observed even when the apoMb scavenging agent was added in great excess. Baek et al. (15) observed a similar result using apohemopexin as a scavenging agent and suggested that a key role of haptoglobin is to take up metHb dimers and prevent hemin loss until the complex can be processed by macrophages.
By analogy with the results for autooxidation, our interpretation of the effects of quaternary structure is that the formation of αβ subunit interfaces or the Hp:HbA dimer complex dampens fluctuations in the globin tertiary structure, which markedly reduces water penetration into the proximal portion of the heme pocket and inhibits hydration of the Fe-His(F8) bond. Using a large library of recombinant variants, we were able to identify more specific interactions that inhibit hemin dissociation from myoglobin and most of which involve favorable electrostatic interactions with the heme propionates (79). However, in the case of hemoglobin these interactions either don’t exist or are weaker, accounting in part for the 30 to 100-fold higher rates of hemin dissociation from metHb versus from metMb. Introducing more favorable electrostatic interactions in the β subunits of hemoglobin does appear to inhibit hemin loss from HbA dimers but the effects are small (≤ 2-fold) and not observed in Hb tetramers. As in the case of autooxidation, we have not been able to engineer rationally single point mutants with rates of hemin loss that are smaller than those for native HbA tetramers. This result is emphasized in the last two columns of Table 1, where all the HBOCs, even when polymerized, have higher rates of hemin dissociation than tetrameric HbA.
Again, we feel that the key to reducing both autooxidation and hemin loss is to understand the dynamics of water penetration into the heme pocket and then to engineer α and β subunits with reduced rates and extents of solvent movement into protein. The Hp:HbA dimer complex serves as a remarkable example of slowing hemin loss. However, a simple static comparison of the crystal structures of the Hp:dimer complex and tetrameric Hb shows almost no differences in the positions of the α and β polypeptide chains with respect to their heme groups. In both cases, the heme propionates are still exposed to solvent (80). Thus, the dramatic differences shown in Fig. 7A must be due to differences in the fluctuations of the structures that allow water to transiently penetrate and hydrolyze the Fe-His(F8) bond. The dilemma is that hemin loss is slow (as is autooxidation) so the amount of water inside the pocket at anytime is very small and undetectable by conventional NMR and crystallographic methods. Molecular dynamics simulations are needed to identify regions of the structure that enhance or inhibit fluctuations. Then these regions need to be mutated to try to inhibit water penetration.
APOGLOBIN STABILITY AND EXPRESSION
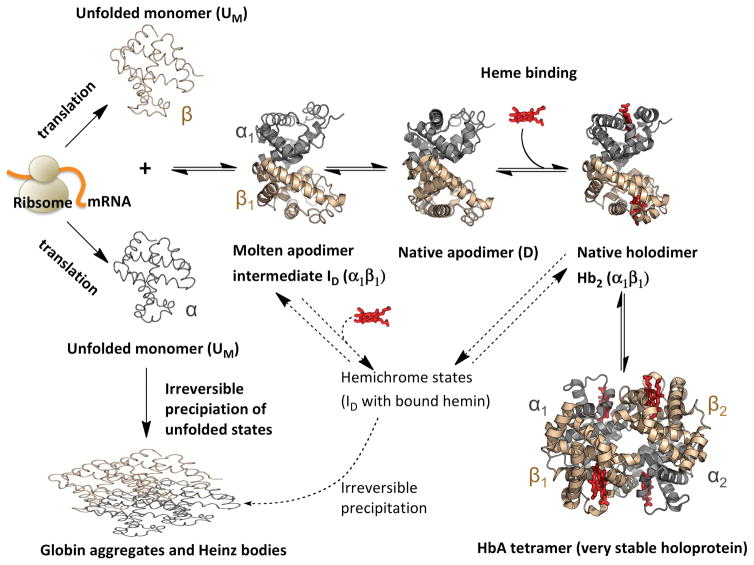
The final process in the degradation of hemoglobin is the unfolding and precipitation of the apoprotein (Reaction 4 in Figs. 1 and 3). In general, apohemoglobin is difficult to work with at temperatures above 10° C and rapidly precipitates under physiological conditions at 37°C. Almost all previous studies of apoHb denaturation involved irreversible studies of thermal or acid denaturation, dehydration, or solublization in sodium dodecyl sulfate. Recently, we examined for the first time reversible unfolding of apoHb at pH 7, 10° C using GdnHCl as a denaturant, which prevents the unfolded subunits from precipitating (81). Results for apoHbA are summarized in Fig. 8. The process involves two major steps. Loss of hemin generates an apo-dimer (D state), which retains most (~ 70–80%) of the helical structure of the holoprotein tetramer. The first unfolding step for this apodimer involves melting of the heme pocket with loss of most of the B, C, D, E, and F helices but retention of the helices (A, G, and H) that are involved in the α1β1 interface. The second step involves dissociation of the dimer interface and formation of almost completely unfolded monomers. The molten globule intermediate, ID, is a dimer, which can still bind hemin to form hemichrome spectral species that can refold into native holoprotein. As indicated in Fig. 8, mutations that stabilize the heme pocket inhibit the first phase of apoglobin unfolding, whereas mutations that stabilize the α1β1 dimer interface inhibit the second phase of unfolding. The most dramatic of the latter mutations have genetically crosslinked α subunits, i.e. rHb0.1 as described in Fig. 7. In this case, complete denaturation requires disruption of two dimer interfaces to generate two unfolded β subunits and one di-α subunit (81). Thus, crosslinking does inhibit equilibrium unfolding of hemoglobin as would be expected. This mechanism also helps explain why, under most conditions, human fetal hemoglobin is more resistant to denaturation than adult hemoglobin because the α1γ1 interface in HbF is stronger than the α1β1 interface in HbA (81).
Fig. 8. ApoHb unfolding involves two major steps.
These data for apoHbA were taken from Samuel et al. (81) and represent denaturation by GdnHCl in 0.2 M potassium phosphate buffer, pH 7, at 10° C in the presence of 1 mM dithiothreitol. The first phase involves unfolding of the heme pockets in both subunits to form a dimeric molten globule state and the second phase involves dissociation of the dimer intermediate into unfolded monomers.
Because our measurements involve reversible unfolding, the observed mechanism also applies to the folding and assembly of holohemoglobin as shown in Fig. 9, which represents a general scheme for the biosynthesis of hemoglobin either in pre-erythroid cells or in bacteria. The arguments in favor of this scheme for hemoglobin are given in Samuel et al. (81) and Varnado et al. (5), and similar ideas have been proposed for the biosynthesis of myoglobin (82). The scheme in Fig. 9 serves to indicate which factors are crucial for efficient production of holoHb and which are important for preventing denaturation.
Fig. 9.
Mechanisms for the biosynthesis, folding, and assembly of human hemoglobin tetramers.
Assuming heme availability, holoprotein expression yield is governed by native apoglobin stability, which, in the case of hemoglobin, includes both the strength of the dimer interface and the extent of folding of the heme pocket. In a study using myoglobin as a simple model system, we have shown that the highest expression yields are observed for variants in which distal histidine and valine are replaced with large apolar amino acids, i.e. Leu or Phe, which greatly stabilize the folded heme pocket and facilitate the uptake of heme (82). We have observed that qualitatively similar increases in production yields in E. coli occur for His(E7)Leu/Val(E11)Phe mutations in hemoglobin. During biosynthesis the rate of holoprotein formation is governed by the fraction of native apoprotein (native D state in Fig. 9), which is capable of reacting with heme, versus the fraction of unfolded chains (UM states in Fig. 9), which irreversibly self-aggregate. In the case of Mb, we have shown quantitatively that there is strong, direct correlation between holoprotein yield and the overall stability of the folded native apoglobin state obtained from fits to GdnHCl-induced unfolding curves, which are similar to those in Fig. 8. This correlation holds for expression in cell-free systems, E. coli, and in mammalian muscle (82), and we assume the same situation occurs for human hemoglobin.
Thus, the expression yields of holo-myoglobins and hemoglobins depend strongly on the absolute value of the equilibrium constant for formation of the folded native apoglobin state, starting from unfolded chains. This parameter determines the fraction of newly expressed polypeptide chains that are capable of rapidly binding heme versus the fraction that remains unfolded and irreversibly aggregates. In contrast, the yield of holoprotein is little affected by hemin affinity or resistance of the oxygenated complex to autooxidation (82).
However, mutations that enhance expression yields do not always yield holoproteins that are functional or resistant to oxidative degradation. Replacing the distal histidine with Leu enhances the stability of the apoprotein, but markedly decreases O2 affinity (P50 ≥ 100 μM), enhances autooxidation (kautox ≈ 0.5 h−1), and speeds up hemin dissociation (k-H ≥ 20 h−1) in human α subunits (83). The latter detrimental properties are due to loss of hydrogen bonding capacity of the E7 amino acid side chain. His(E7) stabilizes bound O2 and inhibits its protonation, which decreases P50 and kautox, respectively (Fig. 5). The His(E7) side chain also hydrogen bonds to water coordinated to the Fe(III) atom in metHb, inhibiting dissociation of the hemin group. These results provide a good example of how functionality can compromise globin stability and expression and that the evolution of a successful phenotype involves a fine balance of protein properties. It also points out the need to consider simultaneously all the key functional and stability properties when developing protein engineering strategies.
FUTURE DIRECTIONS
The four key characteristics of HBOC products are efficient oxygen delivery, low rates of NO scavenging, resistance to oxidative degradation, and high production yields. O2 affinity is easily engineered with chemical modifications or site-specific mutations based on the well-established mechanisms shown in Fig. 2 (29, 72). Slowing extravasation of the HBOC into the endothelium by increasing its size or by PEGylation reduces the extent of NO scavenging and the hypertensive side effect (26, 29). The bimolecular rate constant for NO dioxygenation can also be reduced more directly by distal pocket mutations that inhibit ligand capture (26, 28, 29).
The key to limiting the oxidative degradation of HBOCs is to inhibit autooxidation. Heme loss and globin denaturation will not occur under physiological conditions if the protein is kept in a reduced holoprotein state. However, once the iron atom is oxidized, hemin loss can be rapid if acceptors (albumin, apohemopexin, membranes, receptors, and lipoproteins) are present, and most of the resultant apoglobins are relatively unstable at 37° C and begin to irreversibly precipitate. Redox reactions with the H2O2 produced by autooxidation can also accelerate degradation (1). Although myoglobins have been engineered with rates of autooxidation and hemin loss that are lower than those for the native or wild-type protein, we have been unable to construct Hb site-specific mutants with rates that are lower than those for native HbA tetramers.
Both autooxidation and hemin loss require interactions of the heme group or bound O2 with water in the heme pocket (Figs. 6 and 7), which in turn requires fluctuations in the tertiary structure that allow solvent penetration into the protein interior. In our view, there are two keys to developing a successful strategy to reduce autooxidation and hemin loss rates. First, computational methods need to be developed and applied systematically to examine where fluctuations and solvent penetration into the heme pocket occurs. The goals would be to identify what regions facilitate fluctuations and which are dampened by dimer and tetramer formation or binding to haptoglobin, and then to examine whether or not these regions are more rigid in myoglobins. Our initial guesses involve regions at the CD, EF, and FG corner, and multiple mutations are probably needed to change these more global motions. Second, high throughput methods need to be developed to screen large libraries of hemoglobin mutants for enhanced resistance to oxidative degradation. In our study of Mb expression, we developed a cell-free translation system for expressing, partially purifying, and characterizing the spectral properties of libraries of myoglobin variants. This assay could easily be adapted to measure rates of autooxidation or hemin loss for hemoglobin in multiple well plates. Similar approaches can be taken to enhance apoglobin stability where again single point mutations normally have only small effects unless they are in crucial areas of the distal pocket or subunit interfaces, but even then multiple substitutions are required to have a large effect.
Acknowledgments
These studies were supported by the U.S. National Institutes of Health (NIH) Grant P01 HL110900 (JSO) and Grant C-0612 from Robert A. Welch Foundation (JSO).
Footnotes
The authors report no conflicts of interest
References
- 1.Alayash AI. Mechanisms of Toxicity and Modulation of Hemoglobin-based Oxygen Carriers(HBOCs) Shock Current series(D-17-00401) 2017 doi: 10.1097/SHK.0000000000001044. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amberson W, Mulder A, Steggerda F, Flexner J, Pankratz D. Mammalian life without red blood corpuscles. Science. 1933;78:106–7. doi: 10.1126/science.78.2014.106. [DOI] [PubMed] [Google Scholar]
- 3.Winslow RM. In: Blood Substitutes, Chapter 1: Historical Background. Winslow RM, editor. San Diego: Academic Press; 2006. pp. 5–16. [Google Scholar]
- 4.Amberson WR. Blood Substitutes. Biological Reviews. 1937;12(1):48–86. [Google Scholar]
- 5.Varnado CL, Mollan TL, Birukou I, Smith BJ, Henderson DP, Olson JS. Development of recombinant hemoglobin-based oxygen carriers. Antioxid Redox Signal. 2013;18(17):2314–28. doi: 10.1089/ars.2012.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santry HP, Alam HB. Fluid resuscitation: past, present, and the future. Shock. 2010;33(3):229–41. doi: 10.1097/SHK.0b013e3181c30f0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahr JS, Nesargi SB, Lewis K, Johnson C. Blood substitutes and oxygen therapeutics: an overview and current status. Am J Ther. 2002;9(5):437–43. doi: 10.1097/00045391-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Alayash AI. Setbacks in blood substitutes research and development: a biochemical perspective. Clin Lab Med. 2010;30(2):381–9. doi: 10.1016/j.cll.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Buehler PW, D’Agnillo F, Schaer DJ. Hemoglobin-based oxygen carriers: From mechanisms of toxicity and clearance to rational drug design. Trends Mol Med. 2010;16(10):447–57. doi: 10.1016/j.molmed.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Alayash AI. Blood substitutes: why haven’t we been more successful? Trends Biotechnol. 2014;32(4):177–85. doi: 10.1016/j.tibtech.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiskopf RB. Hemoglobin-based oxygen carriers: disclosed history and the way ahead: the relativity of safety. Anesth Analg. 2014;119(4):758–60. doi: 10.1213/ANE.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 12.Moore EE, Johnson JL, Cheng AM, Masuno T, Banerjee A. Insights from studies of blood substitutes in trauma. Shock. 2005;24(3):197–205. doi: 10.1097/01.shk.0000180075.76766.fe. [DOI] [PubMed] [Google Scholar]
- 13.Bunn HF, Esham WT, Bull RW. The renal handling of hemoglobin. I. Glomerular filtration. J Exp Med. 1969;129(5):909–23. doi: 10.1084/jem.129.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunn HF, Jandl JH. The renal handling of hemoglobin. II. Catabolism. J Exp Med. 1969;129(5):925–34. doi: 10.1084/jem.129.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122(4):1444–58. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiskopf RB. The efficacy and safety of liquid stored blood and storage duration: a confused subject; are patients confused? Anesth Analg. 2014;119(2):224–9. doi: 10.1213/ANE.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 17.Schaer DJ, Schaer CA, Buehler PW, Boykins RA, Schoedon G, Alayash AI, Schaffner A. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood. 2006;107(1):373–80. doi: 10.1182/blood-2005-03-1014. [DOI] [PubMed] [Google Scholar]
- 18.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121(8):1276–84. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buehler PW, Abraham B, Vallelian F, Linnemayr C, Pereira CP, Cipollo JF, Jia Y, Mikolajczyk M, Boretti FS, Schoedon G, et al. Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification. Blood. 2009;113(11):2578–86. doi: 10.1182/blood-2008-08-174466. [DOI] [PubMed] [Google Scholar]
- 20.Hess JR, MacDonald VW, Brinkley WW. Systemic and pulmonary hypertension after resuscitation with cell-free hemoglobin. J Appl Physiol. 1993;74(4):1769–1778. doi: 10.1152/jappl.1993.74.4.1769. [DOI] [PubMed] [Google Scholar]
- 21.Winslow RM. Cell-free oxygen carriers: Scientific foundations, clinical development, and new directions. Biochim Biophys Acta. 2008;1784(10):1382–6. doi: 10.1016/j.bbapap.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 22.Lee R, Atsumi N, Jacobs EE, Jr, Austen WG, Vlahakes GJ. Ultrapure, stroma-free, polymerized bovine hemoglobin solution: evaluation of renal toxicity. J Surg Res. 1989;47(5):407–11. doi: 10.1016/0022-4804(89)90092-9. [DOI] [PubMed] [Google Scholar]
- 23.Gould SA, Sehgal LR, Rosen AL, Sehgal HL, Moss GS. The development of polymerized pyridoxylated hemoglobin solution as a red cell substitute. Ann Emerg Med. 1986;15(12):1416–9. doi: 10.1016/s0196-0644(86)80931-3. [DOI] [PubMed] [Google Scholar]
- 24.Snyder SR, Welty EV, Walder RY, Williams LA, Walder JA. HbXL99 alpha: a hemoglobin derivative that is cross-linked between the alpha subunits is useful as a blood substitute. Proc Natl Acad Sci U S A. 1987;84(20):7280–4. doi: 10.1073/pnas.84.20.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Looker D, Abbott-Brown D, Cozart P, Durfee S, Hoffman S, Mathews AJ, Miller-Roehrich J, Shoemaker S, Trimble S, Fermi G, et al. A human recombinant haemoglobin designed for use as a blood substitute. Nature. 1992;356(366):258–60. doi: 10.1038/356258a0. [DOI] [PubMed] [Google Scholar]
- 26.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. No scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36(6):685–97. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Brillet T, Marden MC, Yeh JI, Shen TJ, Ho NT, Kettering R, Du S, Vasseur C, Domingues-Hamdi E, Ho C, et al. Interaction of haptoglobin with hemoglobin octamers based on the mutation alphaAsn78Cys or betaGly83Cys. Am J Mol Biol. 2012;2(1):1–10. doi: 10.4236/ajmb.2012.21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol. 1998;16(7):672–6. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 29.Dou Y, Maillett DH, Eich RF, Olson JS. Myoglobin as a model system for designing heme protein based blood substitutes. Biophys Chem. 2002;98(1–2):127–48. doi: 10.1016/s0301-4622(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 30.Vandegriff KD, Olson JS. Morphological and physiological factors affecting oxygen uptake and release by red blood cells. The Journal of biological chemistry. 1984;259:12619–12627. [PubMed] [Google Scholar]
- 31.Lemon DD, Nair PK, Boland EJ, Olson JS, Hellums JD. Physiological factors affecting O2 transport by hemoglobin in an in vitro capillary system. J Appl Physiol. 1987;62(2):798–806. doi: 10.1152/jappl.1987.62.2.798. [DOI] [PubMed] [Google Scholar]
- 32.Boland EJ, Nair PK, Lemon DD, Olson JS, Hellums JD. An in vitro capillary system for studies on microcirculatory O2 transport. J Appl Physiol. 1987;62(2):791–7. doi: 10.1152/jappl.1987.62.2.791. [DOI] [PubMed] [Google Scholar]
- 33.Page TC, Light WR, McKay CB, Hellums JD. Oxygen transport by erythrocyte/hemoglobin solution mixtures in an in vitro capillary as a model of hemoglobin-based oxygen carrier performance. Microvasc Res. 1998;55(1):54–64. doi: 10.1006/mvre.1997.2055. [DOI] [PubMed] [Google Scholar]
- 34.Vandegriff KD. In: Blood Substitutes, Chapter 5: The role of oxygen and hemoglobin diffusioin in oxygen transport by cell-free hemolgobins. Winslow RM, editor. San Diego, CA: Academic Press; 2006. pp. 60–71. [Google Scholar]
- 35.Page TC, Hellums JD. In: Blood Substitutes, Chapter 6: Oxygen transport properties of hemoglobin-based-oxygen-carriers: Studies using artificial capillaries and mathematical simulation. Winslow RM, editor. San Diego: Academic Press; 2006. pp. 72–83. [Google Scholar]
- 36.Tsai AG, Vandegriff KD, Intaglietta M, Winslow RM. Targeted O2 delivery by low-P50 hemoglobin: a new basis for O2 therapeutics. Am J Physiol Heart Circ Physiol. 2003;285(4):H1411–9. doi: 10.1152/ajpheart.00307.2003. [DOI] [PubMed] [Google Scholar]
- 37.Olson JS, Phillips GN. Myoglobin discriminates between O-2, NO, and CO by electrostatic interactions with the bound ligand. Journal of Biological Inorganic Chemistry. 1997;2(4):544–552. [Google Scholar]
- 38.Maillett DH, Simplaceanu V, Shen TJ, Ho NT, Olson JS, Ho C. Interfacial and distal-heme pocket mutations exhibit additive effects on the structure and function of hemoglobin. Biochemistry. 2008;47(40):10551–63. doi: 10.1021/bi800816v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birukou I, Schweers RL, Olson JS. Distal histidine stabilizes bound O2 and acts as a gate for ligand entry in both subunits of adult human hemoglobin. J Biol Chem. 2010;285(12):8840–54. doi: 10.1074/jbc.M109.053934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birukou I, Maillett DH, Birukova A, Olson JS. Modulating distal cavities in the alpha and beta subunits of human HbA reveals the primary ligand migration pathway. Biochemistry. 2011;50(34):7361–74. doi: 10.1021/bi200923k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell KL, Roberts JE, Watson LN, Stetefeld J, Sloan AM, Signore AV, Howatt JW, Tame JR, Rohland N, Shen TJ, et al. Substitutions in woolly mammoth hemoglobin confer biochemical properties adaptive for cold tolerance. Nat Genet. 2010;42(6):536–40. doi: 10.1038/ng.574. [DOI] [PubMed] [Google Scholar]
- 42.Noguchi H, Campbell KL, Ho C, Unzai S, Park SY, Tame JR. Structures of haemoglobin from woolly mammoth in liganded and unliganded states. Acta Crystallogr D Biol Crystallogr. 2012;68(Pt 11):1441–9. doi: 10.1107/S0907444912029459. [DOI] [PubMed] [Google Scholar]
- 43.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Jr, et al. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35(22):6976–83. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 44.Sakai H, Sou K, Horinouchi H, Kobayashi K, Tsuchida E. Review of hemoglobin-vesicles as artificial oxygen carriers. Artif Organs. 2009;33(2):139–45. doi: 10.1111/j.1525-1594.2008.00698.x. [DOI] [PubMed] [Google Scholar]
- 45.Sakai H, Horinouchi H, Tsuchida E, Kobayashi K. Hemoglobin vesicles and red blood cells as carriers of carbon monoxide prior to oxygen for resuscitation after hemorrhagic shock in a rat model. Shock. 2009;31(5):507–14. doi: 10.1097/SHK.0b013e318188f83d. [DOI] [PubMed] [Google Scholar]
- 46.Sakai H, Hara H, Yuasa M, Tsai AG, Takeoka S, Tsuchida E, Intaglietta M. Molecular dimensions of Hb–based O(2) carriers determine constriction of resistance arteries and hypertension. Am J Physiol Heart Circ Physiol. 2000;279(3):H908–15. doi: 10.1152/ajpheart.2000.279.3.H908. [DOI] [PubMed] [Google Scholar]
- 47.Raat NJ, Liu JF, Doyle MP, Burhop KE, Klein J, Ince C. Effects of recombinant-hemoglobin solutions rHb2.0 and rHb1.1 on blood pressure, intestinal blood flow, and gut oxygenation in a rat model of hemorrhagic shock. J Lab Clin Med. 2005;145(1):21–32. doi: 10.1016/j.lab.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 48.Jia Y, Buehler PW, Boykins RA, Venable RM, Alayash AI. Structural basis of peroxide-mediated changes in human hemoglobin: a novel oxidative pathway. J Biol Chem. 2007;282:4894–907. doi: 10.1074/jbc.M609955200. [DOI] [PubMed] [Google Scholar]
- 49.Buehler PW, Karnaukhova E, Gelderman MP, Alayash AI. Blood aging, safety, and transfusion: capturing the “radical” menace. Antioxid Redox Signal. 2011;14(9):1713–28. doi: 10.1089/ars.2010.3447. [DOI] [PubMed] [Google Scholar]
- 50.Alayash AI. Oxidative pathways in the sickle cell and beyond. Blood Cells Mol Dis. 2017 doi: 10.1016/j.bcmd.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alayash AI. Hemoglobin-Based Blood Substitutes and the Treatment of Sickle Cell Disease: More Harm than Help? Biomolecules. 2017;7(1) doi: 10.3390/biom7010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reeder BJ, Grey M, Silaghi-Dumitrescu RL, Svistunenko DA, Bulow L, Cooper CE, Wilson MT. Tyrosine residues as redox cofactors in human hemoglobin: implications for engineering nontoxic blood substitutes. J Biol Chem. 2008;283(45):30780–7. doi: 10.1074/jbc.M804709200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reeder BJ, Svistunenko DA, Cooper CE, Wilson MT. Engineering tyrosine-based electron flow pathways in proteins: the case of aplysia myoglobin. J Am Chem Soc. 2012;134(18):7741–9. doi: 10.1021/ja211745g. [DOI] [PubMed] [Google Scholar]
- 54.Silkstone GG, Silkstone RS, Wilson MT, Simons M, Bulow L, Kallberg K, Ratanasopa K, Ronda L, Mozzarelli A, Reeder BJ, et al. Engineering tyrosine electron transfer pathways decreases oxidative toxicity in hemoglobin: implications for blood substitute design. Biochem J. 2016;473(19):3371–83. doi: 10.1042/BCJ20160243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silkstone RS, Silkstone G, Baath JA, Rajagopal B, Nicholls P, Reeder BJ, Ronda L, Bulow L, Cooper CE. The betaLys66Tyr Variant of Human Hemoglobin as a Component of a Blood Substitute. Adv Exp Med Biol. 2016;876:455–60. doi: 10.1007/978-1-4939-3023-4_57. [DOI] [PubMed] [Google Scholar]
- 56.Brooks J. The oxidation of haemoglobin to methaemoglobin by oxygen. Proceedings of the Royal Society of London. 1931;109:35–50. [Google Scholar]
- 57.Brooks J. The Oxidation of Haemoglobin to Methaemoglobin by Oxygen. Ii-The Relation between the Rate of Oxidation and the Partial Pressure of Oxygen. Proc R Soc Lond B Biol Sci. 1935;118:560–577. [Google Scholar]
- 58.Brooks J. The oxidation of haemoglobin to methaemoglobin by oxygen. J Physiol. 1948;107(3):332–5. doi: 10.1113/jphysiol.1948.sp004277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wallace WJ, Houtchens RA, Maxwell JC, Caughey WS. Mechanism of autooxidation for hemoglobins and myoglobins. Promotion of superoxide production by protons and anions. J Biol Chem. 1982;257(9):4966–77. [PubMed] [Google Scholar]
- 60.Shikama K. A controversy on the mechanism of autoxidation of oxymyoglobin and oxyhaemoglobin: oxidation, dissociation, or displacement? Biochem J. 1984;223(1):279–80. doi: 10.1042/bj2230279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brantley RE, Jr, Smerdon SJ, Wilkinson AJ, Singleton EW, Olson JS. The mechanism of autooxidation of myoglobin. J Biol Chem. 1993;268(10):6995–7010. [PubMed] [Google Scholar]
- 62.George P, Stratmann CJ. The oxidation of myoglobin to metmyglobin by oxygen. 2. The relation between the first order rate constant and the partial pressure of oxygen. Biochem J. 1952;51(3):418–25. doi: 10.1042/bj0510418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wallace WJ, Maxwell JC, Caughey WS. The mechanisms of hemoglobin autoxidation. Evidence for proton-assisted nucleophilic displacement of superoxide by anions. Biochem Biophys Res Commun. 1974;57(4):1104–10. doi: 10.1016/0006-291x(74)90810-9. [DOI] [PubMed] [Google Scholar]
- 64.Weiss JJ. Nature of the Iron-Oxygen Bond in Oxyhaemoglobin. Nature. 1964;202:83–4. doi: 10.1038/202083b0. [DOI] [PubMed] [Google Scholar]
- 65.Olson JS, Phillips GN., Jr Kinetic pathways and barriers for ligand binding to myoglobin. J Biol Chem. 1996;271(30):17593–6. doi: 10.1074/jbc.271.30.17593. [DOI] [PubMed] [Google Scholar]
- 66.Kakar S, Hoffman FG, Storz JF, Fabian M, Hargrove MS. Structure and reactivity of hexacoordinate hemoglobins. Biophys Chem. 2010;152(1–3):1–14. doi: 10.1016/j.bpc.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang L, Levy A, Rifkind JM. Autoxidation of hemoglobin enhanced by dissociation into dimers. J Biol Chem. 1991;266(36):24698–701. [PubMed] [Google Scholar]
- 68.Mollan TL, Jia Y, Banerjee S, Wu G, Kreulen RT, Tsai AL, Olson JS, Crumbliss AL, Alayash AI. Redox properties of human hemoglobin in complex with fractionated dimeric and polymeric human haptoglobin. Free Radic Biol Med. 2014;69:265–77. doi: 10.1016/j.freeradbiomed.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MacQuarrie RA, Gibson QH. Functional heterogeneity of the alpha and beta chains in the oxidation-reduction reaction of human hemoglobin. J Biol Chem. 1971;246(2):517–22. [PubMed] [Google Scholar]
- 70.Tsuruga M, Matsuoka A, Hachimori A, Sugawara Y, Shikama K. The molecular mechanism of autoxidation for human oxyhemoglobin. Tilting of the distal histidine causes nonequivalent oxidation in the β chain. J Biol Chem. 1998;273(15):8607–15. doi: 10.1074/jbc.273.15.8607. [DOI] [PubMed] [Google Scholar]
- 71.Draghi F, Miele AE, Travaglini-Allocatelli C, Vallone B, Brunori M, Gibson QH, Olson JS. Controlling ligand binding in myoglobin by mutagenesis. J Biol Chem. 2002;277(9):7509–19. doi: 10.1074/jbc.M109206200. [DOI] [PubMed] [Google Scholar]
- 72.Olson JS, Ghosh A. In: The Smallest Biomolecules: Perspectives on Heme-Diatomic Interactions, Chapter 1: Mammalian Myoglobin as a Model for Understanding Ligand Affinities and Discrimination in Heme Proteins. Ghosh A, editor. London: Elsevier; 2007. pp. 2–17. [Google Scholar]
- 73.Carver TE, Brantley RE, Jr, Singleton EW, Arduini RM, Quillin ML, Phillips GN, Jr, Olson JS. A novel site-directed mutant of myoglobin with an unusually high O2 affinity and low autooxidation rate. J Biol Chem. 1992;267(20):14443–50. [PubMed] [Google Scholar]
- 74.Wiltrout ME, Giovannelli JL, Simplaceanu V, Lukin JA, Ho NT, Ho C. A biophysical investigation of recombinant hemoglobins with aromatic B10 mutations in the distal heme pockets. Biochemistry. 2005;44(19):7207–17. doi: 10.1021/bi048289a. [DOI] [PubMed] [Google Scholar]
- 75.Tam MF, Rice NW, Maillett DH, Simplaceanu V, Ho NT, Tam TC, Shen TJ, Ho C. Autoxidation and oxygen binding properties of recombinant hemoglobins with substitutions at the alphaVal-62 or betaVal-67 position of the distal heme pocket. J Biol Chem. 2013;288(35):25512–21. doi: 10.1074/jbc.M113.474841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hargrove MS, Olson JS. The stability of holomyoglobin is determined by heme affinity. Biochemistry. 1996;35(35):11310–8. doi: 10.1021/bi9603736. [DOI] [PubMed] [Google Scholar]
- 77.Hargrove MS, Whitaker T, Olson JS, Vali RJ, Mathews AJ. Quaternary structure regulates hemin dissociation from human hemoglobin. J Biol Chem. 1997;272(28):17385–9. doi: 10.1074/jbc.272.28.17385. [DOI] [PubMed] [Google Scholar]
- 78.Hargrove MS, Singleton EW, Quillin ML, Ortiz LA, Phillips GN, Jr, Olson JS, Mathews AJ. His64(E7)-->Tyr apomyoglobin as a reagent for measuring rates of hemin dissociation. J Biol Chem. 1994;269(6):4207–14. doi: 10.2210/pdb1mgn/pdb. [DOI] [PubMed] [Google Scholar]
- 79.Hargrove MS, Wilkinson AJ, Olson JS. Structural factors governing hemin dissociation from metmyoglobin. Biochemistry. 1996;35(35):11300–9. doi: 10.1021/bi960372d. [DOI] [PubMed] [Google Scholar]
- 80.Andersen CB, Torvund-Jensen M, Nielsen MJ, de Oliveira CL, Hersleth HP, Andersen NH, Pedersen JS, Andersen GR, Moestrup SK. Structure of the haptoglobin-haemoglobin complex. Nature. 2012;489(7416):456–9. doi: 10.1038/nature11369. [DOI] [PubMed] [Google Scholar]
- 81.Samuel PP, Ou WC, Phillips GN, Jr, Olson JS. Mechanism of Human Apohemoglobin Unfolding. Biochemistry. 2017;56(10):1444–1459. doi: 10.1021/acs.biochem.6b01235. [DOI] [PubMed] [Google Scholar]
- 82.Samuel PP, Smith LP, Phillips GN, Jr, Olson JS. Apoglobin Stability Is the Major Factor Governing both Cell-free and in Vivo Expression of Holomyoglobin. J Biol Chem. 2015;290(39):23479–95. doi: 10.1074/jbc.M115.672204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bisse E, Schaeffer-Reiss C, Van Dorsselaer A, Alayi TD, Epting T, Winkler K, Benitez Cardenas AS, Soman J, Birukou I, Samuel PP, et al. Hemoglobin Kirklareli (alpha H58L), a New Variant Associated with Iron Deficiency and Increased CO Binding. J Biol Chem. 2017;292(6):2542–2555. doi: 10.1074/jbc.M116.764274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCarthy MR, Vandegriff KD, Winslow RM. The role of facilitated diffusion in oxygen transport by cell-free hemoglobins: implications for the design of hemoglobin-based oxygen carriers. Biophys Chem. 2001;92(1–2):103–17. doi: 10.1016/s0301-4622(01)00194-6. [DOI] [PubMed] [Google Scholar]
- 85.Chen JY, Scerbo M, Kramer G. A review of blood substitutes: examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics (Sao Paulo) 2009;64(8):803–13. doi: 10.1590/S1807-59322009000800016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299(19):2304–12. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gulati A, Singh G, Rebello S, Sharma AC. Effect of diaspirin crosslinked and stroma-reduced hemoglobin on mean arterial pressure and endothelin-1 concentration in rats. Life Sci. 1995;56(17):1433–42. doi: 10.1016/0024-3205(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 88.Vandegriff KD, Malavalli A, Minn C, Jiang E, Lohman J, Young MA, Samaja M, Winslow RM. Oxidation and haem loss kinetics of poly(ethylene glycol)-conjugated haemoglobin (MP4): dissociation between in vitro and in vivo oxidation rates. Biochem J. 2006;399(3):463–71. doi: 10.1042/BJ20060809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katz LM, Manning JE, McCurdy S, Sproule C, McGwin G, Jr, Moon-Massat P, Cairns CB, Freilich D. Nitroglycerin attenuates vasoconstriction of HBOC-201 during hemorrhagic shock resuscitation. Resuscitation. 2010;81(4):481–7. doi: 10.1016/j.resuscitation.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 90.Vandegriff KD, Malavalli A, Wooldridge J, Lohman J, Winslow RM. MP4, a new nonvasoactive PEG-Hb conjugate. Transfusion. 2003;43(4):509–16. doi: 10.1046/j.1537-2995.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 91.Abuchowski A. SANGUINATE (PEGylated Carboxyhemoglobin Bovine): Mechanism of Action and Clinical Update. Artif Organs. 2017;41(4):346–350. doi: 10.1111/aor.12934. [DOI] [PubMed] [Google Scholar]
- 92.Abuchowski A, Sloshberg S, O’hare K. Hemoglobin Compositions. USA: Prolong Pharmaceuticals; 2010. [Google Scholar]
- 93.Hermann J, Corso C, Messmer KF. Resuscitation with recombinant hemoglobin rHb2.0 in a rodent model of hemorrhagic shock. Anesthesiology. 2007;107(2):273–80. doi: 10.1097/01.anes.0000270756.11669.64. [DOI] [PubMed] [Google Scholar]