1. Introduction
The US Congress passed the Food and Drug Administration Modernization Act and Amendments Act, mandating that investigators post protocols and the results of all clinical studies enrolling human subjects in the United States in the trial registry clinicaltrials.gov within 1 year after study completion [1], [2]. Sponsors and principal investigators who do not comply with the federal law are subject to financial penalties up to $10,000/day for failing to register or submit the results of trials. Numerous previous analyses suggested poor compliance with this policy [3], [4], [5], [6]. However, previous analyses were not able to precisely identify studies' applicability to federal law, because this information is not in the publicly available Web version of the trial registry, clinicaltrials.gov [4], [7]. The Aggregate Analysis of ClincalTrials.gov (AACT) database enabled us to analyze the entire set of registered studies and precise information about studies' applicability to federal law. We aimed to investigate sponsor compliance with federal law to post complete high-quality information about study and participant characteristics and outcomes. We assumed noncompliance when sponsors did not post the results or posted ambiguous data. The law clearly states a sponsor's responsibility for posting timely, complete, and accurate information about the study, sponsors, investigators, and participants [1], [2]. Therefore, we assumed sponsor responsibility was the sole reason and did not speculate on other reasons for not posting information about studies labeled by registry administrators as “applicable clinical trial.” We analyzed sponsorship, conflict of interest, study design and risk of bias, and reporting of participants' flow and baseline demographics based on provided information in the database extracts from the Clinical Trials Transformation Initiative (CTTI) (https://www.ctti-clinicaltrials.org/aact-database).
2. Methods
We downloaded, reformatted, and analyzed all studies available in the CTTI database as of May 2016 in the HPCC platform (High-Performance Computing Cluster, https://hpccsystems.com/). The CTTI database has 2 variables indicating whether a study is an “applicable clinical trial” as defined in US Public Law 110-85, Title VIII, Section 801 and whether a trial is a Food and Drug Administration–regulated intervention (Appendix Table 2). We relied on straightforward law language regarding sponsor responsibility for compliance. Therefore, in contrast with previous publications, we did not speculate or hypothesize what other factors can result in missing or ambiguous data [3], [5], [8].
Appendix Table 2.
Definitions of the data elements that are available for downloading from www.clinicaltrials.gov (* mandatory fields required by the federal law).
| Field name | Definition of the data element | Utilization in our analysis |
|---|---|---|
| NCT ID* | The ClinicalTrials.gov identifier | Unique study identifier |
| Other IDs | Other identification numbers assigned to the protocol, including unique identifiers from other registries and NIH grant numbers | Not used |
| Title* | Official name of the protocol provided by the study principal investigator or sponsor | Not used |
| Acronym | Acronym or initials used to identify this study | Not used |
| FDA Regulated Intervention? |
FDA Regulated Intervention? (FDAAA) Yes/No Definition: Indicate whether this trial includes an intervention subject to US Food and Drug Administration regulation under section 351 of the Public Health Service Act or any of the following sections of the Federal Food, Drug and Cosmetic Act: 505, 510(k), 515, 520(m), and 522. |
Analyzed |
| Is section 801? |
Section 801 Clinical Trial? (FDAAA) Yes/No Definition: If this trial includes an FDA regulated intervention, indicate whether this is an “applicable clinical trial” as defined in US Public Law 110-85, Title VIII, Section 801. Briefly, applicable drug trials include controlled clinical investigations, other than Phase I investigations, of a drug or biologic subject to US FDA regulation. Applicable device clinical trials are controlled trials with health outcomes of devices subject to FDA regulation, other than small feasibility studies, and pediatric postmarket surveillance. |
Analyzed |
| Funded* | Funding source as industry, NIH, U.S. Federal Government, Network, or other | We categorized as industry funded or other funding |
| Sponsors* | Name of primary organization that oversees implementation of study and is responsible for data analysis | Analyzed |
| Recruitment* |
|
We used the exact categories as reported in trial registry |
| Conditions* | Primary disease or condition being studied, or focus of the study. Diseases or conditions should use the National Library of Medicine's Medical Subject Headings (MeSH) controlled vocabulary when possible. | Normalized and analyzed |
| Study Types* | Interventional or observational studies | We used the exact categories as reported in trial registry |
| Study Designs | Purpose, phase, treatment allocation, masking of the treatment status; type of primary outcome or endpoint that the protocol is designed to evaluate | Analyzed |
| Phases* | Phase of investigation, as defined by the US FDA for trials involving investigational new drugs | We used the exact categories as reported in trial registry |
| Study Results |
|
We categorized the studies into 2 categories: with posted results and without posted results. We analyzed reporting of the number of participants who completed studies and the number of participants who discontinued the study due to adverse effects |
| Interventions* |
|
We categorized interventions as drug, procedure, radiation, biologics, or behavioral according to the categories in ClinicalTrials.gov |
| Outcome Measures | Specific key measurement(s) or observation(s) used to measure the effect of experimental variables in a study, or for observational studies, to describe patterns of diseases or traits or associations with exposures, risk factors or treatment. | Not used |
| Gender* | Physical gender of individuals who may participate in the protocol | We used the exact categories as reported in trial registry |
| Age Groups | Age of participants | We used the exact categories as reported in trial registry |
| Enrollment* | Number of subjects in the trial | We used the reported numbers, excluding ambiguous values e.g., enrollment values of more than 99,999 participants registry |
| First Received | Date the protocol information was received | Not used |
| Start Date* | Date that enrollment to the protocol begins | We calculated the length of studies as the time period between start and completion dates |
| Completion Date | Final date on which data was (or is expected to be) collected | |
| Last Updated | Date the protocol information was updated | Not used |
| Last Verified* | Date the protocol information was last verified | Not used |
| Primary Completion Date* | The date that the final subject was examined or received an intervention for the purposes of final collection of data for the primary outcome, whether the clinical trial concluded according to the prespecified protocol or was terminated | We calculated the length of studies as the time period between start and primary completion date when completion dates were missing. |
| Has Expanded Access?* | Indicate whether any non-protocol access is to be provided for the investigational drug or device. If so, an Expanded Access record should also be created for this IND/IDE. | Analyzed |
| Accepts Healthy Volunteers?* | Indicate if persons who have not had the condition(s) being studied or otherwise related conditions or symptoms, as specified in the eligibility requirements, may participate in the study. Select Yes/No. | Analyzed |
| Maximum age* | Maximum age of participants. | Analyzed |
| Minimum age* | Minimum age of participants. | Analyzed |
| Organization's Unique Protocol id* | Unique identification assigned to the protocol by the sponsoring organization, usually an accession number or a variation of a grant number. Multiple studies conducted under the same grant must each have a unique number. | Analyzed |
| Primary Completion Date Type* | A “Type” menu is also included, with options Anticipated and Actual. For active studies, set Type to Anticipated and specify the expected completion date, updating the date as needed over the course of the study. Upon study completion, change Type to Actual and update the date if necessary. | Analyzed |
| Lead Sponsor or Collaborators* | Name of primary organization that oversees implementation of study and is responsible for data analysis. For applicable clinical trials, sponsor is defined in 21 CFR | Analyzed |
We conducted frequency analysis of all fields required by the World Health Organization for the registration of clinical studies (Appendix Table 1). We identified clinical trials and conducted frequency analysis of the fields describing study sponsorship, conflict of interest by principal investigators, risk of bias in study design, and the reporting of participants' flow and baseline demographics. We also analyzed reporting of all mandatory fields required by the federal law (Appendix Table 2). For each study applicable to Section 801, we calculated proportions of missing data among all mandatory fields required by federal law.
Appendix Table 1.
| The minimum amount of trial information that must appear in a register in order for a given trial to be considered fully registered. There are currently 20 items in the WHO Trial Registration Data Set. It is sometimes referred to as the TRDS.
|
Quality assurance of the trial registry uses automated business rules to detect missing data or inconsistencies in the data elements [9]. However, this methodology has limitations, including the lack of an independent source of study data to verify each data element [9]. As stated by the registry director, “posting does not guarantee that the record is fully compliant with either ClinicalTrials.gov or legal requirements.” [9] We detected missing or ambiguous data but did not contact the institutional review boards to confirm the accuracy of the data in ClinicalTrials.gov.
Following the federal financial disclosure guidance [10], we concluded that there was a conflict of interest when study sponsors were employers of principal investigators. We categorized study funding by industry for all studies funded by pharmaceutical or device companies exclusively or in combination with individuals, universities, or community-based organizations.
We defined high risk of bias in study design according to the criteria outlined by the Agency for Healthcare Research and Quality, including termination status, non-random allocation of study subjects, and open allocation status [11].
We identified all completed studies that did not post results on clinicaltrials.gov in compliance with federal law (Section 801 and criteria without posted results). We estimated the fee for noncompliance with federal law as a minimum $1/day up to a maximum $10,000/day for all days between the primary completion day and the end of 2014. We excluded from the cost-analysis studies completed in 2015–2016 to address a possible time lag between posting the results on the Web and in the downloadable database.
3. Results
We downloaded 217,258 studies from the database in the format of related tables. We linked all related tables by unique study, treatment, and outcome identifiers. We were able to analyze 211,437 studies. We identified 44,635 studies applicable to Section 801 (Table 1). Industry was involved in sponsoring 63% of these studies. The majority of the studies (75%) did not report employment of principal investigators by a sponsoring organization, and obvious conflict of interest was identified in 4% of the studies in which principal investigators were employed by sponsoring organizations (Table 1). The majority of the studies were randomized clinical trials (60%), but only 38% of all studies applicable to Section 801 were double-blind studies. More than 3000 studies (9%) applicable to Section 801 were terminated, mostly due to poor recruitment. Information about attrition of study subjects (18%) and treatment discontinuation due to adverse effects (8%) was available only in a small proportion of studies (Table 1). Baseline participant age was available only in 18% of studies, and enrollment of racial and ethnic minorities was available in less than 5% of studies (Table 1).
Table 1.
Sponsorship, conflict of interest, risk of bias, and reporting of participant's flow and baseline demographic information in studies that must comply with the US Public Law 110-85 to post the results in clinicaltrials.gov.
| Study characteristics | Number of studies | % of total applicable studies |
|---|---|---|
| Applicable clinical trial as defined in US Public Law 110-85, Title VIII, Section 801 | 44,635 | |
| Industry involvement in sponsoring the study | 27,995 | 62.7 |
| Principal investigators are employed by sponsoring organization | 1910 | 4.3 |
| Principal investigators are not employed by sponsoring organization | 9325 | 20.9 |
| Employment of principals investigators by sponsoring organization is not reported | 33,400 | 74.8 |
| Risk of bias | ||
| Allocation of subjects: randomized | 26,635 | 59.7 |
| Allocation of subjects: non-random | 5526 | 12.4 |
| Allocation of subjects not reported | 12,474 | 27.9 |
| Terminated | 3789 | 8.5 |
| Double-blind study | 17,089 | 38.3 |
| Open Label study | 22,748 | 51.0 |
| Single Blind study | 2357 | 5.3 |
| Masking not reported | 2441 | 5.5 |
| Study design | ||
| Phase not reported | 6998 | 15.7 |
| Phase 0 | 292 | 0.7 |
| Phase 1 | 3890 | 8.7 |
| Phase 1/Phase 2 | 3063 | 6.9 |
| Phase 2 | 14,534 | 32.6 |
| Phase 2/Phase 3 | 1051 | 2.4 |
| Phase 3 | 9553 | 21.4 |
| Phase 4 | 5254 | 11.8 |
| Participants flow | ||
| Reported attrition ( # of subjects not completed the study) | 8133 | 18.2 |
| Reported study discontinuation due to adverse effects | 3366 | 7.5 |
| Baseline patient characteristics | ||
| Reported baseline age of enrolled patients | 8218 | 18.4 |
| Both genders enrolled | 38,379 | 86.0 |
| Female studies | 3782 | 8.5 |
| Male studies | 1855 | 4.2 |
| Gender not reported | 619 | 1.4 |
| Reported # of Asian patients | 1918 | 4.3 |
| Reported # of African-American patients | 1928 | 4.3 |
| Reported # of Hispanic patients | 486 | 1.1 |
| Reported # of Native American patients | 1481 | 3.3 |
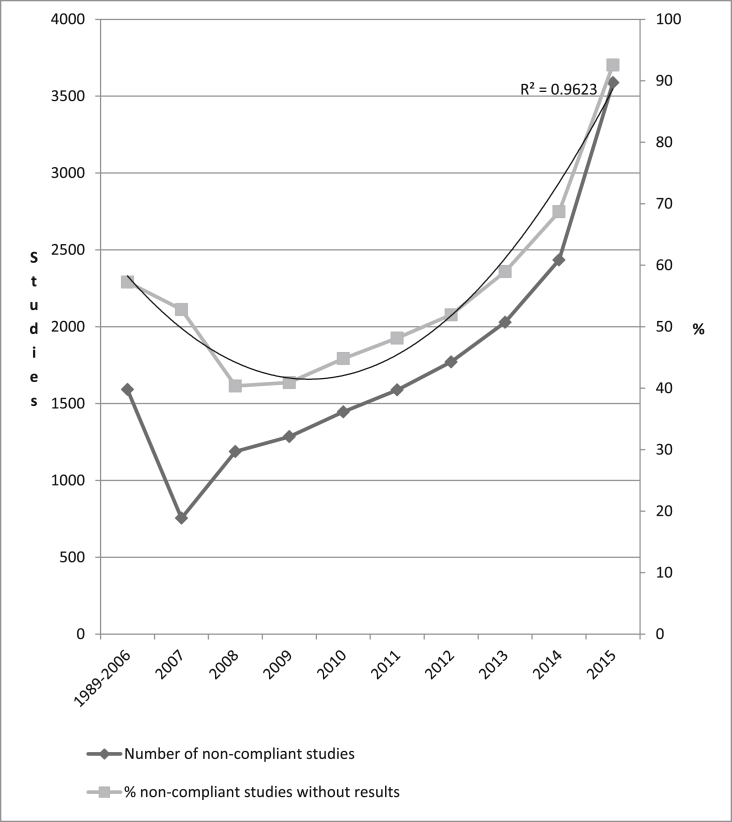
We identified 29,992 applicable clinical trials (according to Section 801) that did not post results on clinicaltrials.gov. We restricted our analysis to 14,476 studies completed before 2015 that failed to post results in clinicaltrials.gov and therefore did not comply with federal law (Fig. 1). These 14,476 studies enrolled 3,660,385 participants. The results from 12,479 studies have not been published in journals; therefore, they are unavailable to the public. We estimated that the penalty of noncompliance would be from a minimum of $28.6 million to a maximum of $286 billion. We identified 22 sponsors that failed to post the results from >50 studies each (the list is available upon request). The majority of noncomplying studies were industry-sponsored (65.4%) randomized clinical trials (67.5%).
Fig. 1.
The number of the studies that did not comply with the federal US Public Law 110-85, Title VIII, Section 801about posting the results in clinicaltrials.gov by the primary completion dates as reported in clinicaltrials.gov (only studies completed before 2015 are included).
4. Discussion
In concordance with multiple previous publications, our findings indicated poor compliance with the federal law requiring certain studies to make results available [3], [5], [8]. There are several ongoing policy improvement efforts, including the Trial and Experimental Studies Transparency (TEST) Act, National Institutes of Health (NIH) policy, and growing public discussion about emergent need in sharing of clinical trial results [12], [13], [14]. The NIH recently issued a final policy mandating the registration and posting of the results of all NIH-funded clinical trials regardless of coverage by the Food and Drug Administration Amendments Act requirements [15]. The final rule is expected to promote public trust in clinical research, to fulfill an ethical obligation to the public and trial participants, to optimize the public investment in research, and to ensure accountability via the public reporting of information [16]. Much better enforcement is needed. Lack of accountability mechanisms result in enormous reporting bias in evidence analyses that inform policy, coverage, and clinical decisions [17], [18]. Policy efforts must address enrollment of racial and ethnic minorities and members of other underrepresented populations including elderly people, children, and women.
Our study has several limitations. We relied on information submitted by principal investigators and did not contact sponsors or investigators requesting submission of the missing data. We relied on the trial registry for identification of applicable studies and assumed noncompliance when the results were not available or ambiguous. We projected ranges of financial penalties and found no publicly available evidence of sponsor payments in cases of delayed posting.
Nevertheless, our analysis demonstrates a clear need for better accountability from sponsors and investigators who do not comply with the federal law about posting results in clinicaltrials.gov. The final rule estimated an additional cost of $56 million for the public and the sponsors related to study registration and the posting of results [16]. However, modern technology (http://www.lexisnexis.com/risk/) allows harmonization of institutional review board databases with trial registries. This harmonization will allow sponsors to submit study information once, providing instant analysis of compliance and the ability to check the quality control of the data as it is entered.
References
- 1.US Food and Drug Administration Modernization Act of 1997. http://www.fda.gov/RegulatoryInformation/Legislation/SignificantAmendmentstotheFDCAct/FDAMA/FullTextofFDAMAlaw/default.htm (Acccessed August 2, 2016)
- 2.US Food and Drug Administration Amendments Act of 2007. https://www.gpo.gov/fdsys/pkg/PLAW-110publ85/pdf/PLAW-110publ85.pdf (Accessed August 2, 2016)
- 3.Miller J.E., Korn D., Ross J.S. Clinical trial registration, reporting, publication and FDAAA compliance: a cross-sectional analysis and ranking of new drugs approved by the FDA in 2012. BMJ Open. 2015;5(11):e009758. doi: 10.1136/bmjopen-2015-009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson M.L., Chiswell K., Peterson E.D., Tasneem A., Topping J., Califf R.M. Compliance with results reporting at ClinicalTrials.gov. N. Engl. J. Med. 2015;372(11):1031–1039. doi: 10.1056/NEJMsa1409364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prayle A.P., Hurley M.N., Smyth A.R. Compliance with mandatory reporting of clinical trial results on ClinicalTrials.gov: cross sectional study. BMJ. 2012;344:d7373. doi: 10.1136/bmj.d7373. [DOI] [PubMed] [Google Scholar]
- 6.Shamliyan T.A., Kane R.L. Availability of results from clinical research: failing policy efforts. J. Epidemiol. Glob. Health. 2014;4(1):1–12. doi: 10.1016/j.jegh.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huser V., Cimino J.J. Linking ClinicalTrials.gov and PubMed to track results of interventional human clinical trials. PLoS One. 2013;8(7):e68409. doi: 10.1371/journal.pone.0068409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Withycombe B., Ovenell M., Meeker A., Ahmed S.M., Hartung D.M. Timing of pivotal clinical trial results reporting for newly approved medications varied by reporting source. J. Clin. Epidemiol. April 22, 2016 doi: 10.1016/j.jclinepi.2016.04.007. [published online ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Zarin D.A., Tse T., Williams R.J., Califf R.M., Ide N.C. The ClinicalTrials.gov results database—update and key issues. N. Engl. J. Med. 2011;364(9):852–860. doi: 10.1056/NEJMsa1012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services, Food and Drug Administration, Office of Good Clinical Practice, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health . February 2013. Guidance for Clinical Investigators, Industry, and FDA Staff.http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM341008.pdf Financial Disclosure by Clinical Investigators. (Accessed October 2016) [Google Scholar]
- 11.Viswanathan M., Ansari M.T., Berkman N.D. Agency for Healthcare Research and Quality; Rockville, MD: 2008. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. [PubMed] [Google Scholar]
- 12.Logvinov I. Clinical trials transparency and the trial and experimental studies transparency (TEST) act. Contemp. Clin. Trials. 2014;37(2):219–224. doi: 10.1016/j.cct.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Zarin D.A., Tse T., Sheehan J. The proposed rule for US clinical trial registration and results submission. N. Engl. J. Med. 2015;372(2):174–180. doi: 10.1056/NEJMsr1414226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudson K.L., Collins F.S. Sharing and reporting the results of clinical trials. JAMA. 2015;313(4):355–356. doi: 10.1001/jama.2014.10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarin D.A., Tse T., Williams R.J., Carr S. Trial reporting in ClinicalTrials.gov—the final rule. N. Engl. J. Med. September 16, 2016 doi: 10.1056/NEJMsr1611785. [published online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institutes of Health, Department of Health and Human Services. Clinical Trials Registration and Results Information Submission; Final Rule. https://www.gpo.gov/fdsys/pkg/FR-2016-09-21/pdf/2016-22129.pdf. (Accessed October 2, 2016). [PubMed]
- 17.Lampert A., Hoffmann G.F., Ries M. Ten years after the international committee of medical journal editors' clinical trial registration initiative, one quarter of phase 3 pediatric epilepsy clinical trials still remain unpublished: a cross sectional analysis. PLoS One. 2016;11(1):e0144973. doi: 10.1371/journal.pone.0144973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito H., Gill C.J. How frequently do the results from completed US clinical trials enter the public domain?—A statistical analysis of the ClinicalTrials.gov database. PLoS One. 2014;9(7):e101826. doi: 10.1371/journal.pone.0101826. [DOI] [PMC free article] [PubMed] [Google Scholar]