Abstract
In experimental studies the assigned intervention measures the received intervention if full protocol adherence is achieved, but this is rarely the case in public health. The objective of this study was to estimate the effect of a brief counseling intervention delivered in Swedish dental clinics on tobacco use cessation, taking non-adherence into account. We conducted three secondary analyses. In a per-protocol analysis the experimental counseling delivered as intended was contrasted to usual care (control). In an as-treated analysis individuals were compared according to the counseling components actually received, disregarding randomization. In an instrumental variable analysis the effect of the intervention among those who would always be treated as assigned was estimated. Logistic regression was used to examine the association between tobacco cessation outcomes (seven-day abstinence, three-month abstinence, half-reduction, quit attempts) and the defined exposure to the intervention. Protocol adherence in the intervention group was 73.4%. The per-protocol analysis closely replicated the results of the intention-to-treat analysis, showing a statistically significant effect of the brief counseling on the reduction in tobacco consumption OR = 1.81, 95% CI [1.06, 3.07], but no significant effect for other outcomes. In the as-treated analysis, receiving more counseling components compared with no tobacco counseling increased the likelihood of half-reduction. The instrumental variable yielded biased results. We conclude that despite application problems, conducting per-protocol, as-treated and instrumental variable analyses in randomized trials where experimental conditions are not strictly standardized strengthens and puts in context the inference based on intention-to-treat analysis.
Keywords: Tobacco use cessation, Adherence, Per-protocol, As-treated, Instrumental variable
1. Introduction
Brief counseling complemented by oral examination in dental settings is effective in assisting patients to achieve tobacco use cessation [1]. In Sweden, the effectiveness of a brief structured counseling for tobacco use cessation delivered in dental clinics to unselected tobacco users was evaluated in the cluster randomized controlled trial FRITT (Swedish acronym for “Free from Tobacco in Dentistry”). The intention-to-treat (ITT) analysis of this trial showed a statistically significant effect on reduction by half of tobacco consumption from baseline to follow-up, but not on complete abstinence [2].
The “intention-to-treat” (ITT) analysis preserves the benefits of randomization in the comparison of alternative interventions. Therefore, it is the primary analytic approach in randomized clinical trials [3], [4]. This approach estimates the effect of being assigned to a specific treatment, irrespective of whether or not the individual received, took or completed the assigned treatment. In case of non-adherence, the assigned intervention is a misclassified measure of the received intervention and the results could be a biased estimate of the treatment's effect [4].
Adherence patterns are important for an appraisal of the extent to which the ITT analysis yields a valid measure of the effect of the intervention. A review of randomly selected trials published in high-impact medical journals showed that protocol non-adherence was reported in 98% of the studies, whereas methods to address non-adherence in only 51% of them [5]. In many of the studies, “as-treated” (AT) or per-protocol (PP) analyses were the methods usually applied to account for non-adherence, but there was no discussion on the potential biases introduced by these methods [5]. The PP and AT analyses do not capture the causal effects if the sources of systematic error (i.e confounding) are not dealt with [6]. Instrumental variable (IV) analysis has been proposed as a method yielding an unbiased estimate of the effect of receiving the alternative treatment even in the presence of unmeasured confounders, if some central assumptions are met [4], [7].
Analyses following the PP, AT or IV approaches are often overlooked in RCTs of non-pharmacological interventions. Several studies have underlined the benefits of reporting additional analysis besides ITT [6], [8], [9], [10]. For instance, estimating the effect of interventions taking non-adherence into account may help to extrapolate the results to settings where the adherence pattern is different from that in the trial [8], [9]. Also, results may be more informative for patients and clinicians, who are interested in the true effect of the intervention rather than in the effect of the assigned intervention [8].
Therefore, the objective of this study was to conduct secondary analyses in order to estimate the effect corrected for non-adherence of the brief counseling intervention evaluated in the FRITT study on cessation of tobacco use. We analyzed the effect of (a) receiving the intervention as-intended among patients randomized to the intervention group (per-protocol analysis), (b) receiving different components of the intervention (as-treated analysis), (c) receiving always the intervention assigned among patients of dental practitioners with similar propensity to adherence (instrumental variable analysis).
2. Material and methods
2.1. Study design
Details on the study design are provided elsewhere [2]. Twenty-seven dental clinics in two Swedish regions willing to be included in the study constituted the unit of randomization. Simple randomization was performed using a computer-generated random sequence with 1:1 ratio to either deliver the novel intervention (structured brief advice) or to continue with the usual practice of tobacco prevention, if any (control condition). The intervention was delivered by dentists or dental hygienists individually to patients who were tobacco users. Given the nature of the control condition, there was no standardization of intervention in this group. The follow-up time was six months.
2.2. Intervention
The intervention was developed by the Swedish National Institute of Public Health in line with the Swedish National Board of Health and Welfare guidelines on brief standardized advice in dental care for disease prevention and health promotion. It was based on the “5 A's” model centered around readiness to quit, and acknowledging the chronic nature of tobacco dependence [11], [12]. It consisted of a 5 min, single-session counseling delivered during a dental visit. According to the written instructions given to the dental professional the following components had to be delivered to all patients: (a) Ask about tobacco use, (b) Advise to quit relating tobacco use with the patient's oral health (c) Assess willingness to quit (“Have you thought about quitting?”, “Are you interested in quitting?”) and (d) Assist by offering information about available support for tobacco use cessation and/or a leaflet about the quitting process (minimal Assist). For patients considering quitting, Assist included more specific components (setting a quit date, discussion about abstinence problems, suggestion or prescription of pharmacological treatment) and should have been followed by (e) Arrange, i.e. referral to Smoking Quit Line, or to other available smoking cessation resources.
2.3. Sample and data collection
2.3.1. Participants
A total of 467 patients participated in the FRITT study. The mean age was 45.6 years (SD 14.9), 63.4% were males, the majority had at least secondary school degree (78.9%), were full-time employed (62.5%) and were unmarried (53.1%). Concerning tobacco use, 43.6% used snus (Swedish moist smokeless tobacco product), 47.5% smoked cigarettes while only 8.9% were dual users. The average duration of use was 24.4 (SD 14.0) years, being highly correlated with patients' age. The majority of the participants were light or moderate tobacco users (51.2% used less than 10 cigarettes/snus pouches daily), used tobacco within 30 min after awakening (58.4%), had a history of at least one previous quit attempt (86.7%), were not considering quitting tobacco at all or in the next six months (81.1%) and never received a diagnosis of chronic disease (69.9%) [2].
The analytical sample for this study included the 452 patients (97%) who participated in the six-month follow-up. There was no significant difference in the proportions of lost to follow-up between experimental groups. The patients lost to follow-up did not differ from those retained in the study, therefore the baseline characteristics of the entire sample also apply to the analytical sample in this study.
2.3.2. Data collection
Information at the patient level was self reported both at baseline (paper-and-pencil questionnaires filled in at the clinic) and at follow-up (paper-and-pencil questionnaires filled in at the clinic, sent via postal mail or during a telephone interview). Information on the content of the intervention was collected from the dental professional both in intervention and control clinics using the same structured form, with pre-coded intervention components. The form also included information about the counseling's length (minutes). We refer to a previous paper from this study for detailed information about data collection, the eligibility and exclusion criteria for patients and clinics and the recruitment process [2].
2.4. Measures
2.4.1. Outcomes
The primary outcome was defined as complete abstinence from tobacco during the seven days prior to the follow-up survey (seven-day abstinence). Secondary outcomes were (a) sustained abstinence during the three months prior to the follow-up survey (three-month abstinence), (b) reporting at follow-up half or less of the daily average of cigarettes smoked or snus portions used reported at baseline (half-reduction) and (c) at least one quit attempt lasting 24 h or longer during the six months follow-up (quit attempts). All outcome variables were derived from self-reported information.
2.4.2. “As-intended” (“per-protocol”) intervention
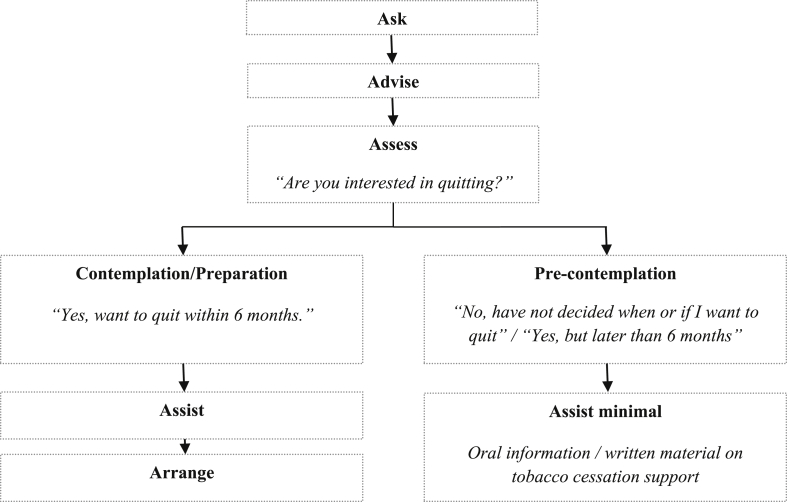
Adherence in this study was measured at the health care provider's level. In Table 1, the definition of the Ask, Assist, Assess, Assist and Arrange components is reported, being largely in accordance with the clinical interventions described in Treating Tobacco Use and Dependence Guidelines [12]. Readiness to quit was included in the protocol definition since it was an important feature for tailoring the counseling. The answer to the patient's baseline question: “How do you look upon your future tobacco use?” was used as an indicator for the readiness to quit. In accordance with the Stages of Change framework [11] and the timeline of the study, the patients were classified as being in pre-contemplation stage (“I want to quit completely, but later than six months” or “Have not yet decided, if and when I will quit completely”) or in contemplation/preparation stage (“I have decided to quit completely within six months”). As shown in Fig. 1, patients in the intervention group were considered treated as-intended if they were in (a) pre-contemplation stage and their dental practitioner declared to have delivered Ask, Advise, Assess and minimal Assist or (b) contemplation/preparation stage and their dental practitioner declared to have delivered Ask, Advise, Assess, Assist and Arrange.
Table 1.
Proportion of patients receiving tobacco cessation counseling components according to treatment group.
| Components of the counseling | Intervention (N = 219) |
Usual care (N = 233) |
|---|---|---|
| n (%) | n (%) | |
| Ask (Ask about current use, type and daily amount of tobacco used) | 219 (100.0) | 156 (67.0) |
| Advise (Explain how tobacco use affects patient's oral health) | 208 (95.0) | 95 (40.8) |
| Assess readiness to quit (Identify readiness to quit) | 202 (92.2) | 85 (36.5) |
| Assist (At least one of the following) | 210 (95.9) | 49 (21.0) |
| Offer information about available support for quitting tobacco | 170 (77.6) | 16 (6.9) |
| Offer leaflet about tobacco use cessation process | 139 (63.5) | 0 (0.0) |
| Present motivational arguments to quit tobacco | 159 (72.6) | 29 (12.4) |
| Ask about decision regarding quitting date | 35 (16.0) | 5 (2.1) |
| Discuss abstinence problems | 86 (39.3) | 13 (5.6) |
| Offer information about pharmacological treatment | 154 (70.3) | 9 (3.9) |
| Prescribe/suggest pharmacological treatment | 41 (18.7) | 3 (1.3) |
| Arrange (At least one of the following) | 121 (55.3) | 17 (7.3) |
| Make appointment for tobacco cessation with the same provider | 16 (7.3) | 2 (0.9) |
| Refer to tobacco cessation with other care provider at the clinic | 11 (5.0) | 3 (1.3) |
| Refer to tobacco cessation with external care provider outside the clinic | 60 (27.4) | 5 (2.1) |
| Refer to the Tobacco Quit Line (Sluta Röka Linjen) | 76 (34.7) | 8 (3.4) |
| Other | 9 (4.1) | 1 (0.4) |
| Type of counselling delivered | ||
| “As-intended” intervention | 160 (73.4) | – |
| Usual care similar with the “as-intended” intervention | – | 4 (1.9) |
| No intervention delivered | 0 (0.0) | 77 (33.0) |
| Less than 4 components | 23 (10.5) | 135 (57.9) |
| 4 components (Any combination of 4 A's) | 86 (39.3) | 18 (7.7) |
| 5 components (All 5 A's) | 110 (50.2) | 3 (1.3) |
Note. Each of the 5A's steps and the frequency of their delivery are in boldface.
Fig. 1.
Definition of the intervention “as-intended” (per-protocol).
2.4.3. Potential confounders
Prognostic factors of long-term tobacco abstinence have been identified, such as gender, age, income, amount of daily smoking, time to first cigarette after awakening, alcohol consumption, motivation to quit [11], [13], [14]. In this study, we considered potential confounders patients' characteristics that may have influenced dental practitioners' conduct, i.e. age, sex, occupation, education, disease status and tobacco-related characteristics, i.e. readiness to quit, length of tobacco use, time to tobacco use after awakening, amount of tobacco used daily, previous quit attempts.
2.5. Analytical methods
In the PP analysis, we compared the study outcomes of individuals randomized to the intervention arm and receiving the counseling “as-intended” with all those randomized to control group. The selection of the entire control group as the reference group was done because there was no univocal definition of “usual care”, therefore, no manualized protocol for the control group. The PP comparison could be affected by confounding if common prognostic factors for the receipt of the assigned intervention and the occurrence of the outcomes are not adjusted for (e.g. motivation to quit). Additionally, the restriction to the “per-protocol” subgroup introduces selection bias [6].
In the AT analysis, we compared the effect of being exposed to different number of counseling components with no tobacco counseling. The different exposure levels were (a) five components - all the 5 A's (b) four components – any combination of 4 A's (c) less than 4 components - any type of tobacco cessation counseling not described in (a) or (b). Therefore, we ignored the random assignment to intervention or control group as well as the protocol requirement to adapt the counseling to the patient's readiness to quit. Similarly to the PP, the AT could be affected by confounding.
To address the observed heterogeneity in terms of counseling received and outcomes achieved in the PP analysis, we additionally compared patients randomized to the intervention but non-receiving it “as-intended” with the “usual care” group. This analysis would inform on the “additional effect of being randomized to intervention”, i.e. effects in the intervention group not attributable to the intervention. In fact, non-receivers of the “intended” intervention would not differ from the control condition under the alternative hypothesis.
All the prognostic factors described previously, except readiness to quit were considered as potential confounders in the PP, AT analyses and the additional analysis described above. Readiness to quit was considered as potential confounder only in the AT analysis, as in the PP analysis it was included in the definition of the “as-intended” treatment.
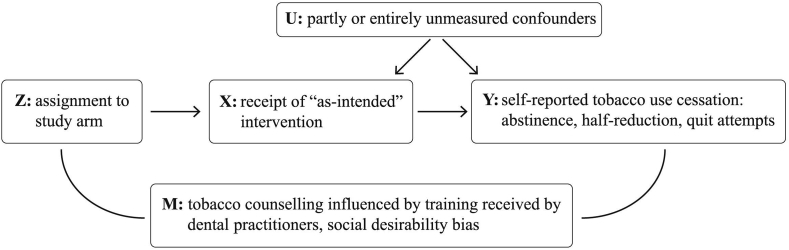
The Instrumental Variable (IV) analysis is an approach proposed to estimate the intervention effect in the sub-sample for which the received treatment was fully determined by the instrumental variable (i.e. the random assignment). By contrast to PP and AT, this method would implicitly control for confounding introduced by factors related to non-adherence because it compares groups for which the assignment will always have the same (positive) effect on delivery. A formal description of the underlying concepts and the general assumptions of IV analysis together with practical applications are available in the literature [7], [8], [10], [15]. The three assumptions essential for a valid instrument (Z), illustrated in Fig. 2 are presented below together with the monotonicity assumption under which IV analysis renders an effect estimate that can be interpreted as causal.
-
1)
Z (Instrumental variable, here the randomized treatment assignment) is independent of U (confounders of the association between intervention and outcome).
-
2)
Z is associated with X (receipt of the intervention) which is influenced, but not fully determined by treatment assignment, i.e. patients assigned to the intervention group receive to a higher extent the “as-intended” intervention relative to control.
-
3)
Z is independent of Y given X and U (exclusion restriction assumption). Assignment to intervention should not cause the outcomes through any other path than receiving the treatment. In this study, assignment to the intervention may theoretically influence the outcomes also through M, not only through X. Dental practitioners' behavior could be influenced by the awareness of being assigned to a specific group. For instance, they may have delivered additional care components or a more intensive and structured counseling than suggested. Also, patients can be prompted to underreport their tobacco use to a larger extent in intervention than in the control group.
-
4)
For the part of the sample for which Z has an effect on X, the direction of the effect is the same for everyone in the respective sub-sample (monotonicity). If Z increase X for one person, it must either increase or not affect X for all other people as well. In this study, there were no dental practitioners who would always deliver the opposite of what they were assigned to. Dental practitioners in control group did not have access to the training and the counseling they delivered (if any) differed substantially from the manualized intervention.
Fig. 2.
Simplified causal diagram for the FRITT cluster randomized controlled trial adapted from Hernán & Hernández-Díaz [6].
In our study, the exclusion restriction assumption was not met. However, for the purpose of sensitivity analyses, we calculated the average success ratio for the tobacco cessation outcomes using the IV method for non-compliance described by Greenland [7].
2.6. Statistical analysis
Proportions and means with corresponding standard deviations (SD) are presented for categorical and continuous variables, respectively. For comparisons, Chi-square test was used for categorical variables and t-test for continuous variables. Odds Ratios (OR), with corresponding 95% confidence intervals, calculated through logistic regression models, were used to estimate the association between the study outcomes and the “as-intended” intervention in PP and the exposure to different components of the intervention in the AT analyses. Each prognostic factor at baseline was used in adjusted models one at the time. In no case the crude estimates of association changed with more than 10%, therefore we retained for adjustments only time to tobacco use after awakening as indicator of dependence. In the AT analysis we adjusted also for readiness to quit. Sensitivity analyses were performed for missing data for the outcomes (assuming unchanged tobacco use from baseline). The average success ratios for the tobacco use cessation outcomes in IV analysis were calculated with hand calculator. All statistical analyses using ordinary logistic regression were performed using the statistical software Statistical Package for the Social Sciences (SPSS) version 22. To account for the clustering effect, the association between the exposure and outcomes was also estimated using multilevel logistic regression with random intercept (SAS v. 9.4). We conducted a formal test of the model with random effects by Likelihood Ratio Test. In no case the test indicated a significantly better fit of the multilevel model compared to ordinary logistic regression. The Intra-Cluster Correlation Coefficient (ICC) for each model was close to 0 for all the associations when including relevant covariates. Accordingly, the results from the multilevel logistic regression produced results very close to the ordinary logistic regression, but these latter models were more stable, as indicated by the −2 log likelihood. Therefore the results of the ordinary logistic regression are presented.
3. Results
3.1. Intervention's delivery
In the intervention group, 160 (73.4%) patients received an intervention appropriate to their readiness to quit (“as-intended” intervention) as shown in Table 1. In the control group (usual care) only 4 (1.9%) patients received counseling similar with the “as-intended” intervention. Also, 110 (50.2%) and 3 (1.3%) received all the 5 A's in the intervention and control group, respectively (Table 1). In general, it appeared that the alternative protocol was followed to a high extent. For instance, patients in the intervention group who intended to quit within six months received more often an Arrange component compared with those who were planning to do so later than six months or had no intention to quit (69% vs. 52%, p = 0.043) (not shown in the table).
None of the baseline characteristics differed at a statistically significant level between patients treated and not treated “as-intended”; only previous quit attempts differed statistically at borderline significance level, those with at least one quit attempt being more likely to receive the “as-intended” intervention compared with those who had no previous quit attempt (90.6% vs.81%, p = 0.054) (Table 2). Receivers of the intervention “as-intended” did not substantially differ from patients in the control group.
Table 2.
Baseline characteristics of patients According to treatment group and receipt of the “as-intended” intervention.
| Baseline characteristic | Intervention n (%) |
Control n (%) | P-value |
||
|---|---|---|---|---|---|
| Treated “as-intended” | Not treated “as-intended” | Treated vs. not treated “as-intended” | |||
| N | 160 | 58 | 233 | ||
| Sex | Male | 103 (64.4) | 32 (55.2) | 151 (64.8) | 0.216 |
| Female | 57 (35.6) | 26 (44.8) | 82 (35.2) | ||
| Age, mean (SD) | 44.84 (14.84) | 42.31 (14.32) | 47.36 (14.63) | 0.262 | |
| Education | Elementary school | 29 (19.3) | 9 (17.0) | 49 (23.4) | 0.831 |
| Secondary | 92 (61.3) | 35 (66.0) | 125 (59.8) | ||
| Post-secondary | 29 (19.3) | 9 (17.0) | 35 (16.7) | ||
| Occupation | Employed full-time | 110 (69.6) | 30 (52.6) | 132 (60.8) | 0.070 |
| Self-employed | 12 (7.6) | 7 (12.3) | 21 (9.7) | ||
| Not employed | 36 (22.8) | 20 (35.1) | 64 (29.5) | ||
| Readiness to quit | Pre-contemplation | 131 (81.9) | 42 (72.4) | 178 (82.4) | 0.127 |
| Contemplation/Preparation | 29 (18.1) | 16 (27.6) | 38 (17.6) | ||
| Duration of tobacco use in years mean (SD) | 24.31 (13.91) | 20.84 (13.76) | 25.72 (13.95) | 0.104 | |
| Time to tobacco use after awakening | <=30 min | 93 (58.1) | 37 (63.8) | 122 (56.5) | 0.451 |
| >30 min | 67 (41.9) | 21 (36.2) | 94 (43.5) | ||
| Daily cigarettes/snus pouches or both for dual users | <=10 | 80 (50.0) | 34 (58.6) | 117 (52.0) | 0.260 |
| >10 | 80 (50.0) | 24 (41.4) | 108 (48.0) | ||
| 24-h previous quit attempts | None | 15 (9.4) | 11 (19.0) | 34 (15.1) | 0.054 |
| At least one | 145 (90.6) | 47 (81.0) | 191 (84.9) | ||
| Chronic diagnosis | Yes | 24 (15.0) | 6 (10.3) | 38 (17.5) | 0.378 |
| No | 136 (85.0) | 52 (89.7) | 179 (82.5) | ||
Note. Numbers may not sum up to the total because of missing values. Data is missing for the following variables: Education 8.6% (n = 39), Occupation 4.2% (n = 19), Readiness to quit 4.0% (n = 18), Duration of tobacco use 2.2% (n = 10), Time to tobacco use after awakening 3.8% (n = 17), Daily cigarettes/snus pouches1.8% (n = 8), 24-h previous quit attempts 1.8% (n = 8), Chronic diagnosis 3.5% (n = 16).
3.2. Secondary analyses
3.2.1. Per-protocol (PP) analysis
The Odds Ratio (OR) of complete abstinence from tobacco (either seven-day or sustained three-month prior to the follow-up) was higher for the receivers of the intervention “as-intended” compared with the control group, but these results were not statistically significant. The likelihood of cutting by half the amount of tobacco consumed daily was 81% higher among the receivers “as-intended” compared with the patients in the control group OR = 1.81, 95% CI [1.06, 3.07]. The intervention “as-intended” was associated with a marginally significant increased likelihood of making at least one quit attempt (Table 3).
Table 3.
Odds ratios of tobacco cessation outcomes among receivers of the counseling “As-Intended”compared to control group (Per-Protocol Analysis).
| Outcome | Receivers intervention Patients with successful outcome/Total N |
Control group Patients with successful outcome/Total N |
Unadjusted |
Adjusteda |
||
|---|---|---|---|---|---|---|
| OR | [95% CI] | OR | [95% CI] | |||
| Seven-day abstinence | 11/160 (6.9%) | 14/233 (6.0%) | 1.15 | [0.51, 2.61] | 1.09 | [0.48, 2.51] |
| Three-month abstinence | 6/160 (3.8%) | 8/233 (3.4%) | 1.10 | [0.37, 3.22] | 1.03 | [0.35, 3.06] |
| Half-reduction | 36/160 (22.5%) | 31/224 (13.8%) | 1.81 | [1.06, 3.07] | 1.76 | [1.03, 3.00] |
| Quit attempts | 83/160 (51.9%) | 100/233 (42.9) | 1.43 | [0.96, 2.15] | 1.59 | [1.03, 2.45] |
Adjusted for time to tobacco use after awakening.
3.2.2. As-treated (AT) analysis
The receipt of any combination of 4 A's or of 5 A's was associated with significantly increased odds of reduction by half of tobacco consumed daily when compared with no tobacco counseling, in the crude model and after discounting for readiness to quit and time to tobacco use after awakening. Receiving 4 A's or 5 A's increased the likelihood of quit attempts, but the results were not statistically significant. For 7-days abstinence and 3-months abstinence, the similar association could not be studied because these outcomes were not achieved by any of the patients in the reference category, i.e. those exposed to no tobacco counseling (Table 4).
Table 4.
Odds ratios of tobacco cessation outcomes According to counseling components Actually received (as-treated analysis).
| Outcome | Received counseling | Patients with successful outcome/Total N | Unadjusted |
Adjusteda |
||
|---|---|---|---|---|---|---|
| OR | [95% CI] | OR | [95% CI] | |||
| Half-reduction | No counseling | 8/77 (10.4%) | 1 | 1 | ||
| Less than 4 components | 26/153 (17.0%) | 1.77 | [0.76, 4.11] | 1.83 | [0.77, 4.30] | |
| 4 components (Any combination of 4 A's) | 25/75 (25.0%) | 2.87 | [1.22, 6.80] | 2.61 | [1.09, 6.27] | |
| 5 components (All 5 A's) | 28/113 (24.8%) | 2.84 | [1.22, 6.63] | 2.63 | [1.11, 6.22] | |
| Quit attempts | No counseling | 33/77 (42.9%) | 1 | 1 | ||
| Less than 4 components | 63/158 (39.9%) | 0.88 | [0.51, 1.54] | 0.94 | [0.52, 1.73] | |
| 4 components (Any combination of 4 A's) | 57/104 (54.8%) | 1.62 | [0.89, 2.93] | 1.64 | [0.86, 3.14] | |
| 5 components (All 5 A's) | 58/113 (51.3%) | 1.41 | [0.78, 2.52] | 1.51 | [0.80, 2.84] | |
Note. None of the patients in the “No counseling” category achieved 7-day abstinence or 3-month abstinence, therefore the results are not presented for these outcomes.
Adjusted for readiness to quit and time to tobacco use after awakening.
3.2.3. Instrumental variable (IV) analysis
The success ratio of cessation outcomes was estimated by IV analysis among patients of dental practitioners who would always adhere to the assigned task, i.e. controlling for non-adherence effect. The success ratio intervention to control was 1.56 for seven-day abstinence, 2.57 for three-month continuous abstinence, 3.32 for reduction by half of tobacco consumption and 1.27 for quit attempts (Supplementary material).
The additional sensitivity analysis showed that being assigned to the intervention group, but not receiving the intervention as intended tripled the odds of half-reduction compared with the control condition (Supplementary material).
4. Discussion
We conducted secondary analyses of a randomized trial of tobacco cessation, with the aim to assess the effect of being exposed to a preventive intervention as opposed to the effect of being assigned to the same intervention estimated through intention-to-treat analysis. This distinction is of great importance for the estimation of a potential population impact of a novel technology, therefore for decisions about its dissemination.
The results of the per-protocol (PP) analysis were in good agreement with those obtained through the previously conducted intention-to-treat analysis (ITT) [2]. In essence, the proportions of patients reporting successful outcomes were higher among the receivers of the intervention “as-intended” than among the patients treated as usual, but significantly so only for the reduction of consumption. In the as-treat (AT) analysis, the effect of being exposed to different components of the intervention was evaluated. The AT results were in line with those of the other analyses, indicating that structured counseling consisting of 4 A's or 5 A's had an effect on facilitating a substantial reduction of tobacco consumption when compared with no tobacco counseling. The success ratios calculated through the instrumental variable (IV) analysis were similar to the odds ratios estimated by ITT for two of the outcomes (seven-day abstinence and quit-attempts), but were higher for continuous three-month abstinence and for half-reduction. However, it should be kept in mind that one central assumption underlying this analysis (exclusion restriction assumption) was most likely violated in this study.
ITT is the standard method of analysis in RCTs yielding the least biased estimate in placebo RCTs [6]. Also, it is the reference method to estimate effectiveness in pragmatic trials, as it simulates a real life situation, allowing for a certain degree of protocol deviation [16], [17]. However, in randomized controlled trials when the comparator is usual care or an active treatment, the ITT analysis may bias the effect of the alternative intervention in any direction [6], [18]. The least biased results of alternative intervention's true effect may be obtained using any of the four approaches (ITT, PP, AT, IV) depending on the expected size of the effect, the magnitude of and the reasons for non-adherence [6], [17]. As these methods have both common and individual sources of bias, conducting supplementary analyses of the kind presented in this article increases the understanding of the true effect of the intervention.
In this study, the good agreement between the results from ITT and PP methods can be explained by several factors. First, adherence was high both in the intervention group and in the usual care group, the latter delivering just minimal components compared with the experimental counseling. Secondly, the prognostic factors for tobacco cessation outcomes were evenly distributed between receivers of the intervention and patients assigned to usual care. Therefore, the comparison was not confounded by these baseline characteristics as it was also confirmed after discounting their possible effect through adjustment in multiple logistic regression models. Thirdly, selection bias which could severely affect the results of the PP seemed not to be an issue at least regarding the measured baseline characteristics associated with prognosis. However, selective delivery of the intervention may be caused by unmeasured prognostic factors at the patient level, such as self-efficacy [19]. This may lead to under-estimation of the effect of the manualized intervention if patients with low self-efficacy would be targeted with the complete intervention to a higher extent than those with high self-efficacy.
In a classical approach of AT analysis the comparison is done between the adherent group (the same as in the PP analysis) and the rest of the sample (including the non-adherent group excluded from the PP analysis) [4]. We used an adaptation of this format, contrasting patients reached by different qualitative and quantitative levels of the counseling, irrespective of the experimental group they were assigned to. The decision to use an adaptation of the classical AT approach was determined by two factors. Firstly, using the formal AT definition, we would have had a very heterogeneous comparison group, merging individuals receiving counseling components approximating the intended protocol and individuals receiving no or just minimal counseling (those initially randomized to the control condition). Secondly, in a pragmatic trial with a multi-component experimental intervention, some components could be offered spontaneously in “care as usual”, therefore the difference between “treated” and “non-treated” according to the protocol is likely to constitute an artifact in the AT analysis [8], [10], [18]. In fact, some of the components of the manualized intervention are likely to be delivered in “care as usual” in Sweden, a country in which dental clinics have a strong mandate on prevention and health promotion (i.e. guidelines for tobacco control) [20]. Therefore, we considered that an analysis contrasting individuals receiving specific intervention components with those who did not receive any tobacco counseling, would be more informative, conditionally on baseline characteristics representing indications to those components (i.e. patient readiness to quit).
In behavioral interventions, the IV assumption that the only pathway between the assignment to the intervention and the projected outcome goes through full-delivery of the intervention itself is usually violated, because patients targeted with a high level of care may be prompted to change their behavior in other domains, thus increasing the success of favorable outcomes [10]. In this study, even the patients randomly assigned to the treatment, but not receiving it as intended presented more favorable outcomes than the control group. A possible reason could be that the counseling offered by dental practitioners in the intervention group differed in intensity and quality from the “usual care”, even when not delivered as intended.
4.1. Strengths and limitations
The careful monitoring of the implementation process was a major strength in the FRITT trial, making it possible to carry PP and AT analyses and to provide the empirical base for the assumptions underlying the IV analysis. The balanced distribution between experimental groups of baseline characteristics representing important prognostic factors for tobacco use cessation was also a notable feature of the study.
We acknowledge some major limitations, first and foremost that adherence was not defined a priori as part of the protocol [5]. However, the definition used in this study was driven by theory and previous empirical evidence, not by the study results. Furthermore, adherence had different definitions in the intervention and control group. As adherence to “usual care” was not manualized and could not be univocally defined, this condition was considered to have virtually full adherence. The level of support offered in “usual care” followed a pattern recognizable in other studies where a majority of health practitioners actually declare no action beyond asking about tobacco use and advising about its negative consequences [21], [22], [23], [24].
Further, in assessing adherence we used reports by dental practitioners and by the patients' themselves (readiness to quit in the baseline survey). However, practitioners may have instantaneously adjusted the intervention delivery to additional information gathered during the visit. This would result in a misclassification of the stage-appropriate counseling which could distort the results of the PP and IV analysis. Both dental practitioners' and patients' reports could be affected by social desirability, leading to a misclassification of the readiness to quit and/or to an inflated estimation of the delivered counseling.
Finally, since blindness could not be assured, we cannot exclude the possibility of biased reports, if the individuals in the intervention group underreported their tobacco use at follow up. It is reasonable to assume that the patients report bias would influence in the same way the several outcomes we used. Therefore, the different effect of the intervention on the various outcomes along the continuum of smoking cessation (i.e. effective for half-reduction, but not abstinence) suggest that the results are genuine (i.e. not distorted by patient's report bias). Moreover, in a previous study [2] it was observed that favorable outcomes mostly concerned snus users, who would be less sensitive to social desirability arguments compared to cigarette smokers, at least in a country such Sweden where snus is widely accepted.
5. Conclusions
Defining non-adherence at the intervention provider and at the intervention recipient level and conducting secondary analyses of randomized trials are useful strategies to strengthen and to put in context the inference based on the intention-to-treat analysis. To this end we suggest that:
-
(a)
planners of field RCT of complex public health interventions should develop and test instruments to monitor the implementation process in all experimental groups;
-
(b)
a projection of the “clinically” significant effects of adherence on the outcomes accompany the development of the intervention;
-
(c)
it should be reminded that “usual care” is not an actual assignment. Therefore, if components of the alternative intervention can be delivered spontaneously in usual care, the exploratory analysis of their effect irrespective of the protocol and randomization could be informative.
Funding
This work was supported by the National Board of Health and Welfare [grant number 2.4–43860, 2011]. The funding agencies had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
The authors declare that there are no conflicts of interest. No financial disclosures were reported by the authors of this paper.
Ethical approval
The trial was approved by the Ethical Review Board of Stockholm Region, March 15, 2012 (nr. 2012/237-31/5). On August 28, 2012 it was registered in the International Standard Randomised Controlled Trial Number (ISRCTN) registry with the identification number ISRCTN50627997.
Informed consent
The participants' autonomy was respected by including them in the study only after the informed consent was given. The data was handled and analysed respecting the confidentiality of both patients and the dental clinic staff.
Acknowledgments
The authors are grateful to Zangin Zeebari, PhD for assistance in the statistical analysis.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.conctc.2017.01.005.
Contributor Information
Sinziana I. Oncioiu, Email: sinziana-ioana.oncioiu@ki.se.
Livia Franchetti-Pardo, Email: livia.franchetti.pardo@ki.se.
Suvi E. Virtanen, Email: suvi.virtanen@ki.se.
Fabrizio Faggiano, Email: fabrizio.faggiano@med.uniupo.it.
Maria R. Galanti, Email: rosaria.galanti@ki.se.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Carr A.B., Ebbert J. Interventions for tobacco cessation in the dental setting. Cochrane Database Syst. Rev. 2012;6 doi: 10.1002/14651858.CD005084.pub3. CD005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virtanen S.E., Zeebari Z., Rohyo I., Galanti M.R. Evaluation of a brief counseling for tobacco cessation in dental clinics among Swedish smokers and snus users. A cluster randomized controlled trial (the FRITT study) Prev. Med. 2015;70:26–32. doi: 10.1016/j.ypmed.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 3.International Conference on Harmonisation [ICH], Statistical Principles for Clinical Trials E9. 1998. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/Step4/E9_Guideline.pdf [Google Scholar]
- 4.K.J. Rothman, S. Greenland, T.L. Lash, Modern Epidemiology, third ed., 202–204,.642-651, Lippincott Williams & Wilkins, Philadelphia, USA, 2008.
- 5.Dodd S., White I.R., Williamson P. Nonadherence to treatment protocol in published randomised controlled trials: a review. Trials. 2012;13:84. doi: 10.1186/1745-6215-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernán M.A., Hernández-Díaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin. Trials Lond. Engl. 2012;9:48–55. doi: 10.1177/1740774511420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenland S. An introduction to instrumental variables for epidemiologists. Int. J. Epidemiol. 2000;29:722–729. doi: 10.1093/ije/29.4.722. [DOI] [PubMed] [Google Scholar]
- 8.Shrier I., Steele R.J., Verhagen E., Herbert R., Riddell C.A., Kaufman J.S. Beyond intention to treat: what is the right question? Clin. Trials Lond. Engl. 2014;11:28–37. doi: 10.1177/1740774513504151. [DOI] [PubMed] [Google Scholar]
- 9.Sommer A., Zeger S.L. On estimating efficacy from clinical trials. Stat. Med. 1991;10:45–52. doi: 10.1002/sim.4780100110. [DOI] [PubMed] [Google Scholar]
- 10.Stuart E.A., Perry D.F., Le H.-N., Ialongo N.S. Estimating intervention effects of prevention programs: accounting for noncompliance. Prev. Sci. Off. J. Soc. Prev. Res. 2008;9:288–298. doi: 10.1007/s11121-008-0104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiClemente C.C., Prochaska J.O., Fairhurst S.K., Velicer W.F., Velasquez M.M., Rossi J.S. The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change. J. Consult. Clin. Psychol. 1991;59:295–304. doi: 10.1037//0022-006x.59.2.295. [DOI] [PubMed] [Google Scholar]
- 12.Fiore M.C., Jaen C., Baker T. US Department of Health and Human Services; 2008. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. [Google Scholar]
- 13.Freund K.M., D'Agostino R.B., Belanger A.J., Kannel W.B., Stokes J. Predictors of smoking cessation: the framingham study. Am. J. Epidemiol. 1992;135:957–964. doi: 10.1093/oxfordjournals.aje.a116407. [DOI] [PubMed] [Google Scholar]
- 14.Hyland A., Li Q., Bauer J.E., Giovino G.A., Steger C., Cummings K.M. Predictors of cessation in a cohort of current and former smokers followed over 13 years. Nicotine Tob. Res. 2004;6:S363–S369. doi: 10.1080/14622200412331320761. [DOI] [PubMed] [Google Scholar]
- 15.Glymour M.M. 2009. Natural Experiments and Instrumental Variables Analyses in Social Epidemiology.http://www.tc.umn.edu/∼alonso/Glymour_2006.pdf [Google Scholar]
- 16.Altman D.G., Schulz K.F., Moher D., Egger M., Davidoff F., Elbourne D., Gøtzsche P.C., Lang T. CONSORT GROUP (Consolidated Standards of Reporting Trials), the revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann. Intern. Med. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 17.Ye C., Beyene J., Browne G., Thabane L. Estimating treatment effects in randomised controlled trials with non-compliance: a simulation study. BMJ Open. 2014;4:e005362. doi: 10.1136/bmjopen-2014-005362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White I.R. Uses and limitations of randomization-based efficacy estimators. Stat. Methods Med. Res. 2005;14:327–347. doi: 10.1191/0962280205sm406oa. [DOI] [PubMed] [Google Scholar]
- 19.Vangeli E., Stapleton J., Smit E.S., Borland R., West R. Predictors of attempts to stop smoking and their success in adult general population samples: a systematic review. Addict. Abingdon Engl. 2011;106:2110–2121. doi: 10.1111/j.1360-0443.2011.03565.x. [DOI] [PubMed] [Google Scholar]
- 20.The National Board of Health and Welfare . 2010. Befolkningens Tandhälsa 2009.http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/18049/2010-6-5.pdf [Google Scholar]
- 21.Steele R.J., Shrier I., Kaufman J.S., Platt R.W. Simple estimation of patient-oriented effects from randomized trials: an open and shut CACE. Am. J. Epidemiol. 2015;182:557–566. doi: 10.1093/aje/kwv065. [DOI] [PubMed] [Google Scholar]
- 22.Amemori M., Korhonen T., Michie S., Murtomaa H., Kinnunen T.H. Implementation of tobacco use cessation counseling among oral health professionals in Finland. J. Public Health Dent. 2013;73:230–236. doi: 10.1111/jphd.12019. [DOI] [PubMed] [Google Scholar]
- 23.Gordon J.S., Andrews J.A., Albert D.A., Crews K.M., Payne T.J., Severson H.H. Tobacco cessation via public dental clinics: results of a randomized trial. Am. J. Public Health. 2010;100:1307–1312. doi: 10.2105/AJPH.2009.181214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prakash P., Belek M.G., Grimes B., Silverstein S., Meckstroth R., Heckman B., Weintraub J.A., Gansky S.A., Walsh M.M. Dentists' attitudes, behaviors, and barriers related to tobacco-use cessation in the dental setting. J. Public Health Dent. 2013;73:94–102. doi: 10.1111/j.1752-7325.2012.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.