Abstract
Background/Objectives
Obesity (body mass index (BMI) ≥ 30) is associated with an increased risk of estrogen-dependent breast cancer after menopause. Levels of aromatase, the rate-limiting enzyme in estrogen biosynthesis, are elevated in breast tissue of obese women. Recently, the regulation of aromatase by the p53-HIF1α/PKM2 axis was characterized in adipose stromal cells (ASCs) of women with Li-Fraumeni Syndrome, a hereditary cancer syndrome that predisposes to estrogen-dependent breast cancer. The current study aimed to determine whether stimulation of aromatase by obesity-associated adipokine leptin involves the regulation of the p53-HIF1α/PKM2 axis.
Subjects/Methods
Human breast ASCs were used to characterize the p53-HIF1α/PKM2-aromatase axis in response to leptin. The effect of pharmacological or genetic modulation of PKC, MAPK, p53, Aha1, Hsp90, HIF1α and PKM2 on aromatase promoter activity, expression and enzyme activity was examined. Semi-quantitative immunofluorescence and confocal imaging were used to assess ASC-specific protein expression in FFPE sections of breast of women and mammary tissue of mice following a low fat (LF) or high fat (HF) diet for 17 weeks.
Results
Leptin-mediated induction of aromatase was dependent on PKC/MAPK signaling and the suppression of p53. This, in turn, was associated with an increase in Aha1 protein expression, activation of Hsp90 and the stabilization of HIF1α and PKM2, known stimulators of aromatase expression. Consistent with these findings, ASC-specific immunoreactivity for p53 was inversely associated with BMI in breast tissue, while HIF1α, PKM2 and aromatase were positively correlated with BMI. In mice, HF feeding was associated with significantly lower p53 ASC-specific immunoreactivity compared to LF feeding, while immunoreactivity for HIF1α, PKM2 and aromatase were significantly higher.
Conclusions
Overall, findings demonstrate a novel mechanism for the obesity-associated increase in aromatase in ASCs of the breast and support the study of lifestyle interventions, including weight management, which may reduce breast cancer risk via effects on this pathway.
Introduction
Estrogen receptor positive (ER+) breast cancers account for over 70% of breast cancer diagnoses (1). Despite low levels of circulating estrogens, the majority of ER+ breast cancers occur after menopause (2). In these women, it is hypothesized that estrogens synthesized locally within the breast fat are responsible for driving the growth of hormone-dependent cancers. The local biosynthesis of estrogens is dependent on the expression of the aromatase enzyme which catalyzes the final and key step in estrogen biosynthesis. Recently, it was demonstrated that breast aromatase is increased as a function of both body mass index (BMI) and menopausal status (3, 4). Consistently, obesity, defined as having a BMI of 30 or above, increases the risk of ER+ breast cancer in postmenopausal women, may impair the efficacy of breast cancer treatment, and increases the risk of recurrence and cancer-associated death (5, 6). More specifically, the risk of ER+ breast cancer is approximately doubled in obese compared to healthy weight postmenopausal women (7). With obesity, the excessive storage of fat in adipocytes leads to an unhealthy expansion of adipose tissue which is associated with endoplasmic reticulum stress, adipose tissue fibrosis and localized hypoxia, leading to the initiation of an inflammatory response (8, 9). Increased fat mass is also associated with dramatic changes in adipokine secretion, including the increased production of the appetite-suppressing peptide hormone leptin. Inflammatory mediators and leptin are key stimulators of aromatase transcript expression in adipose stromal cells (ASCs) of the breast, and metabolic pathways have recently been implicated in this regulation (reviewed in 10). For example, inflammatory mediator PGE2 and leptin have been shown to drive aromatase expression via the suppression of the metabolic regulators LKB1/AMPK (11). This leads, in turn, to the nuclear translocation of CREB-coactivators, the CRTC proteins, and increased expression of aromatase. PGE2 was also demonstrated to stimulate aromatase via effects on tumor suppressor p53 and oncogene hypoxia-inducible factor-1α (HIF1α). Specifically, p53 was identified as a transcriptional repressor of the aromatase gene via binding to a p53 response element on promoter PII, the main promoter used to drive aromatase expression in the ovary and obesity- or cancer-associated breast adipose tissue (12), while PGE2 stabilized HIF1α, leading to cooperative binding with CREB to aromatase promoter PII and stimulation of aromatase expression (13). Findings in Li-Fraumeni Syndrome (LFS) patients, who have germline mutations in the TP53 gene and are at increased risk of ER+ breast cancer (14), were consistent with in vitro studies (12, 15). Namely, that mutation of p53 was associated with elevated aromatase expression in the breast adipose stroma. Further studies demonstrated that in addition to direct effects on promoter activity, loss of p53 function in LFS, leads to the stabilization of HIF1α and pyruvate kinase M2 (PKM2) proteins via Hsp90-dependent mechanisms (15). PKM2 is another metabolic regulator that has been implicated in the shift in mechanism of energy metabolism from mitochondrial respiration to aerobic glycolysis referred to as the Warburg effect, a phenomenon that occurs frequently in cancer. HIF1α and PKM2 were shown to co-localize to the nucleus of ASCs, bind to the aromatase promoter and stimulate its expression (13, 15). This novel axis, first characterized in a hereditary cancer syndrome and implicating p53-HIF1α/PKM2-aromatase has, to date, not been examined in the context of obesity, and despite numerous studies examining the impact of obesity-associated factors on isolated ASCs, little is known about the in situ expression of aromatase in relation to obesity. The aim of the current study was to determine whether aromatase regulation by leptin involves the p53-HIF1α/PKM2-aromatase axis and to examine, in situ, the relationship between BMI and the breast ASC-specific immunoreactivity of members of the p53-HIF1α/PKM2-aromatase axis.
Material and Methods
Materials
MTT assay kits, glucose-6-phosphate, pepstatin, leupeptin, glucose-6-phosphate dehydrogenase, DMSO, L-LDH, leptin, calphostin C, phosphoenolpyruvate, pyruvate kinase, NADH, antibodies to β-actin and primers for aromatase were obtained from Sigma. SP600125, SB202190, PD98059, and 17-Allylamino-17demethoxygeldanamycin (17-AAG) were obtained from Cayman Chemicals. PU-H71 was purchased from Tocris Bioscience. Monoclonal aromatase antibody 677 was obtained from the Baylor College of Medicine. Antibodies to ERK1/2 (1:1000; #9102), pERK1/2 (1:1000; #4370), p38 (1:1000; #8690), phospho-p38 (1:1000; #4511), JNK (1:1000; #9252), phospho-JNK (1:500; #9255), PKM2 (1:1000; #4053) and HIF-1α (1:1000; #14179) were from Cell Signaling Technology. The p53 antibody (1:1000; SC-6243) was from Santa Cruz. Antibody to Aha1 was purchased from Abcam (1:1000; EPR13888). Lipofectamine 2000 and PKC activity kits were from (Invitrogen). Control siRNA or siRNAs to Aha1, HIF-1α and PKM2 were from Thermo Scientific. Primers for p53 (QT00060235) and β-actin (QT01680476) were purchased from Qiagen. Western blotting detection reagents were from Perkin Elmer. Reagents for the luciferase assay and pSVβgal were from Promega. The aromatase promoter (CYP19PII)-luciferase construct was kindly provided by Dr. S. Chen (City of Hope, Duarte, CA). 1β-[3H]-androstenedione and [32P]-ATP were from Perkin-Elmer Life Science. Expression vectors for p53 were from Addgene.
Human adipose stromal cells and breast tissue
Immortalized human mammary ASCs HMS32-hTERT were provided by Dr. Brittney-Shea Herbert (Indiana University School of Medicine) (16). Human primary preadipocytes (ASCs) derived from subcutaneous fat and preadipocyte growth medium were purchased from Cell Applications, Inc. Cellular cytotoxicity was assessed by measurements of cell number, lactate dehydrogenase release, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. No evidence of cell toxicity was detected in any of the experiments described below.
The study and sample collection for immunofluorescence-based studies was approved by the Institutional Review Boards of Memorial Sloan Kettering Cancer Center (MSKCC) and Weill Cornell Medical College in New York, USA (#1004010984). Women undergoing mastectomy at MSKCC for the treatment or prevention of breast cancer (n= 52) were consented under a standard tissue acquisition protocol. Normal breast tissue from a quadrant uninvolved by tumor was collected in 4% neutral buffered formaldehyde. Formaldehyde-fixed tissue was later processed and embedded in paraffin. Patient medical records were reviewed to record patient information including age and menopausal status. Height (in meters) and weight (kg) recorded on the day of surgery were used to calculate BMI as kg/m2. Clinicopathologic features are reported in Supplemental Table 1. Sample size was determined based on both the availability of tissue samples and the statistical power for detecting expected correlation between aromatase levels and BMI. With 50 subjects we have >85% power to detect a Pearson correlation of around 0.45 using a two-sided hypothesis test with a significance level of 0.05.
PKC, aromatase and Hsp90 ATPase activity assays
Total PKC activity was measured in cell lysates. To determine cytosolic and membrane-bound PKC activity, cell lysates were centrifuged at 100,000 × g for 30 min. The resulting supernatant contains cytosolic PKC; membrane-bound PKC activity is present in the pellet. DEAE cellulose columns were used to partially purify PKC enzymes. PKC activity was then measured by incubating partially purified PKC with [32P]-ATP (3000–6000 Ci/mmol) and the substrate myelin basic protein for 20 min at room temperature. The activity of PKC is expressed as cpm incorporated/μg of protein. Aromatase activity was measured as described previously (3). Aromatase activity is expressed as femtomoles/μg protein. The Hsp90 ATPase assay was based on a regenerating coupled enzyme assay and was performed as described previously (17). Hsp90 ATPase activity is expressed as pmol/min/mg protein.
Western Blotting
Cells were sonicated using a lysis buffer (150 mM NaCl, 100 mM Tris, pH 8.0, 1% Tween 20, 50 mM diethyldithiocarbamate, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml trypsin inhibitor, and 10 μg/ml leupeptin). Cell lysates were centrifuged and the supernatants were used to determine the protein concentration according to Lowry et al. (18). Cell lysates were subjected to SDS-PAGE to separate proteins under reducing conditions on 10% polyacrylamide gels and transferred onto nitrocellulose sheets. The blots were then probed with the ECL western blot detection system after incubation with primary and secondary antibodies.
Quantitative Real-time PCR
RNA was isolated from cells using the RNeasy mini kit (Qiagen). RNA was reversed transcribed to cDNA using murine leukemia virus reverse transcriptase and oligo (dT)16 primer and the cDNA was used for amplification. Primers for aromatase have been described previously (3). Real-time PCR was done using 2x SYBR green PCR master mix on a 7500 Real-time PCR system (Applied Biosystems). Using the ddCT (relative quantification) analysis protocol, relative fold induction was determined.
Transient Transfections
Cells were grown to 60–70% confluence in 6-well dishes and were transfected using Lipofectamine 2000 (Invitrogen) for 24h. Following transfection, the medium was replaced with serum-free medium for another 24h. Luciferase and β-galactosidase enzyme activities were measured in cellular extracts. Luciferase activity in cell lysates was normalized to β-galactosidase enzymatic activity. For experiments involving gene silencing, cells were transfected with 2μg of siRNA oligonucleotides using DharmaFECT 4 transfection reagent according to the manufacturer’s instructions.
Diet-induced obesity mouse model
Sixteen female C57BL/6J mice (n=8/group) were purchased from The Jackson Laboratory (JAX) and ovariectomized at 4 weeks of age. At the age of 5 weeks, mice were randomly assigned to be fed a 10% low fat diet (LFD, 12450Bi) or a 60% high fat diet (HFD, D12492i) for 17 weeks. All mice were sacrificed at 22 weeks of age. Mouse mammary fat pads were obtained and fixed in 4% neutral buffered formaldehyde. Fixed tissue sections were used to assess the levels of p53, HIF1α, PKM2 and aromatase in adipose stromal cells using immunofluorescence. Sample size was selected based on investigators’ previous experience in conducting animal studies. Mouse experiments were conducted in accordance with protocols approved by the Institutional Animal Care and MMaterial Use Committee at Weill Cornell Medicine (New York, NY). Investigators were not blinded.
Immunofluorescence, confocal microscopy and the adipose stromal cell-specific assessment of immunofluorescent staining
Formalin-fixed paraffin-embedded sections from human breast tissue and mouse mammary glands were deparaffinized with xylene and rehydrated in descending grades of ethanol. Immunofluorescence was performed after antigen retrieval. For p53 and aromatase, sections were subjected to microwave in 10mM Citric Acid for 2 min at high power followed by 5 min at 50% power, while staining for HIF1α and PKM2 was performed after heating sections in 10mM Tris/1mM EDTA buffer for 30min in a 100°C water bath. Sections were then cooled for 30 min at room temperature and washed in PBS and blocked with 0.5% BSA/PBS for 30min. Sections were incubated with primary antibodies overnight at 4°C, followed by the application of the appropriate secondary antibody, either 1:750 of anti-mouse Alexa Fluor 546 or 1:1000 anti-rabbit Alexa Fluor 488 and 1:2000 Hoechst 33342 (Invitrogen) for 2 h. Finally, the sections were washed and mounted with ProLong® Gold Antifade Mountant (P36934-Thermo Fisher Scientific) and stored in dark at 4°C until imaging. Imaging was done using a Nikon inverted confocal microscope (1024 × 1024 pixels, 3 × 3 tiled images, 3 images per case). Identical confocal settings were used to limit intra-experimental variability. Metamorph® software (Molecular Devices, USA) was used to quantify nuclear immunoreactivity for p53, HIF1α and PKM2 and peri-nuclear aromatase immunoreactivity in ASCs specifically, as previously described (19). Investigators were blinded to BMI or experimental group when performing these studies.
Statistical analyses
Differences in patient characteristics across BMI categories were examined using the non-parametric Kruskal-Wallis rank sum test for continuous variables and Fisher’s exact test for categorical variables. Continuous experimental data were expressed as mean ± standard deviation (SD). Comparisons between two groups were made by Student’s t test. If multiple treatment groups were examined, statistical analysis was done using one-way ANOVA followed by Dunnett’s test whereby the effect of treatment was compared to control. Statistical significance was defined as *p ≤ 0.05; **p ≤ 0.005; ***p ≤ 0.0005; ****p ≤ 0.0001. Correlations between protein immunoreactivity and BMI or immunoreactivity of two proteins, as well as the strength of the correlation between the aromatase expression and each blood parameter in the study cohort was examined using the non-parametric Spearman method. Data analysis was performed using GraphPad Prism version 5.02 (GraphPad Software, San Diego, CA, USA) and R version 3.3.1[ref: R Core Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/.].
Results
Leptin induces aromatase gene expression via effects on PKC and ERK1/2
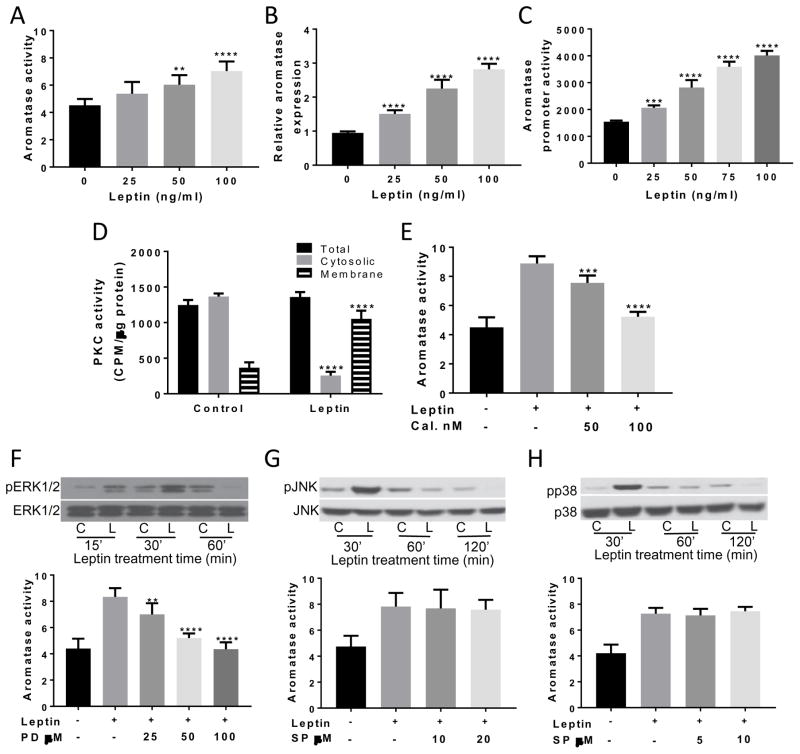
Treatment with leptin caused a dose-dependent increase in aromatase activity in both immortalized (Figure 1A) and primary human ASCs (data not shown). Consistent with a hypothesized effect on aromatase gene transcription, leptin also caused a dose-dependent increase in aromatase mRNA levels (Figure 1B) and promoter activity (Figure 1C). The signal transduction pathway by which leptin stimulated aromatase gene expression was examined. Treatment of ASCs with leptin for 30 min activated PKC and the increase in PKC activity was found in the membrane fraction (Figure 1D). To determine if the observed increase in PKC activity contributed to the induction of aromatase, calphostin C, an inhibitor of PKC activity, was used. Calphostin C blocked the leptin-mediated induction of aromatase activity (Figure 1E). PKC can activate MAPK signaling. Interestingly, leptin activated ERK1/2, p38 and JNK MAPKs (Figure 1F–H). Selective inhibitors of these MAPKs were tested for their ability to block the leptin-mediated induction of aromatase. PD98059, an inhibitor of MAP kinase kinase, was the only inhibitor to block the induction of aromatase by leptin (Figure 1F).
Figure 1. Leptin induces aromatase gene expression via effects on PKC and ERK1/2.
Immortalized ASCs were treated for 24h with 0–100 ng/ml leptin. Following treatment, aromatase (A) activity, (B) mRNA expression and (C) promoter PII activity were examined and found to be increased in a dose-dependent manner. (D) Leptin stimulated the translocation of cytosolic PKC to the membrane. (E) Treatment of cells with PKC inhibitor calphostin (Cal.) led to the dose-dependent inhibition of the leptin-mediated increase in aromatase activity. ASCs were pre-treated with vehicle or the indicated concentrations of calphostin (Cal.) for 2h. Subsequently, cells were treated with leptin or leptin plus calphostin for 24 h. (F; top) Leptin treatment caused a transient increased in ERK1/2 phosphorylation. ASCs were treated with vehicle or 100 ng/ml leptin for the indicated times. Cell lysates were subjected to Western Blotting. (F; bottom) Treatment with MEK1/2 inhibitor PD98059 caused a dose-dependent decrease in the leptin-mediated induction of aromatase activity. ASCs were pretreated with vehicle or the indicated concentrations of PD98059 for 2h. Subsequently, the cells received vehicle, 100ng/ml leptin or leptin + 25–100 uM PD98059 for 24h. (G,H; top) Western blotting demonstrating time-dependent effects of leptin on phosphorylation of JNK (G) and p38 (H). No effect of inhibitors of JNK or p38 MAPK on aromatase activity was observed (G,H; bottom). ASCs were pretreated with indicated concentrations of SP600125 or SB202190, respectively for 2h. Subsequently, cells received vehicle, 100ng/ml leptin or leptin + the indicated concentrations of SP600125 or SB202190 for 24h. Aromatase activity is expressed as femtomoles/μg protein. Data are presented as mean ± SD, n=6. C: control; L: leptin.
Downregulation of p53 contributes to the leptin-dependent induction of aromatase
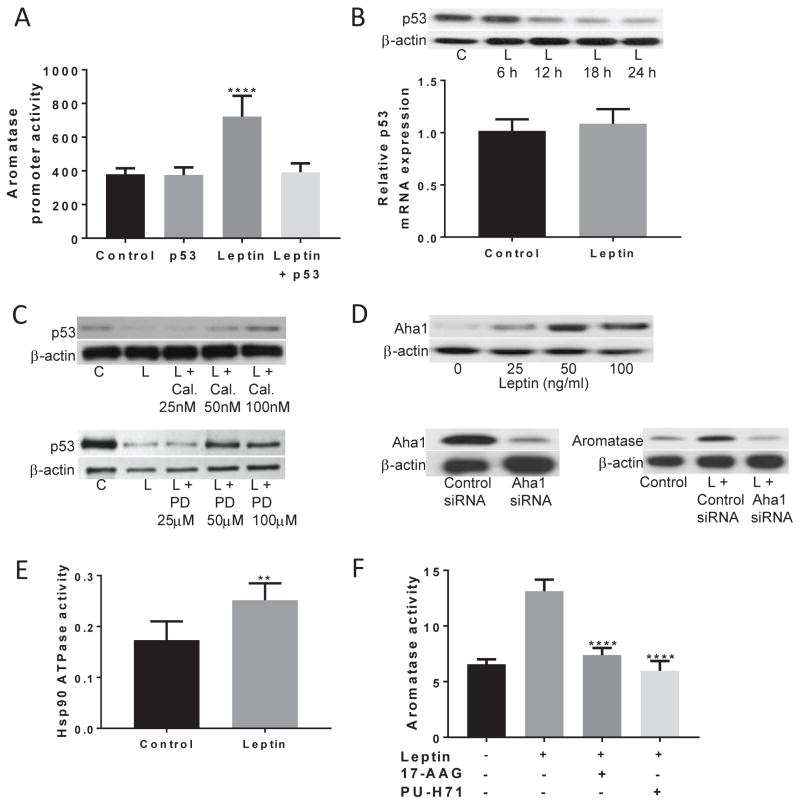
Previously, we reported that p53 suppresses aromatase expression in ASCs (15, 19). In the current study, transfection of ASCs with a p53 expression vector prevented the induction of aromatase promoter activity by leptin (Figure 2A). Activation of ERK1/2 MAPK has previously been shown to downregulate p53 protein levels (20). Hence, the effect of leptin on p53 was examined. Treatment with leptin suppressed p53 protein levels without affecting p53 mRNA levels (Figure 2B). The downregulation of p53 by leptin was dependent on PKC and ERK1/2, as use of calphostin C and PD98059 prevented the leptin-mediated decreased in p53 levels in a dose-dependent manner (Figure 2C). Loss of p53 has been shown to stimulate Hsp90 ATPase activity via the induction of Aha1, a co-chaperone of Hsp90, resulting in induction of aromatase (15). Leptin caused a dose-dependent increase in Aha1 protein levels and silencing Aha1 blocked the leptin-mediated induction of aromatase (Figure 2D). In addition to inducing Aha1, leptin stimulated Hsp90 ATPase activity (Figure 2E) and the importance of the leptin-mediated induction of Hsp90 ATPase on aromatase was defined using prototypic inhibitors of Hsp90 ATPase, 17-AAG and PU-H71. Each of these Hsp90 ATPase inhibitors blocked the leptin-mediated induction of aromatase activity (Figure 2F).
Figure 2. Downregulation of p53 contributes to the leptin-dependent induction of aromatase.
(A) p53 overexpression abolishes the leptin-mediated induction of aromatase promoter activity. ASCs were transfected with 0.9 μg aromatase promoter PII-luciferase and 0.2 μg psvβ-gal constructs. Cells also received 0.9 μg p53 expression vector or 0.9 μg control vector. 48h after transfection, cells were treated with vehicle or 100 ng/ml leptin for 24h and aromatase promoter activity was assessed. (B) Leptin caused a time-dependent decrease in p53 protein levels (top) with no effect on p53 mRNA expression (bottom). (C) Suppression of p53 protein levels in response to leptin were dependent on PKC and ERK1/2 activity. ASCs were pretreated with vehicle or the indicated doses of calphostin C (Cal.; top) or PD98059 (bottom) for 2h. Subsequently, cells were treated with vehicle, 100 ng/ml leptin or 100 ng/ml leptin plus the indicated concentrations of calphostin C or PD98059 for 24h. (D) The leptin-mediated increase in aromatase expression is dependent on the increased expression of Aha1. Top, Leptin induced Aha1 protein levels in a dose-dependent manner. Bottom, ASCs were transfected with 2 μg of control or Aha1 siRNA. 48h later, cell lysates were subjected to Western Blotting (left hand panel). In the right hand panel, following transfection cells were treated with vehicle or 100 ng/ml leptin for 24h, and aromatase protein levels were examined. (E) 24h treatment with 100ng/ml leptin caused a significant increase in Hsp90 ATPase activity. (F) Leptin-mediated induction of aromatase activity was dependent on Hsp90. ASCs were pre-treated with vehicle, 1 μM 17-AAG or 0.5 μM PU-H71 for 2h. Subsequently, the cells received vehicle, 100ng/ml leptin, leptin and 17-AAG or leptin and PU-H71 for an additional 24h. Data are presented as mean ± SD, n=6. C: control; L: leptin
Leptin-mediated induction of aromatase expression is dependent on HIF1α and PKM2
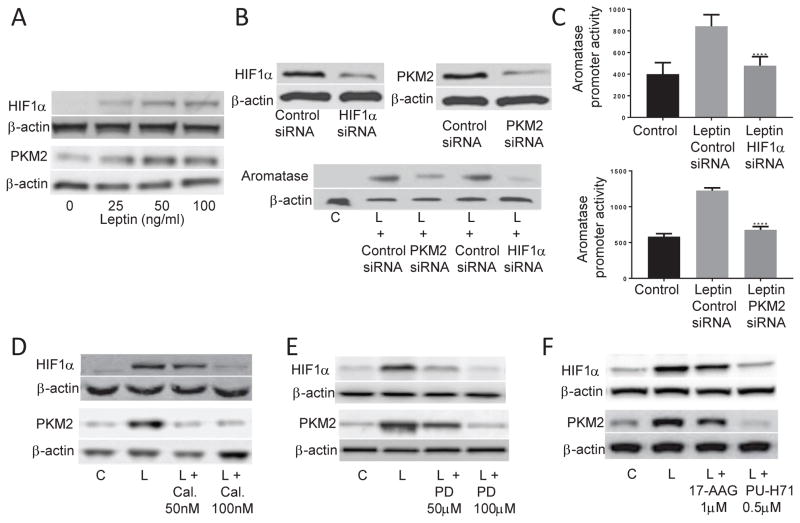
HIF1α and PKM2 are Hsp90 client proteins that regulate aromatase expression (13, 15). Leptin induced the levels of both HIF1α and PKM2 proteins (Figure 3A). Silencing either HIF1α or PKM2 blocked the leptin-mediated induction of aromatase protein (Figure 3B) and aromatase promoter activity (Figure 3C). The inductive effects of leptin on HIF1α and PKM2 proteins were inhibited by treatment with calphostin C and PD98059 (Figure 3D & E, respectively). Inhibition of Hsp90 ATPase also attenuated the leptin-mediated induction of HIF1α and PKM2 (Figure 3F).
Figure 3. Leptin-mediated induction of aromatase is dependent on HIF1α and PKM2.
(A) 24h treatment of ASCs with leptin caused a dose-dependent increase in HIF1α (top) and PKM2 (bottom) protein levels. (B) Stimulation of aromatase protein expression and (C) promoter activity by leptin is dependent on HIF1α and PKM2 expression. ASCs were transfected with 2 μg of control siRNA or siRNAs to HIF1α or PKM2 for 48h. Cells were then lysed and subjected to Western Blotting for HIF1α and PKM2. Following transfection, cells received vehicle or 100 ng/ml leptin for 24h. Western blotting for aromatase was then carried out. For aromatase promoter activity, cells were transfected with 0.9 μg of the indicated siRNA, 0.9 μg of aromatase promoter PII-luciferase and 0.2μg of psvβ-gal constructs. 48h after transfection, cells received vehicle or 100 ng/ml leptin for 24h. Luciferase activity was normalized to β-galactosidase activity. Effects of leptin on HIF1α and PKM2 are dependent on PKC, ERK1/2 and Hsp90 ATPase activity. ASCs were pretreated with vehicle or the indicated doses of (D) calphostin C, (E) PD98059 or (F) 17-AAG or PU-H71, for 2h. Then, cells were treated with vehicle, 100 ng/ml leptin or 100 ng/ml leptin plus the indicated concentrations of respective inhibitors for 24h. Western blotting was performed on whole cell lysates. β-actin used as a loading control. Data are presented as mean ± SD, n=6. C: control; L: leptin
BMI and high fat feeding are associated with immunoreactivity of proteins in the p53-HIF1α/PKM2-aromatase axis in ASCs of women and mice
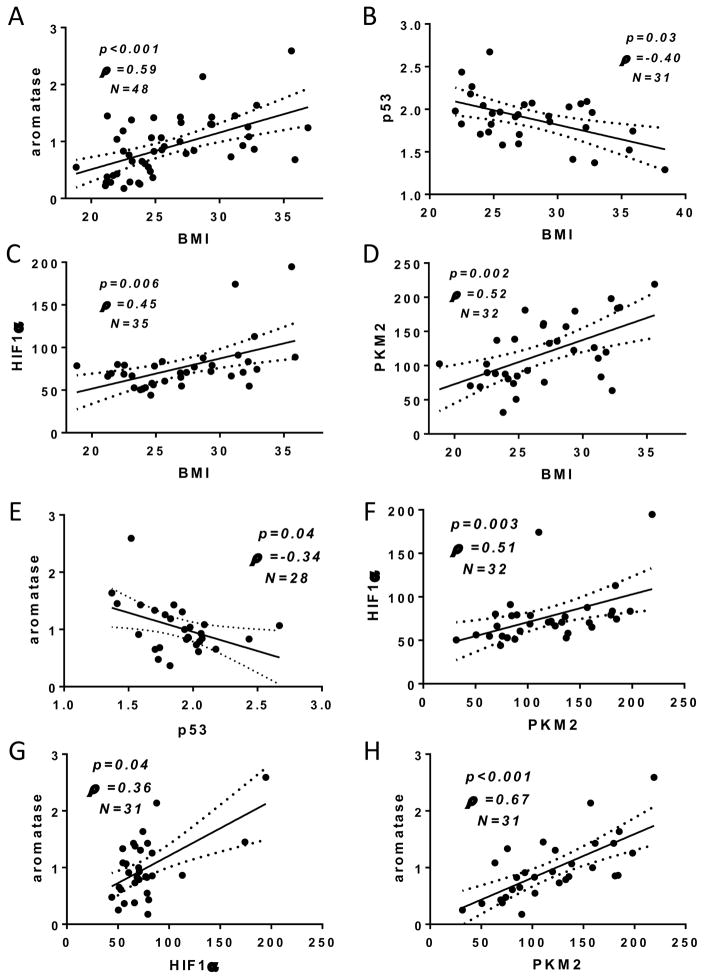
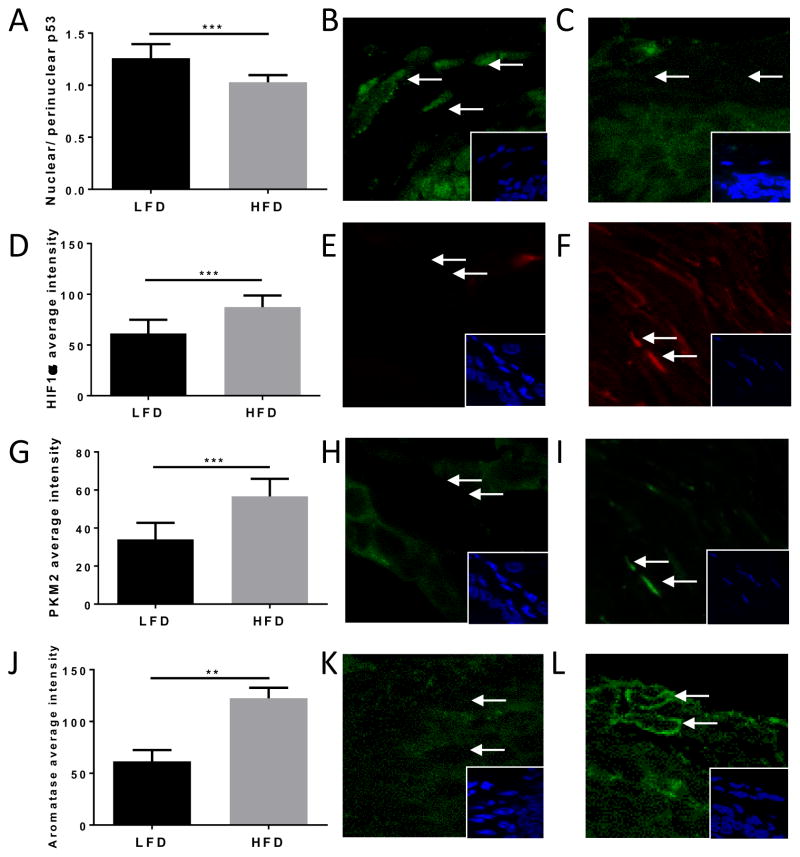
A total of 52 women were enrolled with median BMI 24.9 (IQR 23–29.42). Average fluorescent staining intensity for p53, HIF1α, PKM2 and aromatase was measured in ASCs of FFPE tissue sections. Aromatase immunoreactivity was positively correlated with BMI (Figure 4A), while nuclear p53 levels were inversely correlated with BMI (Figure 4B). Immunoreactivity for both HIF1α and PKM2 were positively correlated with BMI (Figure 4C & D, respectively). Associations between the average fluorescence intensity for each protein were also examined on a case-by-case basis. Aromatase immunoreactivity was found to be inversely correlated with p53 nuclear expression (Figure 4E). Consistent with the hypothesis that HIF1α and PKM2 are co-regulated in ASCs, a positive correlation was also observed between both proteins (Figure 4F). Aromatase was positively correlated with both HIF1α and PKM2 (Figures 4G & H). In C57BL/6J mice, high fat feeding was associated with ASC-specific differences in levels of proteins of the p53-HIF1α/PKM2-aromatase axis (Figure 5). Specifically, immunoreactivity for aromatase, HIF1α and PKM2 were significantly higher in high fat-fed mice compared to low fat-fed mice. Conversely, nuclear p53 levels were significantly lower in the HFD group compared to the LFD group.
Figure 4. BMI is positively correlated with immunoreactivity of proteins in the p53-HIF1α/PKM2-aromatase axis in ASCs of women.
Breast ASC-specific immunoreactivity for aromatase, p53, HIF1α, and PKM2 are correlated with BMI. (A) Average staining intensity for aromatase is positively correlated with BMI (n=48; ρ=0.59; p<0.001). (B) Average nuclear p53 immunoreactivity is negatively correlated with BMI (n=31; ρ=−0.40; p=0.03). Nuclear staining intensity for (C) HIF1α and (D) PKM2 are positively correlated with BMI (n=35; ρ=0.45; p=0.006 and n=32; ρ=0.52; p=0.002, respectively). Per case, aromatase is correlated with p53, HIF1α and PKM2, and HIF1α levels are correlated with PKM2. (E) Aromatase is inversely associated with nuclear p53 immunoreactivity (n=28; ρ= −0.34; p=0.04). (F) HIF1α and PKM2 are positively correlated in ASCs (n=32; ρ=0.51; p=0.003). Aromatase is positively correlated with (G) HIF1α (n=31; ρ=0.36; p=0.04) and (H) PKM2 (n=31; ρ=0.67; p<0.001).
Figure 5. Effect of high fat diet on ASC-specific p53, PKM2, HIF1α and aromatase.
(A) p53 average staining intensity was significantly higher in LFD mammary fat pad ASCs compared to HFD. (B) Nuclear p53 (green) staining is present in ASCs in LFD mice and (C) lower in HFD-fed mice. Nuclear HIF1α (D) and PKM2 (G) average staining intensity was significantly lower in LFD-fed mice compared to HFD-fed mice. Levels of nuclear HIF1α (E; red) and PKM2 (H; green) immunoreactivity in LFD mice compared to HFD (F, I). (J) Aromatase average staining intensity was significantly lower in LFD compared to HFD mice. Lower aromatase (green) staining in (K) LFD compared to (L) HFD. Hoechst 33342 nuclear stain (blue). Error bars represent mean ± SD.
Discussion
The current study provides novel mechanistic insights into the regulation of aromatase by leptin. Furthermore, it also characterizes the ASC-specific immunoreactivity of aromatase and related pathways in relation to obesity in breast tissue from women and in the mammary fat pad of mice.
Obesity is associated with an increase in aromatase expression and activity in the breast tissue of women (3, 4). The current study supports these findings and demonstrates for the first time that the obesity-associated increases in aromatase observed in human breast tissue occur, at least in part, due to the increased in situ expression of aromatase in ASCs. A number of factors known to be altered in obesity have been shown to affect aromatase expression in isolated ASCs. These range from inflammatory mediators associated with white adipose tissue inflammation and adipokines, to gut-derived peptide hormones (10, 21).
Mechanistic insights into the regulation of aromatase have come from in vitro studies where isolated ASCs are manipulated either pharmacologically or through gene silencing, or from the analysis of hereditary cancer syndromes. One example is the identification of the increased expression of aromatase in the ovaries, testis and breast of Peutz Jeghers Syndrome patients (22–24). The majority of Peutz Jeghers cases occur as a consequence of mutations in the STK11 gene that encodes LKB1 and these studies paved the way to the identification of the LKB1/AMPK/CRTC axis as a novel regulator of aromatase in the context of breast cancer (11). These studies also suggested that pathways that were dysregulated in hereditary cancer syndromes could be affected by environmental factors, including inflammatory and adipokine changes that occur as a consequence of obesity and tumor formation (11). More recently, we have demonstrated that patients with Li-Fraumeni Syndrome, a hereditary cancer syndrome that predisposes to many cancers including ER+ breast cancer, is associated with an increase in aromatase expression in the breast adipose stroma (15, 19). Elucidation of the p53-HIF1α/PKM2-aromatase axis was undertaken in breast tissue from these women, leading to the hypothesis that aromatase may also be regulated by this axis in response to host changes, including obesity. Leptin is a well-characterized obesity-associated factor that has been causally linked to the increased growth of breast cancer cells, and despite some studies showing an inverse or no association, most epidemiological studies support a positive association between circulating leptin levels and breast cancer risk (25, 26). Leptin has also been shown to be associated with breast aromatase levels in women with breast cancer in the context of obesity (27), as well as being a potent stimulator of aromatase expression and activity in isolated adipose stromal cells (11, 28, 29), MCF-7 breast cancer cells (30), endometrial cancer cells (31) and luteinized granulosa cells (32). The current study therefore aimed to characterize the regulation of aromatase in breast adipose stromal cells by leptin via effects on the p53-HIF1α/PKM2 axis.
Consistent with previous studies, leptin was shown to stimulate aromatase expression in ASCs via effects on promoter PII (11). The current study, however, also demonstrates that the effects of leptin are dependent on activation of PKC at the plasma membrane and ERK1/2. This mechanism of action is similar to that described in MCF-7 breast cancer cells, whereby the induction of aromatase transcript expression by leptin was attenuated when MAPK inhibitor PD98059 or ERK dominant negative constructs were used (30). In the present study, leptin also led to a decrease in p53 protein levels. Multiple studies have reported that leptin downregulates p53 in other cell types, including hepatic and breast cancer cells (33, 34), trophoblastic cells (20), granulosa cells (35) and prostate cancer cells (36). Previously, p53 was found to bind to a p53 response element on aromatase promoter PII and suppress aromatase expression (19). This may be one mechanism whereby suppression of p53 by leptin leads to the increased expression of aromatase. Interestingly, the decreases in p53 protein levels seen in response to leptin treatment were also associated with an increase in Aha1 protein expression, Hsp90 ATPase activity and the stabilization of HIF1α and PKM2 proteins, consistent with findings in Li-Fraumeni patients (15). Moreover, the increase in aromatase in response to leptin was found to be dependent on the stabilization of both HIF1α and PKM2. In addition to directly interacting with PKM2, HIF1α has previously been shown to bind to a hypoxia response element on aromatase promoter PII and act cooperatively with CREB to increase aromatase expression (13). Leptin has previously been shown to increase HIF1α and PKM2 levels in the vasculature and breast cancer cells, respectively (37, 38). Leptin levels have also been shown to be positively correlated with HIF1α in endometrial and colorectal cancer (39, 40).
In vitro findings suggest that leptin is a driver of the obesity-associated increase in ASC-specific expression of aromatase, and that the p53-HIF1α/pKM2-aromatase axis is actively regulated by environmental cues, including obesity (Figure 6). Importantly, changes in the levels of proteins in the p53-HIF1α/PKM2-aromatase axis observed in vitro in response to leptin were reflected in studies of normal breast tissue in relation to BMI or in the mammary fat pad of a diet-induced mouse model of obesity. Leptin is not the only factor that may be responsible for changes in the expression of the proteins in vivo. Inflammatory mediators increased in obesity, including PGE2, have been shown to regulate p53 and HIF1α levels in adipose stromal cells, and obese adipose tissue is recognized to be more hypoxic than healthy adipose tissue, a major driver of HIF1α stabilization. Nevertheless, it is likely that obesity-associated factors, including leptin, converge to regulate metabolic pathways and aromatase in ASCs, leading to a hormonal milieu conducive to tumor growth.
Figure 6. Proposed model for the leptin-mediated induction of aromatase in ASCs via the p53-HIF1α/PKM2 axis in obesity.
Leptin stimulates PKC translocation to the plasma membrane and ERK1/2 activity, leading to a decrease in p53 levels. p53 has been shown to act as a transcriptional repressor of Aha1 and aromatase in breast ASCs. Loss of p53 leads to an increase in Aha1 expression and Hsp90 ATPase activity that results in the stabilization of HIF1α and PKM2. HIF1α and PKM2 have been shown to bind to aromatase promoter PII and increase transcriptional activity. The resultant increase in aromatase expression is hypothesized to drive estrogen production and breast cancer formation and growth in obese women.
Taken together, results from the current study further emphasize the effect of obesity and obesity-associated factors on the local expression of aromatase and associated increase in estrogen production. Efforts aimed at reducing weight or maintaining a healthy weight may have profound impact on inhibiting the development and growth of hormone-dependent breast cancers. Better understanding the mechanisms of aromatase regulation offer the possibility to explore novel therapeutic approaches, including targeting of leptin signaling.
Supplementary Material
Acknowledgments
Funding: NHMRC project grant (GNT1061800), Mavis Robertson Fellowship from the National Breast Cancer Foundation (ECF-16-004), NIH R01 CA215797, NIH R01 CA185293, NIH/NCI U54 CA210184-01, the Breast Cancer Research Foundation, the Botwinick-Wolfensohn Foundation (in memory of Mr. and Mrs. Benjamin Botwinick), the Victorian Government Operational Infrastructure Support Program, Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748), Conquer Cancer Foundation of the American Society of Clinical Oncology and by Myrna and Bernard Posner.
This work was supported by NHMRC project grant (GNT1061800) and Mavis Robertson Fellowship from the National Breast Cancer Foundation (ECF-16-004), as well as NIH R01 CA215797, NIH R01 CA185293, NIH/NCI U54 CA210184-01, the Breast Cancer Research Foundation, the Botwinick-Wolfensohn Foundation (in memory of Mr. and Mrs. Benjamin Botwinick), the Victorian Government Operational Infrastructure Support Program, Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748), and by Myrna and Bernard Posner. NMI is supported by the Conquer Cancer Foundation of the American Society of Clinical Oncology.
Footnotes
Conflicts of Interest
The authors have nothing to declare.
References
- 1.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5) doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ACS. Breast Cancer. American Cancer Society; 2014. [Available from: http://www.cancer.org/acs/groups/cid/documents/webcontent/003090-pdf.pdf. [Google Scholar]
- 3.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4(7):1021–9. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown KA, Iyengar NM, Zhou XK, Gucalp A, Subbaramaiah K, Wang H, et al. Menopause Is a Determinant of Breast Aromatase Expression and Its Associations With BMI, Inflammation, and Systemic Markers. J Clin Endocrinol Metab. 2017;102(5):1692–701. doi: 10.1210/jc.2016-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114–36. doi: 10.1093/epirev/mxt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nechuta S, Chen WY, Cai H, Poole EM, Kwan ML, Flatt SW, et al. A pooled analysis of post-diagnosis lifestyle factors in association with late estrogen-receptor-positive breast cancer prognosis. Int J Cancer. 2016;138(9):2088–97. doi: 10.1002/ijc.29940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohan TE, Heo M, Choi L, Datta M, Freudenheim JL, Kamensky V, et al. Body fat and breast cancer risk in postmenopausal women: a longitudinal study. J Cancer Epidemiol. 2013;2013:754815. doi: 10.1155/2013/754815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutkowski JM, Stern JH, Scherer PE. The cell biology of fat expansion. J Cell Biol. 2015;208(5):501–12. doi: 10.1083/jcb.201409063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YS, Kim J-w, Osborne O, Oh DY, Sasik R, Schenk S, et al. Increased Adipocyte O(2) Consumption Triggers HIF-1α Causing Inflammation and Insulin Resistance in Obesity. Cell. 2014;157(6):1339–52. doi: 10.1016/j.cell.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Simpson ER, Brown KA. Aromatase overexpression in dysfunctional adipose tissue links obesity to postmenopausal breast cancer. J Steroid Biochem Mol Biol. 2015;153:35–44. doi: 10.1016/j.jsbmb.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Brown KA, McInnes KJ, Hunger NI, Oakhill JS, Steinberg GR, Simpson ER. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer Res. 2009;69(13):5392–9. doi: 10.1158/0008-5472.CAN-09-0108. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Docanto MM, Sasano H, Lo C, Simpson ER, et al. Cancer KCFCfRiFB. Prostaglandin E2 Inhibits p53 in Human Breast Adipose Stromal Cells: A Novel Mechanism for the Regulation of Aromatase in Obesity and Breast Cancer. Cancer Research. 2015 doi: 10.1158/0008-5472.CAN-14-2164. [DOI] [PubMed]
- 13.Samarajeewa NU, Yang F, Docanto MM, Sakurai M, McNamara KM, Sasano H, et al. HIF-1alpha stimulates aromatase expression driven by prostaglandin E2 in breast adipose stroma. Breast Cancer Res. 2013;15(2):R30. doi: 10.1186/bcr3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masciari S, Dillon DA, Rath M, Robson M, Weitzel JN, Balmana J, et al. Breast cancer phenotype in women with TP53 germline mutations: a Li-Fraumeni syndrome consortium effort. Breast Cancer Res Treat. 2012;133(3):1125–30. doi: 10.1007/s10549-012-1993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subbaramaiah K, Brown KA, Zahid H, Balmus G, Weiss RS, Herbert BS, et al. Hsp90 and PKM2 Drive the Expression of Aromatase in Li-Fraumeni Syndrome Breast Adipose Stromal Cells. J Biol Chem. 2016;291(31):16011–23. doi: 10.1074/jbc.M115.698902. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Shay JW, Tomlinson G, Piatyszek MA, Gollahon LS. Spontaneous in vitro immortalization of breast epithelial cells from a patient with Li-Fraumeni syndrome. Mol Cell Biol. 1995;15(1):425–32. doi: 10.1128/mcb.15.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okayama S, Kopelovich L, Balmus G, Weiss RS, Herbert BS, Dannenberg AJ, et al. p53 protein regulates Hsp90 ATPase activity and thereby Wnt signaling by modulating Aha1 expression. J Biol Chem. 2014;289(10):6513–25. doi: 10.1074/jbc.M113.532523. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75. [PubMed] [Google Scholar]
- 19.Wang X, Docanto MM, Sasano H, Lo C, Simpson ER, et al. Kathleen Cuningham Foundation Consortium for Research into Familial Breast C. Prostaglandin E2 inhibits p53 in human breast adipose stromal cells: a novel mechanism for the regulation of aromatase in obesity and breast cancer. Cancer Res. 2015;75(4):645–55. doi: 10.1158/0008-5472.CAN-14-2164. [DOI] [PubMed] [Google Scholar]
- 20.Toro AR, Perez-Perez A, Corrales Gutierrez I, Sanchez-Margalet V, Varone CL. Mechanisms involved in p53 downregulation by leptin in trophoblastic cells. Placenta. 2015;36(11):1266–75. doi: 10.1016/j.placenta.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Au CC, Furness JB, Brown KA. Ghrelin and Breast Cancer: Emerging Roles in Obesity, Estrogen Regulation, and Cancer. Front Oncol. 2016;6:265. doi: 10.3389/fonc.2016.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ham S, Meachem SJ, Choong CS, Charles AK, Baynam GS, Jones TW, et al. Overexpression of aromatase associated with loss of heterozygosity of the STK11 gene accounts for prepubertal gynecomastia in boys with Peutz Jeghers Syndrome. J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2013-2291. [DOI] [PubMed] [Google Scholar]
- 23.Coen P, Kulin H, Ballantine T, Zaino R, Frauenhoffer E, Boal D, et al. An aromatase-producing sex-cord tumor resulting in prepubertal gynecomastia. N Engl J Med. 1991;324(5):317–22. doi: 10.1056/NEJM199101313240507. [DOI] [PubMed] [Google Scholar]
- 24.Bulun SE, Rosenthal IM, Brodie AM, Inkster SE, Zeller WP, DiGeorge AM, et al. Use of tissue-specific promoters in the regulation of aromatase cytochrome P450 gene expression in human testicular and ovarian sex cord tumors, as well as in normal fetal and adult gonads. J Clin Endocrinol Metab. 1993;77(6):1616–21. doi: 10.1210/jcem.77.6.8263150. [DOI] [PubMed] [Google Scholar]
- 25.Engin A. Obesity-associated Breast Cancer: Analysis of risk factors. Adv Exp Med Biol. 2017;960:571–606. doi: 10.1007/978-3-319-48382-5_25. [DOI] [PubMed] [Google Scholar]
- 26.Barone I, Giordano C, Bonofiglio D, Ando S, Catalano S. Leptin, obesity and breast cancer: progress to understanding the molecular connections. Curr Opin Pharmacol. 2016;31:83–9. doi: 10.1016/j.coph.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Hosney M, Sabet S, El-Shinawi M, Gaafar KM, Mohamed MM. Leptin is overexpressed in the tumor microenvironment of obese patients with estrogen receptor positive breast cancer. Exp Ther Med. 2017;13(5):2235–46. doi: 10.3892/etm.2017.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dieudonne MN, Sammari A, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Sex steroids and leptin regulate 11beta-hydroxysteroid dehydrogenase I and P450 aromatase expressions in human preadipocytes: Sex specificities. J Steroid Biochem Mol Biol. 2006;99(4–5):189–96. doi: 10.1016/j.jsbmb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Magoffin DA, Weitsman SR, Aagarwal SK, Jakimiuk AJ. Leptin regulation of aromatase activity in adipose stromal cells from regularly cycling women. Ginekol Pol. 1999;70(1):1–7. [PubMed] [Google Scholar]
- 30.Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, et al. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem. 2003;278(31):28668–76. doi: 10.1074/jbc.M301695200. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Wang L, Zheng J, Tang G. Leptin promotes human endometrial carcinoma cell proliferation by enhancing aromatase (P450arom) expression and estradiol formation. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):198–201. doi: 10.1016/j.ejogrb.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Kitawaki J, Kusuki I, Koshiba H, Tsukamoto K, Honjo H. Leptin directly stimulates aromatase activity in human luteinized granulosa cells. Mol Hum Reprod. 1999;5(8):708–13. doi: 10.1093/molehr/5.8.708. [DOI] [PubMed] [Google Scholar]
- 33.Shrestha M, Park PH. p53 signaling is involved in leptin-induced growth of hepatic and breast cancer cells. Korean J Physiol Pharmacol. 2016;20(5):487–98. doi: 10.4196/kjpp.2016.20.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C, Chang YC, Liu CL, Chang KJ, Guo IC. Leptin-induced growth of human ZR-75-1 breast cancer cells is associated with up-regulation of cyclin D1 and c-Myc and down-regulation of tumor suppressor p53 and p21WAF1/CIP1. Breast Cancer Res Treat. 2006;98(2):121–32. doi: 10.1007/s10549-005-9139-y. [DOI] [PubMed] [Google Scholar]
- 35.Sirotkin AV, Benco A, Tandlmajerova A, Vasicek D. Involvement of transcription factor p53 and leptin in control of porcine ovarian granulosa cell functions. Cell Prolif. 2012;45(1):9–14. doi: 10.1111/j.1365-2184.2011.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mistry T, Digby JE, Desai KM, Randeva HS. Leptin and adiponectin interact in the regulation of prostate cancer cell growth via modulation of p53 and bcl-2 expression. BJU Int. 2008;101(10):1317–22. doi: 10.1111/j.1464-410X.2008.07512.x. [DOI] [PubMed] [Google Scholar]
- 37.Wei L, Li K, Pang X, Guo B, Su M, Huang Y, et al. Leptin promotes epithelial-mesenchymal transition of breast cancer via the upregulation of pyruvate kinase M2. J Exp Clin Cancer Res. 2016;35(1):166. doi: 10.1186/s13046-016-0446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitade M, Yoshiji H, Kojima H, Ikenaka Y, Noguchi R, Kaji K, et al. Leptin-mediated neovascularization is a prerequisite for progression of nonalcoholic steatohepatitis in rats. Hepatology. 2006;44(4):983–91. doi: 10.1002/hep.21338. [DOI] [PubMed] [Google Scholar]
- 39.Koda M, Sulkowska M, Kanczuga-Koda L, Cascio S, Colucci G, Russo A, et al. Expression of the obesity hormone leptin and its receptor correlates with hypoxia-inducible factor-1 alpha in human colorectal cancer. Ann Oncol. 2007;18(Suppl 6):vi116–9. doi: 10.1093/annonc/mdm238. [DOI] [PubMed] [Google Scholar]
- 40.Koda M, Sulkowska M, Wincewicz A, Kanczuga-Koda L, Musiatowicz B, Szymanska M, et al. Expression of leptin, leptin receptor, and hypoxia-inducible factor 1 alpha in human endometrial cancer. Ann N Y Acad Sci. 2007;1095:90–8. doi: 10.1196/annals.1397.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.