Abstract
Extant stick and leaf insects commonly imitate twigs or leaves, with lateral lamellae used to enhance crypsis or achieve mimicry for protection. However, the origin and early evolution of such lateral expansions among Phasmatodea are unknown, because all known Mesozoic phasmatodeans hitherto lack preserved evidence of such structures. We report here the first Mesozoic stick insect, Elasmophasma stictum gen. et sp. nov., with well-preserved, thin, lateral lamellae on the thoracic pleura, the terga of abdominal segments I–X and the ventrolateral margins of all femora. This new species, from the mid-Cretaceous amber of northern Myanmar, has a clear, stick-like body and is assigned to Euphasmatodea. The abdominal structures of E. stictum exhibit traces of multiple expansions of the terga, suggesting that such structure might have been an early development of body expansions used to improve crypsis for stick or leaf insects when they sprawled on twigs or leaves.
Keywords: Elasmophasma, Euphasmatodea, lamella, mimicry, crypsis, Myanmar
1. Introduction
Phasmatodea, stick and leaf insects, are a rather small insect order, comprising approximately 3000 described extant species [1,2]. Phasmatodeans typically live on trees or shrubs throughout the world, especially in the tropics and subtropics [3–5]. Most extant stick insects imitate the shape and colour of thin twigs, but some stick insects in the genera Cotylosoma and Extatosoma (Phasmatidae) (figure 1a,b) have abdominal lobes expanded from terga and/or legs as mimicry of leaves to improve crypsis. Living leaf insects (Phylliidae), comprising only 1% of modern phasmatodean diversity, imitate leaves by means of specialized expansive structures on the legs, thorax, wings and abdomen [6].
Figure 1.
(a,b) Photographs of Extatosoma tiaratum Macleay, 1826 by Runzhi Zhang at Durban, South Africa on 10 July 2008. (c,d) E. stictum gen. et sp. nov. Holotype CNU-PHA-MA2017004. (c) Photograph of lateral view as preserved. (d) Line drawing of lateral view. Scale bars: (a,b) 10 mm and (c,d) 2 mm.
Many fossil ‘stick insects' have been described from the Mesozoic, and most of these species were erected based on isolated wings or wing fragments. Owing to significant differences between Mesozoic and extant stick insects, the classification and relationships among these groups remain contentious [7,8]. Furthermore, none of the Mesozoic lineages show evidence for the lateral body expansions associated with adaptive crypsis, instead exhibiting elongate forms [8,9]. Two mid-Cretaceous species, Pseudoperla scapiforma and P. leptoclada from Myanmar, have elongate bodies and slightly curved profemora, but aside from their superficial form relative to sticks, nothing more could be concluded regarding potential mimicry [10].
Here we describe from the mid-Cretaceous amber of northern Myanmar a well-preserved, slender stick insect with thin, lateral lobe-like structures expanded from the body and femora.
2. Material and methods
The amber type specimen described in this paper is housed in the Key Lab of Insect Evolution and Environmental Changes, College of Life Sciences, Capital Normal University, Beijing, China (CNU; Dong Ren, Curator).
The specimen CNU-PHA-MA2017004 is preserved in a round triangular piece of yellow amber, about 40 mm in maximum length, 35 mm in maximum width and 10 mm in maximum thickness. The amber specimen was studied under a Leica M205C dissecting microscope. The habitus photographs were taken with a Nikon SMZ 25 microscope with a Nikon DS-Ri 2 digital camera system, while the magnified images of details of the specimen were taken using a Nikon ECLIPSE Ni microscope with a Nikon DS-Ri 2 digital camera system. Line drawing was prepared by using Adobe Illustrator CC and Adobe Photoshop CC graphics software.
3. Results
Systematic palaeontology
Order Phasmatodea Jacobson & Bianchi, 1902
Suborder Euphasmatodea Bradler, 1999
Family Incertae sedis
Elasmophasma gen. nov.
Type species. Elasmophasma stictum sp. nov.
Etymology. The new generic name is a combination of the Greek word of elasmos, meaning ‘plate', and phasma, meaning ‘ghost', stem of the ordinal name of Phasmatodea. The name refers to the lateral lamellae of the legs, thorax and abdomen. The gender of the name is neuter.
Diagnosis. Antenna much longer than body and foreleg. Cervix with a median plate. Prosternum with paired sensory areas. Mesonotum more than three times as long as pronotum. Metanotum and abdominal tergum I separated. Legs long, nearly equal to the length of body. Metafemora just similar to the other femora. Area apicalis present on all tibiae and without spines. Femora, thorax and abdomen with lateral expanded lamellae. Abdominal tergum X undivided. Cerci straight, undivided, circular in cross section, without prominence at base or thorns.
Remarks. The current classification of Phasmatodea is confusing [7,11–13]. Two broad groups, ‘Areolatae' and ‘Anareolatae', distinguished from each other based on the presence or absence of the area apicalis, were historically recognized as two main clades, which are now known to be polyphyletic [11,12,14–16]. Elasmophasma stictum gen. et sp. nov. shares apomorphies with Phasmatodea as labrum emarginate [11,17,18], prothorax with defensive glands [5,11,18,19], metasternum and abdominal sternum I fused [11,20], and cerci unsegmented [6,11,16,19,21]. Elasmophasma stictum sp. nov. can be further assigned within Euphasmatodea based on the following apomorphies: profurca largely reduced [5,11,17,18,21,22], pro- and mesospina absent [5,11,23], profemur distinctly curved basally [11,18] and tarsus with five articles [5,11,21,23], although five-segmented tarsi are generally considered to be plesiomorphic among Polyneoptera [1]. In addition, the antenna of E. stictum is much longer than body and foreleg, which is uncommon in stick insects. However, because the amber specimen is immature and lacks some diagnostic details, it cannot be placed further within that group and is deemed as family Incertae sedis. This systematic relationship is consistent with our phylogenetic analysis documented and presented in the electronic supplementary material.
The new species has seven annuli in the flagellum, and each flagellomere is greatly elongate. The eighth abdominal sternum lacks paired tubercles and a transverse fold medially, but the ninth abdominal sternum has a slight mounding, which suggest that the new specimen may be a male first-instar nymph [24]. The new genus is differentiated from other groups of Euphasmatodea mainly by the three characters femora, mesothorax and metathorax, and abdominal segments I–X with lateral expanded lamellae; prosternum with paired sensory areas and metanotum separated from the first abdominal tergum. The fusion of the metanotum with the first abdominal tergum is another apomorphy shared by Euphasmatodea [5,11,20,21,23,25]. However, the well-differentiated boundary between the metanotum and the first abdominal tergum in the new species indicates that they are separated, similar to some first-instar nymphs of living groups of Phasmatodea [24,25].
Elasmophasma stictum sp. nov.
Etymology. The epithet is from the Greek word ‘stictos', referring to the lateral lamellae of thorax and abdomen with a large number of spots.
Diagnosis. As for the genus.
Holotype. A male first-instar nymph, no. CNU-PHA-MA2017004, deposited in Capital Normal University, Beijing.
Type locality and horizon. The amber specimen was collected from Kachin (Hukawng Valley) of northern Myanmar, which was dated at 98.79 ± 0.62 Ma [26,27].
Description. Body elongate, slender (figures 1c,d and 2a), with a longitudinal median carina in ventral view; surface smooth. Head subglobose, longer than wide; ocelli absent; compound eyes ovoid, exophthalmic; antenna filiform, with nine antennomeres, almost twice as long as body and foreleg, bearing abundant setae; scape cylindrical, longer than wide (figure 2f); pedicel cylindrical, as long as scape; each flagellomere significantly elongate; first flagellomere more than twice as long as scape and pedicel combined; mandibles broad, massive (figure 2f); labrum emarginate; maxillary palps pentamerous, with numerous setae; paraglossa and glossa clearly visible; labial palps trimerous, bearing numerous setae; cervix with a median plate and lateral cervical sclerites bipartite.
Figure 2.
Elasmophasma stictum gen. et sp. nov. Photographs of Holotype CNU-PHA-MA2017004. (a) Lateral view as preserved. (b) Left mesotarsus in lateral view. (c) T8–10, epiproct and cerci in dorsal view. (d) Mesonotum, metanotum and the first abdominal tergum. (e) Thoracic sterna. (f) Head in lateral view. (g) The area apicalis (arrows) of right protibia in the ventral view. Scale bars: (a) 2 mm; (b–f) 0.2 mm and (g) 0.1 mm. Af, antennal field; Apg, aperture of the pronotal gland; Ar, arolium; Bs1, prothoracic basisternum; Ce, cercus; Cerv, cervix; Cx, coxa; Ep, epiproct; Fla I, flagellomere I; Gl, glossa; Lb, labrum; Md, mandible; Mst, mesothorax; Mtt, metathorax; Pd, pedicellus; Pgl, paraglossa; Plb, labial palpus; Pmx, maxillary palpus; Prt, prothorax; Sc, scape; T1, the first abdominal tergum (median segment); T8–T10, abdominal tergum 8–10; Ta1–Ta5, tarsomere I–V; Tr, trochanter; Un, claw.
Thorax smooth in dorsal view (figure 2d), but ventrally with a longitudinal median carina, lateral edges obvious (figure 2e); prothorax and anterior one-third of mesothorax apparently transparent (due to preservation); pronotum rectangular, lateral margin straight, shorter than head, apertures of prothoracic defensive glands present (figure 2f); prosternum shorter than pronotum, with paired sensory areas, profurca largely reduced; mesonotum strongly elongate; pleuron with lamellate expansions (figure 3a); mesosternum slightly longer than mesonotum, pro- and mesospina absent; metanotum rectangular, almost as long as mesonotum; pleuron bearing lamellate expansions (figure 3b); metasternum longer than metanotum; abdominal tergum I (median segment) rectangular, shorter than metanotum, separated from metanotum and with a well-differentiated boundary; abdominal sternum I distinctly fused with metasternum.
Figure 3.
The lateral lamellae of E. stictum gen. et sp. nov. Photographs of holotype CNU-PHA-MA2017004. (a) The posterior of mesothorax, (b) metathorax, (c) abdominal segments V and VI in dorsal view, (d) abdominal segments VI and VII in ventral view, and (e) abdominal segment VII in ventral view. Scale bars: (a–e) 0.2 mm.
Legs slender and elongate; procoxa large; profemur distinctly curved basally; trochanter small and fused with femur; all femora round in cross section, with lateral lamellae on ventral margins, and bearing distinct and regular bristles (figure 4d–f,i,j); all tibiae round in cross section, with V-shaped area apicalis and spines absent (figure 2g); basitarsus elongate, longer than remaining tarsomeres combined (figure 2b); arolia present and pretarsal claws without teeth; pro- and mesofemora longer than corresponding tibiae, but metafemur shorter than metatibia.
Figure 4.
Elasmophasma stictum gen. et sp. nov. Holotype CNU-PHA-MA2017004. Photographs (a–f). (a) Abdominal segment VIII in dorsal view, (b) abdominal segment VIII in ventral view, (c) the lateral view of abdominal segment III, (d) the dorsal view of right mesofemur, (e) the ventral view of right mesofemur, (f) the lateral view of left mesofemur and (g–j) diagrammatic representation of the lateral lamellae. Scale bars: (a–f) 0.2 mm.
Abdomen smooth in dorsal view, but ventrally with a longitudinal median carina, lateral edges obvious (figure 3c,d); 11 complete abdominal segments preserved; segments III to VII apically with slight lateral expansions; both sides of abdominal segments I–X with lamellate expansions from terga (figures 3c–e, 4a–c,g,h), apparently transparent, with some setae and spots (figure 3c–e); segments II–VII of similar length and width; segment VIII and IX shorter; the sterna of segment VIII and IX slightly projecting; segment X undivided, much longer than segment IX and apically concave; epiproct apically curving and with numerous short setae (figure 2c); cerci unsegmented, straight, cylindrical and long, without prominence at the base or thorns, bearing abundant elongate setae.
Measurements (in millimetres). Body 11.76 (excluding antennae); head 1.13; antenna 21.06, scape 0.24, pedicel 0.24, flagellomeres I–VII 2.69, 2.51, 2.85, 2.74, 3.05, 3.20, 3.54; pronotum 0.74, prosternum 0.65; mesonotum 2.33, mesosternum 2.41; metanotum 0.66, metasternum 1.10; abdominal tergum I 0.51, abdomen 6.90; profemur 4.55, protibia 4.09, protarsus 2.97; mesofemur 4.03, mesotibia 3.88, mesotarsus 2.46; metafemur 4.51, metatibia 4.97, metatarsus 3.46.
4. Discussion and conclusion
(a). The earliest twig mimesis by stick insects
Stick mimesis has already been demonstrated for archipseudophasmatids based on an unnamed phasmid nymph from Eocene Baltic amber [9,28]. In addition, an undescribed male of Euphasmatodea was figured in 1990 from the Eocene Messel pit in Germany [9]. Both of these fossils have elongate bodies and similarly curved profemora, which form a notch for the head when the stick insect is at rest and pressed against vegetation, and such features are considered adaptive for such mimicry [9]. Elasmophasma stictum gen. et sp. nov. shares these traits but is even more similar to modern stick insects owing to the more elongate and slender body. The morphological characters of E. stictum are quite different from those of other known Mesozoic, fully winged stick insects [4,29–31], as well as the more squat, robust and compressed Echinosomiscus primoticus, also known from Burmese amber [7]. Elasmophasma stictum extends the origin of stick mimesis by Phasmatodea at least to the earliest epoch of the mid-Cretaceous and broadens the diversity and known disparity of Mesozoic stick insects.
(b). The structure of lateral lamellae
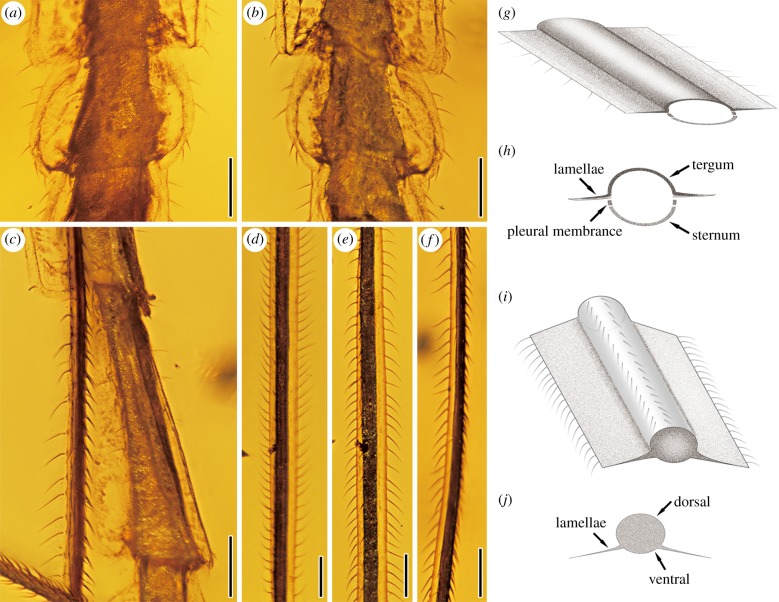
Lamellae, albeit not as greatly expanded, are common in many extant Phasmatodea, usually where carinae are particularly developed and enlarged on the legs or abdomen. The more complex forms are related to mimicry and defence; for example, leaf insects, Extatosoma Gray, 1833 of Phasmatidae (figure 1a,b) and Trychopeplus Shelford, 1909 of Diapheromerinae, mimicking leaves, moss or lichen [7,32]. The fossil E. stictum gen. et sp. nov. has lateral lamellate structures expanded from both sides of all femora and the thoracic and abdominal segments, which look like a thin film consisting of chitin (figures 3 and 4a–f). On the femora, the lamellae are ventrolaterally positioned (figure 4d–f,i,j), while on the thorax these extend from the pleura (figure 3a,b).
Furthermore, on the abdomen, such lamellate lobes independently stretch out from the terga of each segment (very clear on the 8th segment; figure 4a,b) and exhibit a synchronous constriction on every abdominal segment (figures 3c–e, 4a,b). Two longitudinal, dark, irregular lines originate from one point at the base, running through each lamella and ending at the distal edge (very clear on the 6th and 7th segments, figure 3c–e). On the 7th and 8th abdominal segments, it is clear that the upper layer curves and folds at the anterior and posterior of the abdominal segments, and the edges of the expansion form two clear black lines at both sides (figure 4a,b). The bottom layer expansion overlaps with each other, which are not completely synchronous with each abdominal segment. We conclude that the lateral lamellae of the abdomen are composed of at least two layers and that the bottom layer, with a row of setae at both sides, is larger than the upper layer, and that the longitudinal dark line is the boundary of the upper layer. We hypothesize that the inner longitudinal dark line might also be a boundary from the expansion of the tergum (on the 6th and 7th abdominal segments). The first-instar nymph of Extatosoma tiaratum (figure 1a,b) also has the lateral expansions which grow larger and wider as the insect matures [32]. Therefore, we believe that the lateral lamellae of E. stictum gen. et sp. nov. would be much larger and wider for the adults.
(c). Multiple expansions of the terga might have been an early development used by phasmatodeans in their cryptic strategies
The expansions of the abdomen lateral lamellae of living stick insects (e.g. Extatosoma Gray 1833) are normally produced by the terga [33,34], but in extant leaf insects, both the abdominal terga and sterna are extended simultaneously, converging and forming the lamellate structures involved in their mimicry, a condition unique among Phasmatodea [33,34]. Wedmann et al. [6] reported the oldest fossil record of leaf insects, Eophyllium messelensis, from the Eocene of Messel and the foliaceous appearance suggested its lateral abdominal expansions originated from both the terga and sterna. It seems that the mechanism and structure of the lamellate lobes of E. stictum gen. et sp. nov. are similar to those of stick insects rather than leaf insects.
In summary, it is suggested that the lateral lamellae of the stick-like E. stictum probably provided the insect with improved crypsis to elude predators, and/or allowed for a flattening of the body sprawled against the substrate to protect the insect. As E. stictum had a different mechanism and structure in the composition of the lateral lamellae from those of extant stick or leaf insects, it is likely that such lateral protuberances in living stick or leaf insects might have occurred and experienced multiple evolutionary and developmental changes. The stick-like body shape clearly demonstrates that E. stictum was already imitating twigs of trees and bushes during the mid-Cretaceous, around 99 Ma. In addition, the lateral lamellae on the thorax, abdomen and femora further enhanced the crypsis of E. stictum. This study highlights the presence of early plant-mimicking and adaptive crypsis in the complex forest ecosystems of the mid-Cretaceous, and the various contrivances by which the Phasmatodea achieved their successful elusion amid plants during the Mesozoic and Cenozoic.
Supplementary Material
Acknowledgements
We are grateful to Dr Michael S. Engel (University of Kansas) for providing guidance and advices. We thank the editorial board of Proceedings B, and in particular Dr Sasha Dall and Dr Jakob Vinther for their dedication. We express our gratitude to Dr Marco Gottardo (University of Siena) and two anonymous reviewers for their critical but valuable reviews of the manuscript. We appreciate M-Y Ren and Dr Y-J. Wang for their helpful advice and discussions regarding this study. We thank Dr Thies Büscher (Kiel University), Dr Paul Brock (Natural History Museum) and Dr Sven Bradler (Georg-August-University Göttingen) for their helpful comments with the morphological analysis of this specimen.
Data accessibility
The data supporting the conclusions of this article are included within the article and the result of phylogenetic analyses are available in the electronic supplementary material.
Authors' contributions
D.R. and T.G. conceived and designed the experiments; S.C., X.Y., X.L., C.S., R.Z., T.G. and D.R. performed the analyses and experiments; S.C. prepared photographs and line drawings; S.C., X.L., C.S., T.G. and D.R. wrote the manuscript. All authors read and approve the final manuscript.
Competing interests
We declare that we have no competing interests.
Funding
D.R. was supported by grants from the National Natural Science Foundation of China (grant nos. 31730087, 41688103 and 31672323), the Program for Changjiang Scholars and Innovative Research Team in University (IRT-17R75) and Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five-year Plan (grant no. IDHT20180518); T.G. was supported by the Young Elite Scientist Sponsorship Program by CAST (YESS), Beijing Natural Science Foundation (grant no. 5182004) and Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five-year Plan (grant no. CIT&TCD201704090).
References
- 1.Bragg PE. 2001. Phasmids of Borneo. Kota Kinabalu, Malaysia: Natural History Publications. [Google Scholar]
- 2.Brock PD, Hasenpusch JW.. 2009. Complete field guide to stick and leaf insects of Australia. Melbourne, Australia: CSIRO Publishing. [Google Scholar]
- 3.Bragg PE. 1995. The phasmid database version 1.5. Phasmid Stud. 3, 41–42. [Google Scholar]
- 4.Ren D. 1997. First record of fossil stick-insects from China with analyses of some palaeobiological features (Phasmatodea: Hagiphasmatidae fam. nov.). Acta Zootaxon. Sin. 22, 268–282 (in Chinese). [Google Scholar]
- 5.Tilgner EH. 2002. Systematics of Phasmida. PhD dissertation, University of Georgia, Athens, Georgia. [Google Scholar]
- 6.Wedmann S, Bradler S, Rust J. 2007. The first fossil leaf insect: 47 million years of specialized cryptic morphology and behavior. Proc. Natl Acad. Sci. USA 104, 565–569. ( 10.1073/pnas.0606937104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engel MS, Wang B, Alqarni AS. 2016. A thorny, ‘anareolate’ stick-insect (Phasmatidae s.l.) in upper cretaceous amber from Myanmar, with remarks on diversification times among Phasmatodea. Cretac. Res. 63, 45–53. ( 10.1016/j.cretres.2016.02.015) [DOI] [Google Scholar]
- 8.Tilgner EH. 2000. The fossil record of Phasmida (Insecta: Neoptera). Insect Syst. Evol. 31, 473–480. ( 10.1163/187631200X00507) [DOI] [Google Scholar]
- 9.Wedmann S. 2010. A brief review of the fossil history of plant masquerade by insects. Palaeontogr. Abt. B 283, 175–182. ( 10.1127/palb/283/2010/175) [DOI] [Google Scholar]
- 10.Chen S, Zhang WW, Shih CK, Ren D. 2017. Two new species of Archipseudophasmatidae (Insecta: Phasmatodea) from upper cretaceous Myanmar amber. Cretac. Res. 73, 65–70. ( 10.1016/j.cretres.2017.01.007) [DOI] [Google Scholar]
- 11.Bradler S. 2009. Phylogenie der Stab- und Gespenstschrecken (Phasmatodea) [Phylogeny of the stick and leaf insects (Phasmatodea)]. Species Phyl. Evol. 2, 3–139. [Google Scholar]
- 12.Bradler S, Robertson JA, Whiting MF. 2014. A molecular phylogeny of Phasmatodea with emphasis on Necrosciinae, the most species-rich subfamily of stick insects. Syst. Entomol. 39, 205–222. ( 10.1111/syen.12055) [DOI] [Google Scholar]
- 13.Buckley TR, Attanayake D, Bradler S. 2009. Extreme convergence in stick insect evolution: phylogenetic placement of the Lord Howe Island tree lobster. Proc. R. Soc. B 276, 1055–1062. ( 10.1098/rspb.2008.1552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kômoto N, Yukuhiro K, Ueda K, Tomita S. 2011. Exploring the molecular phylogeny of phasmids with whole mitochondrial genome sequences. Mol. Phylogenet. Evol. 58, 43–52. ( 10.1016/j.ympev.2010.10.013) [DOI] [PubMed] [Google Scholar]
- 15.Kômoto N, Yukuhiro K, Tomita S. 2012. Novel gene rearrangements in the mitochondrial genome of a webspinner, Aposthonia japonica (Insecta: Embioptera). Genome 55, 222–233. ( 10.1139/g2012-007) [DOI] [PubMed] [Google Scholar]
- 16.Whiting MF, Bradler S, Maxwell T. 2003. Loss and recovery of wings in stick insects. Nature 421, 264–267. ( 10.1038/nature01313) [DOI] [PubMed] [Google Scholar]
- 17.Gottardo M, Vallotto D. 2014. External macro-and micromorphology of the male of the stick insect Hermarchus leytensis (Insecta: Phasmatodea) with phylogenetic considerations. C. R. Biol. 337, 258–268. ( 10.1016/j.crvi.2014.02.005) [DOI] [PubMed] [Google Scholar]
- 18.Friedemann K, Wipfler B, Bradler S, Beutel RG. 2012. On the head morphology of Phyllium and the phylogenetic relationships of Phasmatodea (Insecta). Acta Zool. 93, 184–199. ( 10.1111/j.1463-6395.2010.00497.x) [DOI] [Google Scholar]
- 19.Grimaldi D, Engel MS. 2005. Evolution of the insects. New York, NY: Cambridge University Press. [Google Scholar]
- 20.Bradler S, Buckley TR. 2011. Stick insect on unsafe ground: does a fossil from the early Eocene of France really link Mesozoic taxa with the extant crown group of Phasmatodea? Syst. Entomol. 36, 218–222. ( 10.1111/j.1365-3113.2010.00564.x) [DOI] [Google Scholar]
- 21.Tilgner EH, Kiselyova TG, Mchugh JV. 1999. A morphological study of Timema cristinae Vickery with implications for the phylogenetics of Phasmida. Dtsch. Entomol. Z 46, 149–162. ( 10.1002/mmnd.19990460203) [DOI] [Google Scholar]
- 22.Bradler S, Whiting MF, Klug R. 2003. Basal diversification and the evolution of wings within stick insects (Phasmatodea). Entomol. Abh. 61, 132–133. [Google Scholar]
- 23.Kristensen NP. 1975. The phylogeny of hexapod ‘orders’: a critical review of recent accounts. Z. Zool. Syst. Evol. Forsch. 13, 1–44. [Google Scholar]
- 24.Stringer IAN. 1970. The nymphal and imaginal stages of the bisexual stick insect Clitarchus hookeri (Phasmidae: Phasminae). N. Z. Entomol. 4, 85–95. ( 10.1080/00779962.1970.9722927) [DOI] [Google Scholar]
- 25.Gottardo M. 2011. A new genus and new species of Philippine stick insects (Insecta: Phasmatodea) and phylogenetic considerations. C. R. Biol. 334, 555–563. ( 10.1016/j.crvi.2011.04.003) [DOI] [PubMed] [Google Scholar]
- 26.Cruickshank RD, Ko K. 2003. Geology of an amber locality in the Hukawng Valley, northern Myanmar. J. Asian Earth Sci. 21, 441–455. ( 10.1016/S1367-9120(02)00044-5) [DOI] [Google Scholar]
- 27.Shi GH, Grimaldi DA, Harlow GE, Wang J, Wang J, Yang MC, Lei WY, Li QL, Li XH. 2012. Age constraint on Burmese amber based on U-Pb dating of zircons. Cretac. Res. 37, 155–163. ( 10.1016/j.cretres.2012.03.014) [DOI] [Google Scholar]
- 28.Zompro O. 2001. The Phasmatodea and Raptophasma n. gen., Orthoptera incertae sedis, in Baltic amber (Insecta: Orthoptera). Mitt. Geol-Pal. Inst. Hamburg 85, 229–261. [Google Scholar]
- 29.Nel A, Delfosse E. 2011. A new Chinese Mesozoic stick insect. Acta Palaeontol. Pol. 56, 429–432. ( 10.4202/app.2009.1108) [DOI] [Google Scholar]
- 30.Shang LJ, Béthoux O, Ren D. 2011. New stem-Phasmatodea from the Middle Jurassic of China. Eur. J. Entomol. 108, 677–685. ( 10.14411/eje.2011.086) [DOI] [Google Scholar]
- 31.Wang MM, Béthoux O, Bradler S, Jacques FMB, Cui YY, Ren D. 2014. Under cover at pre-angiosperm times: a cloaked phasmatodean insect from the Early Cretaceous Jehol Biota. PLoS ONE 9, e91290 ( 10.1371/journal.pone.0091290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brock PD. 2001. Studies on the Australasian stick-insect genus Extatosoma Gray (Phasmida: Phasmatidae: Tropoderinae: Extatosomatini). J. Orthopt Res. 10, 303–313. ( 10.1665/1082-6467(2001)010%5B0303:SOTASI%5D2.0.CO;2) [DOI] [Google Scholar]
- 33.Zompro O. 2004. Revision of the genera of the Areolatae, including the status of Timema and Agathemera (Insecta: Phasmatodea). Abh. Natwiss. Ver. Hamburg 37, 1–327. [Google Scholar]
- 34.Zompro O, Größer D. 2003. A generic revision of the insect order Phasmatodea: the genera of the areolate stick insect family Phylliidae (walking leaves) (Insecta, Orthoptera). Spixiana 26, 129–141. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the conclusions of this article are included within the article and the result of phylogenetic analyses are available in the electronic supplementary material.