Abstract
Crocodilians are important for understanding the evolutionary history of amniote neural systems as they are the nearest extant relatives of modern birds and share a stem amniote ancestor with mammals. Although the crocodilian brain has been investigated anatomically, functional studies are rare. Here, we employed functional magnetic resonance imaging (fMRI), never tested in poikilotherms, to investigate crocodilian telencephalic sensory processing. Juvenile Crocodylus niloticus were placed in a 7 T MRI scanner to record blood oxygenation level-dependent (BOLD) signal changes during the presentation of visual and auditory stimuli. Visual stimulation increased BOLD signals in rostral to mid-caudal portions of the dorso-lateral anterior dorsal ventricular ridge (ADVR). Simple auditory stimuli led to signal increase in the rostromedial and caudocentral ADVR. These activation patterns are in line with previously described projection fields of diencephalic sensory fibres. Furthermore, complex auditory stimuli activated additional regions of the caudomedial ADVR. The recruitment of these additional, presumably higher-order, sensory areas reflects observations made in birds and mammals. Our results indicate that structural and functional aspects of sensory processing have been likely conserved during the evolution of sauropsids. In addition, our study shows that fMRI can be used to investigate neural processing in poikilotherms, providing a new avenue for neurobiological research in these critical species.
Keywords: poikilotherms, reptile, audio-visual stimulation, dorsal ventricular ridge, hierarchical processing
1. Introduction
Crocodilians are a key species for understanding the evolutionary history of amniote neural systems as they are the closest extant relatives of modern birds and share a stem amniote ancestor with mammals [1]. Based on mitochondrial genomic evidence, crocodilians diverged from birds approximately 240 million years ago [2]. It appears that the crocodilian phenotype has remained relatively stable over time [3], making them a useful species to evaluate shared and divergent features of amniote brain structure and function. The sub-telencephalic sensory regions have remained similar in structure and function in all vertebrates [4]. The telencephalon, however, has undergone significant modifications, leading to a layered, cortical organization of the pallium in mammals and a non-layered, nuclear pallium in birds [5]. The organizational principles of sensory neural processing are highly similar in birds and mammals, despite the very different architecture of their forebrains [6,7]. In both classes, sensory information reaching the pallium is processed in a hierarchical fashion, with relatively simple stimuli activating downstream, or lower-order, regions within the sensory pallium, while presentation of more complex stimuli leads to the recruitment of additional higher-order regions upstream [8,9]. Consequently, simple auditory stimuli, like pure tones, only activate auditory core regions within the temporal lobe of primates [10] or the nidopallial Field L of songbirds, while more complex stimuli, like music or vocalizations, also activate the temporal belt and parabelt regions in primates and the caudomedial nidopallium (NCM) in birds [11]. These functional similarities appear to have been conserved despite the obvious gross anatomical differences of the telencephalon between these classes and the phylogenetic distance between them [12]. However, to more accurately reconstruct the evolutionary history of these neural organizational principles in mammals and birds, similar information regarding the crocodilian telencephalon is needed [5,13].
Previous studies that investigated telencephalic sensory systems in crocodilians [14–19], provided the foundation in the interpretation of a general systems-level assessment of the functional organization of sensory processing. However, functional magnetic resonance imaging (fMRI) has the advantage of providing a more holistic account of how the anatomical regions are integrated functionally. By applying fMRI techniques to the crocodile, we have attempted to overcome technical limitations. To date, fMRI has only been used in homoeothermic vertebrates such as humans [20], non-human mammals [21] and birds [22–24]. Here, we present the results of our blood oxygenation level-dependent (BOLD) fMRI study in a poikilothermic species (Crocodylus niloticus), revealing telencephalic activation patterns resulting from visual and auditory stimulation.
The aims of our study were: (i) to establish a comprehensive fMRI protocol for reptiles; (ii) to use this protocol to investigate the functional organization of visual and auditory systems in a living crocodile; and (iii) to draw conclusions regarding the organizational principles of sensory system processing networks in ancestral amniotes.
2. Results
(a). Establishment of an functional magnetic resonance imaging method for the crocodile
We used a multi-slice single shot gradient echo (GE) EPI sequence to demonstrate activity-dependent BOLD signals in the visual and auditory systems of five mildly sedated Nile crocodiles. Sequence parameters, T1, T2 and T2* relaxometry measurements, and experimental results used to optimize the fMRI sequence and the BOLD response are presented in electronic supplementary material, figures S01, S02 and S03. Animals were fixed in a custom-made restrainer to minimize body and head movements (figure 1a). The estimated motion parameters (see electronic supplementary material, figure S03 and movie S01) demonstrated that all animals were immobile during the scanning process and the motion parameters were much smaller than the used voxel size of 0.46 × 0.46 mm2 (mean motion across all animals in any direction 0.018 ± 0.003 mm (mean ± s.d.)).
Figure 1.
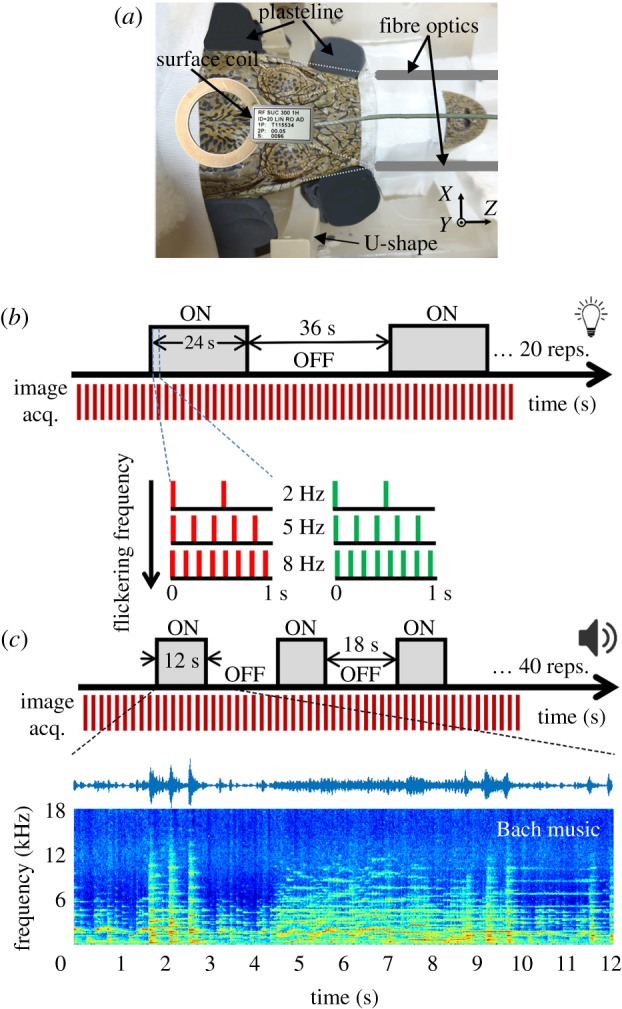
Experimental set-up and stimulation procedure. (a) Custom-made restrainer. Mildly sedated crocodiles were placed in an MRI compatible tube. To immobilize the animal in Y and Z directions, the snout, tail and back of the animal were taped to the tube. To control motion artefacts in X direction, the jaw and head were secured by blocks of Plasticine. (b) Timing of visual stimuli. The visual stimuli consisted of binocular visual stimulation using two different colours (green and red), with a flickering frequency at 2, 5 or 8 Hz. Each stimulus was presented in 20 repeated blocks consisting of 24 s light followed by 36 s darkness. (c) Timing of auditory stimuli. Three different stimuli were used to stimulate the auditory system. Random chords centred around 1000 or 3000 Hz were used as simple stimuli, while classical music (by Johann Sebastian Bach) was used as a complex stimulus. Stimuli were played using a speaker in front of the animals. Stimulation was done in 40 repeated blocks with each block consisting of 12 s sound followed by 18 s silence. As an example, the spectrogram of the classical music stimulus is shown.
We employed an ON/OFF block design to measure BOLD fMRI signals during auditory and visual stimulation at 7.0 T. The visual stimuli were flashing red/green lights at 2, 5 and 8 Hz flickering frequency (figure 1c) for 24 s with a 36 s inter stimulus interval. Stimuli were presented both monocularly and binocularly. The auditory stimuli consisted of: (i) simple sounds, being random chord noise centred around 1000 Hz (bandwidth: 500 Hz to 1500 Hz) and 3000 Hz (bandwidth: 1500 to 4500 Hz); and (ii) complex sounds (part of Brandenburg Concerto No. 4 by Johann Sebastian Bach), which has been previously used as a complex stimulus in bird fMRI studies [11] (figure 1b). All stimuli lie in the hearing range of crocodiles [13].
The BOLD signal variation was visible in average time courses in the corresponding regions specifically related to the visual and auditory stimuli in all of the subjects (see electronic supplementary material, figure S04 for visual and auditory stimuli). Using the general linear model (GLM) analysis method, all subjects represented significant and reproducible evoked BOLD activation patterns from the external visual and auditory stimuli within the telencephalon.
(b). Blood oxygenation level-dependent response topography for visual stimulation
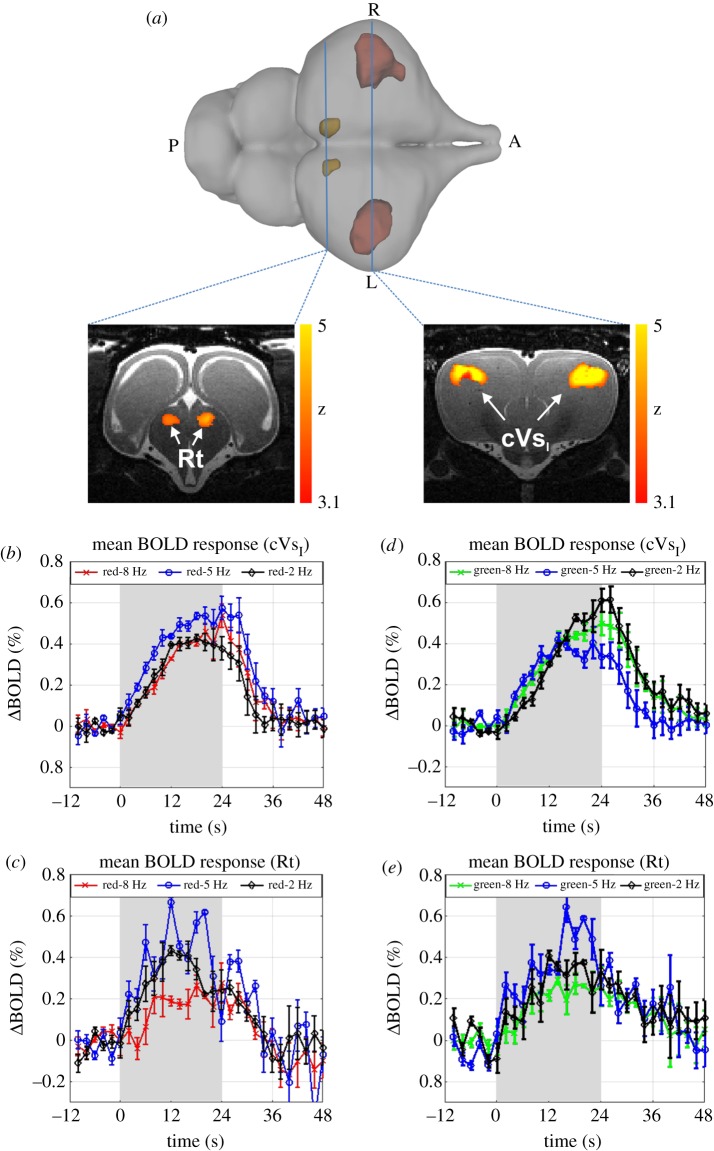
Binocular visual stimulation at 2, 5 and 8 Hz flickering frequencies evoked robust BOLD responses bilaterally in regions known to form parts of the reptilian visual pathway [25]. These regions included the thalamic nucleus rotundus (Rt) and rostral portions of the dorsolateral, anterior dorsal ventricular ridge (ADVR) in the pallium [26,27]. As this pallial area does not have a specific nomenclature, we refer to it as area cVsI (crocodilian primary pallial visual area, figures 2a and electronic supplementary material, figure S07). The dynamic properties of mean BOLD responses in cVsI and Rt were identical for all three temporal frequencies and for both red and green colour light pulses (figure 2b,d).
Figure 2.
Activation of the Nile crocodile forebrain after binocular visual stimulation. (a) 3D view and two axial slices illustrating the spatial pattern of significant signal changes during binocular visual stimulation with red light flickering at 8 Hz. Significant activations were found in the thalamic nucleus rotundus (Rt, left slice) and a forebrain area strongly resembling the avian entopallium (cVsI, right slice) (group results, mixed-effect model, FLAME1 + 2, p < 0.05, N = 5). (b–e) Mean BOLD response of all five crocodiles in cVsI and Rt measured after stimulation with red or green light at 2, 5 or 8 Hz flickering frequency. As the shape of BOLD responses did not differ between left and right hemisphere, the average of both hemispheres is plotted. The larger variation of BOLD responses in Rt in comparison with cVsI were caused by the higher distance between the surface coil and Rt, causing an increase in the noise level. Stimulation intervals are marked in grey. Error bars represent s.e.m.
Differences between the mean BOLD responses obtained from the different light pulse frequencies were evaluated using a two-way ANOVA for each colour with the BOLD response amplitude (the average of three-time points around the peak of BOLD response for each stimulus) as the dependent variable and with frequency and brain region as independent factors. The results indicate a significant effect of brain region (F1,10 = 7.66, p < 0.025) and frequency (F2,10 = 6.56, p < 0.021) in the green light condition and a significant effect of frequency (F2,16 = 5.01, p < 0.045) for red light. Post hoc test results for cVsI demonstrated that the red 5 Hz stimulus evoked a higher BOLD response than the 2 Hz (p < 0.031) and 8 Hz (p < 0.039) stimulus, respectively. By contrast, the green 2 Hz stimulus elicited a higher mean BOLD response than the 5 Hz (p < 0.004) and the 8 Hz (p < 0.036) stimuli in cVsI. Statistical comparison of mean BOLD responses in Rt revealed that 2 Hz (p < 0.033/0.019, red/green) and 5 Hz (p < 0.045/0.008, red/green) stimuli evoked higher responses in comparison with 8 Hz, irrespective of colour. Besides these differences, BOLD responses in cVsI and Rt also differed with respect to other parameters. Namely, the BOLD responses in cVsI persisted after the stimulation period, only decreasing 4 s after the stimulation had ended. By contrast, the stimulus-dependent BOLD signal in Rt was restricted to the stimulation period. Furthermore, BOLD responses in Rt reached their peak value earlier than in cVsI (14 ± 2.5 s and 22.6 ± 2.4 s after stimulus onset, mean ± s.d., n = 5, p < 0.001). There was also a larger variation of the BOLD responses in Rt in comparison with cVsI, which was likely caused by the larger distance between the surface coil and the ventrally positioned diencephalon, resulting in an increased noise level. To confirm our findings, we also performed monocular visual stimulation using the red 8 Hz stimulus in four subjects which revealed comparable but unilateral activation (see electronic supplementary material for further information).
(c). Blood oxygenation level-dependent response topography for different auditory stimulation
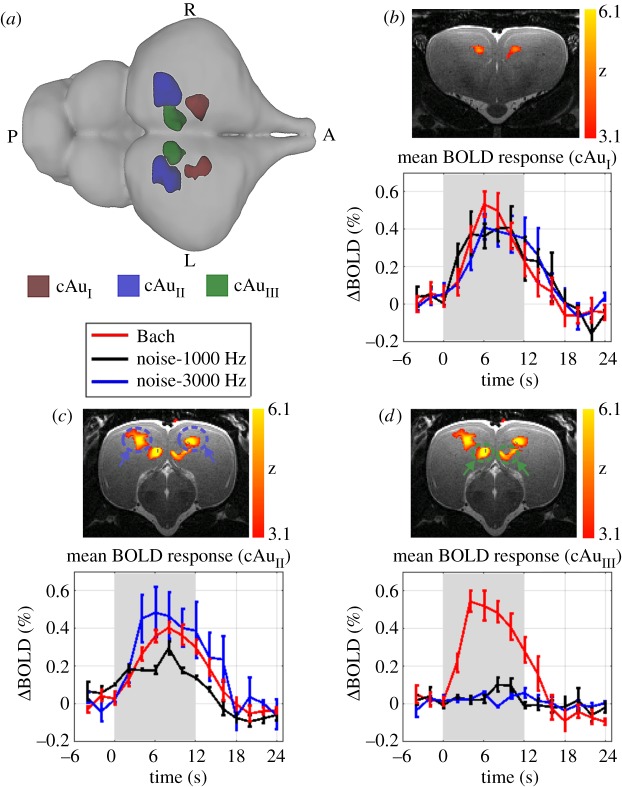
To identify brain regions sensitive to auditory stimuli, we used a sound versus silence contrast. For this, acoustic stimuli were presented in 40 repeated blocks of 12 s stimulation followed by 18 s of silence. When simple auditory stimuli were presented, Z-score maps revealed that the BOLD signal increased bilaterally in the rostromedial and caudocentral ADVR. Presentation of the complex stimulus also activated these areas, but in addition a bilateral activation of a region within the caudomedial ADVR close to the lateral ventricle was observed (figure 3 and electronic supplementary material, figure S07). As these pallial regions have no specific nomenclature, we refer to them as cAuI (crocodilian auditory region in the rostromedial ADVR), cAuII (crocodilian auditory region in the caudo-centrodorsal ADVR) and cAuIII (crocodilian auditory region in the caudo-medioventral ADVR). A two-way ANOVA was performed with the mean BOLD signal as the dependent variable and stimulus type and brain area as the independent variables. The results revealed a significant effect of brain area (F2,28 = 8.69, p < 0.001) and stimulus type (F2,8 = 10.79, p < 0.001) as well as a significant interaction between brain area and stimulus type (F4,28 = 6.85, p < 0.001). Post hoc tests demonstrated that there were no significant differences in evoked BOLD responses between different stimuli for cAuI (all, p > 0.307). By contrast, the amplitude of evoked BOLD signals in cAuII in the 3000 Hz condition was significantly higher than in the 1000 Hz condition (p < 0.021), while there was no significant difference between complex and simple sounds (p > 0.32). A multiple comparison analysis of cAuIII revealed that the BOLD signal amplitude in response to the complex stimulus was significantly higher compared to the amplitude evoked by the simple stimuli (both low and high frequencies, p < 3−05). For all auditory regions identified, BOLD responses were restricted to the stimulus period and reached their peak at 7.2 ± 1.8 s, 8.5 ± 2.1 s and 5.3 ± 1.6 s (mean ± s.d., N = 4) after stimulus onset in cAuI, cAuII and cAuIII, respectively. The peak of BOLD activation in the auditory areas in all cases occurred significantly earlier than in the visual areas cVsI and Rt (p < 0.001).
Figure 3.
Auditory activation in the Nile crocodile brain in response to different stimuli. (a) 3D dorsal view representing the location of significant BOLD activations after stimulation. While auditory areas cAuI and cAuII responded to all stimuli, cAuIII was only activated in response to the complex stimulus (music by Bach). Mean BOLD response of all four crocodiles in areas cAuI (b), cAuII (c), cAuIII (d) measured after stimulation with random chords, centred around 1000 (black line) or 3000 Hz (blue line) and classical music (red line). As the shape of BOLD activations did not differ between left and right hemisphere, the average of both hemispheres is plotted. Stimulation intervals are marked in grey. Error bars represent the standard error of the mean (s.e.m.). Z-score maps were acquired during classical music presentation (group results, mixed-effect model, FLAME1 + 2, p < 0.05, N = 4).
3. Discussion
Here, we conducted, to our knowledge, the first fMRI experiment in the brain of a poikilothermic vertebrate. The established protocol allowed us to record BOLD signals generated within the crocodile's forebrain in response to visual and auditory stimulation. The main evoked BOLD responses were identified within the ADVR, a neuroanatomical structure present in birds and reptiles [28], and known to process visual, auditory and somatosensory neural information [13,29,30]. BOLD activation induced by visual stimuli was found in rostral dorsolateral aspects of the ADVR, a region we label cVsI—crocodilian primary pallial visual area. Furthermore, visual stimulation elicited diencephalic activation in the nucleus Rt. Auditory stimulation led to an increase in BOLD signal in the rostromedial ADVR (cAuI), caudo-central ADVR (cAuII) and caudo-medial ADVR (cAuIII), with cAuIII being activated only after exposure to complex acoustic stimuli. This spatial and functional segregation of activated areas in response to sensory stimuli differing in modality and complexity closely resembles the situation observed in birds and mammals. This suggests that the presence of hierarchically organized, sensory processing networks may be a shared feature of amniote brains, possibly present in the common ancestor of amniotes that existed more than 300 million years ago.
(a). Functional magnetic resonance imaging as a reliable neuroimaging tool in crocodiles
fMRI in reptiles represents a serious challenge due to ectothermia and irregular respiratory rates [31], both of which substantially affect fMRI outcomes. We addressed these problems by first maintaining the body temperature of the crocodile at 23°C. To counter the effects of motion artefacts, caused by movement or respiration, we built a restrainer (figure 1a), in which we secured the mildly sedated animal during scanning. To counteract motion artefacts caused by respiration (e.g. intermittent breathing pattern: respiratory bursts followed by a low respiratory rate, [31]), we designed a U-shaped device, to minimize the transfer of respiratory movements to the skull (motion parameters represented in electronic supplementary material, figure S03 and movie S01; see electronic supplementary material section for more detailed information). Hence, we were able to successfully counteract the above-mentioned challenges and produce a reliable and biologically relevant BOLD activation sequence.
(b). Functional organization of the crocodile's visual system
Amniotes share two main parallel image-forming visual pathways: the tectofugal and thalamofugal pathways. The tectofugal system includes the retina, the mesencephalic optic tectum (sauropsids) or superior colliculus (mammals), the diencephalic nucleus rotundus (sauropsids) or lateral posterior/pulvinar (mammals) and terminates in the telencephalon (entopallium: embedded in the ADVR of birds, reptiles: dorsolateral ADVR; mammals: extrastriate and partly primary visual cortex) [6,8,14,25,32–34]. The thalamofugal system for mammals and sauropsids bypasses the midbrain and projects directly to the diencephalic lateral geniculate nuclei and on to the telencephalon.
The current study demonstrated a prominent BOLD activation after visual stimulation in the tectofugal visual system of the Nile crocodile in a telencephalic area we refer to as area cVsI. This area corresponds to the termination region of fibres carrying visual information from the nucleus rotundus to the ADVR in crocodilians [14–17]. Furthermore, studies in other reptiles, such as gecko and iguana [35], turtle [36] and snake [37], have identified visual ADVR regions broadly matching the localization of cVsI in crocodiles. Two recent studies by Briscoe et al. [38,39], using genetic markers to identify homologues of avian pallial subareas in American alligators, found areas highly resembling the avian meso- and nidopallium in genetic expression patterns. Within the putative nidopallium, they also identified primary sensory areas, including a putative entopallium. The identified entopallium matches cVsI very well, further strengthening our finding. Besides cVsI, we found BOLD activation in the diencephalic nucleus rotundus (Rt) in response to light stimulation. Many studies have shown that Rt is a central part of the tectofugal visual system in reptiles and birds and relays information from the optic tectum to the telencephalon [14–17,22]. These data correlate with our findings, confirming that we recorded functional correlates of the crocodilian tectofugal visual system.
A recent fMRI study in pigeons using a similar stimulation paradigm revealed activations in the telencephalic entopallium [22], which matches cVsI of the current study. In this pigeon study, no activation of the thalamofugal visual system was found [22]. This is identical to the findings of the present study in crocodiles (see electronic supplementary material for further discussion on this point).
Crocodiles are trichromatic with photoreceptors associated with green, violet and red [40]. It is likely that their tectofugal system is involved in colour perception similar to birds [32,41]. In pigeons, entopallial neurons even show colour preferences to green and red stimuli [42], resembling our findings in the Nile crocodile. In addition, we found significant frequency differences in cVsI between red and green: the red stimulus elicited highest BOLD responses at 5 and green at 2 Hz. No such difference was found for Rt. Indeed, recent studies in mammals demonstrated that cortical visual BOLD activations depended on stimulus frequency and differed according to anatomical region (mesencephalon, diencephalon and telencephalon) and their internal subdivisions [43,44]. Thus, it is possible that the crocodilian visual system displays a similar suite of functional similarities like those observed in birds and mammals.
(c). Functional organization of crocodile's telencephalic auditory system
Simple and complex auditory stimulation revealed robust BOLD activations in three distinct bilateral pallial regions: cAuI rostro-medial ADVR, cAuII caudo-centrodorsal ADVR and cAuIII caudo-medioventral ADVR. cAuIII was only activated by the complex auditory stimulus (figure 3).
The cAuI, localized in the rostromedial region of the ADVR (figure 3b), showed similar BOLD responses to all three sound stimuli (1000 Hz, 3000 Hz, classical music), suggesting a generalized auditory responsivity. The position of cAuI at the medial border of cVsI resembles topographically the nidopallium intermedium medialis (NIM) of pigeons which resides dorsomedially to the entopallium [45] and receives multimodal thalamic input [45–48]. Electrophysiological recordings from the thalamic relay to NIM in pigeons show robust and frequency-independent responses to auditory stimuli [48]. This correlates with the BOLD activation pattern identified for cAuI in our crocodile study. In pigeons, these structures respond to visual stimuli [48], but this was not the case for crocodile cAuI; however, it is likely that our visual stimuli were not suited to activate a response, as the strongest signals in pigeon studies were elicited to moving lights [48]. A visual activation within an area corresponding to cAuI has also been described in the Iguana [30] and cAuI lies in the putative nidopallium identified in the American alligator [39]. Thus, the crocodilian cAuI might resemble the avian multimodal NIM.
Like cAuI, cAuII also showed bilateral activation in response to all stimuli, but was localized in the caudo-central ADVR (figure 3c). A primary auditory zone in the caudomedial ADVR has been described in several reptilian species [18,49–51]. At least in the caiman (Caiman crocodilus), this area is even constituted by two overlapping regions with afferents from the thalamic n. reuniens pars centralis and pars diffusa that receive ascending auditory input from the tonotopically organized midbrain torus semicircularis [18,19]. Furthermore, cAuII seems to overlap with field L identified by Briscoe et al. in the American alligator [39]. Tonotopy is the hallmark of both the mammalian [52] and the avian [53] primary auditory telencephalic areas. Although our resolution was insufficient to reveal frequency-specific activity patterns, our frequency-dependent BOLD-signals of cAuII could hint at tonotopy within this region, which we suggest is possibly the primary auditory telencephalic region in crocodiles.
In contrast to cAuI and cAuII, cAuIII was selectively active during the presentation of complex auditory stimuli, and did not show BOLD signal increases to simple auditory stimuli (figure 3d). fMRI studies in songbirds also demonstrate that the primary auditory Field L responds to a broad range of sound stimuli such as white noise, music and birdsong, while the auditory-associative caudomedial nidopallium (NCM) and caudal mesopallium [11] only respond to more temporally complex sounds including classical music and song. Thus, we suggest cAuIII to be a higher order auditory area, specifically processing auditory information with complex spectrotemporal patterns. cAuIII appears to topologically overlap with auditory areas identified in caiman [16,18] and other reptiles [29,30,49,54–56]. In addition, cAuIII seems to be located in an area resembling the caudo-medial aspects of the putative nidopallium homologue in the American alligator, which makes it likely that cAuIII is indeed a structure resembling NCM.
Cumulatively, the auditory data in the current study reveal three regions in the crocodile pallium: first, a potentially multimodal region that is broadly activated by most sounds (cAuI); second, a primary and possibly tonotopically organized auditory region specific to a wide range of frequencies (cAuII); third, an adjacent higher-order auditory subdivision which selectively responds to complex sounds (cAuIII). This pattern of organization specific to auditory processing in crocodilians could possibly resemble the hierarchical organized auditory processing system evident in birds and mammals. In both of these classes of vertebrates, several multimodal pallial/cortical areas integrate broadly tuned auditory, visual and tactile senses [57,58]. In addition, both birds and mammals have tonotopically organized primary pallial/cortical auditory regions (Field L for birds, primary auditory cortex for mammals) that then transfers information to more broadly tuned higher-order auditory association areas (NCM/CM for birds, several cortical areas creating a belt around the primary cortex in mammals) [11,53,59]. These associative auditory areas mainly respond to more temporally complex sounds, including classical music and song [11].
(d). Is hierarchical processing of sensory information a conserved feature among amniotes?
Crocodilians represent a distinct and evolutionarily conservative phylogenetic clade closely related to modern birds, collectively identified as the archosaurians, and share a common amniote ancestor with mammals [1,6,60]. Thus, neural traits that are found in crocodiles, birds and mammals are likely to have been present in the shared amniote ancestor and have remained relatively unchanged for the past 300 million years [6]. The current study affords the possibility that the hierarchical processing of sensory information could be such a conserved neural trait of the amniote brain. Hierarchical processing is based on the premise that sensory information is broken down into basic features and later integrated into more complex stimulus categories or other sensory systems [58,59,61]. As outlined above, these organizational principles are present in the pallial sensory systems of birds and mammals [7,62–64]. The existence of unimodal and multimodal auditory areas within the reptilian brain makes it possible that this feature of neural information processing might have been present in the common amniote ancestor of reptiles, birds and mammals. If this is the case, this would imply that, despite approximately 300 million years of independent evolutionary trajectories, notwithstanding major changes in the structure of the brains of these three major classes, hierarchical processing networks would be a primitive and conserved principle of sensory processing in the amniote brain. However, to prove this assumption, further functional evidence, especially from potential higher-order visual areas in reptilians are required, which is currently lacking.
4. Conclusion
Our study demonstrates that fMRI is an applicable and useful technique in poikilothermic vertebrate ectotherms, allowing insights into the functional organization of sensory processing systems. We revealed the precise location and the functional organization of both auditory and visual areas within the ADVR of the Nile crocodile forebrain which are in line with previous anatomical studies. At least for the auditory system, we could also collect evidence for a possible hierarchical organization, which would be in line with organizational principles described for the sensory systems of mammals and birds, and thus may represent a conserved feature of the amniote brain.
5. Material and methods
(a). Preparation of crocodiles
Five juvenile Nile crocodiles (Crocodylus niloticus, body length 61–73 cm), supplied by the crocodile farm ‘La Ferme Aux Crocodiles’ (Pierrelatte, France) and housed in the zoo of Bochum were used in this study. All animals were returned to the crocodile farm after the experiments. (Information on housing can be found in the electronic supplementary material.) Before the scans, animals were mildly sedated with an intramuscular injection of medetomidine (0.5–1 mg kg−1) on the ventral aspect of the tail. Previous studies have shown that medetomidine, an α-2 adrenergic agonist, has only a weak effect on BOLD signals and is thus widely used in fMRI studies [65]. The animals were then secured in a custom-made holding device which was placed in the scanner (figure 1a). Post scanning, animals received intramuscular injections of atipamezole (Antisedan©, 1 mg kg−1) in the forelimb to counteract the effects of sedation.
(b). Stimuli
Visual stimuli consisted of either green or red light, flickering at a frequency of 2, 5 or 8 Hz presented binocularly with two optic fibres positioned 2 cm in front of the eyes (compare figure 1). Each stimulus was presented in 20 repeated blocks of 24 s light followed by 36 s of no stimulation. Furthermore, we conducted monocular visual stimulation with red light at an 8 Hz flickering frequency (see electronic supplementary material for further information). The dominant wavelengths for red/green light stimuli were 630 and 517 nm, while the intensity of stimuli (measured 1 cm in front of the fibre optic) was 18.7/28.9 millicandela, respectively. Auditory stimuli included a complex stimulus, namely classical music (first 12 s of Brandenburg Concerto No. 4 of Johann Sebastian Bach, compare supplementary sound S01) and simple stimuli, two random chord stimuli centred at 1000 Hz (500–1500 Hz) and 3000 Hz (1500–4500 Hz). Auditory stimuli were presented using a speaker (SoundCraft) placed in front of the animal at a distance of 8 cm from the tip of the snout. Stimulation occurred in 40 repeated blocks of 12 s of sound followed by 18 s silence. All acoustic stimuli were digitized at 44.1 kHz and were delivered with a maximum sound pressure level (SPL) of approximately 100 dB (measured at a 1 cm distance from the speaker).
(c). Magnetic resonance imaging protocols
The imaging was performed on a horizontal-bore small animal MRI scanner (7.0 T, Bruker BioSpec, 70/30 USR, Germany) using an 80 mm transmit quadrature birdcage resonator. A 20 mm ring surface coil was positioned on the dorsal aspect of the head directly over the braincase. Owing to the limited field of view granted by the coil, we centred the coil over the telencephalic hemispheres, excluding the majority of the olfactory tracts and the olfactory bulb from our analysis. Temperature within the scanner was kept at a constant 23–24°C. Body temperature was monitored using a temperature probe (Small Animal Instruments, Inc., SAII) taped to the abdominal skin of the animals.
Initial scanning sessions included acquisition of coronal, horizontal and sagittal scans using multi-slice rapid acquisition (RARE) with the following parameters: TR = 4 s; effective TE = 40.37 ms; RARE factor= 8; no average; acquisition matrix = 256 × 128; field of view (FOV) = 32 × 32 mm2; spatial resolution = 0.125 × 0.25 mm2; slice thickness = 1 mm; number of slices = 20 coronal, 17 sagittal and 15 horizontal slices; read orientation = left to right. Based on these images, 15 coronal slices with no gap between slices, which were positioned in a way to cover the entire telencephalon, were acquired using a single shot GE-EPI with following parameters: TR/TEeff = 2000/30 ms, matrix size = 96 × 96, FOV = 40 × 40 mm2, flip angle 60°, 15 coronal slices each 1 mm thickness. All experiments had a length of 1240 s (620 repetitions). The first 20 volumes were discarded for magnetic field equilibration. Anatomical images (as expanded functional images) of these slices were acquired using a RARE sequence with the following parameters: TR = 2 s, TE = 43.59 ms, RARE factor = 8, FOV = 40 × 40 mm2, acquisition matrix = 128 × 128. Images were later used to register individual functional data to the previously obtained high-resolution anatomical images. Prior to anatomical and functional image acquisition, a second-order local shim was realized using static magnetic field mapping (Bruker Mapshim). The volume for shimming (3 × 4 × 4 mm3) covered the entire telencephalon. Typical water-line (FWHM) inside the shimmed voxel was approximately 35 Hz.
For spatial normalization, an anatomical high-resolution T2-weighted MRI image was acquired with the same orientation as for the fMRI images using a multiple slice 2D RARE sequence with following parameters: TR/TEeff = 2000/60 ms; number of averages = 2; FOV = 40 × 40 mm2; matrix size = 256 × 256; number of slices = 108, slice thickness = 0.25 mm, RARE factor = 8.
(d). Functional magnetic resonance imaging data processing
All fMRI data pre-processing and fitting to general linear models (GLM) was performed using FSL 5.0.9 [66]. Data pre-processing steps consisted of: (i) upscaling the voxel size by a factor of 10, (ii) motion correction using the MCFLIRT tool [67], (iii) slice time correction, (iv) removing of non-brain tissues from functional data (using a brain mask), (v) spatial smoothing (FWHM = 8 mm, after upscaling), (vi) temporal filtering (high-pass filter with cut-off at 60 and 30 s for visual and auditory experiments, respectively) and (vii) registration to the high-resolution anatomical images. After analysing individual subjects, the results were normalized to one of the animal's anatomical scans for group analysis. To improve the registration, functional data were first aligned to the expanded functional image using affine linear registration (six degrees-of-freedom). Then, expanded functional images were registered to the high-resolution T2-weighted RARE anatomical images using affine linear registration (12 degrees-of-freedom). Thereafter, RARE images from all individuals were normalized to the anatomical image of the first animal.
A GLM model implanted in FSL (FEAT) was used to perform whole-brain statistical analysis. A first-level analysis was constructed for each subject to examine regions of the brain sensitive to visual or auditory stimulation. A custom input file (onsets of stimuli in seconds, duration of stimuli = 12 s or 24 s (sound/light), weighting = 1) and six motion parameters (as confound regressors) were used to create a design matrix (TN, T representing the time points and N the number of regressors, here 7) to convolve with the canonical haemodynamic response (Double-Gamma HRF function, phase = 5 s) to model each explanatory variable (light or sound) in each analysis. Two contrasts were specified to investigate positive and negative effect of the stimuli on brain functions (stimulus versus rest and rest versus stimulus). The threshold of computed statistics was set to Z > 3.1 to identify contiguous clusters, and a cluster extend of p < 0.05 was applied to control family error wise (FEW). The product of first-level analysis served as input for group-level analysis. For group-level analysis, mixed-effect model (FLAME1 + 2) was used with thresholding at Z > 3.1 (p < 0.001).
(e). Region of interest analysis
To avoid the circularity problem mentioned by Kriegeskorte [68], we ran localizer sessions for the 8 Hz flickering frequency condition for both red and green light and the classical music condition for each individual. To quantify the BOLD response, ROIs were delineated on high-resolution anatomical images based on group Z-score maps of the localizer session and then (using a transformation matrix) projected onto a second session of each individual for further analysis. The percentage signal change (PSC) within the selected voxels was calculated by subtracting and dividing each voxel's response by the average of the last three baseline time points of the respective block. The mean PSC was calculated by averaging the PSC over all blocks without artefacts.
Supplementary Material
Acknowledgements
Animal imaging was conducted at the Ruhr-University Bochum Imaging Centre. We thank Samuel Martin and Antoine Soler of La Ferme Aux Crocodiles for supplying us with the crocodiles used in this study and Ralf Slabik, Wilfried Werner and Jens Stirnberg of the Tierpark Bochum for housing the animals during the experiment.
Ethics
All procedures were carried out in accordance with the guidelines for care and use of animals provided by the State of North Rhine-Westphalia, Germany and were approved by a national ethics committee (LANUV, Application number: 84-02.04.2015.A488).
Data accessibility
Data are available from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.6107210) [69].
Authors' contributions
M.B., B.K.B., P.R.M., O.G. and F.S. designed research; M.B., B.K.B, X.H. and F.S. performed research; M.B. and X.H. analysed data; M.B., B.K.B. and F.S. wrote the paper with input from X.H., P.R.M. and O.G.
Competing interests
We declare we have no competing interests.
Funding
The research was supported, in part, by National Research Foundation of South Africa Thuthuka Grant (TTK14051567366) to B.K.B. and through Gu227/16-1 to O.G. and SFB 874.
References
- 1.Güntürkün O, Stacho M, Ströckens F. 2017. The brains of reptiles and birds. In Evolution of nervous systems (ed. Kaas J.), pp. 171–221. Amsterdam, Netherlands: Elsevier. [Google Scholar]
- 2.Janke A, Arnason U. 1997. The complete mitochondrial genome of Alligator mississippiensis and the separation between recent archosauria (birds and crocodiles). Mol. Biol. Evol. 14, 1266–1272. (doi:10.1093/oxfordjournals.molbev.a025736) [DOI] [PubMed] [Google Scholar]
- 3.Bird CM, Berens SC, Horner AJ, Franklin A. 2014. Categorical encoding of color in the brain. Proc. Natl Acad. Sci. USA 111, 4590–4595. (doi:10.1073/pnas.1315275111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosman CA, Aboitiz F. 2015. Functional constraints in the evolution of brain circuits. Front. Neurosci. 9, 303 (doi:10.3389/fnins.2015.00303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis ED. 2009. Evolution of the pallium in birds and reptiles. In Encyclopedia of neuroscience (eds Binder MD, Hirokawa N, Windhorst U), pp. 1390–1400. Berlin, Heidelberg, Germany: Springer Berlin Heidelberg. [Google Scholar]
- 6.Shimizu T, Bowers AN. 1999. Visual circuits of the avian telencephalon: evolutionary implications. Behav. Brain Res. 98, 183–191. (doi:10.1016/S0166-4328(98)00083-7) [DOI] [PubMed] [Google Scholar]
- 7.Shanahan M, Bingman VP, Shimizu T, Wild M, Güntürkün O. 2013. Large-scale network organization in the avian forebrain: a connectivity matrix and theoretical analysis. Front. Comput. Neurosci. 7, 89 (doi:10.3389/fncom.2013.00089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu T, Patton TB, Husband SA. 2010. Avian visual behavior and the organization of the telencephalon. Brain. Behav. Evol. 75, 204–217. (doi:10.1159/000314283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada K, Rong F, Venezia J, Matchin W, Hsieh IH, Saberi K, Serences JT, Hickok G. 2010. Hierarchical organization of human auditory cortex: evidence from acoustic invariance in the response to intelligible speech. Cereb. Cortex 20, 2486–2495. (doi:10.1093/cercor/bhp318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Recanzone GH, Cohen YE. 2010. Serial and parallel processing in the primate auditory cortex revisited. Behav. Brain Res. 206, 1–7. (doi:10.1016/j.bbr.2009.08.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Meir V, et al. 2005. Spatiotemporal properties of the BOLD response in the songbirds' auditory circuit during a variety of listening tasks. Neuroimage 25, 1242–1255. (doi:10.1016/j.neuroimage.2004.12.058) [DOI] [PubMed] [Google Scholar]
- 12.Ahumada-Galleguillos P, Fernández M, Marin GJ, Letelier JC, Mpodozis J. 2015. Anatomical organization of the visual dorsal ventricular ridge in the chick (Gallus gallus): layers and columns in the avian pallium. J. Comp. Neurol. 523, 2618–2636. (doi:10.1002/cne.23808) [DOI] [PubMed] [Google Scholar]
- 13.Vergne AL, Pritz MB, Mathevon N. 2009. Acoustic communication in crocodilians: from behaviour to brain. Biol. Rev. 84, 391–411. (doi:10.1111/j.1469-185X.2009.00079.x) [DOI] [PubMed] [Google Scholar]
- 14.Pritz MB. 1975. Anatomical identification of a telencephalic visual area in crocodiles: ascending connections of nucleus rotundus in Caiman crocodilus. J. Comp. Neurol. 164, 323–338. (doi:10.1002/cne.901640305) [DOI] [PubMed] [Google Scholar]
- 15.Pritz MB, Northcutt RG. 1977. Succinate dehydrogenase activity in the telencephalon of crocodiles correlates with the projection areas of sensory thalamic nuclei. Brain Res. 124, 357–360. (doi:10.1016/0006-8993(77)90893-9) [DOI] [PubMed] [Google Scholar]
- 16.Pritz MB. 1995. The thalamus of reptiles and mammals: similarities and differences. Brain. Behav. Evol. 46, 197–208. (doi:10.1159/000113274) [DOI] [PubMed] [Google Scholar]
- 17.Pritz MB. 2015. Crocodilian forebrain: evolution and development. Integr. Comp. Biol. 55, 949–961. (doi:10.1093/icb/icv003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pritz MB. 1974. Ascending connections of a thalamic auditory area in a crocodile, Caiman crocodilus. J. Comp. Neurol. 153, 199–213. (doi:10.1002/cne.901530205) [DOI] [PubMed] [Google Scholar]
- 19.Pritz MB, Stritzel ME. 1992. A second auditory area in the non-cortical telencephalon of a reptile. Brain Res. 569, 146–151. (doi:10.1016/0006-8993(92)90381-I) [DOI] [PubMed] [Google Scholar]
- 20.Ogawa S, Lee TM, Kay AR, Tank DW. 1990. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl Acad. Sci. USA 87, 9868–9872. (doi:10.1073/pnas.87.24.9868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu JV, Hirano Y, Nascimento GC, Stefanovic B, Leopold DA, Silva AC. 2013. FMRI in the awake marmoset: somatosensory-evoked responses, functional connectivity, and comparison with propofol anesthesia. Neuroimage 78, 186–195. (doi:10.1016/j.neuroimage.2013.03.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Groof G, Jonckers E, Güntürkün O, Denolf P, Van Auderkerke J, Van der Linden A. 2013. Functional MRI and functional connectivity of the visual system of awake pigeons. Behav. Brain Res. 239, 43–50. (doi:10.1016/j.bbr.2012.10.044) [DOI] [PubMed] [Google Scholar]
- 23.Behroozi M, Chwiesko C, Ströckens F, Sauvage M, Helluy X, Peterburs J, Güntürkün O. 2017. In vivo measurement of T1 and T2 relaxation times in awake pigeon and rat brains at 7T. Magn. Reson. Med. 79, 1090–1100. (doi:10.1002/mrm.26722) [DOI] [PubMed] [Google Scholar]
- 24.Behroozi M, Ströckens F, Stacho M, Güntürkün O. 2017. Functional connectivity pattern of the internal hippocampal network in awake pigeons: a resting-state fMRI study. Brain. Behav. Evol. 90, 62–72. (doi:10.1159/000475591) [DOI] [PubMed] [Google Scholar]
- 25.Guirado S, Real MÁ, Dávila JC. 2005. The ascending tectofugal visual system in amniotes: new insights. Brain Res. Bull. 66, 290–296. (doi:10.1016/j.brainresbull.2005.02.015) [DOI] [PubMed] [Google Scholar]
- 26.Rose M. 1923. Histologische Lokalisation des Vorderhirns der Reptilien. J. Psychol. Neurol. 29, 219–227. [Google Scholar]
- 27.Riss W, Halpern M, Scalia F. 1969. The quest for clues to forebrain evolution—the study of reptiles. Brain. Behav. Evol. 2, 1–25. (doi:10.1159/000125810) [Google Scholar]
- 28.Jarvis ED, et al. 2005. Avian brains and a new understanding of vertebrate brain evolution. Nat. Rev. Neurosci. 6, 151–159. (doi:10.1038/nrn1606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiner A, Northcutt RG. 2000. Succinic dehydrogenase histochemistry reveals the location of the putative primary visual and auditory areas within the dorsal ventricular ridge of Sphenodon punctatus. Brain. Behav. Evol. 55, 26–36. (doi:10.1159/000006639) [DOI] [PubMed] [Google Scholar]
- 30.Manger PR, Slutsky DA, Molnár Z. 2002. Visual subdivisions of the dorsal ventricular ridge of the iguana (Iguana iguana) as determined by electrophysiologic mapping. J. Comp. Neurol. 453, 226–246. (doi:10.1002/cne.10373) [DOI] [PubMed] [Google Scholar]
- 31.Perry SF. 1988. Functional morphology of the lungs of the Nile crocodile, Crocodylus niloticus: non-respiratory parameters. J. Exp. Biol. 117, 99–117. [Google Scholar]
- 32.Michael N, Löwel S, Bischof HJ. 2015. Features of the retinotopic representation in the visual wulst of a laterally eyed bird, the zebra finch (Taeniopygia guttata). PLoS ONE 10, e0124917 (doi:10.1371/journal.pone.0124917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiner A, Powers AS. 1980. The effects of extensive forebrain lesions on visual discriminative performance in turtles (Chrysemys picta picta). Brain Res. 192, 327–337. (doi:10.1016/0006-8993(80)90887-2) [DOI] [PubMed] [Google Scholar]
- 34.Casagrande VA., Kaas JH. 1994. The afferent, intrinsic, and efferent connections of primary visual cortex in primates. Cereb. Cortex 10, 201–259. (doi:10.1007/978-1-4757-9628-5_5) [Google Scholar]
- 35.Bruce LL, Butler AB. 1984. Telencephalic connections in lizards. II. Projections to anterior dorsal ventricular ridge. J. Comp. Neurol. 229, 602–615. (doi:10.1002/cne.902290412) [DOI] [PubMed] [Google Scholar]
- 36.Belekhova MG, Kosareva AA. 1971. Organization of the turtle thalamus: visual, somatic and tectal zones. Brain. Behav. Evol. 4, 337–375. (doi:10.1159/000125444) [DOI] [PubMed] [Google Scholar]
- 37.Berson DM, Hartline PH. 1988. A tecto-rotundo-telencephalic pathway in the rattlesnake: evidence for a forebrain representation of the infrared sense. J. Neurosci. 8, 1074–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briscoe SD, Albertin CB, Rowell JJ, Ragsdale CW. 2018. Neocortical association cell types in the forebrain of birds and alligators. Curr. Biol. 28, 686–696.e6. (doi:10.1016/j.cub.2018.01.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briscoe SD, Ragsdale CW. 2018. Molecular anatomy of the alligator dorsal telencephalon. J. Comp. Neurol. (doi:10.1002/cne.24427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagloo N, Collin SP, Hemmi JM, Hart NS. 2016. Spatial resolving power and spectral sensitivity of the saltwater crocodile, Crocodylus porosus, and the freshwater crocodile, Crocodylus johnstoni. J. Exp. Biol. 219, 1394–1404. (doi:10.1242/jeb.135673) [DOI] [PubMed] [Google Scholar]
- 41.Medina L. 2010. Do birds and reptiles possess homologues of mammalian visual, somatosensory, and motor cortices? In Evolution of nervous systems (ed. Kaas J.), pp. 163–194. Amsterdam, Netherlands: Elsevier. [Google Scholar]
- 42.Verhaal J, Kirsch JA, Vlachos I, Manns M, Güntürkün O. 2012. Lateralized reward-related visual discrimination in the avian entopallium. Eur. J. Neurosci. 35, 1337–1343. (doi:10.1111/j.1460-9568.2012.08049.x) [DOI] [PubMed] [Google Scholar]
- 43.Van Camp N, Verhoye M, De Zeeuw CI, Van der Linden A. 2006. Light stimulus frequency dependence of activity in the rat visual system as studied with high-resolution BOLD fMRI. J. Neurophysiol. 95, 3164–3170. (doi:00400.2005[pii]\r10.1152/jn.00400.2005) [DOI] [PubMed] [Google Scholar]
- 44.Yen CCC, Fukuda M, Kim SG. 2011. BOLD responses to different temporal frequency stimuli in the lateral geniculate nucleus and visual cortex: insights into the neural basis of fMRI. Neuroimage 58, 82–90. (doi:10.1016/j.neuroimage.2011.06.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wild JM, Gaede AH. 2016. Second tectofugal pathway in a songbird (Taeniopygia guttata) revisited: tectal and lateral pontine projections to the posterior thalamus, thence to the intermediate nidopallium. J. Comp. Neurol. 524, 963–985. (doi:10.1002/cne.23886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Güntürkün O, Kröner S. 1999. A polysensory pathway to the forebrain of the pigeon: the ascending projections of the nucleus dorsolateralis posterior thalami (DLP). Eur. J. Morphol. 37, 185–189. (doi:10.1016/B978-0-7216-0334-6.50124-2) [PubMed] [Google Scholar]
- 47.Korzeniewska E. 1987. Multisensory convergence in the thalamus of the pigeon (Columba livia). Neurosci. Lett. 80, 55–60. (doi:10.1016/0304-3940(87)90494-0) [DOI] [PubMed] [Google Scholar]
- 48.Korzeniewska E, Güntürkün O. 1990. Sensory properties and afferents of the N. dorsolateralis posterior thalami of the pigeon. J. Comp. Neurol. 292, 457–479. (doi:10.1002/cne.902920311) [DOI] [PubMed] [Google Scholar]
- 49.Foster RE, Hall WC. 1978. The organization of central auditory pathways in a reptile, Iguana iguana. J. Comp. Neurol. 178, 783–831. (doi:10.1002/cne.901780412) [DOI] [PubMed] [Google Scholar]
- 50.Belekhova MG. 1985. An underestimated visual pathway in reptiles. Neurosci. Lett. 58, 111–116. [DOI] [PubMed] [Google Scholar]
- 51.Balaban CD, Ulinski PS. 1981. Organization of thalamic afferents to anterior dorsal ventricular ridge in turtles. I. Projections of thalamic nuclei. J. Comp. Neurol. 200, 95–129. (doi:10.1002/cne.902000108) [DOI] [PubMed] [Google Scholar]
- 52.De Martino F, Moerel M, Ugurbil K, Goebel R, Yacoub E, Formisano E. 2015. Frequency preference and attention effects across cortical depths in the human primary auditory cortex. Proc. Natl Acad. Sci. USA 112, 16 036–16 041. (doi:10.1073/pnas.1507552112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terleph TA, Mello CV, Vicario DS. 2006. Auditory topography and temporal response dynamics of canary caudal telencephalon. J. Neurobiol. 66, 281–292. (doi:10.1002/neu.20219) [DOI] [PubMed] [Google Scholar]
- 54.Bruce LL, Butler AB. 1984. Telencephalic connections in lizards. I. Projections to cortex. J. Comp. Neurol. 229, 585–601. (doi:10.1002/cne.902290411) [DOI] [PubMed] [Google Scholar]
- 55.Belekhova MG, Chudinova TV, Repérant J, Ward R, Jay B, Vesselkin NP, Kenigfest NB. 2010. Core-and-belt organisation of the mesencephalic and forebrain auditory centres in turtles: expression of calcium-binding proteins and metabolic activity. Brain Res. 1345, 84–102. (doi:10.1016/j.brainres.2010.05.026) [DOI] [PubMed] [Google Scholar]
- 56.Belekhova MG, Zharskaja VD, Khachunts AS, Gaidaenko GV, Tumanova NL. 1985. Connections of the mesencephalic, thalamic and telencephalic auditory centers in turtles. Some structural bases for audiosomatic interrelations. J. Hirnforsch. 26, 127–152. [PubMed] [Google Scholar]
- 57.Downar J, Crawley AP, Mikulis DJ, Davis KD. 2000. A multimodal cortical network for the detection of changes in the sensory environment. Nat. Neurosci. 3, 277–283. (doi:10.1038/72991) [DOI] [PubMed] [Google Scholar]
- 58.Mouritsen H, Heyers D, Güntürkün O. 2016. The neural basis of long-distance navigation in birds. Annu. Rev. Physiol. 78, 133–154. (doi:10.1146/annurev-physiol-021115-105054) [DOI] [PubMed] [Google Scholar]
- 59.Kaas JH. 1989. The evolution of complex sensory systems in mammals. J. Exp. Biol. 146, 165–176. [DOI] [PubMed] [Google Scholar]
- 60.Derobert Y, Médina M, Rio JP, Ward R, Repérant J, Marchand MJ, Miceli D. 1999. Retinal projections in two crocodilian species, Caiman crocodilus and Crocodylus niloticus. Anat. Embryol. 200, 175–191. (doi:10.1007/s004290050271) [DOI] [PubMed] [Google Scholar]
- 61.Wessinger CM, VanMeter J, Tian B, Pekar J, Rauschecker JP. 2001. Hierarchical organization of the human auditory cortex revealed by functional magnetic resonance imaging. J. Cogn. Neurosci. 13, 1–7. (doi:10.1162/089892901564108) [DOI] [PubMed] [Google Scholar]
- 62.Theunissen FE, Shaevitz SS. 2006. Auditory processing of vocal sounds in birds. Curr. Opin. Neurobiol. 16, 400–407. (doi:10.1016/j.conb.2006.07.003) [DOI] [PubMed] [Google Scholar]
- 63.Sadagopan S, Temiz-Karayol NZ, Voss HU. 2015. High-field functional magnetic resonance imaging of vocalization processing in marmosets. Sci. Rep. 5, 10950 (doi:10.1038/srep10950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grill-Spector K, Malach R. 2004. The human visual cortex. Annu. Rev. Neurosci. 27, 649–677. (doi:10.1146/annurev.neuro.27.070203.144220) [DOI] [PubMed] [Google Scholar]
- 65.Pawela CP, Biswal BB, Hudetz AG, Schulte ML, Li R, Jones SR, Cho YR, Matloub HS, Hyde JS. 2009. A protocol for use of medetomidine anesthesia in rats for extended studies using task-induced BOLD contrast and resting-state functional connectivity. Neuroimage 46, 1137–1147. (doi:10.1016/j.neuroimage.2009.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith SM, et al. 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl. 1), S208–S219. (doi:10.1016/j.neuroimage.2004.07.051) [DOI] [PubMed] [Google Scholar]
- 67.Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. (doi:10.1006/nimg.2002.1132) [DOI] [PubMed] [Google Scholar]
- 68.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. 2009. Circular analysis in systems neuroscience: the dangers of double dipping. Nat. Neurosci. 12, 535–540. (doi:10.1038/nn.2303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Behroozi M, Billings BK, Helluy X, Manger PR, Güntürkün O, Ströckens F.2018. Data from: Functional MRI in the Nile crocodile: a new avenue for evolutionary neurobiology. Dryad Digital Repository. (https://doi.org/10.5061/dryad.6107210. ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Behroozi M, Billings BK, Helluy X, Manger PR, Güntürkün O, Ströckens F.2018. Data from: Functional MRI in the Nile crocodile: a new avenue for evolutionary neurobiology. Dryad Digital Repository. (https://doi.org/10.5061/dryad.6107210. ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.6107210) [69].