Abstract
Elucidating how ecological and evolutionary mechanisms interact to produce and maintain biodiversity is a fundamental problem in evolutionary ecology. Here, we focus on how physiological evolution affects performance and species coexistence along the thermal niche axis in replicated radiations of Anolis lizards best known for resource partitioning based on morphological divergence. We find repeated divergence in thermal physiology within these radiations, and that this divergence significantly affects performance within natural thermal environments. Morphologically similar species that co-occur invariably differ in their thermal physiology, providing evidence that physiological divergence facilitates species coexistence within anole communities. Despite repeated divergence, phylogenetic comparative analyses indicate that physiological traits have evolved more slowly than key morphological traits related to the structural niche. Phylogenetic analyses also reveal that physiological divergence is correlated with divergence in broad-scale habitat climatic features commonly used to estimate thermal niche evolution, but that the latter incompletely predicts variation in the former. We provide comprehensive evidence for repeated adaptive evolution of physiological divergence within Anolis adaptive radiations, including the complementary roles of physiological and morphological divergence in promoting community-level diversity. We recommend greater integration of performance-based traits into analyses of climatic niche evolution, as they facilitate a more complete understanding of the phenotypic and ecological consequences of climatic divergence.
Keywords: thermal biology, physiology, thermal tolerance, thermal performance, evolutionary rates, sympatry
1. Introduction
A mechanistic understanding of how divergent phenotypic evolution facilitates species coexistence is a fundamental problem in ecology and evolution [1]. This problem comes into particular focus in the study of adaptive radiation, a process for which a hallmark is the repeated evolution of niche differences that allow closely related species to partition resources and stably co-occur [2–4]. Traditionally, most research on trait evolution during adaptive radiation has focused on morphological traits associated with dietary resource acquisition or structural habitat use, due to the clear role such adaptations can play in mediating competition and facilitating coexistence [3,5–7]. Physiological adaptation to abiotic conditions has received comparatively less attention in adaptive radiation studies, particularly in animals [3,8]. Such adaptations are more typically studied for their role in generating divergence among geographically disjunct lineages experiencing different climates [9,10]. However, physiological divergence can also play an important role in adaptive radiation by facilitating fine-scale partitioning of the local climatic niche [11], providing an additional resource axis along which a radiating clade may diversify [12,13]. Indeed, such abiotic niche divergence has been hypothesized to underlie many impressive radiations, including bolitoglossine salamanders [14,15], Liolaemus lizards [16–18], Drosophila flies [19], Petrolisthes crabs [20] and lake whitefish [21].
If physiological adaptation to abiotic conditions contributes to adaptive radiation, physiological divergence should affect performance in the different abiotic regimes that species occupy. Therefore, knowledge of both physiological traits related to the fundamental abiotic niche (broadly, the range of conditions a species could occupy) and abiotic conditions (at scales appropriate for the organisms under study [22]) is required to make the important phenotype-to-performance linkages established for many classic adaptive radiations driven by morphological divergence [3,5,23]. Despite the power of performance-based analyses, one of the most common tools applied in recent investigations of abiotic niche divergence is correlative ecological niche modelling (ENM), which estimates species' realized niches (i.e. the range of conditions that species occupy) using information about species occurrences and broad-scale climate conditions [24–28]. Though correlative ENMs are powerful for addressing many questions, they also have characteristics that limit the inferences that can be made from their application [29–34]. In particular, correlative ENMs provide no information about phenotypic traits (physiological or otherwise) linked to performance [30]. Additionally, ENMs are limited in their ability to elucidate the role of abiotic niche divergence in coexistence, as they do not provide fine-scale information about microclimate use. Such factors can be of paramount importance for coexistence in radiating clades, as species with identical or largely overlapping ranges may be able to partition resources via adaptation to different microenvironments [11,35].
Here, we investigate the contribution of thermal niche divergence to the replicated Anolis lizard adaptive radiations of Puerto Rico and Jamaica by analysing patterns and performance consequences of physiological divergence across 16 species (figure 1). Greater Antillean Anolis are best known for morphological diversification along the fundamental structural niche axis [36–38], but morphological evolution cannot fully explain the patterns of divergence and community composition observed across the Greater Antilles [23]. Divergence in the thermal niche is also proposed to be an important component of Anolis diversification based primarily on realized niche estimates from natural history and ENM studies [12,13,24,39–44]. However, we still know little about how physiology evolves during realized niche divergence [45] and the extent to which physiological evolution facilitates species coexistence.
Figure 1.
Phylogenetic relationships of the species included in this study, along with the islands from which they were sampled.
We first use data from the Puerto Rican cristatellus species group to investigate repeated divergence in thermal physiology and performance between species that occupy distinct thermal environments. Next, we integrate these data with measurements of operative thermal conditions in Puerto Rico to explore the performance consequences of physiological divergence. We then consider spatial patterns of physiological variation to evaluate the contribution of physiological evolution to fine-scale niche partitioning and community-level species coexistence across Puerto Rico and Jamaica. Finally, we conduct phylogenetic analyses of rates of physiological and morphological evolution to compare the pace of disparification between traits associated with the fundamental thermal niche and the fundamental structural niche, and we employ phylogenetic methods to estimate the strength of the association between fundamental and broad-scale realized thermal niche divergence.
2. Methods
We measured physiological traits for 304 individuals across 16 species from three islands (figure 1; see electronic supplementary material, table S1 for species information). Experiments in Puerto Rico were conducted 20 May–20 June 2011, 8–21 October 2011, and 13–28 May 2012 at the Mata de Plátano field station. In Jamaica, we sampled five of the six extant endemic species, plus the introduced A. sagrei. Experiments in Jamaica occurred from 25 February to 13 March 2013 at Green Castle Estates, a privately owned farm and nature preserve in St. Mary Parish. Endemic Puerto Rican and Jamaican anoles evolved in situ from single colonization events on their respective islands [46–49]. Prior to experiments, lizards were kept individually in plastic cages (18 × 11 × 15 cm) with a wooden dowel perch on an approximately 12 L : 12 D light schedule. Lizards were watered daily and fed crickets or Phoenix worms every other day. The exception was A. acutus from St Croix, which were brought to Durham, NC, and housed following Gunderson & Leal [50]. For a subset of analyses, we use only the eight endemic Puerto Rican species, for two reasons. First, phylogenetic and natural history data indicate that these species form four sister-species pairs [46–48], with each pair representing an independent divergence in realized thermal niche based on the degree to which they occupy open versus shaded perches (we refer to these species as ‘warm-niche’ and ‘cool-niche’, respectively) [12,41,51,52]. Second, we have detailed measurements of operative thermal environments in Puerto Rico to investigate in situ performance consequences of physiological divergence.
Heat tolerance (CTmax) was measured following Leal & Gunderson [53], with N = 9–11 individuals/species (electronic supplementary material, table S1). Briefly, lizards (all captured 3 days prior to measurements) were warmed under a heat lamp while monitoring their body temperatures with a wire thermocouple probe placed inside the cloaca. Lizards were flipped onto their backs at 1°C body temperature intervals starting at 34°C, and CTmax was recorded as the body temperature at which they lost righting ability. Warming rates were very similar among species, with mean rates ranging from 1.8 to 2.6°C min−1 (electronic supplementary material, figure S1). We modelled variation in log-transformed CTmax among species using analysis of variance (ANOVA). Planned orthogonal contrasts were applied to test a priori predictions about divergence in CTmax between sister species (in Puerto Rico) and divergence among sympatric, morphologically similar species (Puerto Rico and Jamaica).
Temperature-dependent sprint speeds were measured for 13 species (N = 9–13 individuals/species; electronic supplementary material, table S1) following Gunderson & Leal [52]. Briefly, we analysed high-speed video (120 frames s−1) of lizards running up a 2 m wooden racetrack set at a 37° angle and marked with a line every 12.5 cm. Lizards were run two to four times at each temperature (two runs minimum, additional runs were added if the lizard stopped or jumped off of the track during a trial). Speed for a lizard at a given temperature was taken as the fastest 25 cm interval at that temperature [54,55]. Puerto Rican lizards were run at five body temperatures in the following randomized order: 32°C, 22°C, 27°C, 17°C and 35°C. Conditions were the same for Jamaican lizards, except the 17°C temperature was excluded. All individuals were captured 1 day prior to the start of trials, and each temperature treatment was administered on a different day over five straight days (4 days for Jamaican lizards). No animals were used for both heat tolerance and sprint performance. Target body temperatures were achieved by placing lizards into a calibrated chilling-heating incubator (Cole-Parmer®, Vernon Hills, IL, USA) for 30 min prior to a run [52].
The body temperature of maximum performance (the ‘optimal temperature’, Topt) was estimated for each individual (sensu [56]). Thermal performance curves are nonlinear [57], and we initially fitted two different nonlinear models to the sprint data for each individual: a second-order polynomial and a modified Gaussian function ( ) commonly used to fit performance curves for ectotherms [58], including Anolis sprinting [52,59]. The Gaussian model provided relatively poor fits to the data (much higher residual standard errors than the second-order polynomial model; electronic supplementary material, figure S2). Therefore, Topt estimates from the second-order polynomial models were used in analyses. Topt estimates were constrained such that Topt had to occur within the range of experimental temperatures. This is a conservative assumption that likely leads us to underestimate divergence in thermal physiology between species (see Results). Topt data were heavily skewed (see Results), so we compared Topt among species by assessing overlap in bootstrapped 95% CIs (999 bootstrap replicates using the adjusted bootstrap percentile method due to the skew in our data [60]). Analyses were conducted with the ‘boot’ and ‘boot.ci’ functions, respectively, from the ‘boot’ package in R [61].
) commonly used to fit performance curves for ectotherms [58], including Anolis sprinting [52,59]. The Gaussian model provided relatively poor fits to the data (much higher residual standard errors than the second-order polynomial model; electronic supplementary material, figure S2). Therefore, Topt estimates from the second-order polynomial models were used in analyses. Topt estimates were constrained such that Topt had to occur within the range of experimental temperatures. This is a conservative assumption that likely leads us to underestimate divergence in thermal physiology between species (see Results). Topt data were heavily skewed (see Results), so we compared Topt among species by assessing overlap in bootstrapped 95% CIs (999 bootstrap replicates using the adjusted bootstrap percentile method due to the skew in our data [60]). Analyses were conducted with the ‘boot’ and ‘boot.ci’ functions, respectively, from the ‘boot’ package in R [61].
We calculated the expected physiological performance of Puerto Rican species under natural warm (open xeric forest) and cool (shaded mesic forest) operative thermal regimes found on Puerto Rico [62]. Operative temperature distributions provide a quantitative description of a thermal environment incorporating physical features of the organism [63], representing the distribution of available body temperatures in a habitat. Operative temperature distributions came from a previous study [52] and were measured with copper lizard models [50] during the peak breeding season (summer) for Puerto Rican anoles [64]. Mean operative temperature in the mesic forest (28.9°C) is cooler than that in the xeric forest (33.4°C) by 4.5°C [52].
We estimated two performance metrics under the different operative environments: (i) relative sprint performance capacity [52,59,63] and (ii) probability of overheating (the percentage of operative temperature observations over CTmax). For relative performance capacity, we generated species-level thermal performance curves using our sprint speed and CTmax data. The curves were a combination of two functions fit to the data: one describing performance below Topt, and the other describing performance from Topt to CTmax [52,58,59]. The former function was a second-order polynomial fit to the sprint data, and the latter function took the form:  [52,59]. Curves were scaled to relative performance by setting sprint speed at Topt to 1.0 (see electronic supplementary material, figure S3 for fitted curves). Performance in each habitat was calculated by applying the curves to operative temperatures [52,59,63].
[52,59]. Curves were scaled to relative performance by setting sprint speed at Topt to 1.0 (see electronic supplementary material, figure S3 for fitted curves). Performance in each habitat was calculated by applying the curves to operative temperatures [52,59,63].
To compare rates of evolution of thermal physiology to ecomorphological traits involved in fundamental structural niche partitioning, we used a phylogenetic comparative approach. Rate of evolution of heat tolerance (CTmax, with expanded species sampling, see electronic supplementary material, table S1) was compared to rates for body size (snout–vent–length, or SVL), head length, femur length and adhesive toepad width for the 4th hindtoe. We used the maximum clade credibility phylogeny and morphological data of Mahler et al. [47] for these analyses. Shape data (head length, femur length and toepad width) consisted of residuals from phylogenetic regressions of each shape variable on SVL, a standard measure of lizard body size; these residuals were obtained using the phyl.resid function in phytools [65]. We used the approach of Adams [66] to conduct pairwise rate comparisons for all combinations of these five traits. We used likelihood ratio tests to compare a model in which the two traits could evolve at different rates to a model in which the traits were constrained to evolve at a single rate. Rates were estimated using species means of natural log-transformed values for all traits (such that rate variation is represented in terms of relative change in proportion to the trait mean; this is essential for comparison of traits measured using different units [66]). For these analyses, we co-estimated among-trait covariances (similar results were obtained with covariances fixed at zero).
To test for an association between fundamental and broad-scale realized thermal niche estimates, we conducted a phylogenetically controlled correlation test between CTmax and geo-referenced Worldclim temperature data for each species based on collection localities. We used the first principal component axis from the Anolis climate temperature dataset of Algar & Mahler [44], in which a phylogenetic principal component analysis (PCA) was used to condense the 11 Worldclim variables related to temperature (at 1 km resolution) into PCA axes (electronic supplementary material, table S2). The first PC axis loaded positively for all variables related to temperature (e.g. mean annual temperature, mean maximum temperature; electronic supplementary material, table S2), and can therefore, be considered a composite estimate of habitat temperature. Phylogenetically controlled correlation analyses between CTmax and individual Worldclim variables that loaded strongly with PC1 yielded similar results (electronic supplementary material, figure S4).
3. Results
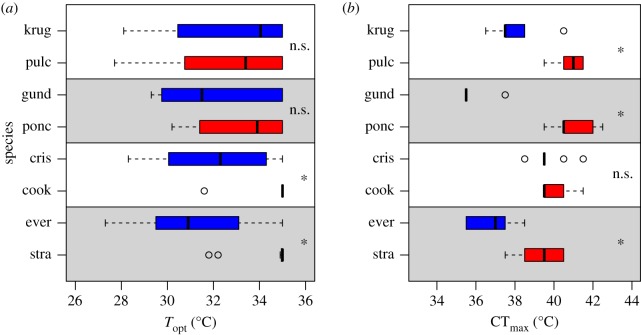
All Puerto Rican sister-species pairs have diverged in at least one physiological trait (Topt, CTmax or both), and in the predicted direction in all cases (figure 2). Topt differed between the A. evermanni/A. stratulus and A. cristatellus/A. cooki species pairs (non-overlapping bootstrapped 95% CIs), but not between the A. poncensis/A. gundlachi or A. pulchellus/A. krugi species pairs (figure 2a). We note that the Topt of A. cooki and A. stratulus were likely underestimated because most individuals had a Topt of 35°C solely because that was our maximum test temperature. CTmax differed among Puerto Rican species (F7,72 = 42.74; p < 0.001), with significant divergence in three of the four species pairs: A. evermanni/A. stratulus, A. poncensis/A. gundlachi and A. pulchellus/A. krugi (all p < 0.001), but not in the A. cristatellus/A. cooki species pair (p = 0.490; figure 2b).
Figure 2.
Thermal physiology of four sister-species pairs of Puerto Rican anole. Each species pair is represented in alternating grey and white regions of the plot with warm-niche species (the bottom species in each pair) in red and cool-niche species in blue. (a) Optimal sprint performance temperatures (Topt). (b) Heat tolerance limits (CTmax). ‘*’, significant difference between members of a species pair; n.s., no significant difference between members of a species pair. See Methods for analysis details. (Online version in colour.)
Physiological divergence influenced predicted performance in natural environments. In three of four species pairs, the cool-niche species had higher predicted performance than the warm-niche species in the shaded operative thermal environment, with mean performance advantages of 8–14% depending on the species pair and time of day (figure 3). The lone exception was the A. pulchellus/A. krugi species pair, for which both have similar performance estimates (figure 3g). Warm-niche species tend to have a performance advantage in the open operative environment, particularly during midday hours (from 10.00 to 13.00; figure 3). The A. cristatellus/A. cooki species pair is the exception (figure 3d). However, A. cooki midday performance is likely underestimated due to our probable underestimation of its Topt (see above).
Figure 3.
Predicted relative physiological performance of sister-species pairs of Puerto Rican Anolis under shaded and open habitat operative thermal environments found on Puerto Rico during morning, midday and afternoon/evening hours. Warm-niche species in red squares, cool-niche species in blue circles. (a,b) A. stratulus/A. evermanni, (c,d) A. cooki/A. cristatellus, (e,f) A. poncensis/A. gundlachi and (g,h) A. pulchellus/A. krugi. (Online version in colour.)
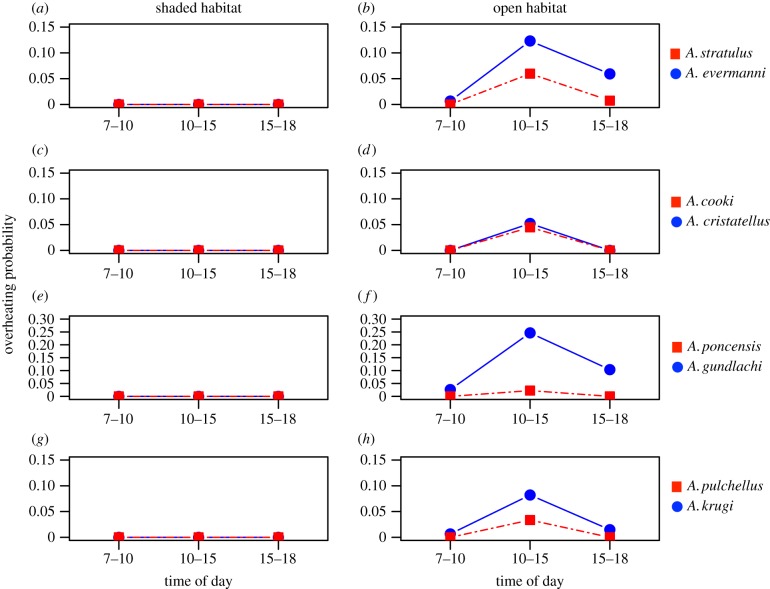
No species is under threat of overheating in the shaded operative environment (figure 4a,c,e,g). However, all species have overheating risk in the open operative environment (figure 4b,d,f,h). For three of the four sister-species pairs (A. stratulus/A. evermanni, A. poncensis/A. gundlachi and A. pulchellus/A. krugi), the cool-niche species has a higher probability of overheating (figure 4b,f,h). Difference in overheating probability are particularly acute during midday, when cool-niche species have overheating probabilities at least twice that of their warm-niche counterparts (G-tests, all p < 0.05). The A. cristatellus/A. cooki species pair, which had no divergence in CTmax, was the only exception (figure 4d).
Figure 4.
Predicted overheating probability of sister-species pairs of Puerto Rican Anolis under shaded and open habitat operative thermal environments found on Puerto Rico during morning, midday and afternoon/evening hours. Warm-niche species in red squares, cool-niche species in blue circles. (a,b) A. stratulus/A. evermanni, (c,d) A. cooki/A. cristatellus, (e,f) A. poncensis/A. gundlachi and (g,h) A. pulchellus/A. krugi. (Online version in colour.)
Significant physiological divergence is also apparent among Jamaican species. Among species mean Topt ranges from 29.5°C to 34.6°C (figure 5a), while CTmax ranges from 35.8°C to 41.5°C (figure 5b; ANOVA, F3,36 = 13.6, p < 0.001). Note that Topt values of A. grahami and A. opalinus are likely underestimated due to our upper experimental limit of 35°C (see above).
Figure 5.
Thermal physiology of endemic Jamaican anoles. (a) Optimal sprinting temperature (Topt). Anolis valencienni does not have a Topt estimate because this species could not be induced to run. (b) Heat tolerance (CTmax). (Online version in colour.)
Across Puerto Rico and Jamaica, sympatric species that share the same ecomorphology invariably differ in thermal physiology (table 1). Thermal tolerance has evolved significantly more slowly than some, but not all, ecomorphological traits (table 2). CTmax evolved significantly more slowly than body size (SVL; p < 0.001) and relative limb length (femur; p = 0.029), but did not differ from head length (p = 0.105) or toepad width (p = 0.112).
Table 1.
Physiological differences between sympatric species that share the same or similar structural niches (i.e. ecomorphology). For CTmax, p-values are based on one-way ANOVA with planned orthogonal contrasts. For Topt, “*” indicates that the bootstrapped 95% confidence intervals did not overlap, “n.s.” indicates no significant difference. “†” indicates that the species is an introduced member of the community.
| physiological trait |
||||
|---|---|---|---|---|
| co-occurring species | ecomorph | CTmax | Topt | |
| A. stratulus | A. evermanni | trunk-crown | <0.001 | * |
| A. cristatellus | A. cooki | trunk-ground | 0.490 | * |
| A. cristatellus | A. gundlachi | trunk-ground | <0.001 | n.s. |
| A. krugi | A. pulchellus | grass-bush | <0.001 | n.s. |
| A. lineatopis | A. grahami | trunk-ground/trunk-crown | <0.001 | n.s. |
| A. lineatopis | A. sagrei† | trunk-ground | <0.001 | n.s. |
Table 2.
Estimated rates of evolution for heat tolerance (CTmax) and morphological traits related to ecomorphological divergence. Rate estimates (italics) are in the diagonal of the matrix. Upper off-diagonals contain estimates of pairwise evolutionary covariances among traits, lower off-diagonals contain p-values for likelihood ratio test comparisons of 2-rate versus 1-rate models for each trait pair. p-values less than 0.05 indicate support for a 2-rate model over a 1-rate model.
| CTmax | SVL | head length | femur length | toepad width | |
|---|---|---|---|---|---|
| CTmax | 5.80 × 10−5 | −4.90 × 10−5 | −1.60 × 10−5 | −5.50 × 10−5 | −9.90 × 10−6 |
| SVL | <0.001 | 2.30 × 10−3 | 8.90 × 10−6 | −1.80 × 10−5 | 2.70 × 10−5 |
| head length | 0.105 | <0.001 | 2.70 × 10−5 | 1.30 × 10−5 | −6.30 × 10−6 |
| femur length | 0.029 | <0.001 | 0.001 | 1.40 × 10−4 | 2.20 × 10−5 |
| toepad width | 0.112 | <0.001 | 0.003 | 0.832 | 1.30 × 10−4 |
CTmax was significantly positively correlated with geo-referenced climatic temperatures as represented by temperature principal component axis 1 (p = 0.006, r2 = 0.45; figure 6; see electronic supplementary material, figure S4).
Figure 6.
Relationship between CTmax and climate temperature PC1 from Algar & Mahler [44].
4. Discussion
Abiotic niche evolution has been invoked as an important component of many evolutionary radiations, but the functional consequences of abiotic niche divergence are generally poorly known. We demonstrate that the Puerto Rican Anolis adaptive radiation is accompanied by physiological divergence in both heat tolerance and sensitivity to sub-lethal temperature variability. Warm-niche species (determined based on preference for open versus shaded perches) have either higher heat tolerance, higher optimal physiological temperatures, or both, compared to cool-niche species. The anoles of Jamaica also diversified physiologically, with species evolving essentially the full range of heat tolerances and optimal temperatures observed in their Puerto Rican counterparts (figure 5). These results highlight the possibility that physiological adaptation can be pervasive within evolutionary radiations, including those for which morphological divergence is widespread.
Repeated divergence in physiological traits under similar conditions is itself evidence of adaptation [67]. However, we provide more direct evidence of adaptation by estimating physiological performance of species in warm and cool operative environments on Puerto Rico. Cool-niche species have higher predicted performance in shaded operative environments than their warm-niche sister species in three of the four species pairs (figure 3), with the lone exception a species pair for which there was no divergence in Topt. The pattern was less pronounced in the open operative environment, with warm-niche species having a sizable performance advantage in only two species pairs (figure 3). However, the performance advantage for warm-niche species becomes apparent when considering overheating risk. During midday hours in the warmer open environment, warm-niche species in three of the four species pairs had less than half the overheating risk of cool-niche species (figure 4). The sole exception was the A. cristatellus/A. cooki species pair, which exhibited no divergence in thermal tolerance (figure 2b). One implication of these results is that what appears to be modest physiological evolution (CTmax and Topt divergence of 2–4°C) can still have significant performance consequences.
The high community-level species diversity associated with Anolis and other adaptive radiations is facilitated by fine-scale resource partitioning [3]. Sympatric anoles are known to behaviourally partition thermal microhabitats [12,13], and our findings indicate that this is facilitated by physiological divergence. When co-occurring anole species share the same fundamental structural niche (i.e. ecomorphological microhabitat specialization), they invariably differ in thermal physiology (table 1). This pattern is also maintained for introduced species: in Jamaica, the endemic trunk-ground species A. lineatopus is found in sympatry with the introduced trunk-ground species A. sagrei, and these species differ significantly in thermal tolerance (table 1). In sum, anoles provide evidence that morphological and physiological variation can simultaneously facilitate coexistence within communities that result from adaptive radiation.
We found that thermal tolerance evolved more slowly than some ecomorphological traits (i.e. SVL and femur length), and evolved at similar rates to others (i.e. head length and toepad width; table 2). In general, divergence along the fundamental thermal niche axis appears to have occurred more slowly than divergence along the fundamental structural niche axis, at least within the Puerto Rican and Jamaican anole radiations. This finding is inconsistent with a recent analysis that found that thermal niche, estimated as field body temperature, evolves more quickly than morphology in Caribbean anoles [43]. Discrepancy between these results may be due to the different traits analysed and/or the different species included, or the fact that the above-mentioned study estimated rates of absolute, rather than proportional, trait change (figure 4). Regardless, the observation that physiology evolves slowly, with repeated divergence of relatively small magnitude, is consistent with our above suggestion that large changes in physiology are not necessary for divergence to be ecologically important. These results also indicate that caution should be exercised when inferring the importance of traits based on comparisons of evolutionary rate estimates in the absence of data on how phenotypic changes map to performance change under natural conditions.
We found that fundamental and broad-scale realized thermal niche estimates were correlated, as CTmax was significantly positively correlated with geo-referenced temperature data across species (figure 6). Therefore, the realized thermal niches estimated in correlative ENM studies can capture some of the underlying variability in the fundamental thermal niche. Nonetheless, the majority of variability in the data (approx. 55%) is left unexplained, and there are clear cases where realized and fundamental niche estimates diverge. For example, A. lineatopus and A. gundlachi have very similar CTmax (mean CTmax = 36.3°C and 36.2°C, respectively), but occur in very different realized thermal niches (temperature PC1 = 0.14 and −2.66, respectively). Conversely, A. opalinus and A. pulchellus occur in very similar realized niches (temperature PC1 = −0.53 and −0.51, respectively) but have very different CTmax (mean CTmax = 37.5°C and 41.3°C, respectively). These results underscore the fact that broad-scale climatic conditions are not necessarily reflective of the underlying thermal biology of the organism being considered. Much of this discrepancy is likely due to behavioural thermoregulation, which allows taxa in the same geographical location to experience very different body temperatures and those in different geographical locations to experience very similar body temperatures [35,69].
We have shown that fundamental thermal niche divergence is important in the Puerto Rican and Jamaican Anolis adaptive radiations. However, the importance of such divergence is likely not restricted to these islands. For example, species of cybotes group trunk-ground anoles in Hispaniola have diverged in thermal tolerance along an elevational gradient [45,70], and variation in thermal physiology also occurs among non-Antillean anoles [71,72]. Water loss rates also differ among anole species and populations [73–79]. In this context, our results suggest that physiological divergence may be as important as morphological divergence for diversification and coexistence in this classic radiation.
Achieving a mechanistic understanding of how evolutionary and ecological processes interact to promote the production and maintenance of biodiversity is a long-standing goal in evolutionary ecology. Given the reality of ongoing climate change, the need to understand these processes has become immediate, particularly with respect to temperature-dependent traits [80]. By focusing on physiological traits that link temperature to performance, we demonstrate that the evolution of thermal physiology can facilitate adaptive radiation by contributing to in situ performance trade-offs and species coexistence. While our trait- and performance-based analyses would not be possible using the type of broad-scale climatic data used in ENM studies alone, we find that climate-scale realized niche features correlate with fundamental niche features. Nonetheless, climatic data provide imperfect signal with respect to the evolution of functional traits, likely due to the ability of species to behaviourally augment their effective thermal environments. We suggest that deeper insights about the contribution of climatic niche divergence to evolutionary radiations will emerge when correlative niche data are used in tandem with experimental physiological studies within an integrative research programme.
Supplementary Material
Acknowledgements
We thank J. Losos and two anonymous reviewers for helpful comments on this manuscript, A. Algar for help with data, D. Steinberg for field help, and El Verde and Mata de Plátano field stations, Ridge to Reef farm and Green Castle Estates for logistical support. Permits were provided by the Puerto Rican Departmento de Recursos Naturales y Ambientales, the St. Croix Division of Fish and Wildlife, and the Jamaican National Environment and Planning Agency.
Ethics
This research was conducted with the approval of the Duke University Animal Care and Use Committee permit no. A106-10-04.
Data accessibility
Physiological data are deposited in Dryad (http://dx.doi.org/10.5061/dryad.688jj72) [81].
Authors' contributions
A.R.G. and M.L. designed the study. A.R.G. collected the physiological data and conducted all statistical analyses that were not phylogenetically controlled. D.L.M. conducted all phylogenetic comparative analyses. A.R.G. led the writing with contributions from M.L. and D.L.M.
Competing interests
We have no competing interests.
Funding
This research was funded by NSF DDIG no. 1110570 to A.R.G.
References
- 1.Hutchinson GE. 1959. Homage to Santa Rosalia or why are there so many kinds of animals? Am. Nat. 93, 145–159. ( 10.1086/282070) [DOI] [Google Scholar]
- 2.Simpson GG. 1953. Major features of evolution. New York, NY: Columbia University Press. [Google Scholar]
- 3.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.McPeek MA. 2008. The ecological dynamics of clade diversification and community assembly. Am. Nat. 172, E270-E284. ( 10.1086/593137) [DOI] [PubMed] [Google Scholar]
- 5.Grant PR. 1999. Ecology and evolution of Darwin's finches. Princeton, NJ: Princeton University Press. [Google Scholar]
- 6.Streelman JT, Danley PD. 2003. The stages of vertebrate evolutionary radiation. Trends Ecol. Evol. 18, 126–131. ( 10.1016/S0169-5347(02)00036-8) [DOI] [Google Scholar]
- 7.Meyer A. 1993. Phylogenetic relationships and evolutionary processes in East African cichlid fishes. Trends Ecol. Evol. 8, 279–284. ( 10.1016/0169-5347(93)90255-N) [DOI] [PubMed] [Google Scholar]
- 8.Nosil P. 2012. Ecological speciation. Oxford, UK: Oxford University Press. [Google Scholar]
- 9.Chown S, Gaston K, Robinson D. 2004. Macrophysiology: large-scale patterns in physiological traits and their ecological implications. Funct. Ecol. 18, 159–167. ( 10.1111/j.0269-8463.2004.00825.x) [DOI] [Google Scholar]
- 10.Gaston KJ, et al. 2009. Macrophysiology: a conceptual reunification. Am. Nat. 174, 595–612. ( 10.1086/605982) [DOI] [PubMed] [Google Scholar]
- 11.Kaspari M, Clay NA, Lucas J, Yanoviak SP, Kay A. 2015. Thermal adaptation generates a diversity of thermal limits in a rainforest ant community. Glob. Change Biol. 21, 1092–1102. ( 10.1111/gcb.12750) [DOI] [PubMed] [Google Scholar]
- 12.Rand AS. 1964. Ecological distribution in anoline lizards of Puerto Rico. Ecology 45, 745–752. ( 10.2307/1934922) [DOI] [Google Scholar]
- 13.Ruibal R. 1961. Thermal relations of five species of tropical lizards. Evolution 15, 98–111. ( 10.1111/j.1558-5646.1961.tb03132.x) [DOI] [Google Scholar]
- 14.Wake DB. 1987. Adaptive radiation of salamanders in Middle American cloud forests. Ann. MI. Bot. Garden 74, 242–264. ( 10.2307/2399397) [DOI] [Google Scholar]
- 15.Kozak KH, Wiens JJ. 2007. Climatic zonation drives latitudinal variation in speciation mechanisms. Proc. R. Soc. B 274, 2995–3003. ( 10.1098/rspb.2007.1106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labra A, Pienaar J, Hansen TF. 2009. Evolution of thermal physiology in Liolaemus lizards: adaptation, phylogenetic inertia, and niche tracking. Am. Nat. 174, 204–220. ( 10.1086/600088) [DOI] [PubMed] [Google Scholar]
- 17.Bonino MF, Azócar DLM, Tulli MJ, Abdala CS, Perotti MG, Cruz FB. 2011. Running in cold weather: morphology, thermal biology, and performance in the southernmost lizard clade in the world (Liolaemus lineomaculatus section: Liolaemini: Iguania). J. Exp. Zool. A 315, 495–503. ( 10.1002/jez.697) [DOI] [PubMed] [Google Scholar]
- 18.Pincheira-Donoso D. 2011. Predictable variation of range-sizes across an extreme environmental gradient in a lizard adaptive radiation: evolutionary and ecological inferences. PLoS ONE 6, e28942 ( 10.1371/journal.pone.0028942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellermann V, van Heerwaarden B, Sgrò CM, Hoffmann AA. 2009. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 325, 1244–1246. ( 10.1126/science.1175443) [DOI] [PubMed] [Google Scholar]
- 20.Stillman JH, Somero GN. 2000. A comparative analysis of the upper thermal tolerance limits of eastern Pacific porcelain crabs, genus Petrolisthes: influences of latitude, vertical zonation, acclimation, and phylogeny. Physiol. Biochem. Zool. 73, 200–208. ( 10.1086/316738) [DOI] [PubMed] [Google Scholar]
- 21.Dalziel AC, Martin N, Laporte M, Guderley H, Bernatchez L. 2015. Adaptation and acclimation of aerobic exercise physiology in Lake Whitefish ecotypes (Coregonus clupeaformis). Evolution 69, 2167–2186. ( 10.1111/evo.12727) [DOI] [PubMed] [Google Scholar]
- 22.Potter KA, Woods HA, Pincebourde S. 2013. Microclimatic challenges in global change biology. Glob. Change Biol. 19, 2932–2939. ( 10.1111/gcb.12257) [DOI] [PubMed] [Google Scholar]
- 23.Losos JB. 2009. Lizards in an evolutionary tree: ecology and adaptive radiation of anoles. Berkeley, CA: University of California Press. [Google Scholar]
- 24.Knouft JH, Losos JB, Glor RE, Kolbe JJ. 2006. Phylogenetic analysis of the evolution of the niche in lizards of the Anolis sagrei group. Ecology 87, S29–S38. ( 10.1890/0012-9658(2006)87%5B29:PAOTEO%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 25.Quintero I, Wiens JJ. 2013. Rates of projected climate change dramatically exceed past rates of climatic niche evolution among vertebrate species. Ecol. Lett. 16, 1095–1103. ( 10.1111/ele.12144) [DOI] [PubMed] [Google Scholar]
- 26.Warren DL, Glor RE, Turelli M. 2008. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62, 2868–2883. ( 10.1111/j.1558-5646.2008.00482.x) [DOI] [PubMed] [Google Scholar]
- 27.Lawing AM, Polly PD, Hews DK, Martins EP. 2016. Including fossils in phylogenetic climate reconstructions: a deep time perspective on the climatic niche evolution and diversification of spiny lizards (Sceloporus). Am. Nat. 188, 138–148. ( 10.1086/687202) [DOI] [PubMed] [Google Scholar]
- 28.Peterson AT, Soberón J, Pearson RG, Anderson RP, Nakamura M, Martinez-Meyer E, Araújo MB. 2011. Ecological niches and geographic distributions. Princeton, NJ: Princeton University Press. [Google Scholar]
- 29.Guisan A, Thuiller W. 2005. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 8, 993–1009. ( 10.1111/j.1461-0248.2005.00792.x) [DOI] [PubMed] [Google Scholar]
- 30.Buckley LB, Urban MC, Angilletta MJ, Crozier LG, Rissler LJ, Sears MW. 2010. Can mechanism inform species' distribution models? Ecol. Lett. 13, 1041–1054. ( 10.1111/j.1461-0248.2010.01506.x) [DOI] [PubMed] [Google Scholar]
- 31.Alvarado-Serrano DF, Knowles LL. 2014. Ecological niche models in phylogeographic studies: applications, advances and precautions. Mol. Ecol. Resour. 14, 233–248. ( 10.1111/1755-0998.12184) [DOI] [PubMed] [Google Scholar]
- 32.Kearney M, Porter W. 2009. Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 12, 334–350. ( 10.1111/j.1461-0248.2008.01277.x) [DOI] [PubMed] [Google Scholar]
- 33.Warren DL, Cardillo M, Rosauer DF, Bolnick DI. 2014. Mistaking geography for biology: inferring processes from species distributions. Trends Ecol. Evol. 29, 572–580. ( 10.1016/j.tree.2014.08.003) [DOI] [PubMed] [Google Scholar]
- 34.Araújo MB, Peterson AT. 2012. Uses and misuses of bioclimatic envelope modeling. Ecology 93, 1527–1539. ( 10.1890/11-1930.1) [DOI] [PubMed] [Google Scholar]
- 35.Bogert CM. 1949. Thermoregulation in reptiles, a factor in evolution. Evolution 3, 195–211. ( 10.1111/j.1558-5646.1949.tb00021.x) [DOI] [PubMed] [Google Scholar]
- 36.Losos JB, Jackman TR, Larson A, de Queiroz K, Rodríguez-Schettino L. 1998. Contingency and determinism in replicated adaptive radiations of island lizards. Science 279, 2115–2118. [DOI] [PubMed] [Google Scholar]
- 37.Williams EE. 1983. Ecomorphs, faunas, island size, and diverse end points in island radiations of Anolis. In Lizard ecology: studies of a model organism (eds Huey RB, Pianka ER, Schoener TW), pp. 326–370. Cambridge, MA: Harvard University Press. [Google Scholar]
- 38.Mahler DL, Ingram T, Revell LJ, Losos JB. 2013. Exceptional convergence on the macroevolutionary landscape in island lizard radiations. Science 341, 292–295. ( 10.1126/science.1232392) [DOI] [PubMed] [Google Scholar]
- 39.Williams EE. 1972. The origin of faunas. Evolution of lizard congeners in a complex island fauna: a trial analysis. Evol. Biol. 6, 47–89. [Google Scholar]
- 40.Heatwole H, Lin T-H, Villalón E, Muñiz A, Matta A. 1969. Some aspects of the thermal ecology of Puerto Rican anoline lizards. J. Herpetol. 3, 65–77. ( 10.2307/1563225) [DOI] [Google Scholar]
- 41.Huey RB, Webster TP. 1976. Thermal biology of Anolis lizards in a complex fauna: the cristatellus group on Puerto Rico. Ecology 57, 985–994. ( 10.2307/1941063) [DOI] [Google Scholar]
- 42.Losos JB, Leal M, Glor RE, de Queiroz K, Hertz PE, Schettino LR, Lara AC, Jackman TR, Larson A. 2003. Niche lability in the evolution of a Caribbean lizard community. Nature 424, 542–545. ( 10.1038/nature01814) [DOI] [PubMed] [Google Scholar]
- 43.Hertz PE, Arima Y, Harrison A, Huey RB, Losos JB, Glor RE. 2013. Asynchronous evolution of physiology and morphology in Anolis lizards. Evolution 67, 2101–2113. ( 10.1111/evo.12072) [DOI] [PubMed] [Google Scholar]
- 44.Algar AC, Mahler DL. 2015. Area, climate heterogeneity, and the response of climate niches to ecological opportunity in island radiations of Anolis lizards. Glob. Ecol. Biogeogr. 25, 781–791. ( 10.1111/geb.12327) [DOI] [Google Scholar]
- 45.Muñoz MM, Stimola MA, Algar AC, Conover A, Rodriguez AJ, Landestoy MA, Bakken GS, Losos JB. 2014. Evolutionary stasis and lability in thermal physiology in a group of tropical lizards. Proc. R. Soc. B 281, 20132433 ( 10.1098/rspb.2013.2433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicholson KE, Glor RE, Kolbe JJ, Larson A, Blair Hedges S, Losos JB. 2005. Mainland colonization by island lizards. J. Biogeogr. 32, 929–938. ( 10.1111/j.1365-2699.2004.01222.x) [DOI] [Google Scholar]
- 47.Mahler DL, Revell LJ, Glor RE, Losos JB. 2010. Ecological opportunity and the rate of morphological evolution in the diversification of Greater Antillean anoles. Evolution 64, 2731–2745. ( 10.1111/j.1558-5646.2010.01026.x) [DOI] [PubMed] [Google Scholar]
- 48.Gamble T, Geneva AJ, Glor RE, Zarkower D. 2014. Anolis sex chromosomes are derived from a single ancestral pair. Evolution 68, 1027–1041. ( 10.1111/evo.12328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jackman TR, Larson A, De Queiroz K, Losos JB. 1999. Phylogenetic relationships and tempo of early diversification in Anolis lizards. Syst. Biol. 48, 254–285. ( 10.1080/106351599260283) [DOI] [Google Scholar]
- 50.Gunderson AR, Leal M. 2012. Geographic variation in vulnerability to climate warming in a tropical Caribbean lizard. Funct. Ecol. 26, 783–793. ( 10.1111/j.1365-2435.2012.01987.x) [DOI] [Google Scholar]
- 51.Hertz PE. 1992. Temperature regulation in Puerto Rican Anolis lizards: a field test using null hypotheses. Ecology 73, 1405–1417. ( 10.2307/1940686) [DOI] [Google Scholar]
- 52.Hertz PE. 1992. Evaluating thermal resource partitioning by sympatric lizards Anolis cooki and A. cristatellus: a field test using null hypotheses. Oecologia 90, 127–136. [DOI] [PubMed] [Google Scholar]
- 53.Leal M, Gunderson AR. 2012. Rapid change in the thermal tolerance of a tropical lizard. Am. Nat. 180, 815–822. ( 10.1086/668077) [DOI] [PubMed] [Google Scholar]
- 54.Huey RB. 1983. Natural variation in body temperature and physiological performance in a lizard (Anolis cristatellus). In Advances in herpetology and evolutionary biology: essays in honor of Ernest E Williams (eds Rhodin GJ, Miyata KI), pp. 484–490. Cambridge, MA: Museum of Comparative Zoology. [Google Scholar]
- 55.Losos JB. 1990. Ecomorphology, performance capability, and scaling of West Indian Anolis lizards: an evolutionary analysis. Ecol. Monogr. 60, 369–388. ( 10.2307/1943062) [DOI] [Google Scholar]
- 56.Logan ML, Cox RM, Calsbeek R. 2014. Natural selection on thermal performance in a novel thermal environment. Proc. Natl Acad. Sci. USA 111, 14 165–14 169. ( 10.1073/pnas.1404885111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 58.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Pérez HJÁ, Garland T. 2009. Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B 276, 1939–1948. ( 10.1098/rspb.2008.1957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Efron B. 1987. Better bootstrap confidence intervals. J. Am. Stat. Assoc. 82, 171–185. ( 10.1080/01621459.1987.10478410) [DOI] [Google Scholar]
- 61.Canty A, Ripley B.. 2017. boot: bootstrap R (S-Plus) functions. R package v 13–20.
- 62.Ewel JJ, Whitmore JL. 1973. Ecological life zones of Puerto Rico and US Virgin Islands. In Forest service research paper ITF-18. US. Department of Agriculture. [Google Scholar]
- 63.Hertz PE, Huey RB, Stevenson R. 1993. Evaluating temperature regulation by field-active ectotherms: the fallacy of the inappropriate question. Am. Nat. 142, 796–818. ( 10.1086/285573) [DOI] [PubMed] [Google Scholar]
- 64.Gorman GC, Licht P. 1974. Seasonality in ovarian cycles among tropical Anolis lizards. Ecology 55, 360–369. ( 10.2307/1935223) [DOI] [Google Scholar]
- 65.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 66.Adams DC. 2013. Quantifying and comparing phylogenetic evolutionary rates for shape and other high-dimensional phenotypic data. Syst. Biol. 62, 181–192. ( 10.1093/sysbio/sys083) [DOI] [PubMed] [Google Scholar]
- 67.Harvey PH, Pagel MD. 1991. The comparative method in evolutionary biology. Oxford, UK: Oxford University Press. [Google Scholar]
- 68.Leal M, Fleishman LJ. 2002. Evidence for habitat partitioning based on adaptation to environmental light in a pair of sympatric lizard species. Proc. R. Soc. Lond. B 269, 351–359. ( 10.1098/rspb.2001.1904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huey RB, Hertz PE, Sinervo B. 2003. Behavioral drive versus behavioral inertia in evolution: a null model approach. Am. Nat. 161, 357–366. ( 10.1086/346135) [DOI] [PubMed] [Google Scholar]
- 70.Hertz PE, Huey RB. 1981. Compensation for altitudinal changes in the thermal environment by some Anolis lizards on Hispaniola. Ecology 62, 515–521. ( 10.2307/1937714) [DOI] [Google Scholar]
- 71.Logan ML, Huynh RK, Precious RA, Calsbeek RG. 2013. The impact of climate change measured at relevant spatial scales: new hope for tropical lizards. Glob. Change Biol. 19, 3093–3102. ( 10.1111/gcb.12253) [DOI] [PubMed] [Google Scholar]
- 72.van Berkum FH. 1986. Evolutionary patterns of the thermal sensitivity of sprint speed in Anolis lizards. Evolution 40, 594–604. ( 10.1111/j.1558-5646.1986.tb00510.x) [DOI] [PubMed] [Google Scholar]
- 73.Gunderson AR, Siegel J, Leal M. 2011. Tests of the contribution of acclimation to geographic variation in water loss rates of the West Indian lizard Anolis cristatellus. J. Comp. Physiol. B 181, 965–972. ( 10.1007/s00360-011-0576-0) [DOI] [PubMed] [Google Scholar]
- 74.Hillman S, Gorman G. 1977. Water loss, desiccation tolerance, and survival under desiccating conditions in 11 species of Caribbean Anolis. Oecologia 29, 105–116. ( 10.1007/BF00345791) [DOI] [PubMed] [Google Scholar]
- 75.Sexton OJ, Heatwole H. 1968. An experimental investigation of habitat selection and water loss in some anoline lizards. Ecology 49, 762–767. ( 10.2307/1935543) [DOI] [Google Scholar]
- 76.Hertz P, Arce-Hernandez A, Ramirez-Vazquez J, Tirado-Rivera W, Vazquez-Vives L. 1979. Geographical variation of heat sensitivity and water loss rates in the tropical lizard, Anolis gundlachi. Comp. Biochem. Physiol. A 62, 947–953. ( 10.1016/0300-9629(79)90033-1) [DOI] [Google Scholar]
- 77.Hertz PE. 1980. Responses to dehydration in Anolis lizards sampled along altitudinal transects. Copeia 1980, 440–446. ( 10.2307/1444519) [DOI] [Google Scholar]
- 78.Hillman S, Gorman GC, Thomas R. 1979. Water loss in Anolis lizards: evidence for acclimation and intraspecific differences along a habitat gradient. Comp. Biochem. Physiol. A 62, 491–493. ( 10.1016/0300-9629(79)90091-4) [DOI] [Google Scholar]
- 79.Dmi'el R, Perry G, Lazell J. 1997. Evaporative water loss in nine insular populations of the lizard Anolis cristatellus group in the British Virgin Islands. Biotropica 29, 111–116. ( 10.1111/j.1744-7429.1997.tb00012.x) [DOI] [Google Scholar]
- 80.Huey RB, Kearney MR, Krockenberger A, Holtum JA, Jess M, Williams SE. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B 367, 1665–1679. ( 10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gunderson AR, Mahler DL, Leal M. 2018. Data from: Thermal niche evolution across replicated Anolis lizard adaptive radiations Dryad Digital Repository. ( 10.5061/dryad.688jj72) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gunderson AR, Mahler DL, Leal M. 2018. Data from: Thermal niche evolution across replicated Anolis lizard adaptive radiations Dryad Digital Repository. ( 10.5061/dryad.688jj72) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Physiological data are deposited in Dryad (http://dx.doi.org/10.5061/dryad.688jj72) [81].