Abstract
Although leopards are the most widespread of all the big cats and are known for their adaptability, they are elusive and little is known in detail about their movement and hunting energetics. We used high-resolution GPS/IMU (inertial measurement unit) collars to record position, activity and the first high-speed movement data on four male leopards in the Okavango Delta, an area with high habitat diversity and habitat fragmentation. Leopards in this study were generally active and conducted more runs during the night, with peaks in activity and number of runs in the morning and evening twilight. Runs were generally short (less than 100 m) and relatively slow (maximum speed 5.3 m s−1, mean of individual medians) compared to other large predators. Average daily travel distance was 11 km and maximum daily travel distance was 29 km. No direct correlation was found between average daily temperature and travel distance or between season and travel distance. Total daily energy requirements based on locomotor cost and basal metabolic rate varied little between individuals and over time. This study provides novel insights into movement patterns and athletic performance of leopards through quantitative high-resolution measurement of the locomotor, energetic, spatial and temporal movement characteristics. The results are unbiased by methodological and observational limitations characteristic of previous studies and demonstrate the utility of applying new technologies to field studies of elusive nocturnal species.
Keywords: locomotion, GPS collars, hunting, speed, running, territory
1. Introduction
Leopards are the most widespread and numerous of the large cats with a distribution ranging widely across Africa and Asia [1]. They occupy a wide variety of habitats including deserts, savannah grasslands, rainforests and high mountain ranges. They are thought to be the most adaptable of the large cats [2], modifying their behaviour, prey selection and ranging patterns according to conditions. However, despite this versatility, like many other species, their numbers have declined dramatically [3], with anthropogenic impact on the environment, poaching, trophy hunting, human conflict, habitat fragmentation and loss and reduced prey availability all contributing factors. An estimated 28–51% of leopard range has been lost in southern Africa [4], while globally leopards have lost 69–75% of their historic range [4]. Conservation of the leopard and other vulnerable species requires insight into how they use their environment: for example, how territory size, movement patterns and hunting strategies correlate with habitat and season. Understanding these issues requires long-term datasets; often gathered by observation [5–9], with the help of VHF collars [1,10–16] or more recently sourced from GPS collars [17–21]. GPS collars can record data 24/7 for months or even years, in any terrain or conditions, at lower cost and without the bias inherent with observational studies. However, the limitation of collar battery life means that most studies use a sample schedule that prolongs collar life at the expense of high-resolution data collection, often relying on daily or hourly measurements and recording no data on the most fine-scale and high-speed movements such as hunting or escape events which are often essential for survival. These limitations have been overcome by a new generation of solar rechargeable GPS/IMU collars, which are able to dynamically switch sample rate based on the animals' activity to provide long-term high-resolution movement monitoring including very detailed data on high-speed events. Such detailed data enable insight into daily locomotor costs, habitat use, hunting strategies and factors that impact hunting success [22–25] and enable investigation of intra- and interspecies interaction between collared individuals [26]. In this study, we obtained the first measurements of athletic performance of leopards and used high-resolution movement data to estimate energetic cost of daily locomotion and analyse territory use.
The leopard's solitary and nocturnal lifestyle and preference for rugged terrain and dense vegetation [5] make direct observations of their hunting and movement difficult [15,21]. Consequently, detailed descriptions of their movement and behaviour often include some type of methodological bias towards spatial or habitat-specific observations. Habitat, sex, age and prey density influence home range size [5]. Adult male leopards are reported to occupy home ranges from 13 km2 [27] to 800 km2 [6]. The most comprehensive study of the African leopard reports variation between 16.4 and 96.1 km2 in habitats associated with the Kruger National Park [5]. Leopards are primarily nocturnal in most areas, while the dominant form of activity during the day is resting [5]. However, all predominantly nocturnal activities, such as walking, feeding, hunting and courting, have been observed during daylight hours, and in some African forest mountain areas leopards are reported to be relatively diurnal [1,13,14]. Leopards rely on sight and sound during hunting [5,28,29], but avoid hunting in the open [30,31]. They are known to either stalk or ambush prey with only short chases (mean 5 m to 10 m [11,30]; mean 10.3 m, range 0–117 m, [15]). However, observations of leopards in the Kalahari [12] suggest, when hunting in more open habitats, chases can be considerably longer (mean ± s.d. 52.3 ± 79.9 m, range 5–450 m). Maximum speed reported for leopards is 60 km h−1 [2]. Males travel further than females and distance travelled per day is thought to correlate to territory size [1,13]; however, this is not always the case [19]. Mean distance travelled is reported to be typically between 2.3 and 4.2 km [1,5,13,19,21], with the exceptions of Stander et al. [15] reporting a mean daily distance travelled of 12.2 km and Bothma et al. [12] of 14.3 km and a maximum distance of 33.0 km for male leopards in the Kalahari. Most leopard studies relied on direct observation and radio telemetry or spoor tracking and few have GPS collar data with at most daily or hourly sample rates. Here, we use high temporal and spatial resolution GPS data on four male leopards in the Okavango Delta in order to (a) determine daily activity and distance travelled (b) estimate the energetic locomotor costs, (c) investigate movement spatially with reference to territory boundaries and (d) quantitatively characterize high-speed locomotion and evaluate athletic performance.
2. Material and methods
(a). Leopards
Four male leopards (CHK, GSE, CAL, LPM) were immobilized by free darting from a vehicle and collared (approved by RVC Ethics & Welfare Committee and under Botswana Department of Wildlife and National Parks research permit EWT8/36/4), in the region of the Okavango Delta in Northern Botswana. The leopards were monitored over different time periods by researchers from the Botswana Predator Conservation Trust as part of an ongoing study. One individual (GSE) was collared three times. Collars were operational for five to eight consecutive months. Data were recorded over a total of 33.5 months (1007 days) at 5-min intervals, with acceleration triggering the collars/data collection into high-resolution mode (5 Hz GPS/50 Hz IMU) for a total of 19 weeks in order to capture run occurrence and performance. CHK and GSE provided data from April to September/October 2012 (CHK 03.04.–20.10.12, GSE 04.04.–02.09.12) and CAL and GSE from September 2015 to April 2016 (CAL 05.09.15–29.4.16, GSE 05.09.15–20.4.16). LPM was recorded between November 2016 and May 2017 (15.11.16–25.05.17). GSE provided additional running data for June 2017 (16.05.17–30.06.17), but 5 min data were not used due to the short period of data collection.
Two research assistants followed CAL and GSE in October 2015, to the best of their abilities, and recorded kill and mating sites.
Data logged on the collars were retrieved opportunistically via UHF radio link from a vehicle. Expected battery life of the deployed data logging collars was 1 year, but collars did not last that long due to physical damage from fighting and/or mechanical factors.
(b). Data recording and analysis
Collars used in this study were designed, engineered and assembled at the RVC [24,25]. They were equipped with a GPS module (M8N GPS module; u-Blox AG, Thalwil, Switzerland), a 6-axis Inertial Measurement Unit incorporating a 3-axis accelerometer and a 3-axis gyroscope (MPU-6050, TDK-Invensense, San Jose, California), a separate low-power 3-axis accelerometer (MMA8652, NXP Semiconductors, Eindhoven, The Netherlands) and an ambient light sensor (TSL2591, Ams AG, Unterpremstaetten, Austria). Solar charging panels [25] were integrated to increase battery life. Collars were able to switch dynamically between three operating states [22,23,25] (resting, moving and running) determined by the animal activity level (based on accelerometer measurements). Switching between different states enabled optimization of power use between relatively low steady-state power consumption and quantity and resolution of data during focal activities. Collars logged GPS position and instantaneous velocity data at 5 min intervals when the animal was moving and hourly when the animal was stationary. A higher resolution data regime was enabled opportunistically to record for a period up to 32 days, based on accessibility of the leopard and collar battery charge. The high-resolution program increased the 12 fix h−1 GPS rate (0.0033 Hz) when moving to five fixes/second (5.0 Hz for GPS and 250 Hz (later changed to 50 Hz) for accelerometers), when acceleration peaks were greater than ± 3g, a conservative threshold indicator of running. Only GPS position and instantaneous velocity data were used for the analysis of movement, energetics and boundary patrol. The GPS module provides an accuracy estimate for each position and instantaneous velocity data point collected (median GPS horizontal position error 1.93 m, median instantaneous velocity error 0.4 m s−1). For analysis of runs, higher accuracy speed and position data were obtained by using Kalman filtering to fuse GPS and IMU data [25].
(c). Activity data
Continuous 24-h activity details were recorded with 50 Hz acceleration data. Maximum change in acceleration was calculated on each axis (X, Y, Z) at 2 s intervals and an activity record was stored every 30 s consisting of: (1) the largest peak–peak acceleration seen in any of the 15 × 2 s windows for each axis; (2) the mean of the mean peak–peak accelerations calculated in each of the 15 × 2 s windows, for each axis.
(d). Movement data
GPS position data with a horizontal accuracy estimate greater than 15 m were excluded from the analysis. Distance moved per day was calculated based on the cumulative distance between consecutive GPS positions. A 99th percentile cut-off was applied to the distance moved per day to exclude outliers. Distances travelled per day were averaged for each animal over the different seasons and subsequently a mean and s.d. for the season calculated.
(e). Energy calculation
Minimum daily energy expenditure was calculated based on the mass-dependent basal metabolic rate (BMR [32,33]) and a minimum locomotor cost depending on distance travelled and cost of transport (COT). The COT estimate was calculated based on [34] COT = 10.7 × mass−0.316. Leopard mass varied between 63 and 68.5 kg and the average of 65.8 kg was assumed for one individual whose body mass was unknown. COT is most probably underestimated, because potential high-speed manoeuvring costs were ignored and preferred speed travel was assumed. Additionally, energy cost of locomotion is higher when mammals move across soft surfaces such as sand, snow and wet areas [35–37]. Energy expenditure increases 1.2 times for reindeer when walking on wet instead of dry tundra [36] and humans walking on sandy surfaces have a 2.5 times higher COT [37]. Owing to the lack of more appropriate studies, we used the conservative human factor of 2.5 to give a higher COT estimate.
(f). Territory boundaries and size
The outline of the territory was calculated in Matlab (The Mathworks Inc., MA, USA) using a convex hull, basic α shape [38] with a probe radius of 800–1000 m. This is comparable to using the local convex hull (LoCoH) method [39] in the free GIS software OpenJUMP HoRAE 1.7.1 (Jump Open source Mapping Platform, http://www.openjump.org) (electronic supplementary material, figure S1). Polygon boundary reduction was performed using perl (perl.org) including a perl CPAN module, (Math:Clipper (http://search.cpan.org)), that implements a wrapper around the C++ Clipper library (https://sourceforge.net/projects/polyclipping). Polygon offsets were generated by parameterized calls representing negative distance (100, 200 and 500 m), using the function's default non-zero fill strategy, thus shrinking the polygons inward. Five-minute position and instantaneous velocity data were used to compare movement speeds inside the 500 m wide strip from the boundary lines with the rest (outside of the boundary area) of the territory. One-hour data (indicating lack of movement) were excluded and 5 Hz fixes down-sampled to 5 min. To assure independence of the samples, the data were then down-sampled to 15 min based on autocorrelation analysis (electronic supplementary material, figure S2). A Wilcoxon rank sum test was performed to test for significant differences between speeds within and outside the territory boundary lines at a significance level of 0.05.
(g). Running data
Runs were characterized by extracting speed, tangential acceleration (fore–aft) and centripetal acceleration. To reduce noise, improve precision and increase temporal resolution in the position and velocity data, GPS and IMU measurements were fused as previously described [22,23,25] using a 12-state extended Kalman filter [40] followed by a Rauch–Tung–Striebel smoother [41] written in Matlab. The accuracy of fused data was estimated from the known error characteristics of the inertial sensors and GPS position and velocity accuracy data for each fix. GPS horizontal position error (median stridewise s.d.) was reduced by data fusion from 1.93 m (pure GPS data) to 0.23 m in the fused data. Speed error was reduced from 0.4 m s−1 to 0.18 m s−1 (electronic supplementary material, figure S3).
Stride timing (beginning and end of a gait cycle) was derived from vertical IMU acceleration data and speed was derived from the Kalman-filtered velocity averaged over strides (from here on referred to as stride speed) in order to remove the effects of speed fluctuation through the stride and collar oscillation relative to the centre of mass. Tangential acceleration and centripetal (turning) acceleration between strides were computed from the change in stride speed and the time between mid-strides. Stride accelerations were weighted by taking the previous and following stride into account [22,23,25] and triggered data were classified as runs when they contained at least three strides with one stride exceeding a speed threshold of 3 m s−1. Of the 2398 trials that were triggered, 422 trials passed the Kalman filter and contained at least three strides. On multiple occasions, trials were triggered in short succession and we combined trials that occurred within 5 min to a single event. This resulted in 270 trials with 162 of those trials exceeding the three strides and greater than 3 m s−1 criterion and being classified as runs.
3. Results
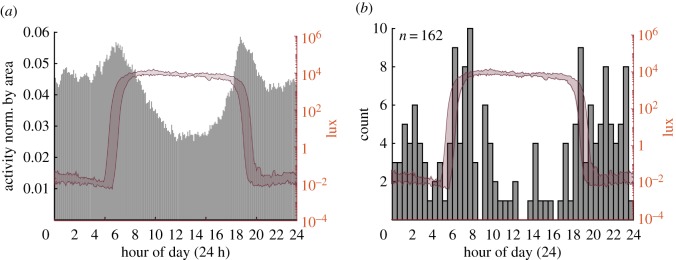
Leopards were predominantly nocturnal, but activity was bimodal with peaks in daylight during the early morning near sunrise and evening after sunset (figure 1a,b). Slow locomotion and periods of resting were interspersed by relatively infrequent runs. Over a period of 134 days, three male leopards ran a total of 162 times with speeds exceeding 3 m s−1 (mean ± s.d.: 1.2 ± 0.09 runs day−1). Few runs occurred between 9.00 and 17.00 local time (figure 1b).
Figure 1.
(a) Leopard activity versus time of day. Figure based on max peak to peak acceleration over 30 s in fore–aft direction. Data averaged over timeslots before being averaged over days. Data normalized by area and averaged over all individuals. (b) Histogram of the time of day when runs above 3 m s−1 occur. (a,b) Red-shaded area represents light level measured by collar, bandwidth reveals the variation over the measurement period.
Data were collected spanning three periods loosely defined by season, but not all individual datasets covered all seasons. We distinguished between dry (post rainy season) cold season (1 April–31 July), dry hot season (1 August–30 November) and the wet (rainy) season (1 December–30 March). Daily travel distances varied within individuals (figure 2a–e), with all individuals reaching up to 20 km per day. During the dry cold season, leopards in this study travelled an average (mean ± s.d.) of 12.0 ± 7.3 km and a maximum distance of 28.7 ± 2.6 m. During the dry hot season, average travel distance was slightly less, 10.2 ± 5.6 km with a maximum distance of 21.7 ± 2.2 km. During the wet season, average travel distance was 10.6 ± 6.3 km with a maximum distance of 19.4 ± 0.6 km. The difference in the collective average travel distance between seasons is not significant (figure 2f), although one individual (GSE) showed a significant difference between seasons (ANOVA, SPSS Statistics v24, IBM, Chicago, IL, USA; GSE: p = 0.003; LPM; p = 0.283; CAL: p = 0.913; CHK: p = 0.147). While the difference in travel distance between seasons is non-significant, there is notable variation in the distances travelled per month (figure 2g). Interestingly, the leopards that were recorded at the same time period show very similar trends in rise and fall of the average distance travelled. No direct correlation was detected between daily distance travelled and average daily temperature (GLMM, SPSS; individual as random effect, p = 0.598).
Figure 2.
Energy expenditure and distance travelled per day after removal of the 99th percentile for four individuals. (a,b) April–October 2012, (c,d,e) September 2015–April 2016. (e) November 2016–April 2017. Abscissa is cut off at 99th percentile. Red line: kernel density estimate, n: number of days for each individual. GSE was recorded in 2012 and 2015. (f) Boxplots showing distance per day versus season (median, Q1, Q3), including the average values for each individual. (g) Distance per day averaged per month for each leopard and s.d. and mean for the respective leopards. (h–l) Energy expenditure due to locomotion (locomotion cost: blue: min. COT, blue + green: COT*2.5) and BMR separated by month and individual; (h,i) dry season (April–September 2012), (j,k) dry hot–wet season (September 2015–April 2016), (l) wet season (November 2016–March 2017).
Minimum daily energy expenditure is shown in figure 2h–l, accounting for BMR and locomotor cost, which was based on preferred speed estimates and a possible increase through sandy conditions, but neglecting potential high speed and manoeuvring costs. Daily locomotor cost varied little with month and individual. Assuming preferred speed and optimal conditions, the contribution of locomotor cost to daily expenditure is on average (mean ± s.d.) 23 ± 4%; while unfavourable sandy conditions might increase it to 42 ± 5%.
The territories of GSE and CHK shared a boundary, even overlapping slightly, in 2012, while GSE and CAL territories were close in 2015/16, but there was a gap between their borders (figure 3). Territory size is fairly consistent among individuals (table 1). GSE's territory changed only slightly between the two observation periods.
Figure 3.
Movement/range use by resident leopards. (a) Territory boundaries for the four leopards during the three collaring periods (April–October 2012, September 2015–February 2016, November 2016–April 2017). (b–f) Positions (for moving animals) and territory boundaries. Boundary strip with 500 m band width located between green lines, red: positions within the boundary strip, grey: positions in the inner range of the territory. (a) CHK (2012), (b) GSE (2012), (c) GSE (2015/16), (d) CAL (2015/16), (e) LPM (2016/17).
Table 1.
Territory size and probe radius for all individuals based on α hull calculation.
| CHK 2012 | GSE 2012 | CAL 2015/16 | GSE 2015/16 | LPM 2016/17 | |
|---|---|---|---|---|---|
| area (km2) | 128.5 | 116.2 | 130.6 | 108.1 | 77.9 |
| probe radius (km) | 0.8 | 0.8 | 1 | 0.8 | 0.8 |
We compared the instantaneous velocity values of movement fixes situated within the 500 m boundary strip with those inside the territory (figure 3b–f). Movement within the boundary area occurred at significantly higher velocities (mean ± s.d., averaged over individuals): 0.90 ± 0.09 m s−1 versus 0.85 ± 0.09 m s−1 in all but one example (Wilcoxon rank sum test; figure 3b: p < 0.001; 3c: p = 0.031; 3d: p < 0.001; 3e: p < 0.001; 3f: p = 0.381).
Faster movements (indicated by triggered and Kalman-filtered trials which did not exceed 3 m s−1 threshold) and runs were widely distributed over the territories (figure 4), but there were areas of the territory where they were more tightly clustered.
Figure 4.

Locations of fast moving instances and runs. Display of GPS path for the whole of data collection time (grey) and when the collar was allowed into chase mode (colour coded by individual). (a) GSE and CAL 2015/16. During October 2015, when collars were in chase mode, leopards were followed for prolonged periods of time and one mating and four kill sites were recorded. (b) LPM and GSE, with GSE providing running data for June 2017. GSE previous territory from 2015/16 outlined in (b) with dashed yellow line. Fast moves: triggered trials that passed the Kalman filter, contained three strides, but did not exceed the 3 m s−1 threshold.
The median distance covered in the 5 min preceding the run was only 56 m, and just 78 m during the 10 min preceding the run (figure 5). In comparison, when walking at their preferred speed of 1 m s−1 (based on speed histogram of steady state walking strides, extracted according to [42]), leopards would be predicted to move 300 m in 5 min, while the histogram of distances travelled within 5 min peaks at 200 m (mode: 204 m). Both measures give a clear indication of a much slower stalking mode before the run.
Figure 5.
Histogram of distance travelled before run. (a) Five minutes before, (b) 10 min before, (c) between 5 and 10 min before. Line: median, n: number of runs used for a–c. (Online version in colour.)
Run performance was analysed by extracting duration and distance as well as stride parameters for each run. Data included maximum stride speed, maximum tangential acceleration (fore–aft acceleration and deceleration) and maximum centripetal acceleration, and mean heading rate as an indicator of manoeuvrability (electronic supplementary material, table S1 and dataset 1,2). Maximum speed recorded by a single individual was 11.4 m s−1, all three individuals reached 9.4 m s−1. Median maximum speeds varied between 4.6 and 5.8 m s−1 (figure 6a), with a mean of medians of 5.3 ± 0.8 m s−1. All three individuals reached maximum tangential accelerations of 3.7 m s−2 and decelerations of −7.4 m s−2 (average medians: 1.3 ± 0.1 m s−2 and −1.7 ± 0.6 m s−2), maximum centripetal acceleration of 7.6 m s−2 (average medians: 1.2 ± 0.3 m s−2) and maximum (stride averaged) heading rate of 66° (average medians: 15.5 ± 3.1°). Average median run distance was 58.5 ± 16.3 m with a duration of 26.4 ± 4.4 s. Five run events (by two individuals, stitched together from consecutive runs) exceeded distances of 600 m (not shown in figure).
Figure 6.
Stride parameters for three individuals displayed as violin plots (combining box plot and kernel density plot). Number of runs analysed: n = 162. Violin plots show the density distribution of the values, with each histogram normalized to the same maximum bin width in order to compare distribution shape. The total number of parameter values per individual is given in (a), with the exception of (d) where left and right turns are combined, doubling the count; red cross: mean; white box: median. (a–d) Maximum value for the respective parameter extracted from each trial, (a) maximum stride speed, (b) maximum tangential (fore–aft) acceleration and (c) deceleration, (d) maximum centripetal (turning) acceleration (right + left). (e) Mean absolute heading rate (degree/stride), (f) run distance and (g) run duration.
4. Discussion
The estimated territory size of these Okavango Delta leopards lies between 77.9 and 130.6 km2, putting them at the upper end of the territory sizes measured in the Kruger National Park area [5]. Their activity pattern of mainly nocturnal movement and resting during the day is in agreement with previous observations for leopards in the Kalahari desert [6] and Kruger National Park [5]. The detailed analysis of their activity patterns and run times shows a notable peak in their activity around sunrise and sunset (figure 1a,b) that corresponds with a substantial increase/decrease in light levels. Cats are known for their night vision [43], and being nocturnal hunters it can be assumed leopards can see well at night. However, we are unaware of specific scientific literature about leopards' eyesight. Unlike domestic cats and other, smaller ambush predators, leopards' pupils are round instead of showing the vertically slit configuration associated with ideal night vision [44]. The peak in hunting activity in the early morning and evening hours might indicate that during times when temperatures are low but light levels are significantly higher than night levels, conditions are ideal for spotting and pursuing prey that might be more vulnerable on account of the dim light or their activity pattern [45,46]. Leopards are less active and fewer runs occur during daytime (figure 1a,b).
The four leopards travelled considerable daily distances (mean 11 km), much further than the 1.5 km reported by Bailey [5] and comparable only with those reported by Stander et al. [15] and Bothma et al. [12], who previously reported maximum travel distances of up to 33 km in Kalahari landscapes compared with ours with 29 km. Seasonal changes in average travel distance per day were non-significant. However, average distance travelled varied between month and the fluctuation in distance travelled per month is similar for the respective leopard pairs and might suggest that external factors such as weather or prey abundance influence travel distance. No correlation was found between daily travel distance and average daily temperature, possibly due to the predominant travel during the night when temperatures are lower.
Locomotor energy expenditure varies with total distance travelled and per month, but overall was very similar in all individuals and measurement periods. Despite the extensive travelling, the main source for the daily energetic expense is the BMR. Depending on the factors assumed to influence COT such as sandy surfaces, we calculated the locomotor costs to be a minimum of 26% and a maximum of 65% of the basic metabolic rate, without taking additional costs for running into account or any post-exercise BMR increase. However, we predict both effects to be relatively small.
Like many cats, leopards patrol and scent mark their territory and boundaries [2,5,29]. With an average travel distance of 11 km and at most 29 km day−1 and taking territory size into account, patrolling borders requires several days of travel, raising the question about efficiency of patrolling a large perimeter. We tested the hypothesis that leopards might move more quickly in the outer parts of their territory, moving more purposefully and minimizing time spent on the task or the risk of direct encounters with neighbouring leopards. Excluding running and resting data, we found a small but significant increase in average speed within 500 m of the territory boundaries in all four cases. It is important to note that average speed includes not only purposeful walking, which might occur at preferred speeds independent of location, but also any other kind of movement such as stalking and searching for females, suggesting that leopards might focus more on patrolling close to the boundaries of their territory than on other tasks such as hunting.
Runs and faster movements are widespread over the territories (figure 4), but seem to cluster in some areas, e.g. CAL often runs along the water's edge (figure 4a). The habitat is a mixture of grassland with shrubs and open spaces, mixed woodland and swamp. Overlaying run positions on Google Earth (available historical images from December 2015 and December 2016, respectively) shows that few runs occurred in open areas. Leopards mostly ran in areas with vegetation cover, along the edge of open areas or along the river.
Interspecific competition is common in large predators [47–49]. Leopards in the Moremi area compete for resources with hyena, lion, cheetah and African wild dogs (AWD). Here, we compare their athletic performance with two of their competitors, based on studies in the same area. We recorded an average of 1.2 runs day−1. Leopards (L) run approximately as often as cheetah (C) (1.3 runs day−1 [25]), but less often than AWD (2.4 runs day−1 [22]). However, the number of runs is considerably lower than the 2.7 hunts per day reported for male leopards in the more open Kalahari by Bothma & Coertze [7]. Compared with stride performance data from cheetah and AWD [23,25], and with the requirement that the value must be reached by at least three individuals, leopards show considerably lower maximum stride speeds (L: 9.4 m s−1, C: 22 m s−1, AWD: 19 m s−1) and fore–aft accelerations (L: 3.7 m s−2, C: 9.8 m s−2, AWD: 8 m s−2). Deceleration (L: −7.4 m s−2, C: −15.2 m s−2, AWD: −8 m s−2) and turning performance (centripetal acceleration, L: 7.6 m s−2, C: 13 m s−2, AWD: 8 m s−2) are about half of those in cheetah, but comparable to those in AWD. The maximum speed measured (11.4 m s−1, from a single individual) was slower than the estimated ± 60 km h−1 (16.7 m s−1) found in Schaller [2] which is cited in [50,51] and given in many mainstream reports. However, the measurement method and accuracy of this value are not known.
Reported hunting success rates in leopards vary considerably between 38% in Namibia [15], 20% in South Africa [10], 13.6–27.9% in the Kalahari [7] and 16% in Kruger National Park [5]. Aside from location, success rate varies with sex, the necessity to feed cubs and prey size [7]. We do not know how many of our recorded runs were actual hunting events. Running could be triggered by interactions with other carnivores and/or conspecifics. However, in Kruger National Park leopards eat about one impala per week [5] and with a corresponding success rate of 16% the number of hunts conducted over a period of time can be estimated. With a total of 162 runs recorded (from three leopards) over a total period of 19 weeks, an estimate of 119 runs were actual hunts (19*(1/16*100)), assuming that hunting is always accompanied by at least a short run. The distance covered in the time leading up to the run is very short (figure 5). Leopards only moved a median distance of 56 m in the 5 min before the run, while cheetah and AWD move considerably further, 235 m and 473 m, respectively [22]. This shows a substantial difference in hunting style, where leopards actually more often stalk or ambush their prey as chance encounters arise, perhaps waiting in key prey locations, instead of relying on finding prey as they move around their territory. A study on puma indicates a notable reduction in hunting costs when using the stalk and pounce tactic [52].
Average run distance was 129 ± 202 m (mean ± s.d., all runs), considerably longer than runs previously recorded [12,15]. The results might be biased due to a few very long runs. The median value is 63 m (all runs) and when removing the seven runs over 450 m (longest running distance measured by Bothma et al. [12]) the mean drops to 94 ± 89 m (mean ± s.d., all runs), which fits better with the previously observed distances. Differences could also be caused by the variation in methods between studies; tape measure or pace versus collar GPS data, and the potential error due to missed tortuosity in the former. The seven longest runs (events) contained alternating segments with slower and faster speeds and were highly directional (electronic supplementary material, figure S4).
5. Conclusion
The results provide useful insights into movement patterns and athletic performance of leopards in a habitat characterized by seasonal flooding, open areas and mixed woodland and scrub. The detailed analysis of activity and run occurrence shows similar levels of activity throughout the night hours with distinct spikes in the morning and evening twilight hours. Despite seasonal changes in temperature, daily travel distance and consequently energy expenditure did not change significantly. However, different leopards showed notably similar patterns in average monthly travel distances over time, suggesting that external factors, such as vegetation/grass height, local flooding or food availability, may influence residents' movements to similar extents. In terms of athletic performance, leopards were considerably slower than cheetah and AWD and have lower acceleration; however, their manoeuvring performance, while considerably surpassed by cheetah, is comparable with that of AWD. Comparisons of territory size, hunting style and frequency between these leopards and those inhabiting the broadly similar habitats of Kruger National Park and entirely different habitats in other study areas reinforce the view that leopards are highly adaptable to their environment.
The collar technology deployed for this study has enabled the first measurements of athletic performance of wild leopard, and the most detailed measurements of activity and locomotory behaviour. The recording of such high-resolution activity and behavioural data could provide an efficient way of gaining unbiased insights into animal behaviour, delivering benefits for wildlife management. This knowledge can be used to tailor conservation efforts for specific species and to reduce human–wildlife conflict.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank J. Lowe and S. Amos for fabricating and programming collars, K. Roskilly for GPS/IMU fusion code, N. Jordan, E. Bennitt, H. Bartlam-Brooks, M. Claase and B. Yexley for collar deployment and field assistance, C. Buse for boundary redaction code and S. Wilshin and H. Bartlam-Brooks for very useful contributions and discussions. We thank A.R. Wilson for editorial assistance with the manuscript.
Ethics
This work was approved by RVC Ethics & Welfare Committee (2013 1233) and was carried out under a Government of Botswana Research Permit number EWT 8/36/4 and supplementary permits held by A.M.W.
Data accessibility
Stride data are provided in electronic supplementary material, datasets S1 and S2.
Authors' contributions
A.M.W., T.Y.H., K.A.G. and J.W.M. conceived and designed the methods and study. K.A.G. and K.R. established, maintained and monitored the study population and directed and conducted field data collection. T.Y.H. analysed and interpreted the data and wrote the paper with input from all authors.
Competing interests
The authors declare no competing financial interests.
Funding
This study was supported by EPSRC (EP/H013016/1), BBSRC (BB/J018007/1) and ERC (323041). The BPCT field research programme is supported by grants from the Paul G. Allen Family Foundation, Wild Entrust International, Tusk Trust and numerous private donors.
References
- 1.Norton P, Henley S. 1987. Home range and movements of male leopards in the cedarberg wilderness area, Cape Province. S. Afr. J. Wildl. Res. 17, 41–48. [Google Scholar]
- 2.Schaller GB. 1972. The serengeti lion: a study of predator–prey relations. Chicago, IL: University of Chicago Press. [Google Scholar]
- 3.IUCN. 2017. The IUCN Red List of Threatened Species. Version 2017-3 2017.
- 4.Jacobson AP, et al. 2016. Leopard (Panthera pardus) status, distribution, and the research efforts across its range. PeerJ 4, e1974 ( 10.7717/peerj.1974) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey TN. 1993. The african leopard: ecology and behavior of a solitary felid. New York, NY: Columbia University Press. [Google Scholar]
- 6.Bothma J, Le Riche E. 1984. Aspects of the ecology and the behaviour of the leopard Panthera pardus in the Kalahari Desert. Koedoe 27, 259–279. ( 10.4102/koedoe.v27i2.585) [DOI] [Google Scholar]
- 7.Bothma JP, Coertze R. 2004. Motherhood increases hunting success in southern Kalahari leopards. J. Mammal 85, 756–760. ( 10.1644/BNS-010) [DOI] [Google Scholar]
- 8.Henschel P, Abernethy K, White L. 2005. Leopard food habits in the Lope National Park, Gabon, Central Africa. Afr. J. Ecol. 43, 21–28. ( 10.1111/j.1365-2028.2004.00518.x) [DOI] [Google Scholar]
- 9.Ngoprasert D, Lynam AJ, Gale GA. 2007. Human disturbance affects habitat use and behaviour of Asiatic leopard Panthera pardus in Kaeng Krachan National Park, Thailand. Oryx 41, 343–351. ( 10.1017/S0030605307001102) [DOI] [Google Scholar]
- 10.Balme G, Hunter L, Slotow R. 2007. Feeding habitat selection by hunting leopards Panthera pardus in a woodland savanna: prey catchability versus abundance. Anim. Behav. 74, 589–598. ( 10.1016/j.anbehav.2006.12.014) [DOI] [Google Scholar]
- 11.Bertram BCR. 1982. Leopard ecology as studied by radio tracking. Symp. Zool. Soc. Lond. 49, 341–352. [Google Scholar]
- 12.Bothma J, Knight M, Le Riche E, Van Hensbergen H. 1997. Range size of southern Kalahari leopards. S. Afr. J. Wildl. Res. 27, 94–99. [Google Scholar]
- 13.Hamilton PH. 1976. The movements of leopards in tsavo national park, Kenya, as determined by radio-tracking. Nairobi, Kenya: University of Nairobi. [Google Scholar]
- 14.Jenny D, Zuberbühler K. 2005. Hunting behaviour in West African forest leopards. Afr. J. Ecol. 43, 197–200. ( 10.1111/j.1365-2028.2005.00565.x) [DOI] [Google Scholar]
- 15.Stander P, Haden P, Kaqece I, Ghau I. 1997. The ecology of asociality in Namibian leopards. J. Zool. 242, 343–364. ( 10.1111/j.1469-7998.1997.tb05806.x) [DOI] [Google Scholar]
- 16.Stein AB, Bourquin SL, McNutt JW. 2015. Avoiding intraguild competition: leopard feeding ecology and prey caching in northern Botswana. Afr. J. Wildl. Res. 45, 247–257. ( 10.3957/056.045.0247) [DOI] [Google Scholar]
- 17.Odden M, Athreya V, Rattan S, Linnell JD. 2014. Adaptable neighbours: movement patterns of GPS-collared leopards in human dominated landscapes in India. PLoS ONE 9, e112044 ( 10.1371/journal.pone.0112044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balme GA, Slotow R, Hunter LT. 2009. Impact of conservation interventions on the dynamics and persistence of a persecuted leopard (Panthera pardus) population. Biol. Conserv. 142, 2681–2690. ( 10.1016/j.biocon.2009.06.020) [DOI] [Google Scholar]
- 19.Martins Q, Harris S. 2013. Movement, activity and hunting behaviour of leopards in the Cederberg mountains, South Africa. Afr. J. Ecol. 51, 571–579. ( 10.1111/aje.12068) [DOI] [Google Scholar]
- 20.Martins QE. 2010. The ecology of the leopard panthera pardus in the cederberg mountains. PhD thesis University of Bristol, Bristol. [Google Scholar]
- 21.Swanepoel LH. 2008. Ecology and conservation of leopards, panthera pardus, on selected game ranches in the waterberg region. Limpopo, South Africa: University of Pretoria. [Google Scholar]
- 22.Hubel TY, Myatt JP, Jordan NR, Dewhirst OP, McNutt JW, Wilson AM. 2016. Energy cost and return for hunting in African wild dogs and cheetahs. Nat. Commun. 7, 11034 ( 10.1038/ncomms11034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubel TY, Myatt JP, Jordan NR, Dewhirst OP, McNutt JW, Wilson AM. 2016. Additive opportunistic capture explains group hunting benefits in African wild dogs. Nat. Commun. 7, 11033 ( 10.1038/ncomms11033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson AM, et al. 2018. Biomechanics of predator–prey arms race in lion, zebra, cheetah and impala. Nature 554, 183–188. ( 10.1038/nature25479) [DOI] [PubMed] [Google Scholar]
- 25.Wilson AM, Lowe JC, Roskilly K, Hudson PE, Golabek KA, McNutt JW. 2013. Locomotion dynamics of hunting in wild cheetahs. Nature 498, 185–189. ( 10.1038/nature12295) [DOI] [PubMed] [Google Scholar]
- 26.Jordan NR, Buse C, Wilson AM, Golabek KA, Apps PJ, Lowe JC, Van der Weyde LK, Weldon McNutt J. et al. 2017. Dynamics of direct inter-pack encounters in endangered African wild dogs. Behav. Ecol. Sociobiol. 71, 115 ( 10.1007/s00265-017-2338-9) [DOI] [Google Scholar]
- 27.Seidensticker J. 1976. On the ecological separation between tigers and leopards. Biotropica 8, 225–234. ( 10.2307/2989714) [DOI] [Google Scholar]
- 28.Hes L. 1997. The complete book of Southern African mammals. Cape Town, South Africa: Struik. [Google Scholar]
- 29.Sunquist M, Sunquist F. 2002. Wild cats of the world. Chicago, IL: University of Chicago Press. [Google Scholar]
- 30.Kruuk H, Turner M. 1967. Comparative notes on predation by lion, leopard, cheetah and wild dog in the Serengeti area, East Africa. Mammalia 31, 1–27. ( 10.1515/mamm.1967.31.1.1) [DOI] [Google Scholar]
- 31.Ewer RF. 1973. The carnivores. Ithaca, NY: Cornell University Press. [Google Scholar]
- 32.Carbone C, Mace GM, Roberts SC, Macdonald DW. 1999. Energetic constraints on the diet of terrestrial carnivores. Nature 402, 286–288. ( 10.1038/46266) [DOI] [PubMed] [Google Scholar]
- 33.Schmidt-Nielsen K. 1984. Scaling: why is animal size so important? Cambridge, UK: Cambridge University Press. [Google Scholar]
- 34.Taylor CR, Heglund NC, Maloiy G. 1982. Energetics and mechanics of terrestrial locomotion. I. Metabolic energy consumption as a function of speed and body size in birds and mammals. J. Exp. Biol. 97, 1–21. [DOI] [PubMed] [Google Scholar]
- 35.Karasov WH. 1992. Daily energy expenditure and the cost of activity in mammals. Am. Zool. 32, 238–248. ( 10.1093/icb/32.2.238) [DOI] [PubMed] [Google Scholar]
- 36.White R, Yousef M. 1978. Energy expenditure in reindeer walking on roads and on tundra. Can. J. Zool. 56, 215–223. ( 10.1139/z78-031) [DOI] [Google Scholar]
- 37.Lejeune TM, Willems PA, Heglund NC. 1998. Mechanics and energetics of human locomotion on sand. J. Exp. Biol. 201, 2071–2080. [DOI] [PubMed] [Google Scholar]
- 38.Lundgren J. Alphavol 2012 Making data analytics for the mining industry simple with MATLAB. https://uk.mathworks.com/matlabcentral/fileexchange/40165-making-data-analytics-for-the-mining-industry-simple-with-matlab?focused=6795870&tab=function .
- 39.Getz WM, Wilmers CC. 2004. A local nearest-neighbor convex-hull construction of home ranges and utilization distributions. Ecography 27, 489–505. ( 10.1111/j.0906-7590.2004.03835.x) [DOI] [Google Scholar]
- 40.Kalman RE. 1960. A new approach to linear filtering and prediction problems. J. Basic Eng. 82, 35–45. ( 10.1115/1.3662552) [DOI] [Google Scholar]
- 41.Rauch HE, Striebel C, Tung F. 1965. Maximum likelihood estimates of linear dynamic systems. AIAA J. 3, 1445–1450. ( 10.2514/3.3166) [DOI] [Google Scholar]
- 42.Dewhirst OP, Roskilly K, Hubel TY, Jordan NR, Golabek KA, McNutt JW et al. 2016. An exploratory clustering approach for extracting stride parameters from tracking collars on free ranging wild animals. J. Exp. Biol. 220, 341–348. ( 10.1242/jeb.146035) [DOI] [PubMed] [Google Scholar]
- 43.Lamm N.2013. What do cats see? http://nickolaylamm.com/art-for-clients/what-do-cats-see/
- 44.Banks MS, Sprague WW, Schmoll J, Parnell JA, Love GD. 2015. Why do animal eyes have pupils of different shapes? Sci. Adv. 1, e1500391 ( 10.1126/sciadv.1500391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eggermann J, Gula R, Pirga B, Theuerkauf J, Tsunoda H, Brzezowska B, Rouys S, Radler S. 2009. Daily and seasonal variation in wolf activity in the Bieszczady Mountains, SE Poland. Mamm. Biol. -Zeitschrift für Säugetierkunde 74, 159–163. ( 10.1016/j.mambio.2008.05.010) [DOI] [Google Scholar]
- 46.Theuerkauf J, Jędrzejewski W, Schmidt K, Okarma H, Ruczyński I, Śnieżko S, Gula R. 2003. Daily patterns and duration of wolf activity in the Białowieża Forest, Poland. J. Mammal. 84, 243–253. ( 10.1644/1545-1542(2003)084%3C0243:DPADOW%3E2.0.CO;2) [DOI] [Google Scholar]
- 47.Durant SM. 1998. Competition refuges and coexistence: an example from Serengeti carnivores. J. Anim. Ecol. 67, 370–386. ( 10.1046/j.1365-2656.1998.00202.x) [DOI] [Google Scholar]
- 48.Caro T, Stoner C. 2003. The potential for interspecific competition among African carnivores. Biol. Conserv. 110, 67–75. ( 10.1016/S0006-3207(02)00177-5) [DOI] [Google Scholar]
- 49.Hanby J, Bygott J. 1979. Ten Population changes in lions and other predators. In Serengeti: dynamics of an ecosystem. (eds Sinclair A, Norton-Griffiths M), pp. 249–2262. Chicago, IL: University of Chicago Press. [Google Scholar]
- 50.Bertram B. 1979. Serengeti predators and their social systems. In Serengeti: dynamics of an ecosystem (eds Sinclair ARE, Norton-Griffiths M), pp. 221–285. Chicago, IL: University of Chicago Press. [Google Scholar]
- 51.Hayward M, Henschel P, O'brien J, Hofmeyr M, Balme G, Kerley G. 2006. Prey preferences of the leopard (Panthera pardus). J Zool 270, 298–313. [Google Scholar]
- 52.Williams TM, Wolfe L, Davis T, Kendall T, Richter B, Wang Y, Bryce C, Elkaim GH, Wilmers CC. et al. 2014. Instantaneous energetics of puma kills reveal advantage of felid sneak attacks. Science 346, 81–85. ( 10.1126/science.1254885) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Stride data are provided in electronic supplementary material, datasets S1 and S2.