Abstract
Complex life cycles characterized by uncertainty at transitions between larval/juvenile and adult environments could favour irreversible physiological plasticity at such transitions. To assess whether thermal tolerance of intertidal mussels (Mytilus californianus) adjusts to post-settlement environmental conditions, we collected juveniles from their thermally buffered microhabitat from high- and low-shore locations at cool (wave-exposed) and warm (wave-protected) sites. Juveniles were transplanted to unsheltered cages at the two low sites or placed in a common garden. Juveniles transplanted to the warm site for one month in summer had higher thermal tolerance, regardless of origin site. By contrast, common-garden juveniles from all sites had lower tolerance indistinguishable from exposed site transplants. After six months in the field plus a common garden period, there was a trend for higher thermal tolerance at the protected site, while reduced thermal tolerance at both sites indicated seasonal acclimatization. Thermal tolerance and growth rate were inversely related after one but not six months; protected-site transplants were more tolerant but grew more slowly. In contrast to juveniles, adults from low-shore exposed and protected sites retained differences in thermal tolerance after common garden treatment in summer. Both irreversible and reversible forms of plasticity must be considered in organismal responses to changing environments.
Keywords: acclimatization, developmental plasticity, growth, heat stress, rocky intertidal zone
1. Introduction
Animals’ performance in stressful environments could depend on gene–environment ‘match–mismatch’ [1] or on physiological plasticity expressed either during development or in response to variation in the adult environment [2,3]. Delineating the relative roles and magnitudes of these phenomena is particularly relevant to forecasting the effects of global change [4,5]. Reversible adult plasticity can be an effective strategy in sessile organisms exposed to stressful environments after recruitment to the adult population [6], for example, by allowing long-lived organisms to adjust to predictable environmental changes, such as seasonal temperature cycles [7]. Adult plasticity affects thermal tolerance in diverse terrestrial and marine species [8].
By contrast, developmental plasticity is generally considered irreversible and results from individuals experiencing different environments before adulthood [9]. For example, developmental temperature has been shown to induce irreversible phenotypic changes in Drosophila, parasitoid wasps and zebrafish [10–12]. Developmental plasticity may be adaptive in organisms with high gene flow among populations inhabiting disparate environments [13], in cases of high spatial variability within populations [14], and for species that experience considerable temporal environmental variation across generations [14]. These scenarios result in offspring inhabiting environments that vary unpredictably from the parental environment. Complex life cycles, particularly in the numerous species in which different life-history stages occupy distinct environmental niches, could similarly favour the expression of irreversible plasticity during periods of transition between environments [13]. Aside from studies on insects [10,11,15], relatively little is known about the flexibility of physiological mechanisms during these transitions.
Here, we investigate plasticity of thermal tolerance around such a life-history transition—recruitment of post-larval juveniles to adult aggregations—in the ecologically dominant rocky intertidal mussel Mytilus californianus. The dynamic nature and spatial heterogeneity of the rocky intertidal zone [16], along with aspects of this species' life history, may promote both reversible and irreversible forms of plasticity. Adult M. californianus are sessile, can live for 50+ years [17], and experience predictable seasonal temperature changes. Accordingly, adult mussels exhibit seasonal plasticity in gill phospholipid membrane composition [18] and in heat shock protein (Hsp) induction temperature [19]. Mytilus californianus also experience large temperature fluctuations associated with the semidiurnal tidal cycle, corresponding with short-term plasticity in membrane composition [16] and defenses against oxidative stress [20]. Other aspects of this mussel's life history could promote irreversible, developmental plasticity of thermal tolerance [21]. Adults produce long-lived planktonic larvae (up to 45 days; [22]) that can disperse at least 50 km [23], thereby facilitating gene flow [24] and probably increasing environmental variation across generations. There also is pronounced spatial variability in body temperature within mussel beds [16], further reducing the predictability of a settling larva's future adult micro-environment. However, expressing the regulatory mechanisms that allow juveniles to switch between phenotypes may be costly [7], potentially constraining other processes such as growth [12]. Given M. californianus' role as an ecosystem engineer [25], if developmental plasticity does allow juveniles to match their thermal tolerance to their post-recruitment micro-environment—even if such adjustments are limited in magnitude [5,26]—the implications could extend throughout the rocky intertidal community.
We assessed thermal tolerance of juvenile and adult M. californianus from multiple intertidal sites and measured growth rates of juveniles to: (i) determine whether fixed genetic differences or phenotypic plasticity determine juveniles’ thermal tolerance, (ii) examine potential trade-offs between juveniles' thermal tolerance and growth, and (iii) compare plasticity of thermal tolerance in juveniles and adults from the same sites. Our data suggest that juveniles irreversibly adjust thermal tolerance based on post-recruitment environmental conditions. Such plasticity and its associated costs can affect how taxa that experience unique environments throughout development will cope with environmental change.
2. Material and methods
(a). Plasticity of thermal tolerance and growth rate in juvenile mussels
Juvenile mussels (M. californianus, n = 735, shell length 5–14.5 mm) were collected in June 2016 from high- and low-shore beds at both wave-exposed (cool) and wave-protected (warm) microsites at Hopkins Marine Station (HMS) in Pacific Grove, CA, USA (36.6217° N, 121.9043° W) [16,20]. Low- and high-shore sites (approx. 1.5 and approx. 1.75 m above mean lower low water, respectively) within each microsite were situated within 10 m of each other. These exposed and protected microsites differ in wave splash, solar irradiance and mussel body temperatures. Individuals were labelled with coded apiarist tags. Juveniles of this size still inhabit algal tufts and/or interstices of the byssal thread network under and between larger adults [22]; these microhabitats shelter juveniles from the direct solar heating and desiccation risk characteristic of the adult micro-environment [27].
(i). Juvenile one-month and six-month reciprocal transplant studies
Mussels from each of the four origin sites were transplanted to a low-shore location at either their original wave-exposure site or the other site during early summer. Juveniles from each site were placed inside a mesh bag, enclosed within a stainless steel anti-predator cage (3.3 mm mesh), and attached to an acrylic plate (electronic supplementary material, figure S1). The plates were bolted onto open patches of rock, exposing the juveniles to unsheltered conditions, on 21 June 2016. Transplanted individuals were retrieved after one month (July) or six months (December) (electronic supplementary material, figure S2).
Survival was high during the one-month experiments at both transplant sites (99%) (electronic supplementary material, table S1). Owing to tag loss and/or uncertain fate of numerous individuals, survival could not be quantified in the six-month experiment.
(ii). Juvenile one-month common garden study
Shifts in juveniles’ thermal physiology could change with recent environmental experience or simply with age [28]. To begin to tease apart these factors, individuals from each origin site (n = 24 per site) were exposed to a laboratory common garden treatment that ran in parallel with the one-month transplant study. These mussels were continuously submerged in a flow-through seawater tank (temperature 12.4–15.2°C) at HMS. Phytoplankton food in the flow-through seawater was supplemented once per day by adding approximately 1 ml of concentrated Shellfish Diet 1800 (Reed Mariculture) and stopping water flow for approximately 1.5 h.
(iii). Juvenile six-month reciprocal transplant plus common garden study
Juveniles from the six-month transplant study not assayed immediately for thermal tolerance were kept continuously submerged in common-garden conditions (temperature 14.3°C–15.4°C) in a recirculating aquarium system at Loyola Marymount University (LMU) and fed as described above for one month after retrieval from the field. Thermal tolerance assays were then repeated to test for persistent effects of transplant environment. Owing to tag losses, the origin wave exposure was unknown for these individuals (electronic supplementary material, figure S2).
(iv). Juvenile growth rate
Initial and final measurements of shell length, which is correlated with mass in Mytilus [29], were made with digital calipers and used to determine a daily growth coefficient. This coefficient represents proportional change in length per day, assuming a constant growth rate throughout the study. For individuals in the six-month field study, growth coefficients were calculated separately for zero to one month and one to six months. Owing to tag losses, growth could not be quantified in the six-month reciprocal transplant plus common garden study.
(b). Transplant site temperature measurements
To estimate thermal variation between the transplant sites, iButton temperature dataloggers (model DS1921G, Maxim Integrated, San Jose, CA, USA) were embedded in silicon-filled adult mussel shells (approx. 55 mm shell length; [16]) and deployed within the transplant cages (n = 5 per site). Although these adult mimics record different ‘body’ temperatures than those experienced by juveniles [27], they allow between-site comparisons. The dataloggers recorded temperature (0.5°C resolution) every 30 min for 30 days in summer. They were then reprogrammed to record every 120 min (owing to memory constraints) for the six-month field study. This longer sampling interval probably missed many daily maximum temperatures [30], but it provides an overall assessment of thermal differences between the sites. Data from all functional dataloggers at each site and time were averaged for analyses.
(c). Plasticity of thermal tolerance in adult mussels
In July 2016, we collected approximately 50 adult mussels (40–55 mm shell length) from both the exposed-low and protected-low origin sites. Half of these individuals were assayed for thermal tolerance within 6 days of collection. The remaining individuals were placed in common garden conditions for 19–22 days before repeating the thermal tolerance assay.
(d). Thermal tolerance assays
Thermal tolerance assays were conducted in air to mimic hot, low-tide conditions. To determine the thermal tolerance of field-acclimatized juveniles from each of the four origin sites, we exposed individuals to one of three temperatures (n = 6–8 per temperature per site). Each individual was placed in a 0.6 ml microcentrifuge tube, and a 1 × 1 cm seawater-saturated laboratory tissue was placed at the bottom of the tube to maintain humidity. The tubes were placed in a thermalcycler, which ramped temperature up from 20°C at 0.1°C min−1 [16,30]. Upon reaching the target temperature (35.8°C, 37.7°C or 38.6°C), temperature was held constant for 1 h before ramping down to 20°C at 0.3°C min−1. Target temperatures were chosen based on the adult LT50 for this species, approximately 38°C [13]. For subsequent assays, we preserved the ramping profiles but used a larger chamber containing a seawater-saturated laboratory tissue: common-garden and one-month reciprocal transplant juveniles in 1.5 ml microcentrifuge tubes; six-month juveniles in 15 ml tubes; adults in 50 ml tubes. The tubes were submerged in a water bath programmed with the ramp profile. Air temperature inside one to two tubes was monitored with a thermocouple to ensure consistency.
Immediately after the ramp, individuals were returned to a flow-through tank at HMS (or the aquarium system at LMU) and fed once per day as described above. Survival was scored after 7 days. Open mussels that did not respond to mechanical stimulation by closing, as well as mussels that opened upon mechanical stimulation, were scored as dead.
(e). Statistics
(i). Thermal tolerance
Survival data were analysed using the glmulti package in R [31] to perform exhaustive model selection, retaining the terms that provided the most informative binomial general linear model. The full model for juveniles at one month included origin and treatment (field-acclimatized, transplant site (exposed or protected) or common garden) as factors, with assay temperature as a continuous covariate. We included interactions between origin and temperature and between origin and treatment to allow for origin-specific patterns. For the juvenile six months plus common garden analysis, the full model included treatment (exposed or protected), origin shore height (high or low), and temperature, as well as interactions of treatment with the other two predictors. For adults, the full model included origin site and treatment (field-acclimatized or common garden), temperature and interactions of treatment with the other two predictors. The best model was chosen using a modified Akaike's information criterion (AICc) adjusted for small sample sizes [32]. Statistical significance (α = 0.05) and odds ratios are presented from the best model for each analysis. In each analysis, there was another equally parsimonious model (delta AICc < 2.0) [33], but these alternate models did not change the interpretations.
(ii). Growth rate
To analyse growth data, we used the model selection approach described above. For juvenile growth from zero to one month (n = 658, electronic supplementary material, table S2), the full model included origin site, treatment and their interaction. For the 48 individuals measured in both the zero to one month and the one to six month intervals, the full model included origin site, treatment and time interval. We also included interactions between origin site and treatment, origin site and time, and treatment and time to allow for more complex patterns. For both analyses, initial shell length was included as a continuous covariate to account for allometric effects.
3. Results
(a). Transplant site temperatures
(i). One-month transplant
The average daily range of temperature was three times greater at the protected site (8.1°C versus 2.7°C) in the one-month study (electronic supplementary material, figure S3a and table S3). The exposed site reached a maximum of 20.3°C; the protected site exceeded this temperature on 15 days, reaching a maximum of 31.3°C.
(ii). Six-month transplant
During the six-month study the protected site had a greater mean daily temperature range, reaching high temperatures more frequently. Daily temperature range decreased at both sites from summer to autumn, and the absolute difference between the sites grew smaller (electronic supplementary material, table S3).
(b). Origin site and recent environment influence juvenile thermal tolerance
(i). One month
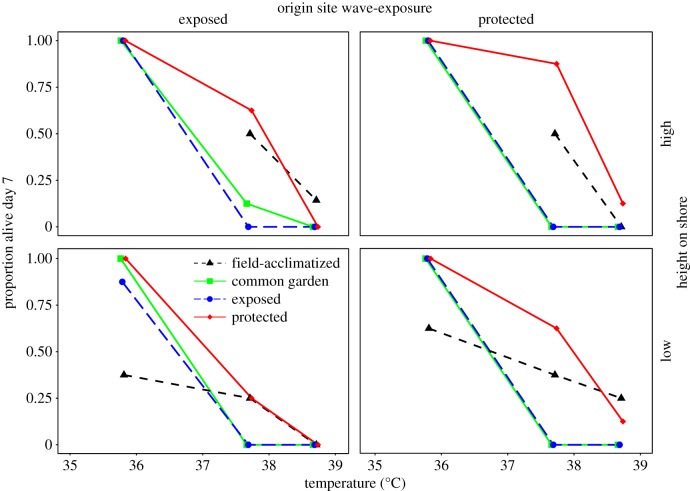
The best model for juvenile survival included significant effects of treatment, origin and temperature (table 1). The odds of survival for transplants to the protected site were estimated at more than 10 times those for individuals held in common garden (p < 0.001) or transplanted to the exposed site (p < 0.001); protected-site transplants had roughly four times the odds of survival as field-acclimatized mussels (p = 0.019) (table 1; post hoc p-values generated using multcomp R package [34]). These treatment differences were most pronounced at 37.7°C (figure 1). It is possible the slightly lower survival of field-acclimatized juveniles (figure 1) was owing to low oxygen content of their small tubes at the end of the heat ramps, but this is unlikely given the propensity of M. californianus to seal its valves upon emersion [30] and thus reduce its oxygen consumption rate [35]. Juveniles from the exposed-low origin site had less than one-fifth the odds of survival relative to the exposed-high site (p = 0.010) and the protected-high site (p = 0.005) (table 1). One other model was supported and included effects of treatment, temperature and the interaction between origin site and temperature (electronic supplementary material, table S4).
Table 1.
Statistical summaries of the best binomial models of thermal tolerance. (The first row for each analysis contains p-values for each of the included predictors (values in bold are significant, α < 0.05), and subsequent rows contain corresponding odds ratios for each term in the model (with 95% confidence intervals). Predictors left blank were excluded from the final model. Odds ratios were produced by exponentiating the model β coefficients and indicate the odds of survival relative to the reference level for each variable. Quasi-complete separation required some model coefficients to be derived by exact conditional logistic regression using the elrm package in R (*). Although the interaction between origin and treatment was initially included in the full model for all analyses, it was not included in any of the best models and is thus not shown here.)
| analysis | origina | treatmentb | temperature | origin × temperaturec |
|---|---|---|---|---|
| juvenile one month |
p = 0.002 highprot = 1.13 (0.42, 3.03) lowexp = 0.17 (0.05, 0.50) lowprot = 0.69 (0.25, 1.88) |
p < 0.001 exp = 0.73 (0.24, 2.22) field = 2.63 (0.90, 8.09) prot = 10.82 (3.84, 34.00) |
p < 0.001 temp = 0.09 (0.05, 0.14) |
|
| juvenile six months + common garden |
p < 0.001 temp* = 0.75 (0.00, 0.85) |
|||
| adult one month |
p < 0.001 temp = 0.04 (0.01, 0.12) |
p < 0.001 temp × prot = 1.12 (1.05, 1.24) |
aReference = high exposed.
bReference = common garden.
cReference = exposed × temperature (adults only).
Figure 1.
Survival of juvenile mussels after exposure to three heat stress temperatures in the one-month experiment. Mussels from each of four origin sites were assayed for thermal tolerance following four treatments (electronic supplementary material, figure S2). Field-acclimatized mussels from the high sites were not tested at 35.8°C. Points are horizontally staggered to aid in interpretation. The proportion alive on day 7 is the y-axis for all panels. ‘High’ and ‘low’ height on shore refer to the top and bottom row of panels, respectively.
To ensure that analysing data for juvenile thermal tolerance that were collected one month apart did not introduce a confounding factor of development time, we reran the analysis without the field-acclimatized treatment. The top two, equally parsimonious models were identical to those derived using the full dataset (not shown).
(ii). Six months
Owing to mussel losses, tolerance was only assessed at the two higher temperatures (37.7°C and 38.6°C) that showed the greatest treatment differences at one month. No individuals survived these exposures.
(iii). Six months plus common garden
Although there was a trend of mussels from the protected transplant site showing higher survival at the lowest temperature, the best model included only temperature (p < 0.001; table 1). An equally parsimonious model included effects of treatment and temperature (p = 0.189 and p < 0.001, respectively; electronic supplementary material, table S4). No mussels survived at 37.7°C or 38.6°C (electronic supplementary material, figure S4).
(c). No evidence for plasticity of thermal tolerance in adults
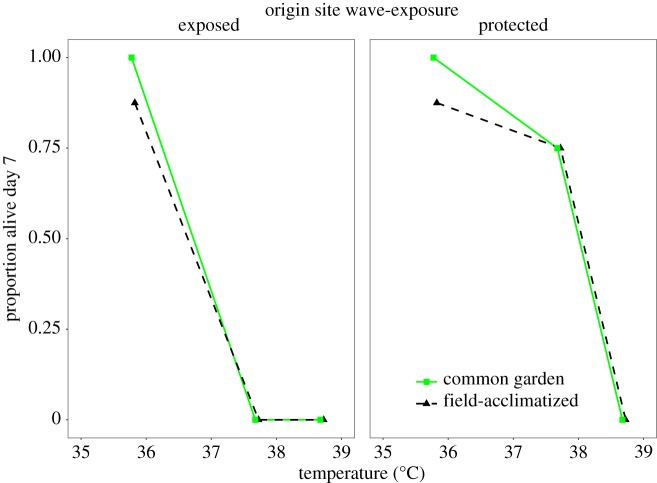
Adults from the protected site were estimated to have 72 times higher odds of survival at 37.7°C than those from the exposed site, both before and after common gardening. Unlike juveniles, adult tolerance did not change with common-garden acclimation (figure 2). The best model included significant effects of temperature and an origin-by-temperature interaction (table 1). An equally parsimonious model included significant effects of temperature and origin (electronic supplementary material, table S4).
Figure 2.
Survival of adult mussels from wave-exposed and wave-protected low sites after exposure to three heat stress temperatures. Adults were assayed directly from the field or after common-garden acclimation.
(d). Juvenile growth rate varies between transplant sites
(i). One month
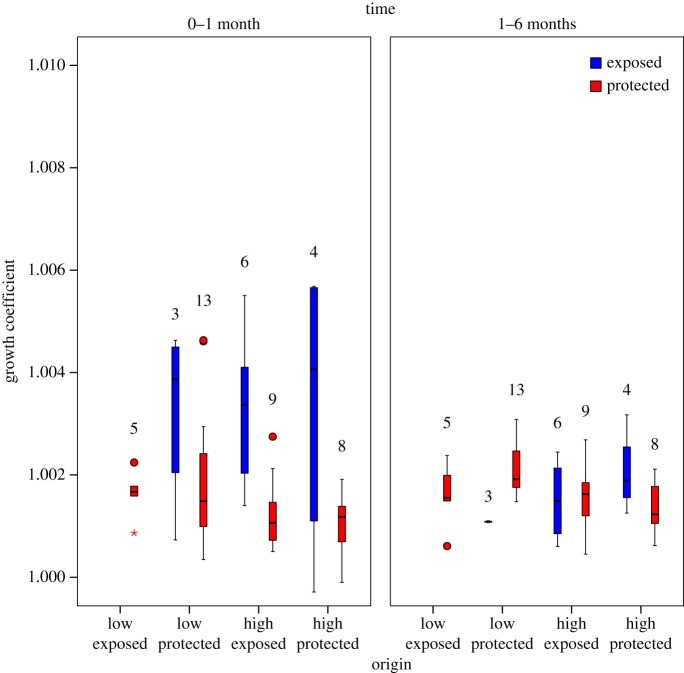
The best model for growth rate over one month included origin (p = 0.992) and three significant terms: treatment, an origin-by-treatment interaction, and a negative effect of shell length (table 2). Overall, transplants to the exposed and protected sites grew 2.45 and 0.75 global standard deviations faster, respectively, than mussels in the common garden (which grew very slowly). For the interaction, juveniles originating from high-shore sites and transplanted to the exposed site grew at more than twice the rate of those at the protected site (p < 0.001 for each post hoc comparison; figure 3). There were no equally parsimonious models.
Table 2.
Statistical summaries of the best models of juvenile growth rate for the one-month and six-month field studies. (The first row for each analysis contains p-values for each of the included predictors (values in bold are significant, α < 0.05). Predictors left blank were excluded from the final model. Partial η2 quantifies variance explained by each predictor after controlling for all other predictors. Model β coefficients were normalized by the global standard deviation (1.679 × 10−3 or 1.195 × 10−3, respectively); resulting values indicate the effect (with 95% confidence intervals) of each term as a multiple of standard deviation, relative to the reference level. No equally parsimonious models were found; model weights were 1.00 and 0.76, respectively.)
| one-month experiment | ||||
|---|---|---|---|---|
| origina | treatmentb | origin × treatmentc | initial shell length | |
p = 0.992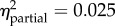
|
p < 0.001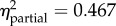 exp = 2.45 (2.12, 2.79) prot = 0.75 (0.41, 1.08) |
p < 0.001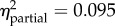 highprot × exp = −0.39 (−0.88, 0.09) lowexp × exp = −0.86 (−1.31, −0.40) lowprot × exp = −0.88 (−1.34, −0.42) highprot × prot = −0.03 (−0.51, 0.45) lowexp × prot = 0.27 (−0.18, 0.73) lowprot × prot = 0.26 (−0.20, 0.72) |
p < 0.001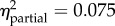 length = −0.10 (−0.13, −0.08) |
|
| six-month experiment | ||||
| treatmentb | time intervald | initial shell length | time × treatmente | |
p < 0.001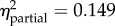
prot = −1.53 (−2.06, −1.01) |
p < 0.001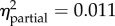
1–6 = −1.32 (−1.97, −0.67) |
p = 0.006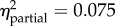 length = −0.12 (−0.20, −0.03) |
p < 0.001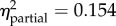
prot × 1–6 = 1.57 (0.82, 2.33) |
|
aReference = high exposed.
bReference = common garden for one-month experiment, exposed site for six-month experiment.
cReference = high exposed, common garden.
dReference = 0–1 month.
eReference = 0–1 month × exposed.
Figure 3.
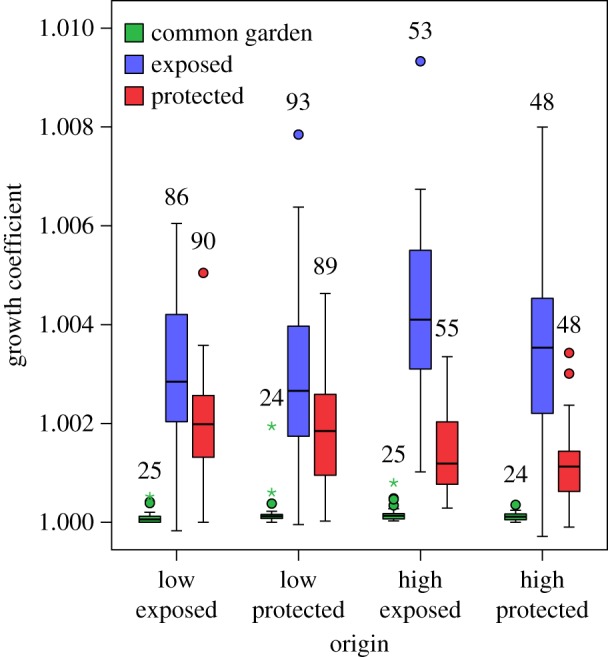
Box plot of daily growth coefficients of juvenile mussels from the four origin sites over one month in summer. Mussels were measured for total shell length immediately after collection from the field and after one month in each treatment. A growth coefficient of 1.0 indicates no growth. Sample sizes are listed above each bar.
(ii). Six months
The best model for juvenile growth over six months included significant effects of transplant site, time interval, their interaction and a negative effect of shell length (table 2). There were no equally parsimonious models. While growth rate at the protected site was consistently slow throughout the study, the interaction term indicated that growth at the exposed site was rapid during the first month (coinciding with reduced thermal tolerance) but slowed to a rate comparable to that of protected-site transplants in the one to six month interval (figure 4).
Figure 4.
Box plots of daily growth coefficients for the subset of juvenile mussels from the four origin sites that were identifiable after both one and six months in the field. The data were separated between the zero to one month and one to six months intervals. A growth coefficient of 1.0 indicates no growth. Sample sizes are listed above each bar. No individuals transplanted from the exposed-low site to the exposed site were included in this analysis.
4. Discussion
Our reciprocal transplant and common garden experiments suggest that recent environmental experience contributes to the thermal tolerance of post-settlement juvenile M. californianus, but not of adults from the same intertidal sites. Seasonal shifts in tolerance are superimposed on these developmental effects. Our results also indicate a potential trade-off between thermal tolerance and growth for juveniles during summer when food is abundant. It is difficult to prove that the changes we observed are irreversible, because one cannot test the thermal tolerance of an individual multiple times. However, these results are consistent with the existence of a developmental window following settlement during which juveniles adjust their thermal tolerance to their micro-environment.
(a). Short-term plasticity of thermal tolerance is restricted to juveniles
Evolution of a thermally sensitive window during development in M. californianus, as has also been observed in lake whitefish and Lepidoptera butterflies [36,37], could be because of unpredictability around the transition between distinct juvenile and adult microhabitats. Juveniles settle in algal tufts or among the byssal threads of adults [22,38]; these microhabitats are moist and sheltered from the sun. The ensuing transition to relatively unsheltered, but spatially variable [16], adult microhabitats as recruits grow could favour physiological plasticity around this period. Subsequently, adult mussels are essentially sessile, with limited capacity to modify their thermal exposure [30]. Consequently, resources devoted to maintaining a plastic juvenile state might best be reallocated to adult growth and reproduction. Analogous trade-offs have been thoroughly examined in plants (e.g. [39]), although rigorous experimental designs are required to quantify the associated costs [40].
The higher thermal tolerance in juveniles transplanted to the wave-protected site in summer is probably owing to more frequent episodes of high body temperature during low tide. The average daily temperature range at the protected site was triple that at the exposed site, although maximum datalogger temperatures never approached within 4.5°C of the lowest assay temperature. Similar correlations between high temperature variability and thermal tolerance have been observed in a range of taxa [12,41–44]. In the six months plus common garden study, there was a similar, albeit non-significant, trend for greater thermal tolerance among mussels transplanted to the warmer, protected site that was evident only at the lowest assay temperature. Seasonal acclimatization (see below) may have obscured evidence for persistent developmental effects on thermal tolerance in our study design; these may have appeared at lower winter assay temperatures (electronic supplementary material, figure S4).
The pattern of thermal tolerance plasticity in juveniles but not adults that we observed in M. californianus is far from universal. Thermal tolerance of adults does respond to recent temperatures in numerous species [15,45–48], although experimental differences necessitate caution in making generalizations [49]. Notably, adult M. californianus seasonally adjust membrane composition and temperature of Hsp induction [18,19]. Similarly, ratios of DNA to RNA in reciprocally transplanted adults quickly adjusted to transplant sites [50]. Adults of other Mytilus congeners acclimate both absolute heart rate and acute temperature thresholds for cardiac function [51]. However, definitive links between these parameters and thermal tolerance have not always been demonstrated. Further comparative work is needed to identify the environmental factors and time-scales of variation driving divergence among life-history stages in plasticity of thermal tolerance and to identify the underlying mechanisms.
(b). Seasonal acclimatization of thermal tolerance curves
Juveniles assayed in summer exhibited moderate survival at 37.7°C and 38.6°C, whereas no juveniles survived these temperatures in the groups assayed in winter. Seasonal acclimatization of thermal tolerance curves has been observed in a wide variety of taxa [8]. The observed seasonal differences in the frequency of warm, low-tide episodes probably drive seasonal variation in mussels' thermal tolerance.
The seasonal differences in thermal tolerance of M. californianus might also be a result of variation in phytoplankton food supply, which influences both growth rates and biochemical status [52,53], or of interactions between food availability and other stressors [54]. Five years of data from the intertidal zone at HMS (2002, 2008–2011) indicate that chlorophyll a levels, a proxy for phytoplankton biomass, are significantly lower during the autumn and winter months (HMS Marine Life Observatory, http://mlo.stanford.edu). Accordingly, daily growth coefficients were significantly lower from 22 July–12 December compared to 21 June–22 July, particularly at the exposed site. Seasonal reductions in autumn and winter growth rate because of reduced food have been observed in other marine invertebrates, including mussels [55–57]. Notably, food restriction decreased thermal tolerance of adult M. californianus, presumably owing to energetic constraints [58].
(c). Plasticity versus genetic constraint in regulation of growth
Growth rates were similarly affected by an interaction between origin shore height and transplant site. Juveniles originally from the high-shore sites grew 94% (exposed-high) and 70% (protected-high) faster at the exposed transplant site compared to the protected transplant site in summer. Similar, context-dependent origin effects on growth have been observed in Cerastoderma edule and Littorina saxatilis [59,60]. Filter-feeding invertebrates like M. californianus at high-shore sites are submerged for shorter durations than conspecifics at low-shore sites, constraining their feeding opportunities. When food is limited, Mytilus edulis adjusts its filtration rate and its ingestion/absorption efficiencies, thereby increasing capacity for growth [61]. Similarly, juvenile M. californianus from high-shore sites could be primed to deal with hotter conditions and lower food availability; when these individuals are moved to favourable conditions, they would be able to grow faster than individuals from low-shore sites.
(d). Thermal tolerance and growth trade-offs
Juveniles transplanted to the warmer, wave-protected site had the highest thermal tolerance, but they grew more slowly in summer. This finding is consistent with previous results from this species [58], as well as from several fishes [12,62,63]. Broadly, this inverse relationship between thermal tolerance and growth is probably a result of energetic constraints among protected-site transplants. For example, induction of the heat shock response—which protects macromolecules but at an appreciable cost [64]—or of other thermoprotective mechanisms could impinge on growth. It is also possible that differences in food quantity and/or time available for feeding exist between the transplant sites, although there is no reason to expect that food concentrations would vary over the short distance between the exposed and protected sites.
Mussels from all origin sites grew slowly and exhibited low thermal tolerance after being constantly submerged in the common garden, where they did not encounter episodes of high temperature that would prime compensatory tolerance mechanisms. Though this pattern is not universal [12], decreased growth under constant temperatures has also been observed in salmon and other intertidal invertebrates [65,66] and may be attributable to a loss of positive metabolic effects resulting from warm, but not stressful, body temperatures [67]. This pattern may also be attributable to higher cumulative metabolic costs; unlike mussels in the field (electronic supplementary material, figure S3), common-garden juveniles did not experience low temperatures, which can reduce metabolic rate via Q10 effects [68]. Furthermore, common-garden mussels did not benefit from reduced rates of energy expenditure via anaerobiosis during emersion [35].
5. Conclusion
Our results imply environmentally driven developmental plasticity in thermal tolerance that is inversely related to growth rate among recent M. californianus recruits. Importantly, the persistent effect of origin site supports the conclusion that both genetics and recent experience contribute to thermal tolerance [4,69,70]. However, there was no evidence for analogous plasticity of thermal tolerance in adults. Without the data for juveniles, the latter result may have been mistaken for fine-scale local adaptation. A more plausible, yet still hypothetical, mechanism for these patterns is irreversible epigenetic programming [71]. The developmental adjustment appears to interact with seasonal acclimatization, evident as horizontal shifts in the thermal tolerance curve. Both irreversible and reversible forms of plasticity should be considered when predicting how species will respond to shifting environments. Under the right circumstances, such plastic responses could mitigate the negative effects of global change.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Mark Denny and HMS staff for hosting our field season. Karina Nielsen and Chris Patton shared chlorophyll data. Brent Lockwood, George Somero and three anonymous reviewers provided constructive suggestions for revision.
Ethics
Animals were collected under California Department of Fish and Wildlife permit no. SC-7955.
Data accessibility
Survival and shell length data are provided in the electronic supplementary material (tables S5 and S6). Temperature data are archived on Dryad (http://dx.doi.org/10.5061/dryad.74sh7) [72].
Authors' contributions
L.U.G., E.L.S., B.J.H. and W.W.D. designed and performed experiments; L.U.G. and W.W.D. analysed data; L.U.G. and W.W.D. drafted the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by NSF grant IOS-1256186 (W.W.D.), a J. Exp. Biol. Travelling Fellowship (L.U.G.), and Alcantar Family Fund and LMU Seaver College of Science and Engineering (E.L.S. and B.J.H.).
References
- 1.Marshall DJ, Monro K, Bode M, Keough MJ, Swearer S. 2010. Phenotype-environment mismatches reduce connectivity in the sea. Ecol. Lett. 13, 128–140. ( 10.1111/j.1461-0248.2009.01408.x) [DOI] [PubMed] [Google Scholar]
- 2.Piersma T, Drent J. 2003. Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 18, 228–233. ( 10.1016/s0169-5347(03)00036-3) [DOI] [Google Scholar]
- 3.Via S. 1993. Adaptive phenotypic plasticity: target or by-product of selection in a variable environment. Am. Nat. 142, 352–365. ( 10.1086/285542) [DOI] [PubMed] [Google Scholar]
- 4.Pereira RJ, Sasaki MC, Burton RS. 2017. Adaptation to a latitudinal thermal gradient within a widespread copepod species: the contributions of genetic divergence and phenotypic plasticity. Proc. R. Soc. B 284, 20170236 ( 10.1098/rspb.2017.0236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunderson AR, Stillman JH. 2015. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B 282, 20150401 ( 10.1098/rspb.2015.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicotra AB, et al. 2010. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 15, 684–692. ( 10.1016/j.tplants.2010.09.008) [DOI] [PubMed] [Google Scholar]
- 7.Fusco G, Minelli A. 2010. Phenotypic plasticity in development and evolution: facts and concepts. Phil. Trans. R. Soc. B 365, 547–556. ( 10.1098/rstb.2009.0267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis, 289 p New York, NY: Oxford University Press. [Google Scholar]
- 9.West-Eberhard MJ. 2003. Developmental plasticity and evolution, 794 p New York, NY: Oxford University Press. [Google Scholar]
- 10.Cooper BS, Tharp JM, Jernberg I, Angilletta MJ. 2012. Developmental plasticity of thermal tolerances in temperate and subtropical populations of Drosophila melanogaster. J. Thermal. Biol. 37, 211–216. ( 10.1016/j.jtherbio.2012.01.001) [DOI] [Google Scholar]
- 11.Hoffmann AA, Hewa-Kapuge S. 2000. Acclimation for heat resistance in Trichogramma nr. brassicae: can it occur without costs? Funct. Ecol. 14, 55–60. ( 10.1046/j.1365-2435.2000.00388.x) [DOI] [Google Scholar]
- 12.Schaefer J, Ryan A. 2006. Developmental plasticity in the thermal tolerance of zebrafish Danio rerio. J. Fish. Biol. 69, 722–734. ( 10.1111/j.1095-8649.2006.01145.x) [DOI] [Google Scholar]
- 13.Minelli A, Fusco G. 2010. Developmental plasticity and the evolution of animal complex life cycles. Phil. Trans. R. Soc. B 365, 631–640. ( 10.1098/rstb.2009.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabriel W, Lynch M. 1992. The selective advantage of reaction norms for environmental tolerance. J. Evol. Biol. 5, 41–59. ( 10.1046/j.1420-9101.1992.5010041.x) [DOI] [Google Scholar]
- 15.Gray EM. 2013. Thermal acclimation in a complex life cycle: the effects of larval and adult thermal conditions on metabolic rate and heat resistance in Culex pipiens (Diptera: Culicidae). J. Insect. Physiol. 59, 1001–1007. ( 10.1016/j.jinsphys.2013.08.001) [DOI] [PubMed] [Google Scholar]
- 16.Denny MW, Dowd WW, Bilir L, Mach KJ. 2011. Spreading the risk: small-scale body temperature variation among intertidal organisms and its implications for species persistence. J. Exp. Mar. Biol. Ecol. 400, 175–190. ( 10.1016/j.jembe.2011.02.006) [DOI] [Google Scholar]
- 17.Gosling E. 1992. The mussel Mytilus: ecology, physiology, genetics and culture. Amsterdam, The Netherlands: Elsevier Science. [Google Scholar]
- 18.Williams E, Somero GN. 1996. Seasonal-, tidal-cycle- and microhabitat-related variation in membrane order of phospholipid vesicles from gills of the intertidal mussel Mytilus californianus. J. Exp. Biol. 199, 1587–1596. [DOI] [PubMed] [Google Scholar]
- 19.Buckley BA, Owen ME, Hofmann GE. 2001. Adjusting the thermostat: the threshold induction temperature for the heat-shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J. Exp. Biol. 204, 3571–3579. [DOI] [PubMed] [Google Scholar]
- 20.Jimenez AG, Jayawardene S, Alves S, Dallmer J, Dowd WW. 2015. Micro-scale environmental variation amplifies physiological variation among individual mussels. Proc. R. Soc. B 282, 20152273 ( 10.1098/rspb.2015.2273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logan CA, Kost LE, Somero GN. 2012. Latitudinal differences in Mytilus californianus thermal physiology. Mar. Ecol. Prog. Ser. 450, 93–105. ( 10.3354/meps09491) [DOI] [Google Scholar]
- 22.Trevelyan GA, Chang ES. 1983. Experiments on larval rearing of the California mussel (Mytilus californianus). J. World Aquac. Soc. 14, 137–148. ( 10.1111/j.1749-7345.1983.tb00068.x) [DOI] [Google Scholar]
- 23.Becker BJ, Levin LA, Fodrie FJ, McMillan PA. 2007. Complex larval connectivity patterns among marine invertebrate populations. Proc. Natl Acad. Sci. USA 104, 3267–3272. ( 10.1073/pnas.0611651104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Addison JA, Ort BS, Mesa KA, Pogson GH. 2008. Range-wide genetic homogeneity in the California sea mussel (Mytilus californianus): a comparison of allozymes, nuclear DNA markers, and mitochondrial DNA sequences. Mol. Ecol. 17, 4222–4232. ( 10.1111/j.1365-294X.2008.03905.x) [DOI] [PubMed] [Google Scholar]
- 25.Seed R, Suchanek TH. 1992. Population and community ecology of Mytilus. In The mussel Mytilus: ecology, physiology, genetics and culture (ed. Gosling EM.), pp. 87–169. Amsterdam, The Netherlands: Elsevier Science. [Google Scholar]
- 26.van Heerwaarden B, Kellermann V, Sgro CM. 2016. Limited scope for plasticity to increase upper thermal limits. Funct. Ecol. 30, 1947–1956. ( 10.1111/1365-2435.12687) [DOI] [Google Scholar]
- 27.Helmuth BST. 1998. Intertidal mussel microclimates: predicting the body temperature of a sessile invertebrate. Ecol. Monogr. 68, 51 ( 10.1890/0012-9615(1998)068%5B0051:IMMPTB%5D2.0.CO;2) [DOI] [Google Scholar]
- 28.Bowler K, Terblanche JS. 2008. Insect thermal tolerance: what is the role of ontogeny, ageing and senescence? Biol. Rev. 83, 339–355. ( 10.1111/j.1469-185X.2008.00046.x) [DOI] [PubMed] [Google Scholar]
- 29.Salkeld PN. 1995. Aspects of reproduction associated with the use of a segmented regression to describe the relationship between body weight and shell length of Mytilus edulis. Mar. Ecol. Prog. Ser. 124, 117–128. ( 10.3354/meps124117) [DOI] [Google Scholar]
- 30.Miller LP, Dowd WW. 2017. Multimodal in situ datalogging quantifies inter-individual variation in thermal experience and persistent origin effects on gaping behavior among intertidal mussels (Mytilus californianus). J. Exp. Biol. 220, 4305–4319. ( 10.1242/jeb.164020) [DOI] [PubMed] [Google Scholar]
- 31.Calcagno V, de Mazancourt C. 2010. glmulti: an R package for easy automated model selection with (generalized) linear models. J. Stat. Softw. 34, 1–29. ( 10.18637/jss.v034.i12) [DOI] [Google Scholar]
- 32.Hurvich CM, Tsai CL. 1989. Regression and time-series model selection in small samples. Biometrika 76, 297–307. ( 10.1093/biomet/76.2.297) [DOI] [Google Scholar]
- 33.Burnham KP, Anderson DR. 2003. Model selection and multimodel inference: a practical information-theoretic approach, 488 p New York, NY: Springer. [Google Scholar]
- 34.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometrical. J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 35.Bayne BL, Bayne CJ, Carefoot TC, Thompson RJ. 1976. The physiological ecology of Mytilus californianus Conrad 2. Adaptation to low oxygen tension and air exposure. Oecologia 22, 229–250. ( 10.1007/BF00344794) [DOI] [PubMed] [Google Scholar]
- 36.Eme J, Mueller CA, Manzon RG, Somers CM, Boreham DR, Wilson JY. 2015. Critical windows in embryonic development: shifting incubation temperatures alter heart rate and oxygen consumption of Lake Whitefish (Coregonus clupeaformis) embryos and hatchlings. Comp. Biochem. Physiol. 179A, 71–80. ( 10.1016/j.cbpa.2014.09.005) [DOI] [PubMed] [Google Scholar]
- 37.Kleynhans E, Conlong DE, Terblanche JS. 2014. Direct and indirect effects of development temperature on adult water balance traits of Eldana saccharina (Lepidoptera: Pyralidae). J. Insect. Physiol. 68, 69–75. ( 10.1016/j.jinsphys.2014.06.018) [DOI] [PubMed] [Google Scholar]
- 38.Petersen JH. 1984. Establishment of mussel beds: attachment behavior and distribution of recently settled mussels (Mytilus californianus). Veliger 27, 7–13. [Google Scholar]
- 39.Kempel A, Schädler M, Chrobock T, Fischer M, van Kleunen M. 2011. Tradeoffs associated with constitutive and induced plant resistance against herbivory. Proc. Natl Acad. Sci. USA 108, 5685–5689. ( 10.1073/pnas.1016508108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auld JR, Agrawal AA, Relyea RA. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503–511. ( 10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliver TA, Palumbi SR. 2011. Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30, 429–440. ( 10.1007/s00338-011-0721-y) [DOI] [Google Scholar]
- 42.Hutchison VH, Ferrance MR. 1970. Thermal tolerances of Rana pipiens acclimated to daily temperature cycles. Herpetologica 26, 1–8. [Google Scholar]
- 43.Otto RG. 1974. Effects of acclimation to cyclic thermal regimes on heat tolerance of western mosquitofish. Trans. Am. Fish. Soc. 103, 331–335. ( 10.1577/1548-8659(1974)103%3C331:TEOATC%3E2.0.co;2) [DOI] [Google Scholar]
- 44.Threader RW, Houston AH. 1983. Heat tolerance and resistance in juvenile rainbow trout acclimated to diurnally cycling temperatures. Comp. Biochem. Physiol. 75A, 153–155. ( 10.1016/0300-9629(83)90062-2) [DOI] [Google Scholar]
- 45.Terblanche JS, Chown SL. 2006. The relative contributions of developmental plasticity and adult acclimation to physiological variation in the tsetse fly, Glossina pallidipes (Diptera, Glossinidae). J. Exp. Biol. 209, 1064–1073. ( 10.1242/jeb.02129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marais E, Terblanche JS, Chown SL. 2009. Life stage-related differences in hardening and acclimation of thermal tolerance traits in the kelp fly, Paractora dreuxi (Diptera, Helcomyzidae). J. Insect. Physiol. 55, 336–343. ( 10.1016/j.jinsphys.2008.11.016) [DOI] [PubMed] [Google Scholar]
- 47.Stillman JH. 2003. Acclimation capacity underlies susceptibility to climate change. Science 301, 65 ( 10.1126/science.1083073) [DOI] [PubMed] [Google Scholar]
- 48.Sgro CM, Overgaard J, Kristensen TN, Mitchell KA, Cockerell FE, Hoffmann AA. 2010. A comprehensive assessment of geographic variation in heat tolerance and hardening capacity in populations of Drosophila melanogaster from eastern Australia. J. Evol. Biol. 23, 2484–2493. ( 10.1111/j.1420-9101.2010.02110.x) [DOI] [PubMed] [Google Scholar]
- 49.Terblanche JS, Hoffmann AA, Mitchell KA, Rako L, le Roux PC, Chown SL. 2011. Ecologically relevant measures of tolerance to potentially lethal temperatures. J. Exp. Biol. 214, 3713–3725. ( 10.1242/jeb.061283) [DOI] [PubMed] [Google Scholar]
- 50.Dahlhoff EP, Menge BA. 1996. Influence of phytoplankton concentration and wave exposure on the ecophysiology of Mytilus californianus. Mar. Ecol. Prog. Ser. 144, 97–107. ( 10.3354/meps144097) [DOI] [Google Scholar]
- 51.Braby CE, Somero GN. 2006. Following the heart: temperature and salinity effects on heart rate in native and invasive species of blue mussels (genus Mytilus). J. Exp. Biol. 209, 2554–2566. ( 10.1242/jeb.02259) [DOI] [PubMed] [Google Scholar]
- 52.Dowd WW, Felton CA, Heymann HM, Kost LE, Somero GN. 2013. Food availability, more than body temperature, drives correlated shifts in ATP-generating and antioxidant enzyme capacities in a population of intertidal mussels (Mytilus californianus). J. Exp. Biol. 449, 171–185. ( 10.1016/j.jembe.2013.09.020) [DOI] [Google Scholar]
- 53.Dahlhoff EP, Stillman JH, Menge BA. 2002. Physiological community ecology: variation in metabolic activity of ecologically important rocky intertidal invertebrates along environmental gradients. Integr. Comp. Biol. 42, 862–871. ( 10.1093/icb/42.4.862) [DOI] [PubMed] [Google Scholar]
- 54.Kroeker KJ, et al. 2016. Interacting environmental mosaics drive geographic variation in mussel performance and predation vulnerability. Ecol. Lett. 19, 771–779. ( 10.1111/ele.12613) [DOI] [PubMed] [Google Scholar]
- 55.Elvin DW, Gonor JJ. 1979. Thermal regime of an intertidal Mytilus californianus Conrad population on the central Oregon coast. J. Exp. Mar. Biol. Ecol. 39, 265–279. ( 10.1016/0022-0981(79)90130-8) [DOI] [Google Scholar]
- 56.Schone BR, Houk SD, Castro ADF, Fiebig J, Oschmann W, Kroncke I, Dreyer W, Gosselck F. 2005. Daily growth rates in shells of Arctica islandica: assessing sub-seasonal environmental controls on a long-lived bivalve mollusk. Palaios 20, 78–92. ( 10.2110/palo.2003.p03-101) [DOI] [Google Scholar]
- 57.Page HM, Hubbard DM. 1987. Temporal and spatial patterns of growth in mussels Mytilus edulis on an offshore platform: relationships to water temperature and food availability. J. Exp. Mar. Biol. Ecol. 111, 159–179. ( 10.1016/0022-0981(87)90053-0) [DOI] [Google Scholar]
- 58.Fitzgerald-Dehoog L, Browning J, Allen BJ. 2012. Food and heat stress in the California mussel: evidence for an energetic trade-off between survival and growth. Biol. Bull. 223, 205–216. ( 10.1086/BBLv223n2p205) [DOI] [PubMed] [Google Scholar]
- 59.DeMontaudouin X. 1996. Factors involved in growth plasticity of cockles Cerastoderma edule (L), identified by field survey and transplant experiments. J. Sea Res. 36, 251–265. ( 10.1016/s1385-1101(96)90794-7) [DOI] [Google Scholar]
- 60.Pardo LM, Johnson LE. 2005. Explaining variation in life-history traits: growth rate, size, and fecundity in a marine snail across an environmental gradient lacking predators. Mar. Ecol. Prog. Ser. 296, 229–239. ( 10.3354/meps296229) [DOI] [Google Scholar]
- 61.Bayne BL, Iglesias JIP, Hawkins AJS, Navarro E, Heral M, Deslouspaoli JM. 1993. Feeding behavior of the mussel, Mytilus edulis: response to variations in quantity and organic content of the seston. J. Mar. Biol. Assoc. UK 73, 813–829. ( 10.1017/S0025315400034743) [DOI] [Google Scholar]
- 62.Donelson JM, Munday PL, McCormick MI, Pankhurst NW, Pankhurst PM. 2010. Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Mar. Ecol. Prog. Ser. 401, 233–243. ( 10.3354/meps08366) [DOI] [Google Scholar]
- 63.Mascaro M, Amaral-Ruiz M, Huipe-Zamora I, Martinez-Moreno G, Simoes N, Rosas C. 2016. Thermal tolerance and phenotypic plasticity in juvenile Hippocampus erectus Perry, 1810: effect of acute and chronic exposure to contrasting temperatures. J. Exp. Mar. Biol. Ecol. 483, 112–119. ( 10.1016/j.jembe.2016.07.005) [DOI] [Google Scholar]
- 64.Feder ME, Hofmann GE. 1999. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243–282. ( 10.1146/annurev.physiol.61.1.243) [DOI] [PubMed] [Google Scholar]
- 65.Biette RM, Geen GH. 1980. Growth of underyearling sockeye salmon (Oncorhynchus nerka) under constant and cyclic temperatures in relation to live zooplankton ration size. Can. J. Fish. Aquat. Sci. 37, 203–210. ( 10.1139/f80-026) [DOI] [Google Scholar]
- 66.Sanford E. 2002. The feeding, growth, and energetics of two rocky intertidal predators (Pisaster ochraceus and Nucella canaliculata) under water temperatures simulating episodic upwelling. J. Exp. Mar. Biol. Ecol. 273, 199–218. ( 10.1016/s0022-0981(02)00164-8) [DOI] [Google Scholar]
- 67.Miller LP, Allen BJ, King FA, Chilin DR, Reynoso VM, Denny MW. 2015. Warm microhabitats drive both increased respiration and growth rates of intertidal consumers. Mar. Ecol. Prog. Ser. 522, 127–143. ( 10.3354/meps11117) [DOI] [Google Scholar]
- 68.Clark DS, Green JM. 1991. Seasonal variation in temperature preference of juvenile Atlantic cod (Gadus morhua), with evidence supporting an energetic basis for their diel vertical migration. Can. J. Zool. 69, 1302–1307. ( 10.1139/z91-183) [DOI] [Google Scholar]
- 69.Neargarder G, Dahlhoff EP, Rank NE. 2003. Variation in thermal tolerance is linked to phosphoglucose isomerase genotype in a montane leaf beetle. Funct. Ecol. 17, 213–221. ( 10.1046/j.1365-2435.2003.00722.x) [DOI] [Google Scholar]
- 70.Luo SQ, Wong SC, Xu CR, Hanski I, Wang RJ, Lehtonen R. 2014. Phenotypic plasticity in thermal tolerance in the Glanville fritillary butterfly. J. Thermal. Biol. 42, 33–39. ( 10.1016/j.jtherbio.2014.02.018) [DOI] [PubMed] [Google Scholar]
- 71.Angers B, Castonguay E, Massicotte R. 2010. Environmentally induced phenotypes and DNA methylation: how to deal with unpredictable conditions until the next generation and after. Mol. Ecol. 19, 1283–1295. ( 10.1111/j.1365-294X.2010.04580.x) [DOI] [PubMed] [Google Scholar]
- 72.Gleason L, Strand E, Hizon B, Dowd W. 2018. Data from: Plasticity of thermal tolerance and its relationship with growth rate in juvenile mussels (Mytilus californianus) Dryad Digital Repository. ( 10.5061/dryad.74sh7) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gleason L, Strand E, Hizon B, Dowd W. 2018. Data from: Plasticity of thermal tolerance and its relationship with growth rate in juvenile mussels (Mytilus californianus) Dryad Digital Repository. ( 10.5061/dryad.74sh7) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Survival and shell length data are provided in the electronic supplementary material (tables S5 and S6). Temperature data are archived on Dryad (http://dx.doi.org/10.5061/dryad.74sh7) [72].