Abstract
Moving animal groups such as schools of fishes or flocks of birds often undergo sudden collective changes of their travelling direction as a consequence of stochastic fluctuations in heading of the individuals. However, the mechanisms by which these behavioural fluctuations arise at the individual level and propagate within a group are still unclear. In this study, we combine an experimental and theoretical approach to investigate spontaneous collective U-turns in groups of rummy-nose tetra (Hemigrammus rhodostomus) swimming in a ring-shaped tank. U-turns imply that fish switch their heading between the clockwise and anticlockwise direction. We reconstruct trajectories of individuals moving alone and in groups of different sizes. We show that the group decreases its swimming speed before a collective U-turn. This is in agreement with previous theoretical predictions showing that speed decrease facilitates an amplification of fluctuations in heading in the group, which can trigger U-turns. These collective U-turns are mostly initiated by individuals at the front of the group. Once an individual has initiated a U-turn, the new direction propagates through the group from front to back without amplification or dampening, resembling the dynamics of falling dominoes. The mean time between collective U-turns sharply increases as the size of the group increases. We develop an Ising spin model integrating anisotropic and asymmetrical interactions between fish and their tendency to follow the majority of their neighbours nonlinearly (social conformity). The model quantitatively reproduces key features of the dynamics and the frequency of collective U-turns observed in experiments.
Keywords: collective animal behaviour, collective motion, fish interaction, conformity, Ising spin model
1. Introduction
The flexible coordination of fishes in schools brings important benefits [1,2]. A striking consequence of this flexibility is the performance of rapid and coherent changes in the swimming direction of schools, for instance as a reaction to a predator in the neighbourhood [3]. In many species, it is only a small number of individuals that detect the danger and change direction and speed, initiating an escape wave that propagates across the entire school [4]. Besides, sudden collective changes of the state of a school may also happen without an external cause as a consequence of stochastic effects [5]. In these cases, local behavioural changes of a single individual can lead to large transitions between collective states of the school, such as between the schooling state (in which individuals are aligned with each other) and the milling state (in which individuals constantly rotate around an empty core). Determining under what conditions fluctuations in individual behaviour, for instance in heading direction, emerge and propagate within a group is key to understanding transitions between collective states in fish schools and in animal groups in general.
Only few theoretical and experimental studies have addressed these questions [6,7]. Calovi et al. [7] used a data-driven model incorporating fluctuations of individual behaviour and attraction and alignment interactions among fishes to investigate the response of a school to local perturbations (i.e. by an individual whose attraction and alignment behaviour differs from that of the rest of the group). They found that the responsiveness of a school is maximum near the transition region between the milling and schooling states, where the fluctuations of the polarization are also maximal. This is entirely consistent with what happens in inert physical systems near a continuous phase transition. For instance, in magnetic systems, the polarization of the atomic spins of a magnet near the transition point has diverging fluctuations and response to a perturbation by a magnetic field. The fluctuations of school polarization are also expected to be strongly amplified at the transition from schooling to swarming observed when the swimming speed of individuals decreases [8,9]. During such a transition, the behavioural changes of a single individual are more likely to affect the collective dynamics of the school. However, the tendency of fishes to conform to the speed and direction of motion of the group can also decrease the fluctuations at the level of the group with increasing group size [10]. Social conformity refers to the nonlinear response of individuals to adjust their behaviour to that of the majority [11–13].
In this work, we analyse in groups of different sizes under which conditions individual U-turns occur, propagate through the group and lead to collective U-turns. We let groups of rummy-nose tetra (Hemigrammus rhodostomus) swim freely in a ring-shaped tank. In this set-up, fish schools only head in two directions, clockwise or anticlockwise, and they regularly switch from one to the other. In a detailed analysis of empirical data, we investigate the effect of group size on both the tendency of individuals to initiate U-turns and the collective dynamics of the U-turns. We develop an Ising-type spin model, a simple model for magnets in the physical context, to investigate the consequences on the dynamics and the propagation of information during U-turns, of the local conformity in heading, of the fish anisotropic perception of their environment, and of the asymmetric interactions between fish. We use tools and quantitative indicators from statistical physics to analyse the model. In particular, we introduce the notion of local (respectively, global) pseudo-energy which, in the context of a fish school, becomes a quantitative measure of the ‘discomfort’ of an individual (respectively, of the group) with respect to the swimming direction of the other fish.
2. Material and methods
(a). Experimental procedures and data collection
Seventy rummy-nose tetras (H. rhodostomus) were used in our experiments. This tropical freshwater species swims in a highly synchronized and polarized manner. Inside an experimental tank, a ring-shaped corridor 10 cm wide with a circular outer wall of radius 35 cm was filled with 7 cm of water (electronic supplementary material, figure S1A). For each trial, n fish were randomly sampled from their breeding tank (n ∈ {1, 2, 4, 5, 8, 10, 20}). Each fish only participated in a single experiment per day. For each group size, we performed between 9 and 14 replications (see the electronic supplementary material, table S1). Trajectories of the fish were recorded by a Sony HandyCam HD camera filming the set-up from above at 50 Hz in HDTV resolution (1920 × 1080 p). Finally, we tracked the positions of each individual using idTracker 2.1 [14], except for groups of 20 fish, for which we recorded the time of individual and collective U-turns. Details about experimental set-up, data extraction and preprocessing are given in the electronic supplementary material.
(b). Detection and quantification of individual and collective U-turns
As fish swim in a ring-shaped tank, their heading can be converted into a binary value: clockwise or anticlockwise. Before a collective U-turn, the fish are all moving in the same direction, clockwise or anticlockwise. When one fish changes its heading to the opposite direction, it can trigger a collective U-turn (electronic supplementary material, movie S1).
From the heading angle φi(t) and angular position θi(t) of an individual i at time t (electronic supplementary material, figure S2), the angle of the fish relative to the wall is computed as
| 2.1 |
and thus the degree of alignment to the circular wall can be defined as
| 2.2 |
The degree of alignment ai(t) between a fish i and the outer wall is 1 when it is moving anticlockwise, −1 when moving clockwise and 0 when it is perpendicular to the wall. When a group of fish makes a collective U-turn, the degree of alignment to the wall averaged over all individuals of the group 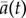 changes sign. We used this as the criterion for detecting collective U-turns automatically from the time series of
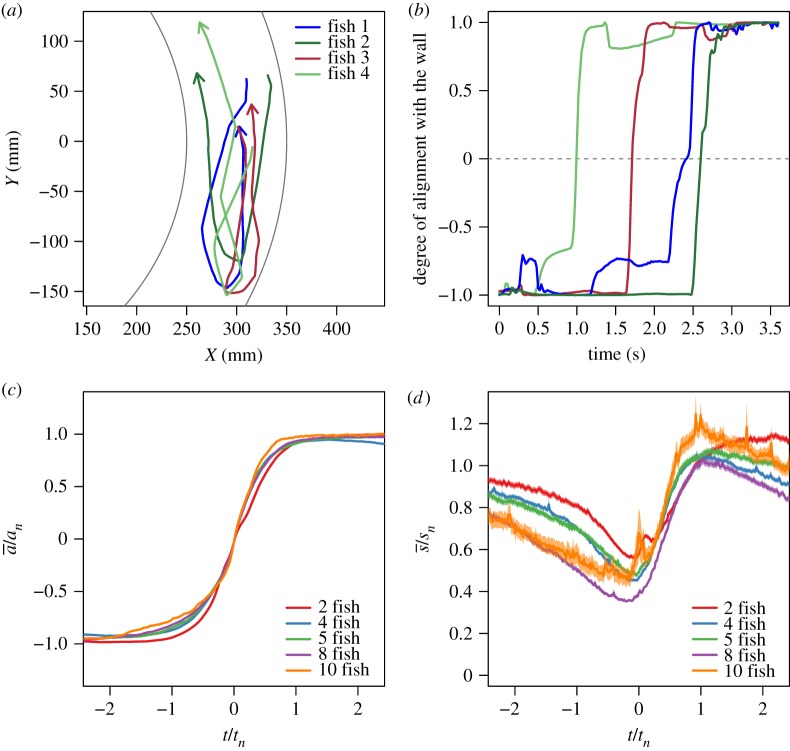
changes sign. We used this as the criterion for detecting collective U-turns automatically from the time series of 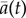 . Figure 1a shows individual trajectories during a typical collective U-turn in a group of four fish and figure 1b reports the corresponding evolution of the degrees of alignment ai(t). Further details about U-turn detection and the calculation of the quantities of interest are detailed in the electronic supplementary material, Material and methods.
. Figure 1a shows individual trajectories during a typical collective U-turn in a group of four fish and figure 1b reports the corresponding evolution of the degrees of alignment ai(t). Further details about U-turn detection and the calculation of the quantities of interest are detailed in the electronic supplementary material, Material and methods.
Figure 1.
Individual trajectories (a) and degree of alignment ai(t) of fish with the wall (b) during a U-turn in a group of four fish. (c) Normalized degree of alignment with the wall, averaged over all fish and U-turns, against the rescaled time t/tn for groups of size n, where tn is a measure of the mean duration of a U-turn. t = 0 is set when 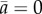 . (d) Average individual speed
. (d) Average individual speed 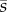 normalized by the average speed sn of the group, against the rescaled time t/tn.
normalized by the average speed sn of the group, against the rescaled time t/tn.
3. Results
(a). Spatio-temporal dynamics of collective U-turn
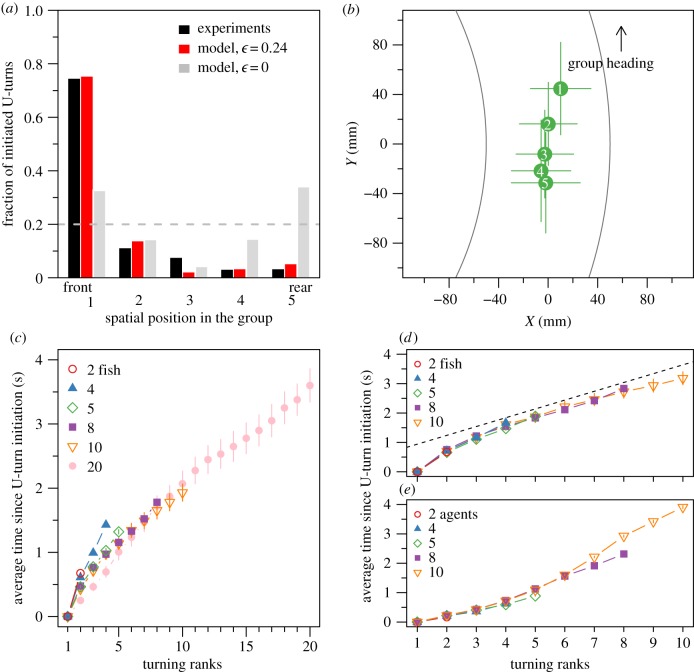
Hemigrammus rhodostomus fish form highly cohesive schools during our experiments (electronic supplementary material, figure S3A) and adjust their speed and heading to that of their group members. In a former study [15], we have shown that this is achieved through attraction and alignment interactions that have been measured. Figure 2 indicates that the average time interval between two U-turns in groups of 10 fish (one U-turn every 20 min) is two orders of magnitude larger that in groups of two fish (one U-turn every 0.2 min). In experiments in which no collective U-turn was observed (grey triangles on figure 2), we took the total period of observation as the interval until the next U-turn. Therefore, the average time λn between U-turns measured in groups of 4, 8, 10 and 20 fish are slightly underestimated. Thus, as group size increases, the number of collective U-turns decreases, because the propensities of a fish to initiate and propagate a U-turn decrease (electronic supplementary material, figure S4). Like in many other species, individual fish tend to adopt the behaviour of the majority of the group members and thus inhibit the initiation of U-turns [10].
Figure 2.
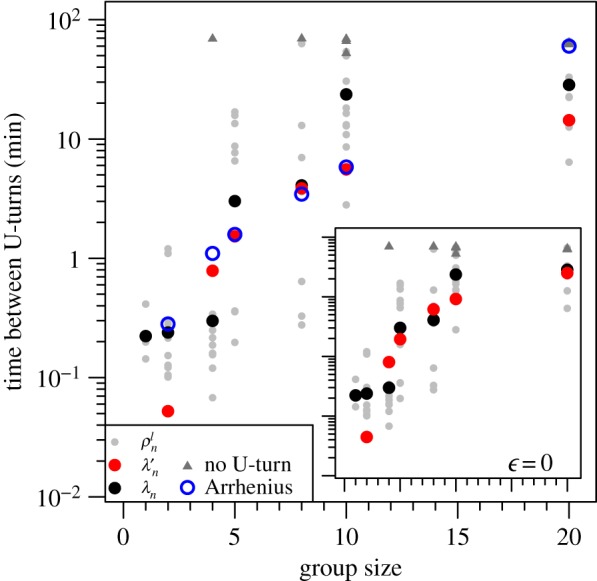
Average time between two consecutive collective U-turns as a function of group size. Average time between collective U-turns ρln in each experiment l with n fish defined as the duration of an experiment Tln divided by the number of collective U-turns performed during this experiment (grey dots). Experiments without any collective U-turn are indicated by grey triangles, with ρln = Tln/1. Average of the log of the time between collective U-turns over all experiments (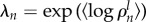 ; black dots) and over 1000 simulations (λ′n; J = 0.95 and ε = 0.24; red dots). Prediction of the Arrhenius Law (open blue circles). Inset: results of the model without asymmetric influence (J = 0.95 and ε = 0).
; black dots) and over 1000 simulations (λ′n; J = 0.95 and ε = 0.24; red dots). Prediction of the Arrhenius Law (open blue circles). Inset: results of the model without asymmetric influence (J = 0.95 and ε = 0).
As shown in figure 1c, the dynamics of collective U-turns, and in particular the evolution of the mean alignment 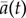 , is similar for all group sizes, once time is rescaled by the mean U-turn duration (see the electronic supplementary material for the Material and methods used to compute the scaling parameter tn, which is an effective measure of the U-turn duration). In the electronic supplementary material, figure S5 shows that tn increases approximately linearly with group size n. In groups of all sizes, fish progressively decrease their speed before turning collectively and accelerating sharply (figure 1d). The duration of this deceleration (and then acceleration) phase is much longer than the time for the group to complete a U-turn (compare figure 1c,d). Moreover, the speed minimum of the group in figure 1d is reached near the midpoint of the U-turn, when t = 0 and the mean alignment is
, is similar for all group sizes, once time is rescaled by the mean U-turn duration (see the electronic supplementary material for the Material and methods used to compute the scaling parameter tn, which is an effective measure of the U-turn duration). In the electronic supplementary material, figure S5 shows that tn increases approximately linearly with group size n. In groups of all sizes, fish progressively decrease their speed before turning collectively and accelerating sharply (figure 1d). The duration of this deceleration (and then acceleration) phase is much longer than the time for the group to complete a U-turn (compare figure 1c,d). Moreover, the speed minimum of the group in figure 1d is reached near the midpoint of the U-turn, when t = 0 and the mean alignment is 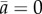 in figure 1c.
in figure 1c.
Collective U-turns are usually initiated at the front of the school and the change of swimming direction propagates towards the rear (figure 3a,b and electronic supplementary material, figures S6 and S7 and tables S2 and S3 for statistical tests). At the time of the turn of each individual, fish almost turn at the same location as the previous ranks, respectively, to the y-coordinates (electronic supplementary material, figure S8 and tables S4 and S5).
Figure 3.
Spatio-temporal propagation of collective U-turns. (a) Spatial position distribution of the initiator in groups of five fish in experiments (black) and in simulations with asymmetric influence (J = 0.95 and ε = 0.24; red) and without asymmetric influence (J = 0.95 and ε = 0; grey). Spatial positions go from 1 (position at the front) to 5 (position at rear). Dashed line shows the uniform distribution 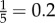 , when spatial position has no effect on the initiation of collective U-turns. (b) Average relative positions (±s.d.) of all individuals at initiation of collective U-turns, ranked by order of turning (i.e. rank 1 is initiator) in groups of five. Positions have been corrected so that all groups move in the same direction, with the outer wall at their right-hand side. The origin of the coordinate system is set to the centroid of the average positions of individuals. Average time interval since the beginning of a collective U-turn as a function of turning rank and group size in experiments ((c) and (d)) and in simulations (e). In (c), data for n = 20 have been manually obtained as the trajectories were not automatically tracked (see the electronic supplementary material). In (d), the time has been scaled by the factor rn = sn/s2, where sn is the average speed of groups of size n (not available for groups of size n = 20), revealing a behaviour almost independent of n (collapse). Dashed line is a guide showing the linear propagation of information from the third fish turning.
, when spatial position has no effect on the initiation of collective U-turns. (b) Average relative positions (±s.d.) of all individuals at initiation of collective U-turns, ranked by order of turning (i.e. rank 1 is initiator) in groups of five. Positions have been corrected so that all groups move in the same direction, with the outer wall at their right-hand side. The origin of the coordinate system is set to the centroid of the average positions of individuals. Average time interval since the beginning of a collective U-turn as a function of turning rank and group size in experiments ((c) and (d)) and in simulations (e). In (c), data for n = 20 have been manually obtained as the trajectories were not automatically tracked (see the electronic supplementary material). In (d), the time has been scaled by the factor rn = sn/s2, where sn is the average speed of groups of size n (not available for groups of size n = 20), revealing a behaviour almost independent of n (collapse). Dashed line is a guide showing the linear propagation of information from the third fish turning.
Although the time interval between the turning of the first and the second fish is longer than it is for others, the time interval between the successive turns of individuals is almost constant in a given group size (figure 3c,d), as illustrated by the fact that the time since the initiation of the collective U-turn increases linearly with the turning rank (greater than 2). The linear propagation of information in all group sizes shows there is no amplification of the individual tendency to perform a U-turn: the time between two successive individuals performing U-turns does not decrease with the number of fish that have already performed a U-turn.
The mean time interval between two successive individual U-turns decreases with group size (see figure 3c where the slopes decrease with n, or the electronic supplementary material, figure S9). However, when these time intervals are multiplied by a factor rn proportional to the average speed sn of groups of size n (rn = sn/s2), they collapse on the same curve (figure 3d). This suggests that the shorter reaction time of fish in larger groups is mostly owing to their faster swimming speed. Larger groups swim faster (electronic supplementary material, figure S3B), presumably because fish are interacting with a greater number of neighbours and are closer to each other (electronic supplementary material, figure S3C).
In summary, our results show that U-turns are mostly initiated by fish located at the front of the school. U-turns are preceded by a decrease in the speed of the group. Once the U-turn has been initiated, the wave of turning propagates in a sequential way, suggesting that fish mainly copy the behaviour of a small number of individuals [16]. Our results show that the propagation of information is on average sequential, both in space and time. This resembles a chain of falling dominoes, for which the time interval between successive falls is constant, without any positive feedback.
4. Modelling collective U-turns
(a). Model description
We now introduce an Ising-type spin model [17,18] to better understand the impact of social conformity, anisotropy and asymmetry of interactions, and group size on the propagation of information during U-turns. Each agent i has a direction of motion di∈{−1, 1}, with di =−1 representing swimming clockwise and di = 1 swimming anticlockwise. A U-turn performed by an agent i corresponds to a transition from di to −di. In the model, the relative positions of individuals and the interaction network (i.e. the influential neighbours ηi of an agent i) are kept fixed in time (electronic supplementary material, figure S10), a simplification justified by the fact that the actual structure of a fish group does not change much in the few seconds before a U-turn, in particular for the fish leading the group (electronic supplementary material, movie S1). Agents are positioned in staggered rows (electronic supplementary material, figure S3D for experimental data supporting an oblong shape that becomes longer when the group size increases, as previously found by others, e.g. [19]) and only interact with their direct neighbours.
The strength of interactions between an agent i and its neighbour j is weighted by a parameter αij that depends on the spatial position of j relative to i. αij controls the anisotropy and asymmetry of the interactions between individuals, assuming that fish react stronger to frontal stimuli, in agreement with previous experimental results on H. rhodostomus [15]. We define αij = 1 + ε when agent j is in front of agent i, αij = 1 if j is at the side of i, and αij = 1 − ε if j is behind i, where the asymmetry coefficient ε ∈ [0, 1] is kept constant for all group sizes.
The propensity of an individual i to make a U-turn depends on the state of its neighbours ηi and on the interaction matrix αij. The ‘discomfort’ Ei of an agent i in a state di is defined as
| 4.1 |
with Jij the coupling constant between two neighbours i and j, set by the two positive parameters of the model, ε and J > 0. When the anisotropy of perception and asymmetry of interactions are ignored (ε = 0), αij = 1 for all neighbouring pairs (i, j). Ei is minimal (and negative) when the focal fish i and its neighbours point in the same direction, and maximal (and positive) if the focal fish points in the opposite direction of its aligned neighbours. A small value of |Ei| corresponds to its neighbours pointing in directions nearly averaging to zero.
If an individual flips (di′ =−di), the new discomfort is 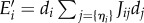 and we have
and we have
| 4.2 |
ΔEi < 0 when the agent i flips to the most common state of its neighbours, whereas ΔEi > 0 when it flips to the state opposite to this most common state. In the ε = 0 case,
| 4.3 |
corresponds to the total actual energy of the magnetic system. In this context, the fully polarized state where all fish are aligned corresponds to the so-called ground state energy, the lowest possible energy of the system. For ε≠0, the asymmetry between the perception of i by j and that of j by i breaks this interpretation in terms of energy [15]. Yet, for ε > 0, it is still useful to define E as a pseudo-energy, as will be discussed later, because it remains a good indicator of the collective discomfort of the group, i.e. the lack of heading alignment within the group.
The dynamics of the model is investigated using Monte Carlo numerical simulations inspired from the Glauber dynamics [20,21]. Within this algorithm, at each time step tk+1 = tk + 1/n (n is the number of agents), an agent is drawn randomly and turns (updates di to di′ =−di) with the acceptance probability
| 4.4 |
which is a sigmoid, going from P → 1 for ΔEi →−∞ (maximal acceptance if the discomfort decreases sharply), to P → 0 for ΔEi →+∞ (no direction switch if the discomfort would increase dramatically). In equation (4.4), T plays the role of the temperature and we chose T = 1. Indeed, as ΔEi is proportional to J, the probability P only depends on the parameter J′ = J/T, and T can then be absorbed in the constant J.
The acceptance probability P represents the social conformity in our model and its strength (i.e. the nonlinearity of P) is mainly controlled by the parameter J (electronic supplementary material, figure S11B). For large J > 0, this dynamics will favour the emergence of strongly polarized states, while for J = 0, all directions will appear with the same probability during the dynamics.
In summary, J controls the directional stiffness of the fish group, while ε describes the fish anisotropic perception of their environment, and the asymmetric interactions between fish. After inspecting the (J, ε) parameter space (see the electronic supplementary material, §1.6.1), we find that the parameter values J = 0.95 and ε = 0.24 lead to a fair agreement between the model and experimental data, as will be shown in the next section.
(b). Simulation results versus experimental data
Our model quantitatively reproduces the effect of group size on the dynamics of collective U-turns (figure 2; electronic supplementary material, S4). This suggests that the tendency of individuals to initiate U-turns and move in the opposite direction of the whole decreases with group size. However, note the lesser agreement between simulations and experimental data in groups of four. One explanation for this may be the age and body size of the fish, because they can influence the strength of interactions between fish [22] (electronic supplementary material, table S1). It is possible to set a different coupling constant Jn for each group size n to account for this effect (electronic supplementary material, §1.6.2 and figures S13, S14, S15, S16). We indeed find that Jn is smaller for the two group sizes with the largest/oldest fish on average (groups of four and eight fish), hence reducing the stiffness of the group and fostering U-turns. Yet, we find that the four models investigated (with constant J or n-dependent Jn, and involving two different topological structures of the n-groups) lead to similar results (electronic supplementary material, figures S12, S13, S15). The model with n-dependent Jn and the alternative topology leads to the best agreement with experiments, but also involves more parameters than the constant J model presented here.
Even though there is no strict notion of energy in our model when ε > 0, we can still compute the mean pseudo-energy barrier ΔEn as a function of group size n. It is defined as the mean difference between the maximum value of the pseudo-energy E during the U-turn and the reference energy computed when all the agents have the same direction (i.e. before and after a U-turn). With the interpretation of E (respectively, Ei) as a quantitative indicator of the discomfort of the group (respectively, of the fish i), the (pseudo) energy barrier ΔEn is hence a measure of the collective effort of the group needed to switch direction. We find that the energy barrier ΔEn increases sublinearly with group size n (electronic supplementary material, figure S17). We then expect that the higher (pseudo) energy barrier ΔEn, the more difficult it will be for the group to perform a U-turn (leading to longer intervals between U-turns), as it must necessarily pass through an intermediate state of greater discomfort as the group size n increases. In fact, for ε = 0, for which E represents a true energy, this mean time interval between direction changes is exactly given by the Arrhenius Law, which can be analytically proved for our spin model. In physical chemistry, the Arrhenius Law describes, for instance, the switching time between two states A and B of a molecule, separated by an energy barrier associated to an intermediate state through which the molecules must necessarily pass to go from A to B. The Arrhenius law stipulates that the mean transition time τ between two states separated by an energy barrier ΔEn grows like
| 4.5 |
where τ0 is a timescale independent of n, and T is the same temperature as the one appearing in equation (4.4) (here, T = 1). Despite the fact that ε > 0, for which E is not anymore a true energy, we still find in figure 2 that the (pseudo) Arrhenius Law reproduces fairly well the experimental mean interval between U-turns as a function of group size n, explaining the wide range of observed time intervals, but with a modified effective temperature T ≈ 4 (and 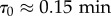 ). It is remarkable that the mean time between U-turns (a purely dynamical quantity) grows exponentially fast with ΔEn (the pseudo-energy difference between two static configurations), considering that both quantities are measured in two completely independent ways.
). It is remarkable that the mean time between U-turns (a purely dynamical quantity) grows exponentially fast with ΔEn (the pseudo-energy difference between two static configurations), considering that both quantities are measured in two completely independent ways.
The sequential propagation of information is also reproduced well by the simulations of the model (and of the alternative models introduced above), both in space (figure 3a; electronic supplementary material, figure S6) and time (figure 3c; electronic supplementary material, figure S18). In particular, the crucial impact of the anisotropic perception of fish (modelled by the parameter ε) in explaining that the initiator of the U-turn is more often located at the front of the group is illustrated by the results of the model for ε = 0.
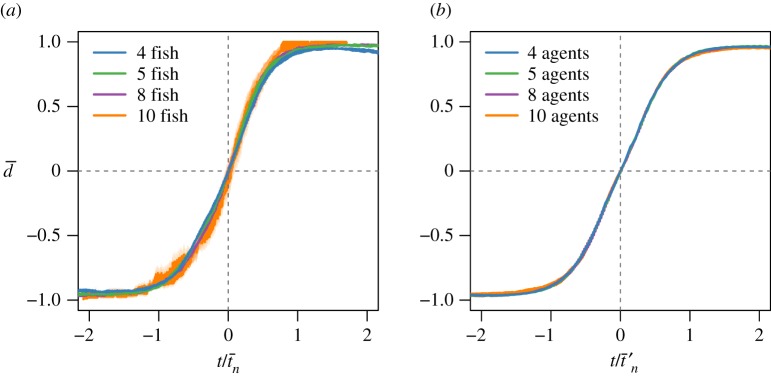
Regarding the propagation in time illustrated in figure 3d,e, the model qualitatively reproduces the linear propagation of information at the individual scale, albeit with a slight upward concavity, contrary to experiment. This disagreement can be probably ascribed to the model oversimplifications (only two free parameters J and ε; fixed topological configurations; only nearest-neighbour interactions). Yet, this result can be improved by changing the topology of the interaction network for n = 8 and 10 (electronic supplementary material, figure S12) and/or allowing the stiffness constant J to depend on the group size (electronic supplementary material, figures S12, S13, S15). Moreover, the durations of collective U-turns are log-normally distributed, both in experiments and in the model (electronic supplementary material, figure S19). Finally, figure 4a shows that, once rescaled by the U-turn duration, the average direction profile is independent of the group size. The model predicts this data collapse and the actual form of the direction profile (figure 4b).
Figure 4.
Mean swimming direction 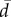 averaged over all collective U-turns as a function of scaled time
averaged over all collective U-turns as a function of scaled time 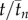 and
and 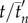 for all group sizes in (a) experiments and (b) model. tn and
for all group sizes in (a) experiments and (b) model. tn and 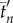 are obtained by data scaling (see the electronic supplementary material, Material and methods). The shadows stand for the standard error. In contrast with figure 1, t = 0 is set to the time (tE − tS)/2 (experiments) or (tE′ − tS′)/2 (model), where tS stands for the start of the collective U-turn (first frame where at least one direction −di × d0 is positive) and tE for the end of the collective U-turn (first frame where all directions −di × d0 are positive). In (a), time has also been shifted so that
are obtained by data scaling (see the electronic supplementary material, Material and methods). The shadows stand for the standard error. In contrast with figure 1, t = 0 is set to the time (tE − tS)/2 (experiments) or (tE′ − tS′)/2 (model), where tS stands for the start of the collective U-turn (first frame where at least one direction −di × d0 is positive) and tE for the end of the collective U-turn (first frame where all directions −di × d0 are positive). In (a), time has also been shifted so that 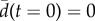 .
.
Despite its simplicity, our model reproduces qualitatively and most often quantitatively the experimental findings, both at the collective scale (the frequency of collective U-turns, average direction profile, duration of U-turns … ) and at the individual scale (the spatio-temporal features of the propagation of information). Note that a linear response of the agents to their neighbours cannot reproduce the order of magnitude of the U-turn durations measured in the experiments (electronic supplementary material, figure S11). Social conformity is thus a good candidate as an individual mechanism underlying the spatio-temporal structure and the decision processes in collective U-turns of fish groups.
5. Discussion
How information propagates among individuals and determines behavioural cascades is crucial to understanding the emergence of collective decisions and transitions between collective states in animal groups [23–26]. Here, we have addressed these questions by analysing the spontaneous collective U-turns in fish schools.
We find that collective U-turns are preceded by a slowing period. It has been shown in other fish species that speed controls alignment between individuals [8], leading slow groups to be less polarized than fast groups [5,9,27,28]. In general, at slower speed, there is less inertia to turn, resulting in weaker polarization [19,29] and thus an increase in the fluctuations in the swimming direction of the fish [30]. Moreover, as the fish speed decreases, the fish school is in a state closer to the transition between the schooling (strong alignment) and swarming (weak alignment) states, where [7] have shown that both fluctuations in fish orientation and the sensitivity of the school to a perturbation increase. It is therefore not surprising that U-turns occur after the group has slowed down.
U-turns are mostly initiated by the fish located at the front of the group. At the front, individuals experience a lesser influence from the other fish. This is owing to the perception anisotropy which results in individuals interacting more strongly with a neighbour ahead than behind. Therefore, frontal individuals are more subject to heading fluctuations and less inhibited to initiate U-turns. Similarly, in starling flocks, the birds that initiate changes in the collective travelling direction are found at the edges of the flock [31].
We found no evidence for dampening or amplification of information as fish adopt a new direction of motion. Moreover, on average, turning information propagates faster in larger groups: 0.19 s per individual in groups of 20 fish, and 0.28 s per individual in groups of five fish (electronic supplementary material, figure S9A). This appears to be the consequence of the increase of the swimming speed with group size, which requires that individuals react faster. Indeed, our results show that the interval between successive turns of individuals during a collective U-turn decreases with swimming speed, although distance between individuals may also play a role [16]. However, the mean time interval between successive individual U-turns is almost constant and independent of the group size, once time has been rescaled by the group velocity. This points to a domino-like propagation of the new direction of motion across the group. This sequential spatio-temporal propagation of information also suggests that each fish interacts with a small number of neighbours.
We found that the level of homogeneity in the direction of motion of the schools increases with group size, resulting in a lower number of collective U-turns. This phenomenon has been previously described in other fish species [10,32] as well as in locusts in a similar set-up [33].
We have developed an Ising-type spin model in which fish adopt probabilistically the direction of the majority of their neighbours, in a nonlinear way (social conformity) influenced by the anisotropic and asymmetrical interactions between fish. As the probability that a fish chooses a direction is a nonlinear function of the number of other fish having already chosen this direction, as previously shown [34,35], it is thus more difficult for a fish to initiate or propagate a U-turn the larger the number of fish swimming in the opposite direction [12]. The model also introduces quantitative indicators of the individual and collective discomfort (lack of alignment of heading among group members), the latter being represented by a measure of global pseudo-energy of the group. Larger groups have to overcome a larger pseudo-energy barrier to switch between the clockwise and anticlockwise fully polarized states. In physics and chemistry, the fast exponential increase of the switching time between two states as a function of this energy barrier is described by the Arrhenius Law, which can be proved using the tools of statistical physics. We find that direct numerical simulations of the model and an effective Arrhenius Law both quantitatively reproduce the sharp increase in the mean time between U-turns as the group size increases. The model also shows that asymmetric interactions and the anisotropic perception of fish are not essential to explain the decrease in collective fluctuations and hence the U-turn frequency as the group size increases. Social conformity [11,13] (controlled by the magnitude of our parameter J) suffices to cause fewer fluctuations with increasing group size, leading to an increased robustness of the polarized state (‘protected’ by increasing pseudo-energy barriers).
Moreover, our model reveals that the front to back propagation of information results from the perception anisotropy and asymmetry of the fish (the ε parameter). Finally, the duration of a U-turn as a function of group size is quantitatively reproduced by the model, while the simulated mean direction temporal profiles during U-turns are very similar to the experimental ones, and are independent of the group size, once time is properly rescaled by the mean U-turn duration for the corresponding group size.
In summary, our work supports that social conformity, asymmetric interactions and the anisotropic perception of fishes are key to the sequential propagation of information without dampening in fish schools, at least in the small group sizes considered. Future work will be needed to disentangle the respective roles of the network topology and the actual functional forms of social interactions between fish in the propagation of information.
Supplementary Material
Acknowledgments
We thank Daniel Calovi for his help in tracking experiments and his insightful comments.
Ethics
Experiments have been approved by the Ethics Committee for Animal Experimentation of the Toulouse Research Federation in Biology No. 1 and comply with the European legislation for animal welfare.
Data accessibility
Supporting data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.9m6d2 [36].
Author's contributions
C.K.H. and G.T. conceived and designed the study. V.L. and P.T. performed research; V.L. and C.S. developed the model. V.L., L.J., C.S. and P.T. analysed data. V.L., C.H.K., C.S. and G.T. wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by grants from the CNRS and Université Paul Sabatier (project Dynabanc). V.L. was supported by doctoral fellowships from the scientific council of the Université Paul Sabatier. L.J. was funded by a grant from the China Scholarship Council (CSC no. 201506040167) and by the National Natural Science Foundation (grant no. 61374165).
References
- 1.Radakov D. 1973. Schooling in the ecology of fish. A Halstead Press book New York, NY: John Wiley & Sons. [Google Scholar]
- 2.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Pitcher TJ, Parrish JK. 1993. Functions of shoaling behaviour in teleosts. In The behaviour of teleost Fishes (ed. Pitcher TJ.), 2nd edn Fish & Fisheries Series, no. 7, pp. 294–337. Dordrecht: The Netherlands: Springer. [Google Scholar]
- 4.Herbert-Read JE, Buhl J, Hu F, Ward AJW, Sumpter DJT. 2015. Initiation and spread of escape waves within animal groups. R. Soc. open. sci. 2, 140355 ( 10.1098/rsos.140355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tunstrøm K, Katz Y, Ioannou CC, Huepe C, Lutz MJ, Couzin ID. 2013. Collective states, multistability and transitional behavior in schooling fish. PLoS Comput. Biol. 9, e1002915 ( 10.1371/journal.pcbi.1002915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolpas A, Moehlis J, Kevrekidis IG. 2007. Coarse-grained analysis of stochasticity-induced switching between collective motion states. Proc. Natl Acad. Sci. USA 104, 5931–5935. ( 10.1073/pnas.0608270104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calovi DS, Lopez U, Schuhmacher P, Chaté H, Sire C, Theraulaz G. 2015. Collective response to perturbations in a data-driven fish school model. J. R. Soc. Interface 12, 20141362 ( 10.1098/rsif.2014.1362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautrais J, Ginelli F, Fournier R, Blanco S, Soria M, Chaté H, Theraulaz G. 2012. Deciphering interactions in moving animal groups. PLoS Comput. Biol. 8, e1002678 ( 10.1371/journal.pcbi.1002678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calovi DS, Lopez U, Ngo S, Sire C, Chaté H, Theraulaz G. 2014. Swarming, schooling, milling: phase diagram of a data-driven fish school model. New. J. Phys. 16, 015026 ( 10.1088/1367-2630/16/1/015026) [DOI] [Google Scholar]
- 10.Herbert-Read JE, Krause S, Morrell LJ, Schaerf TM, Krause J, Ward AJW. 2012. The role of individuality in collective group movement. Proc. R. Soc. B 280, 20122564 ( 10.1098/rspb.2012.2564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latane B. 1981. The psychology of social impact. Am. Psychol. 36, 343–356. ( 10.1037/0003-066X.36.4.343) [DOI] [Google Scholar]
- 12.Efferson C, Lalive R, Richerson PJ, McElreath R, Lubell M. 2008. Conformists and mavericks: the empirics of frequency-dependent cultural transmission. Evol. Hum. Behav. 29, 56–64. ( 10.1016/j.evolhumbehav.2007.08.003) [DOI] [Google Scholar]
- 13.Morgan T, Laland K. 2012. The biological bases of conformity. Front. Neurosci. 6, 87 ( 10.3389/fnins.2012.00087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérez-Escudero A, Vicente-Page J, Hinz RC, Arganda S, de Polavieja GG. 2014. idTracker: tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 11, 743–748. ( 10.1038/nmeth.2994) [DOI] [PubMed] [Google Scholar]
- 15.Calovi DS, Litchinko A, Lecheval V, Lopez U, Pérez Escudero A, Chaté H, Sire C, Theraulaz G. 2018. Disentangling and modeling interactions in fish with burst-and-coast swimming reveal distinct alignment and attraction behaviors. PLoS Comput. Biol. 14, 1–28. ( 10.1371/journal.pcbi.1005933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang L, Giuggioli L, Perna A, Escobedo R, Lecheval V, Sire C, Han Z, Theraulaz G. 2017. Identifying influential neighbors in animal flocking. PLoS Comput. Biol. 13, 1–32. ( 10.1371/journal.pcbi.1005822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castellano C, Fortunato S, Loreto V. 2009. Statistical physics of social dynamics. Rev. Mod. Phys. 81, 591–646. ( 10.1103/RevModPhys.81.591) [DOI] [Google Scholar]
- 18.Brendel K, Barkema GT, van Beijeren H. 2003. Magnetization reversal times in the two-dimensional Ising model. Phys. Rev. E 67, 026119 ( 10.1103/PhysRevE.67.026119) [DOI] [PubMed] [Google Scholar]
- 19.Hemelrijk CK, Hildenbrandt H, Reinders J, Stamhuis EJ. 2010. Emergence of oblong school shape: models and empirical data of fish. Ethology 116, 1099–1112. ( 10.1111/j.1439-0310.2010.01818.x) [DOI] [Google Scholar]
- 20.Glauber RJ. 1963. Time-dependent statistics of the Ising model. J. Math. Phys. 4, 294–307. ( 10.1063/1.1703954) [DOI] [Google Scholar]
- 21.Janke W. 2008. Monte Carlo methods in classical statistical physics. In Computational many-particle physics. Lecture Notes in Physics, vol. 739 (eds Fehske H, Schneider R, Weiße A), pp. 79–140. Berlin, Germany: Springer; ( 10.1007/978-3-540-74686-7). [DOI] [Google Scholar]
- 22.Romenskyy M, Herbert-Read JE, Ward AJW, Sumpter DJT. 2017. Body size affects the strength of social interactions and spatial organization of a schooling fish (Pseudomugil signifer). R. Soc. open. sci. 4, 161056 ( 10.1098/rsos.161056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giardina I. 2008. Collective behavior in animal groups: theoretical models and empirical studies. HFSP J. 2, 205–219. ( 10.2976/1.2961038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumpter D, Buhl J, Biro D, Couzin I. 2008. Information transfer in moving animal groups. Theory Biosci. 127, 177–186. ( 10.1007/s12064-008-0040-1) [DOI] [PubMed] [Google Scholar]
- 25.Wang XR, Miller JM, Lizier JT, Prokopenko M, Rossi LF. 2012. Quantifying and tracing information cascades in swarms. PLoS ONE 7, e40084 ( 10.1371/journal.pone.0040084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attanasi A. et al. 2014. Information transfer and behavioural inertia in starling flocks. Nat. Phys. 10, 691–696. ( 10.1038/nphys3035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viscido SV, Parrish JK, Grünbaum D. 2004. Individual behavior and emergent properties of fish schools: a comparison of observation and theory. Mar. Ecol. Prog. Ser. 273, 239–249. ( 10.3354/meps273239) [DOI] [Google Scholar]
- 28.Hemelrijk CK, Hildenbrandt H. 2008. Self-organized shape and frontal density of fish schools. Ethology 114, 245–254. ( 10.1111/j.1439-0310.2007.01459.x) [DOI] [Google Scholar]
- 29.Kunz H, Hemelrijk CK. 2003. Artificial fish schools: collective effects of school size, body size, and body form. Artif. Life 9, 237–253. ( 10.1162/106454603322392451) [DOI] [PubMed] [Google Scholar]
- 30.Marconi UMB, Puglisi A, Rondoni L, Vulpiani A. 2008. Fluctuation–dissipation: response theory in statistical physics. Phys. Rep. 461, 111–195. ( 10.1016/j.physrep.2008.02.002) [DOI] [Google Scholar]
- 31.Attanasi A. et al. 2015. Emergence of collective changes in travel direction of starling flocks from individual birds fluctuations. J. R. Soc. Interface 12, 20150319 ( 10.1098/rsif.2015.0319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day RL, MacDonald T, Brown C, Laland KN, Reader SM. 2001. Interactions between shoal size and conformity in guppy social foraging. Anim. Behav. 62, 917–925. ( 10.1006/anbe.2001.1820) [DOI] [Google Scholar]
- 33.Buhl J, Sumpter DJT, Couzin ID, Hale JJ, Despland E, Miller ER, Simpson SJ. 2006. From disorder to order in marching locusts. Science 312, 1402–1406. ( 10.1126/science.1125142) [DOI] [PubMed] [Google Scholar]
- 34.Sumpter DJ, Pratt SC. 2009. Quorum responses and consensus decision making. Phil. Trans. R. Soc. B 364, 743–753. ( 10.1098/rstb.2008.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward AJ, Sumpter DJ, Couzin ID, Hart PJ, Krause J. 2008. Quorum decision-making facilitates information transfer in fish shoals. Proc. Natl Acad. Sci. USA 105, 6948–6953. ( 10.1073/pnas.0710344105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lecheval V, Jiang L, Tichit P, Sire C, Hemelrijk CK, Theraulaz G. 2018. Data from: Social conformity and propagation of information in collective U-turns of fish schools Dryad Digital Repository. ( 10.5061/dryad.9m6d2) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lecheval V, Jiang L, Tichit P, Sire C, Hemelrijk CK, Theraulaz G. 2018. Data from: Social conformity and propagation of information in collective U-turns of fish schools Dryad Digital Repository. ( 10.5061/dryad.9m6d2) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Supporting data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.9m6d2 [36].