Abstract
Atherosclerosis is a chronic inflammatory disease characterized by the entrapment of apolipoprotein B–containing lipoproteins in the arterial intima, leading to local inflammation. T helper (Th) cell 1–mediated immune responses have been associated with atherosclerosis, and the cytokine interleukin-25 (IL-25 or IL-17E) has been reported to potentially regulate Th1 cell– and Th17 cell–related immune responses. In this study, we evaluated the effects of complete IL-25 deficiency or of a temporal IL-25 blockade on atherosclerosis development in apolipoprotein E–deficient (Apoe−/−) mice. Mice deficient in both apolipoprotein E and IL-25 (Apoe−/−/IL-25−/−) had more Th1 cells in the spleen, along with elevated plasma levels of IL-17 and an increased release of splenic interferon-γ (INF-γ). In support of this observation, a 4-week-long treatment of young Apoe−/− mice (at 10–14 weeks of age) with an IL-25–blocking antibody increased the release of Th1/Th17-associated cytokines in the spleen. In both mouse models, these findings were associated with increased atherosclerotic plaque formation in the aortic arch. We conclude that complete IL-25 deficiency and a temporal IL-25 blockade during early plaque development aggravate atherosclerosis development in the aortic arch of Apoe−/− mice, accompanied by an increase in Th1/Th17-mediated immune responses. Our finding that endogenous IL-25 has an atheroprotective role in the murine aortic arch has potential implications for atherosclerosis development and management in humans.
Keywords: apolipoprotein E (ApoE), atherosclerosis, antibody, animal model, cellular immune response, cytokine, immunodeficiency, inflammation, T cell, innate lymphoid cells type 2, interleukin-25
Introduction
Atherosclerosis is a chronic inflammatory disease characterized by the entrapment of apolipoprotein B (apoB)4-containing lipoproteins in the arterial intima, leading to induction of local inflammation and to modification of self-antigens, like the oxidative modification of low-density lipoprotein (LDL), targeted by both innate and adaptive immunity (1). To date, Th1 immune responses have been associated with the promotion of atherosclerosis. In particular, a pro-inflammatory Th1-like cytokine profile has been detected in human atherosclerotic plaques (2, 3), and IFN-γ has been found to be expressed from T-cell clones that have been extracted from such plaques (3). The outcomes of murine studies that include genetic deletion of the cytokine, its receptor, and the Th1-related transcription factor T-bet further support this notion (4–7). The role of Th2 responses, on the other hand, is less clear. They have traditionally been associated with B-cell activation and production of antigen-specific antibodies (IgM, IgG) which are oxidized LDL–specific. Anti-oxidized LDL IgM antibodies were reported to be atheroprotective (8, 9); they bind to oxidized LDL particles, leading to inhibition of oxidized LDL uptake by macrophages and prevention of foam cell formation. Additionally, in clinical studies, high levels of IgG autoantibodies to apoB-100 (the major protein in LDL) peptides have been associated with less carotid stenosis as well as lower risk for development of acute myocardial infarction (10, 11). In regard to the Th2-related cytokine IL-4, contradictory reports have been published pointing to either a proatherogenic (12, 13) or anti-atherogenic role (14) or no effect (15) of the cytokine. However, increased atherosclerosis has been observed in LDL receptor–deficient mice, which were also deficient in IL-5 (8). The atheroprotective role of IL-5 is further supported by another study indicating IL-33 to have a protective role in the development of atherosclerosis via the induction of IL-5 and oxLDL antibodies (16). Furthermore, IL-13 deficiency in LDL receptor–deficient mice accelerated atherosclerosis development, whereas exogenous IL-13 administration modulated the morphology of atherosclerotic lesions by increasing the collagen and decreasing the macrophage content of plaques as well as by increasing the balance of M2/M1 cells in that location (17). Last, the role of Th17 cells in atherosclerosis has been contradictory. In murine studies, Th17 cells were reported to be both pro-atherogenic (18–20) and anti-atherogenic (21, 22).
IL-25, or IL-17E, a member of the IL-17 cytokine family, has been implicated in the initiation of Th2-related immunity by driving the expression of IL-4, IL-5, and IL-13 (23). The innate lymphoid type 2 cell population (ILC2s) has previously been found to be targeted by IL-25, resulting in release of IL-5 and IL-13 (24–27). Thus, ILC2s represent an early source of these cytokines in type 2 immune responses. In line with the above, we have previously shown that IL-25 treatment of apoE-deficient mice (Apoe−/−) reduces atherosclerosis through expansion of IL-5–releasing ILC2s, leading to increased levels of atheroprotective B1a-derived IgM antibodies recognizing the oxidized LDL epitope phosphorylcholine (PC) (28). Nevertheless, the biological significance of endogenous IL-25 in atherosclerosis remains an unanswered question. Studies using IL-25–deficient mice have shown that IL-25 can also control the outcome of Th1/Th17 immune responses. IL-25–deficient mice, when infected with Trichuris muris, develop severe intestinal inflammation and increased levels of the pro-inflammatory cytokines IL-17A and IFN-γ (29). In addition, IL-25–deficient mice have been shown to display severe experimental autoimmune encephalomyelitis, which was associated with increased numbers of inflammatory IL-17– and IFN-γ–producing T cells (30). Taken together, it appears that the absence of IL-25 creates an environment where Th1/Th17 immune responses dominate. The aim of this study was to evaluate whether endogenous IL-25 has a supporting influence on atherosclerosis development as exogenous IL-25 administration by using atherosclerosis-prone mice deficient in IL-25 or by blocking IL-25 in Apoe−/− mice using an anti-IL-25 antibody (Ab). We found that double-knockout mice deficient in apoE and IL-25 (Apoe−/−IL-25−/−) shifted the immune response toward Th1/Th17 and aggravated disease progression. In support, treatment of Apoe−/− mice with an IL-25–blocking Ab in an early stage of plaque development showed similar findings.
Results
Experimental outline of the study
The role of endogenous IL-25 in atherosclerosis development was analyzed by using mice deficient in both apoE and IL-25 (Apoe−/−/IL-25−/−). These double-knockout mice were fed a high-fat diet (HFD) from 10 weeks of age and killed at 25 weeks of age (Fig. S1a). In addition, to evaluate the influence of a temporal IL-25 blockade on atherosclerosis progression, atherosclerosis-prone apoE-deficient (Apoe−/−) mice were treated with an anti-IL-25 Ab or an isotype control Ab for 4 weeks during early atherosclerosis development (10–14 weeks of age) and fed an HFD from 10 weeks of age until they were killed at 25 weeks old (Fig. S1b).
IL-25 deficiency or IL-25 blockade increases plaque formation in Apoe−/− mice
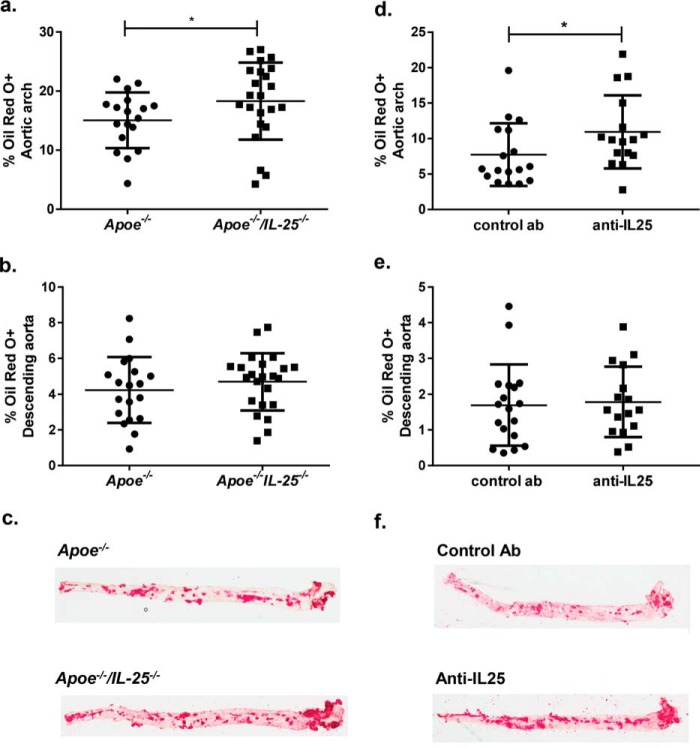
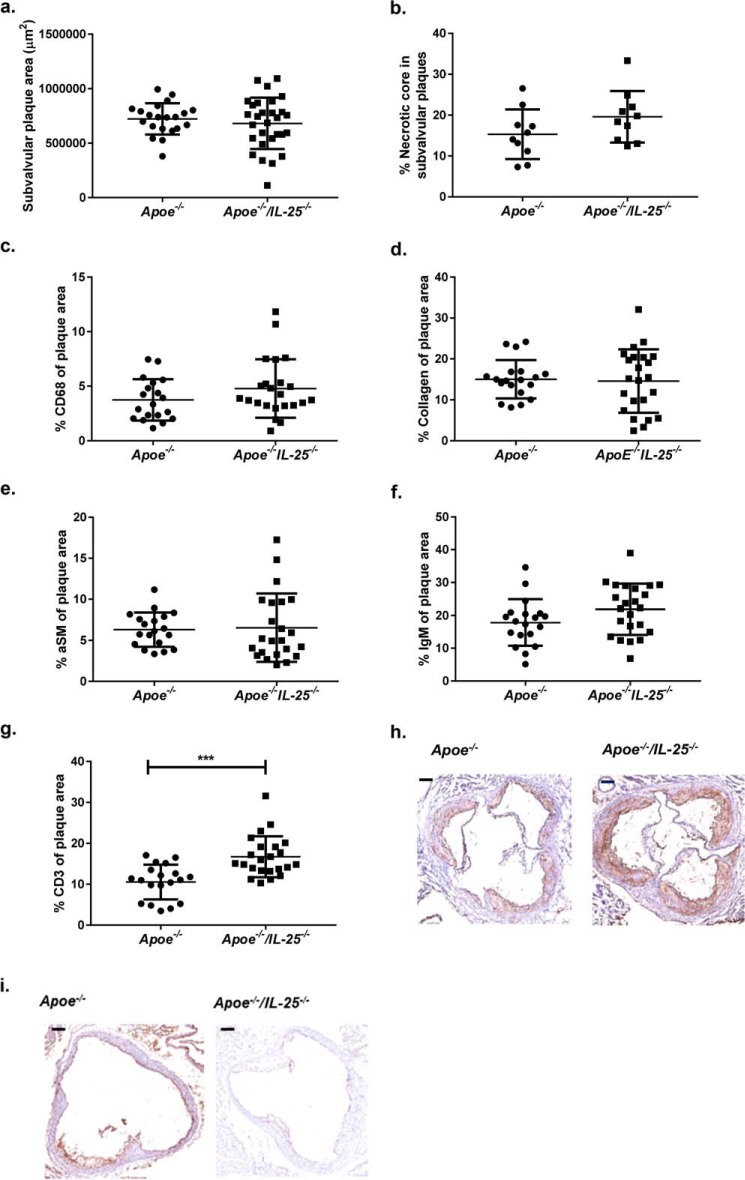
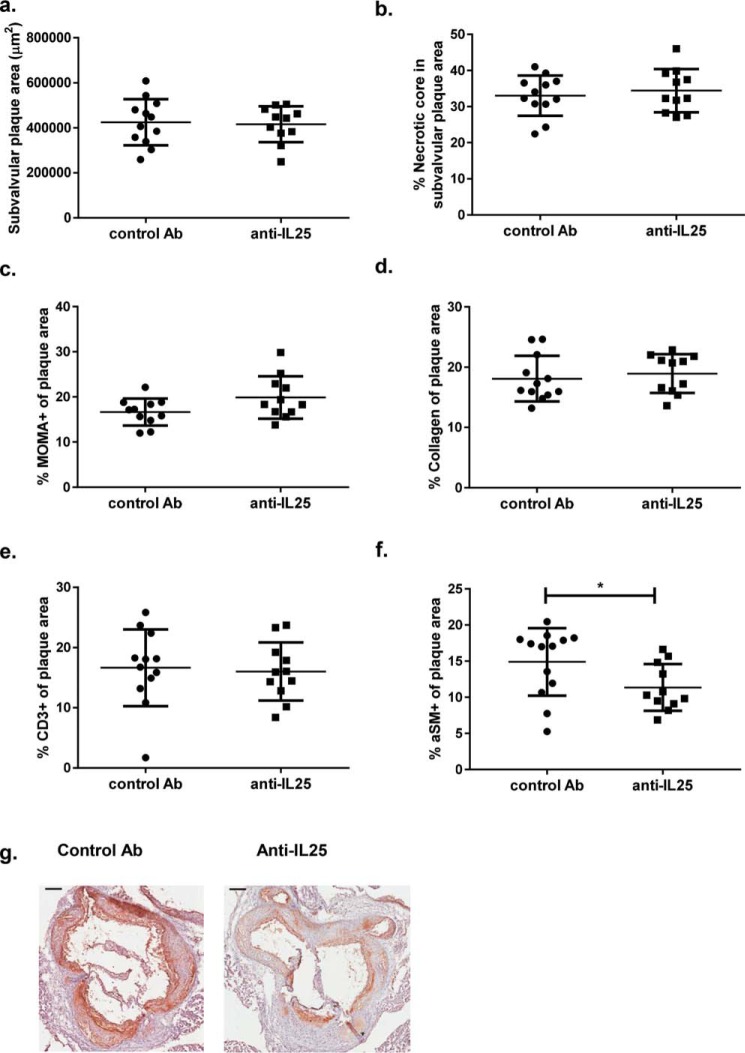
ApoE and IL-25–deficient mice fed HFD for 15 weeks and killed at the age of 25 weeks showed increased plaque areas in the aortic arch (Fig. 1, a and c), a location of enhanced atherosclerotic plaque formation in Apoe−/− mice (31). Additionally, to study the effect of IL-25 blockade during early stages of atherosclerosis, Apoe−/− mice were fed HFD from 10 weeks of age, injected weekly with IL-25–blocking Ab or isotype control Ab the first 4 weeks of HFD (10–14 weeks of age), and killed 11 weeks later (Fig. S1b). Oil Red O staining of the aortas of the mice showed that the anti-IL-25 treatment influenced the disease progression, resulting in increased plaque areas in the aortic arch (Fig. 1, d and f). Treatment of Apoe−/− mice with the IL-25–blocking Ab or complete IL-25 deficiency did not affect plasma lipid levels, indicating that the observed increase in atherosclerotic lesions was not due to an effect on lipid metabolism (Table S1). However, no differences were detected in the plaque area in the descending aorta (Fig. 1, b, c, e, and f) or in subvalvular plaques in these two mouse models (Figs. 2a and 3a). Moreover, there was no difference in necrotic core area (Figs. 2b and 3b). Immunohistochemical staining of subvalvular sections of IL-25–deficient mice showed no differences in macrophage (Fig. 2c), collagen (Fig. 2d), α-smooth muscle actin (Fig. 2e), or IgM (Fig. 2f) content. However, the CD3+ T-cell staining showed increased presence of these cells, indicating formation of more pro-inflammatory plaques (Fig. 2, g and h). IL-25 staining of subvalvular plaques in Apoe−/− mice suggested that IL-25 is expressed by endothelial cells, macrophages, and T cells in the intima, whereas medial smooth muscle cells seem not to be a source of IL-25 (Fig. 2i). In line with the findings in Apoe−/−/IL-25−/− mice, treatment with the IL-25–blocking Ab showed no differences in macrophage (Fig. 3c), collagen (Fig. 3d), and CD3+ T cell (Fig. 3e) content of the subvalvular plaques. Instead, anti-IL-25–treated mice displayed a slightly decreased α-smooth muscle actin staining (Fig. 3, f and g), which may indicate reduced plaque stability.
Figure 1.
IL-25 deficiency and IL-25 blockade aggravate atherosclerosis development in Apoe−/− mice. Atherosclerotic plaque formation in Apoe−/− and Apoe−/−/IL-25−/− mice was assessed with Oil Red O staining in en face preparations of the aortic arch (a) and the descending aorta (b). c, representative Oil Red O stainings in en face preparations of aortas in Apoe−/− and Apoe−/−/IL-25−/− mice. Mann–Whitney test was used; n = 18 for Apoe−/− and n = 23 for Apoe−/−/IL-25−/− mice; *, p = 0.049. Each dot represents one mouse, and the bars represent the mean value ± S.D. Shown is anti-IL-25 treatment of Apoe−/− mice (once a week at 10–14 weeks of age and euthanized at 25 weeks of age). Atherosclerotic plaque formation was assessed with Oil Red O staining in en face preparations of the aortic arch (d), and the descending aorta (e). f, representative Oil Red O stainings in en face preparations of aortas in control Ab and in anti-IL-25–treated mice. Mann–Whitney test was used; n = 18 for control Ab and n = 16 for anti-IL-25–treated mice; *, p = 0.029. Each dot represents one mouse, and the bars show the mean value ± S.D.
Figure 2.
IL-25 deficiency increases the CD3+ T-cell content in subvalvular plaques of Apoe−/− mice. Shown is histochemical and immune-histochemical analysis of subvalvular plaque sections of Apoe−/− and Apoe−/−/IL-25−/− mice fed a high-fat diet for 15 weeks and euthanized at 25 weeks of age. Shown are plaque area (a), necrotic core area (b), macrophage (CD68+) (c), collagen (d), α-smooth muscle cell actin (aSM) (e), IgM (f), and CD3+ T-cell content (g) in Apoe−/−/IL-25−/− and control mice. h, representative CD3+ T-cell stainings of subvalvular lesions; scale bar, 200 μm. i, representative IL-25 stainings of subvalvular lesions; scale bar, 200 μm. Mann–Whitney test was used; n = 19 for Apoe−/− and n = 23 for Apoe−/−/IL-25−/− mice; ***, p = 0.0001. Each dot represents one mouse, and the bars show the mean value ± S.D.
Figure 3.
IL-25 blockade reduces the smooth muscle cell content in subvalvular plaques of Apoe−/− mice. Shown is histochemical and immune-histochemical analysis of subvalvular plaque sections of Apoe−/− mice treated with control Ab or anti-IL-25 once a week at 10–14 weeks of age and euthanized at 25 weeks of age. Shown are plaque area (a), necrotic core area (b), macrophage (MOMA+) (c), collagen (d), CD3+ T cell (e), and α-smooth muscle cell actin (aSM) content (f) in mice treated with the IL-25–blocking or control antibody. g, representative α-smooth muscle cell actin stainings of subvalvular lesions; scale bar, 200 μm. Mann–Whitney test was used; n = 13 for control Ab and n = 11 for anti-IL-25–treated mice; *, p = 0.026. Each dot represents one mouse, and the bars show the mean value ± S.D.
IL-25 deficiency or IL-25 blockade shifts the cytokine balance into a Th1/Th17-related profile in the periphery of Apoe−/− mice
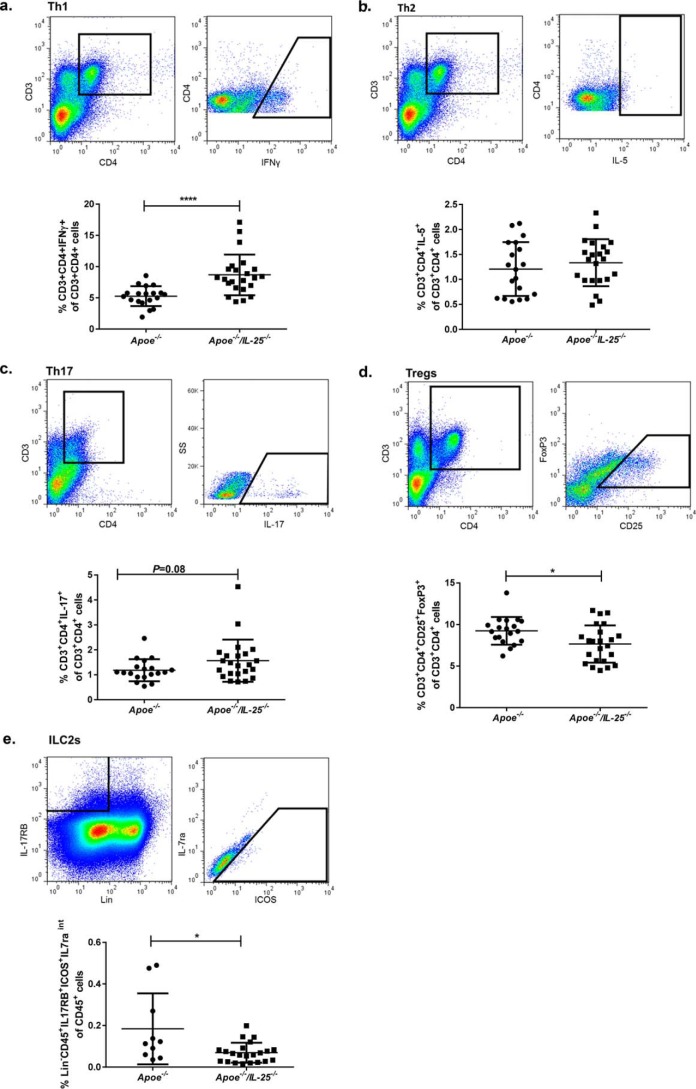
In the assessment of the influence of IL-25 on splenic cell populations, we found that the IL-25–deficient mice have more Th1 cells and a tendency to more Th17 cells and fewer Tregs (Fig. 4, a, c, and d). Because IL-25 is a potent inducer of ILC2s, we also questioned whether IL-25 deficiency would affect this cell subset. Interestingly, IL-25 deficiency reduced splenic ILC2s, as assessed with the use of a flow cytometry (Lin−CD45+IL-17RB+ICOS+IL-7raintermediate), a cell type that previously has been shown to have atheroprotective properties (Fig. 4e) (28). No differences in Th2 cells were detected (Fig. 4b). We also assessed whether the IL-25 blockade at weeks 10–14 of age had resulted in sustained immunological alterations. No differences of the T-cell populations Th1, Th2, Th17, and Tregs were detected between the groups in blood or in spleen at 25 weeks of age (Table S2). Analysis of cytokine levels in the plasma of IL-25–deficient Apoe−/− mice revealed a pro-inflammatory cytokine profile with increased plasma IL-17 levels and tendencies of higher levels of IL-12(p40) as well as TNFα (Table 1). In addition, analysis of supernatants of PMA/ionomycin-stimulated splenocytes of female Apoe−/−/IL-25−/− mice revealed no differences in IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, and IL-17, whereas higher levels of IL-2, IL-12(p40), IFN-γ, and TNFα were detected (Table 2). In line with these findings, IL-25 blockade induced an increase of PMA/ionomycin-stimulated Th1- and Th17-related cytokines (IL-6, IL-12(p40), IFN-γ, and IL-17) in splenocytes isolated from Apoe−/− mice alongside an increased IL-10 and IL-13 release (Table 3). Additionally, anti-IL-25–treated mice revealed decreased plasma levels of the Th2-related cytokines IL-4 and IL-13 (Table S3). No statistically significant differences were detected for the rest of the measured cytokines in plasma (IL-2, IL-5, IL-6, IL-9, IL-10, IL-12(p40), IL-17, IFN-γ, and TNFα) (Table S3). Collectively, the data presented above indicate that IL-25 deficiency and a temporal 4-week-long IL-25 blockade during early atherosclerosis in Apoe−/− mice create an environment with systemic pro-inflammatory and Th1/Th17-related immune responses.
Figure 4.
IL-25 deficiency increases splenic Th1 cells and decreases Tregs and ILC2s. Shown are gating strategies (the graphs show the gating strategy in one Apoe−/−/IL-25−/− mouse) to identify immune cells in the spleen with flow cytometry and the frequencies in the splenic cells. In a and b, the same CD3+CD4+ flow cytometry plot is shown, because it is the same sample from one representative mouse that has been stained for both INF-γ and IL-5. a, to identify Th1 cells, leukocytes were gated as CD3+CD4+IFN-γ+ T cells in the CD3+CD4+ population. Shown is the frequency of Th1 cells in the spleen of Apoe−/− and Apoe−/−/IL-25−/− mice. b, to identify Th2 cells, leukocytes were gated as CD3+CD4+IL-5+ T cells in the CD3+CD4+ population. Shown is the frequency of Th2 cells in the spleen of Apoe−/− and Apoe−/−/IL-25−/− mice. c, to identify Th17 cells, leukocytes were gated as CD3+CD4+IL-17+ T cells in the CD3+CD4+ population. Shown is the frequency of Th17 cells in the spleen of Apoe−/− and Apoe−/−/IL-25−/− mice. d, to identify Tregs, leukocytes were gated as CD3+CD4+CD25+FoxP3+ T cells in the CD3+CD4+ population. Shown is the frequency of Tregs in the spleen of Apoe−/− and Apoe−/−/IL-25−/− mice. e, to identify ILC2s, leukocytes were gated as CD45+, lineage-negative cells (Lin−) expressing IL-17RB, intermediate (int) IL-7ra, and ICOS in the CD45+ population. Shown is the frequency of ILC2s in the spleen of Apoe−/− and Apoe−/−/IL-25−/− mice. Mann–Whitney test was used; n = 10–19 for Apoe−/− and n = 22 for Apoe−/−/IL-25−/− mice; *, p = 0.022; ****, p < 0.0001. Each dot represents one mouse, and the bars show the mean value ± S.D.
Table 1.
IL-25 deficiency increases plasma IL-17 levels
Shown are mean ± S.D. plasma cytokine levels in Apoe−/−IL-25−/− and Apoe−/− mice. **, p < 0.01, Mann–Whitney test.
| Cytokines | Apoe−/−IL-25−/− (n = 23) | Apoe−/− (n = 19) | p |
|---|---|---|---|
| pg/ml | pg/ml | ||
| IL-2 | 22.6 ± 9.6 | 19.7 ± 9.2 | 0.175 |
| IL-4 | 25.8 ± 14.5 | 19.3 ± 8.0 | 0.240 |
| IL-5 | 43.6 ± 14.8 | 37.1 ± 17.3 | 0.538 |
| IL-6 | 34.2 ± 44.2 | 19.1 ± 14.1 | 0.391 |
| IL-9 | 175.4 ± 110.9 | 122.8 ± 113.1 | 0.170 |
| IL-10 | 201.1 ± 62.3 | 177.4 ± 28.9 | 0.230 |
| IL-12(p40) | 337.4 ± 128.7 | 271.6 ± 102.9 | 0.069 |
| IL-13 | 697.5 ± 466.4 | 578.4 ± 360.1 | 0.487 |
| IL-17 | 127.7 ± 41.9 | 88.9 ± 27.4 | 0.001** |
| IFN-γ | 56.0 ± 28.1 | 43.2 ± 24.7 | 0.153 |
| TNFα | 742.5 ± 303.3 | 606.0 ± 300.2 | 0.088 |
Table 2.
IL-25 deficiency increases release of pro-inflammatory cytokines of activated splenocytes
Shown is mean ± S.D. cytokine release (pg/ml) of in vitro cultured and PMA/ionomycin-stimulated splenocytes from Apoe−/−IL-25−/− and Apoe−/− mice. *, p < 0.05; **, p < 0.01, Mann–Whitney test.
| Cytokines | Apoe−/−IL-25−/− (n = 17) | Apoe−/− (n = 19) | p |
|---|---|---|---|
| pg/ml | pg/ml | ||
| IL-2 | 24,917 ± 12,179 | 13,677 ± 8150 | 0.005** |
| IL-4 | 546.4 ± 304.1 | 769.6 ± 433.71 | 0.086 |
| IL-5 | 272.2 ± 184.8 | 248.2 ± 230.6 | 0.471 |
| IL-6 | 284.4 ± 134.5 | 344.1 ± 203.9 | 0.471 |
| IL-9 | 72.6 ± 22.3 | 58.7 ± 27.0 | 0.258 |
| IL-10 | 133.6 ± 81.0 | 87.8 ± 39.8 | 0.14 |
| IL-12(p40) | 46.4 ± 19.9 | 28.7 ± 12.3 | 0.002** |
| IL-13 | 713.4 ± 500.0 | 563.1 ± 353.6 | 0.392 |
| IL-17 | 841.4 ± 1038.0 | 780.7 ± 593.3 | 0.510 |
| IFN-γ | 6968 ± 4238 | 4665 ± 2350 | 0.049* |
| TNFα | 135.7 ± 45.4 | 99.1 ± 31.1 | 0.010* |
Table 3.
IL-25 blockade increases Th1/Th17-related cytokine release from activated splenocytes
Shown is mean ± S.D. cytokine release (pg/ml) of in vitro cultured and PMA/ionomycin-stimulated splenocytes from Apoe−/− mice treated with the IL-25–blocking antibody or a control antibody at 10–14 weeks of age. *, p < 0.05; **, p < 0.01; t test. OR, override; ND, non-detectable.
| Cytokines | Control Ab (n = 12) | Anti-IL-25 (n = 11) | p |
|---|---|---|---|
| pg/ml | pg/ml | ||
| IL-2 | OR | OR | |
| IL-4 | 6322 ± 2381 | 6941 ± 1273 | 0.449 |
| IL-5 | 2715.0 ± 428.6 | 2939.0 ± 953.5 | 0.468 |
| IL-6 | 601.2 ± 219.4 | 919.9 ± 285.2 | 0.007** |
| IL-9 | ND | ND | |
| IL-10 | 1209.0 ± 382.6 | 1552.0 ± 340.8 | 0.034* |
| IL-12(p40) | 227.3 ± 60.8 | 314.6 ± 76.0 | 0.008** |
| IL-13 | 2352.0 ± 604.3 | 3330.0 ± 1072.0 | 0.013* |
| IL-17 | 1938.0 ± 548.6 | 3156.0 ± 971.8 | 0.001** |
| IFN-γ | 11375 ± 9083 | 24708 ± 10329 | 0.003** |
| TNFα | 50.8 ± 25.1 | 64.6 ± 29.0 | 0.222 |
IL-25 deficiency results in less plasma IgM antibody targeting oxidized LDL
Interestingly, the IL-25–deficient mice were found to have fewer plasma IgM antibodies recognizing the oxidized LDL epitope PC (Table S4), antibodies previously found to be atheroprotective and to be induced by ILC2s (28). The plasma levels of other Igs were not influenced by the IL-25 deficiency (Table S4). Assessment of plasma immunoglobulin levels in mice treated for 4 weeks with the anti-IL-25 or the control Ab (IgA, IgG1, IgG2a, IgG2b, IgG3, IgM, anti-PC IgM, and IgE) showed no difference between groups (data not shown).
Discussion
IL-25, which is a member of the IL-17 cytokine family, has been inextricably associated with the promotion of type 2 immune responses (23–27). Mice deficient in IL-25 were reported to suffer from severe intestinal inflammation upon helminthic infection (29) and severe experimental autoimmune encephalomyelitis (30), which in both cases was accompanied by increased Th1- and Th17-related immune responses. Consequently, it is apparent that there is a possible link between endogenous IL-25 and the sustainment of a balance between Th2- and Th1/Th17-related immune responses. In the concept of atherosclerosis, although the role of Th17 immune responses is still debatable (18–22), Th1 immune responses have been uniformly reported to contribute to disease progression (4–7). The opposing role of Th2 compared with Th1/Th17 immune responses in the outcome of the disease is supported by studies investigating the effect of cytokines like IL-5 (8), IL-13 (17), IL-33 (16), and IL-25 (28) in experimental atherosclerosis. Our previous results demonstrating an atheroprotective role of exogenous IL-25 support the findings in the present study (28). In accordance, we report that IL-25 deficiency as well as anti-IL-25 treatment during the early stage of plaque formation in Apoe−/− mice leads to the development of increased atherosclerotic lesions in the aortic arch, a location of enhanced atherosclerotic plaque formation in Apoe−/− mice (31). However, no differences in the plaque area in the descending aorta or in subvalvular plaques were detected. This could be dependent on the age of the mice at sacrifice and thereby the presence of more advanced atherosclerotic plaques. IL-25 may have a greater effect in earlier lesion development. Furthermore, blocking of IL-25 slightly reduced the smooth muscle cell content in subvalvular plaques, whereas an increased lesional CD3+ T-cell infiltration was found in subvalvular plaques of Apoe−/−/IL-25−/− mice. The differences in plaque composition may be reflected by the finding that the genetic deletion of IL-25 in the Apoe−/−/IL-25−/− mouse model results in a complete absence of IL-25 during the whole lifespan, whereas the IL-25 blockade starts later in life and is only temporal. Thus, the absence of IL-25 seemed to affect atherosclerosis development in the aortic arch and to some extent plaque stability because smooth muscle cells are believed to contribute to a more stable plaque phenotype, whereas higher CD3+ T-cell content indicates the formation of more pro-inflammatory plaques (3, 32). However, no phenotyping of the plaque T cells was performed, and therefore the increased infiltration of CD3+ T cells into the lesions may represent both inflammatory and favorable T cells. It is possible that the induced Th1/Th17 cytokine profile in the spleen of Apoe−/− during temporal or complete IL-25 deficiency has influenced the formation of the plaques. In human normal arteries, IL-25 was reported to be expressed by endothelial and smooth muscle cells (33). In Apoe−/− mice, IL-25 appeared to be expressed by endothelial cells and by cells commonly found within atherosclerotic plaques, such as macrophages and T cells, and possibly also smooth muscle cells. However, most of the smooth muscle cells within atherosclerotic lesions have recently been shown to lack expression of conventional markers and exhibit a more macrophage-like phenotype (34–36). In addition, medial smooth muscle cells seem not to be a source for IL-25. In the context of helminth infections and asthma, IL-25 was shown to be produced by epithelial cells promoting type 2 immune responses that can be protective or destructive, respectively, in the aforementioned clinical occasions (37). Several lines of evidence have indicated that Th1-related cytokines promote lesion development and plaque instability. Specifically, Apoe−/− mice with IFN-γ receptor deficiency have decreased atherosclerotic plaques characterized by decreased lesional lipid accumulation and cellularity but increased collagen content (6). IFN-γ deficiency in LDL receptor–deficient mice reduced atherosclerotic plaques and modulated plaque consistency by reducing the macrophage and smooth muscle cell content only in mice that were on an HFD for 8 weeks (representing early lesion formation) and not for mice on an HFD for 20 weeks (5). Moreover, IL-12 treatment of Apoe−/− mice increased atherosclerotic plaque formation and lesional CD3+ T-cell infiltration (38), whereas exogenous administration of IFN-γ to Apoe−/− mice increased the lesion size as well as the number of T lymphocytes and MHC-II–positive cells within lesions (7). Additionally, IL-17A blockade resulted in reduced early plaque formation characterized by increased vascular smooth muscle cell accumulation and collagen content of the fibrous cap as well as reduced lesional apoptosis (19). In the present study, the findings indicate that the absence of IL-25 has a limited effect on plaque phenotype, although we found a pronounced effect on the CD3+ T-cell content in mice with complete IL-25 deficiency.
Several studies have reported an association of the immune responses in the spleen with the outcome of atherosclerosis in mice (39). Splenectomy in Apoe−/− mice was reported to accelerate atherogenesis (40), whereas transfer of splenic T and B cells could reverse the induced pro-atherogenic effect (reviewed by Witztum et al. (39)). Additionally, Emami et al. (41) reported that splenic metabolic activity is increased after acute myocardial infarction in humans and correlates with arterial inflammation. In that particular study, splenic activity appeared as an independent predictor of the risk of cardiovascular disease events (41). Thus, it was of importance to us to record the immunological profile of splenocytes upon temporal and complete IL-25 deficiency in Apoe−/− mice. In accordance with previous findings in IL-25–deficient mice, Apoe−/−/IL-25−/− mice were found to have increased levels of splenic Th1 and a trend of more Th17 cells. No difference in the Th2 cell population was detected. However, one limitation of the study is that only IL-5 was used as a Th2 marker and not IL-4 and IL-13, which may have resulted in an underestimation of the Th2 cell population. Tregs and ILC2s have previously been shown to be atheroprotective and thereby of interest in this study (28, 42–44). In previous work (25), we identified an IL-25–responsive ILC2 population in the spleen as Lin−CD45+IL-17RB+ICOS+IL-7raint (28). In support, these atherosclerosis-prone IL-25–deficient mice revealed lower levels of splenic Tregs and ILC2s. In addition, previous work reported that MHCII-expressing ILC2s interact with T cells and that the cross-talk contributes to their mutual maintenance, expansion, and cytokine production (45). Whether this is also the case in our mouse models must be evaluated. Furthermore, the findings showed a pro-inflammatory cytokine profile in both plasma and spleen as well as reduced plasma levels of atheroprotective anti-PC IgM antibodies. In line with these findings, IL-25 blockade in Apoe−/− mice resulted in increased levels of Th1/Th17-related cytokines in the spleen and also reduced IL-4 and IL-13 Th2 cytokine levels in plasma. Interestingly, alongside the splenic increase of Th1/Th17-related cytokines (IL-6, IL-12, IL-17, and IFN-γ), increased levels of IL-13 and IL-10 were also recorded. Although puzzling, because IL-25 has been shown to induce IL-13 in mice (25), the observed increase of IL-10 and IL-13 might indicate the induction of compensatory immune responses to the pro-inflammatory environment of Th1/Th17 cytokine dominance in the spleens of Apoe−/− mice upon IL-25 blockade. The similar but not identical cytokine responses in the two mouse models may reflect that the measurement of the cytokine levels was done in samples collected 11 weeks after the temporal IL-25 blockade (at 25 weeks of age), whereas the Apoe−/−/IL-25−/− mouse model has a complete absence of IL-25 during the whole lifespan. Altogether, it appears that the absence of IL-25 also during hypercholesterolemia creates an environment where Th1/Th17 immune responses dominate. Although the translation of findings in animal models into human atherosclerosis might be difficult, we herein depict a disease-enhancing effect of complete IL-25 deficiency or a temporal IL-25 blockade at the stage of atherogenesis in the Apoe−/− mouse model.
In conclusion, both complete IL-25 deficiency and IL-25 blockade in Apoe−/− mice during early plaque development lead to increased formation of atherosclerotic plaques in the aortic arch. This phenomenon is accompanied by a systemic pro-inflammatory Th1/Th17 environment. The above-mentioned findings indicate a role of endogenous IL-25 in the maintenance of the T-cell subsets and cytokine balance under hypercholesterolemic conditions and that disturbance of that balance through the absence of IL-25 leads to increased atherosclerotic plaque formation in the aortic arch.
Experimental procedures
Mice and treatment outline
Female Apoe−/−/IL-25−/− mice were bred in-house by crossing Apoe−/− on C57BL/6 background (Jackson Laboratories, B6129P2-Apoetm1Unc/J) and IL-25−/− mice on C57BL/6 background (IL-25tm1Anjm kindly provided by Professor Andrew McKenzie (Cambridge, UK)) (46). Female Apoe−/− mice on C57BL/6 background (Jackson Laboratories) bred in-house were used as control mice. From 10 weeks of age, the Apoe−/−/IL-25−/− mice were fed an HFD (0.15% cholesterol and 21% fat (Lantmännen, Stockholm, Sweden)) until they were killed at 25 weeks of age (Fig. S1a). Upon euthanasia of the mice with an intraperitoneal injection with a mixture of ketamine (50 μg/g mouse weight) and xylazine (10 μg/g mouse weight), the mice were weighed, blood samples were obtained with cardiac puncture, and the plasma samples were snap-frozen in liquid nitrogen and stored at −80 °C until analysis. Spleens were dissected and stored on ice in RPMI 1640 (Gibco, Stockholm, Sweden) and then processed for single-cell suspension preparations. Next, the mice were whole-body–perfused with PBS. Aortas (including the aortic arch) were dissected, mounted en face, and stored in Histochoice (Amresco, Solon, OH). Hearts were dissected and placed in Histochoice or frozen at −80 °C.
To investigate the effect of IL-25 blockade on early plaque development, female Apoe−/− mice on C57BL/6 background (Jackson Laboratories) were injected intraperitoneally once a week with 100 μg of an antibody targeting IL-25 (LEAF purified anti-mouse/human IL-25 (IL-17E), clone 35B, Biolegend) or control Ab (LEAF purified rat IgG1κ, isotype control, clone RTK2071, Biolegend) at 10 weeks of age for a period of 4 weeks. At 10 weeks of age, the mice were placed on an HFD for 15 weeks, after which they were killed (Fig. S1b). The procedure for euthanasia as well as the collection of blood and organs were as described for the Apoe−/−/IL-25−/− mice.
The numbers of mice included in each experiment are shown in the figures and tables. All assessments of outcomes were performed blinded. This study was performed in accordance with the guidelines of the National Institutes of Health. The local animal care and use committee at Lund University (approval M74-14) approved the experimental protocols used in the study.
Flow cytometry
A single-cell suspension of splenocytes was prepared by pressing each spleen through a 70-μm cell strainer (BD Falcon, Franklin Lakes, NJ). After lysis of red blood cells (Red Blood Cell Lysing Buffer, Sigma) and extensive washing, the cells were placed in cell culture medium (RPMI 1640 medium containing 10% heat-inactivated fetal calf serum, 1 mmol liter−1 sodium pyruvate, 10 mmol liter−1 Hepes, 50 units of penicillin, 50 μg ml−1 streptomycin, 0.05 mmol liter−1 β-mercaptoethanol, and 2 mmol liter−1 l-glutamine; Gibco, Paisley, UK). Splenocytes and blood cells were stained with fluorochrome-conjugated antibodies for flow cytometric analysis unless intracellular staining of cytokines was required. In that case, for IL-5 and IFN-γ intracellular staining, the cells were stimulated with 32.4 nmol liter−1 PMA, 1.3 μmol liter−1 ionomycin, and 17.8 μmol liter−1 brefeldin A (Sigma-Aldrich, Stockholm, Sweden) for 4 h before staining, whereas cells that were intended for IL-17 staining were stimulated for 18–24 h with the same stimuli. Fluorochrome-stained cells were run in a CyAn ADP flow cytometer (Beckman Coulter, High Wycombe, UK), and the flow cytometric data obtained were analyzed with FlowJo software (Tree Star, Inc., Ashland, OR). In the case of blood cells, erythrocytes were lysed with lysing buffer (BD Pharm LyseTM, BD Biosciences, Stockholm, Sweden) before all stainings. All of the antibodies were purchased from Biolegend (San Diego, CA) unless indicated otherwise. The extracellular antibodies that were used are CD3-PE/Cy7 (clone 145-2C11), CD4-PB (clone RM4-4), CD8-AF700 (clone YTS156.7.7), CD25-APC (clone 3C7), ICOS-PB (clone C398.4A), CD45-APC/Cy7 (clone 30-F11), IL-7ra-FITC (clone SB/199), and IL-17RB-APC (clone 752101, R&D Systems, Abingdon, UK), whereas the intracellular are Foxp3-PE (clone MF-14), IL-5-APC (clone TRFK5), IFN-γ-PE (clone XMG1.2), and IL-17A-APC (clone TC11-18H10.1). In additional experiments, splenocytes were immunomagnetically enriched in ILC2s with the use of a custom-made kit from Stem Cell Technologies Inc. (Vancouver, Canada) (lineage antibody mixture: CD3, CD4, CD8, CD11b, CD11c, CD45R, CD19, Gr-1, FcϵRI, NK 1.1, and Ter-119). The enriched fraction of cells was next stained with fluorochrome-conjugated antibodies, such as Lin-Streptavidin PE/Cy7, ICOS-PB (clone C398.4A), CD45-APC/Cy7 (clone 30-F11), IL-7ra-FITC (clone SB/199), and IL-17RB-APC (clone 752101, R&D Systems, Abingdon, UK) and analyzed with the use of flow cytometry. All gatings for the investigated cell populations were set according to FMO (fluorescence minus one) samples. Th1 cells were identified as CD3+CD4+IFN-γ+ cells, Th2 as CD3+CD4+IL-5+, Th17 as CD3+CD4+IL-17+, and T regulatory cells (Tregs) as CD3+CD4+CD25+FoxP3+. All of them are reported as a percentage of the T helper cell population (CD3+CD4+) unless indicated otherwise. ILC2s were identified as Lin−CD45+IL-17RB+ICOS+IL-7raint and reported as a percentage of CD45+ cells.
Cytokine analysis
Splenocytes (1.7 × 106 cells/ml) were stimulated with 1.3 μmol liter−1 ionomycin and 32.4 nmol liter−1 PMA for 24 h. Cell culture supernatants were frozen at −80 °C until analysis. Cytokine release by cells and plasma cytokine levels (IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12(p40), IL-13, IL-17, IFN-γ, and TNFα) were assessed with the use of Luminex xMAPTM technology according to the company's instructions (Bio-Plex Pro Mouse Cytokine Group I kit, Bio-Rad).
Aortic plaque area; immunohistochemistry of subvalvular plaques
En face preparations of aortas (descending aorta and aortic arch) were dipped in 78% methanol and stained for 40 min in 0.16% Oil Red O dissolved in 78% methanol containing 0.22 mol liter−1 NaOH. Additionally, 10-μm heart sections were collected after embedding of each tissue in OCT (optimal cutting temperature; Tissue-Tek, Zoeterwoulde, The Netherlands). Subvalvular heart sections were stained for CD3+ T cells (anti-CD3, Dako A0452), macrophages (MOMA-2 antibody, clone MOMA2, BMA Biomedicals, Switzerland; rabbit anti-mouse CD68, Ab125212, Abcam), smooth muscle (α-actin smooth muscle antibody, clone 1A4, Sigma A2547), IgM (biotinylated anti-mouse IgM, Vector Laboratories, BA 2020), IL-25 (rabbit anti-mouse IL-25, 06-1080, Sigma; Signal Stain Boost IHC detection reagent (rabbit), Bio Nordica, 8114), and collagen content. For macrophage and CD3+ T-cell content, rabbit Igs (Dako X0936, Solna, Sweden) were used as negative controls, whereas for IgM, IL-25, and α-actin smooth muscle cell content, a rabbit immunoglobulin (clone EPR25A, ab172730, Abcam) was utilized. A DAB detection kit was used for color development (Vector Laboratories), and the sections were counterstained in hematoxylin. For assessment of the collagen content of the plaques, subvalvular sections were stained with Van Gieson solution acid fuchsin (Sigma-Aldrich). To assess the necrotic core, sections were stained with hematoxylin/eosin, and the area was determined as the acellular area >3000 μm2, lacking nuclei and cytoplasm, under the fibrous cap of lesions. All stainings were quantified with Image-Pro-Plus version 4.5 software (Media Cybernetics, Bethesda, MD).
Plasma Igs
Plasma IgA, IgG1, IgG2a (not expressed in C57BL/6 mice), IgG2b, IgG3, and IgM levels were assessed with the use of the Mouse Isotyping Panel 1 Assay kit from Mesoscale according to the company's instructions (MSD Multi-spot Assays, Mesoscale Discovery, Rockville, MD). Plasma IgE levels were determined with the use of a mouse IgE ELISA kit from Bethyl Laboratories, Inc. (Montgomery, TX), whereas IgM antibodies targeting PC were assessed with an ELISA kit from Athera Biotechnologies (Solna, Sweden) according to the company's instructions with the exception of utilizing peroxidase-conjugated anti-mouse IgM (Jackson ImmunoResearch).
Plasma cholesterol and triglyceride levels
Total cholesterol and triglyceride levels were measured enzymatically using kits from Infinity (Thermo Fisher Scientific).
Statistics
Analysis of data was performed using two-tailed unpaired t test for normally distributed or log-transformed skewed data or a Mann–Whitney test to assess nonnormally distributed variables. Data are presented as mean ± S.D. Analysis was performed using GraphPad Prism version 7.00 (GraphPad Software, La Jolla, CA), and a level of p < 0.05 was considered significant.
Author contributions
P. T. M., P. D., J. N., H. B., and G. N. F. conceived and designed the experiments; P. T. M., P. D., E. B., I. L., L. S., F. T., R. A., I. S., and C. C. performed the experiments; P. M., P. D., I. L., L. S., F. T., J. N., H. B., and G. N. F. analyzed the data; P. T. M. and G. N. F. wrote the paper. All authors analyzed the results and approved the final version of the manuscript.
Supplementary Material
Acknowledgments
We thank Ragnar Alm, Christina Cavala, and Ingrid Söderberg for technical assistance.
This work was supported by Swedish Medical Research Council Grants 521-2010-2929 (to G.N.F.) and 2015-02811 (to J.N.), Swedish Heart-Lung Foundation Grant 20150273 (to G.N.F.), Albert Påhlsson Foundation Grant 27.21.0145 (to G.N.F.), and Skåne University Hospital Foundation Grant 16000109 (to J.N.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Fig. S1 and Tables S1–S4.
- apo
- apolipoprotein
- HFD
- high-fat diet
- ILC2
- innate lymphoid cells type 2
- LDL
- low-density lipoprotein
- PC
- phosphorylcholine
- Tregs
- T regulatory cells
- IFN
- interferon
- Ab
- antibody
- PMA
- phorbol 12-myristate 13-acetate
- TNF
- tumor necrosis factor.
References
- 1. Hansson G. K., and Hermansson A. (2011) The immune system in atherosclerosis. Nat. Immunol. 12, 204–212 10.1038/ni.2001 [DOI] [PubMed] [Google Scholar]
- 2. Frostegård J., Ulfgren A. K., Nyberg P., Hedin U., Swedenborg J., Andersson U., and Hansson G. K. (1999) Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 145, 33–43 10.1016/S0021-9150(99)00011-8 [DOI] [PubMed] [Google Scholar]
- 3. Stemme S., Faber B., Holm J., Wiklund O., Witztum J. L., and Hansson G. K. (1995) T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl. Acad. Sci. U.S.A. 92, 3893–3897 10.1073/pnas.92.9.3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buono C., Binder C. J., Stavrakis G., Witztum J. L., Glimcher L. H., and Lichtman A. H. (2005) T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl. Acad. Sci. U.S.A. 102, 1596–1601 10.1073/pnas.0409015102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buono C., Come C. E., Stavrakis G., Maguire G. F., Connelly P. W., and Lichtman A. H. (2003) Influence of interferon-γ on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 23, 454–460 10.1161/01.ATV.0000059419.11002.6E [DOI] [PubMed] [Google Scholar]
- 6. Gupta S., Pablo A. M., Jiang X., Wang N., Tall A. R., and Schindler C. (1997) IFN-γ potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Invest. 99, 2752–2761 10.1172/JCI119465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whitman S. C., Ravisankar P., Elam H., and Daugherty A. (2000) Exogenous interferon-γ enhances atherosclerosis in apolipoprotein E−/− mice. Am J. Pathol. 157, 1819–1824 10.1016/S0002-9440(10)64820-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Binder C. J., Hartvigsen K., Chang M. K., Miller M., Broide D., Palinski W., Curtiss L. K., Corr M., and Witztum J. L. (2004) IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Invest. 114, 427–437 10.1172/JCI200420479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsimikas S., Brilakis E. S., Lennon R. J., Miller E. R., Witztum J. L., McConnell J. P., Kornman K. S., and Berger P. B. (2007) Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J. Lipid Res. 48, 425–433 10.1194/jlr.M600361-JLR200 [DOI] [PubMed] [Google Scholar]
- 10. Fredrikson G. N., Schiopu A., Berglund G., Alm R., Shah P. K., and Nilsson J. (2007) Autoantibody against the amino acid sequence 661–680 in apo B-100 is associated with decreased carotid stenosis and cardiovascular events. Atherosclerosis 194, e188–e192 10.1016/j.atherosclerosis.2006.12.014 [DOI] [PubMed] [Google Scholar]
- 11. Sjögren P., Fredrikson G. N., Samnegard A., Ericsson C. G., Ohrvik J., Fisher R. M., Nilsson J., and Hamsten A. (2008) High plasma concentrations of autoantibodies against native peptide 210 of apoB-100 are related to less coronary atherosclerosis and lower risk of myocardial infarction. Eur. Heart J. 29, 2218–2226 10.1093/eurheartj/ehn336 [DOI] [PubMed] [Google Scholar]
- 12. Davenport P., and Tipping P. G. (2003) The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 163, 1117–1125 10.1016/S0002-9440(10)63471-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. King V. L., Szilvassy S. J., and Daugherty A. (2002) Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler. Thromb. Vasc. Biol. 22, 456–461 10.1161/hq0302.104905 [DOI] [PubMed] [Google Scholar]
- 14. Huber S. A., Sakkinen P., David C., Newell M. K., and Tracy R. P. (2001) T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation 103, 2610–2616 10.1161/01.CIR.103.21.2610 [DOI] [PubMed] [Google Scholar]
- 15. King V. L., Cassis L. A., and Daugherty A. (2007) Interleukin-4 does not influence development of hypercholesterolemia or angiotensin II-induced atherosclerotic lesions in mice. Am. J. Pathol. 171, 2040–2047 10.2353/ajpath.2007.060857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller A. M., Xu D., Asquith D. L., Denby L., Li Y., Sattar N., Baker A. H., McInnes I. B., and Liew F. Y. (2008) IL-33 reduces the development of atherosclerosis. J. Exp. Med. 205, 339–346 10.1084/jem.20071868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cardilo-Reis L., Gruber S., Schreier S. M., Drechsler M., Papac-Milicevic N., Weber C., Wagner O., Stangl H., Soehnlein O., and Binder C. J. (2012) Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol. Med. 4, 1072–1086 10.1002/emmm.201201374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Butcher M. J., Gjurich B. N., Phillips T., and Galkina E. V. (2012) The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ. Res. 110, 675–687 10.1161/CIRCRESAHA.111.261784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Erbel C., Chen L., Bea F., Wangler S., Celik S., Lasitschka F., Wang Y., Böckler D., Katus H. A., and Dengler T. J. (2009) Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J. Immunol. 183, 8167–8175 10.4049/jimmunol.0901126 [DOI] [PubMed] [Google Scholar]
- 20. Smith E., Prasad K. M., Butcher M., Dobrian A., Kolls J. K., Ley K., and Galkina E. (2010) Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 121, 1746–1755 10.1161/CIRCULATIONAHA.109.924886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danzaki K., Matsui Y., Ikesue M., Ohta D., Ito K., Kanayama M., Kurotaki D., Morimoto J., Iwakura Y., Yagita H., Tsutsui H., and Uede T. (2012) Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 32, 273–280 10.1161/ATVBAHA.111.229997 [DOI] [PubMed] [Google Scholar]
- 22. Taleb S., Romain M., Ramkhelawon B., Uyttenhove C., Pasterkamp G., Herbin O., Esposito B., Perez N., Yasukawa H., Van Snick J., Yoshimura A., Tedgui A., and Mallat Z. (2009) Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 206, 2067–2077 10.1084/jem.20090545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fort M. M., Cheung J., Yen D., Li J., Zurawski S. M., Lo S., Menon S., Clifford T., Hunte B., Lesley R., Muchamuel T., Hurst S. D., Zurawski G., Leach M. W., Gorman D. M., and Rennick D. M. (2001) IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 10.1016/S1074-7613(01)00243-6 [DOI] [PubMed] [Google Scholar]
- 24. Moro K., Yamada T., Tanabe M., Takeuchi T., Ikawa T., Kawamoto H., Furusawa J., Ohtani M., Fujii H., and Koyasu S. (2010) Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463, 540–544 10.1038/nature08636 [DOI] [PubMed] [Google Scholar]
- 25. Neill D. R., Wong S. H., Bellosi A., Flynn R. J., Daly M., Langford T. K., Bucks C., Kane C. M., Fallon P. G., Pannell R., Jolin H. E., and McKenzie A. N. (2010) Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 10.1038/nature08900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Price A. E., Liang H. E., Sullivan B. M., Reinhardt R. L., Eisley C. J., Erle D. J., and Locksley R. M. (2010) Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. U.S.A. 107, 11489–11494 10.1073/pnas.1003988107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saenz S. A., Siracusa M. C., Perrigoue J. G., Spencer S. P., Urban J. F. Jr., Tocker J. E., Budelsky A. L., Kleinschek M. A., Kastelein R. A., Kambayashi T., Bhandoola A., and Artis D. (2010) IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature 464, 1362–1366 10.1038/nature08901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mantani P. T., Dunér P., Bengtsson E., Alm R., Ljungcrantz I., Söderberg I., Sundius L., To F., Nilsson J., Björkbacka H., and Fredrikson G. N. (2015) IL-25 inhibits atherosclerosis development in apolipoprotein E deficient mice. PLoS One 10, e0117255 10.1371/journal.pone.0117255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Owyang A. M., Zaph C., Wilson E. H., Guild K. J., McClanahan T., Miller H. R., Cua D. J., Goldschmidt M., Hunter C. A., Kastelein R. A., and Artis D. (2006) Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 203, 843–849 10.1084/jem.20051496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kleinschek M. A., Owyang A. M., Joyce-Shaikh B., Langrish C. L., Chen Y., Gorman D. M., Blumenschein W. M., McClanahan T., Brombacher F., Hurst S. D., Kastelein R. A., and Cua D. J. (2007) IL-25 regulates Th17 function in autoimmune inflammation. J. Exp. Med. 204, 161–170 10.1084/jem.20061738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Daugherty A. (2002) Mouse models of atherosclerosis. Am. J. Med. Sci. 323, 3–10 10.1097/00000441-200201000-00002 [DOI] [PubMed] [Google Scholar]
- 32. Clarke M. C., Figg N., Maguire J. J., Davenport A. P., Goddard M., Littlewood T. D., and Bennett M. R. (2006) Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat. Med. 12, 1075–1080 10.1038/nm1459 [DOI] [PubMed] [Google Scholar]
- 33. de Boer O. J., van der Meer J. J., Teeling P., van der Loos C. M., Idu M. M., van Maldegem F., Aten J., and van der Wal A. C. (2010) Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J. Pathol. 220, 499–508 [DOI] [PubMed] [Google Scholar]
- 34. Feil S., Fehrenbacher B., Lukowski R., Essmann F., Schulze-Osthoff K., Schaller M., and Feil R. (2014) Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ. Res. 115, 662–667 10.1161/CIRCRESAHA.115.304634 [DOI] [PubMed] [Google Scholar]
- 35. Shankman L. S., Gomez D., Cherepanova O. A., Salmon M., Alencar G. F., Haskins R. M., Swiatlowska P., Newman A. A., Greene E. S., Straub A. C., Isakson B., Randolph G. J., and Owens G. K. (2015) KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 21, 628–637 10.1038/nm.3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vengrenyuk Y., Nishi H., Long X., Ouimet M., Savji N., Martinez F. O., Cassella C. P., Moore K. J., Ramsey S. A., Miano J. M., and Fisher E. A. (2015) Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler. Thromb. Vasc. Biol. 35, 535–546 10.1161/ATVBAHA.114.304029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saenz S. A., Noti M., and Artis D. (2010) Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 31, 407–413 10.1016/j.it.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 38. Lee T. S., Yen H. C., Pan C. C., and Chau L. Y. (1999) The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 19, 734–742 10.1161/01.ATV.19.3.734 [DOI] [PubMed] [Google Scholar]
- 39. Witztum J. L. (2002) Splenic immunity and atherosclerosis: a glimpse into a novel paradigm? J. Clin. Invest. 109, 721–724 10.1172/JCI0215310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Caligiuri G., Nicoletti A., Poirier B., and Hansson G. K. (2002) Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J. Clin. Invest. 109, 745–753 10.1172/JCI7272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Emami H., Singh P., MacNabb M., Vucic E., Lavender Z., Rudd J. H., Fayad Z. A., Lehrer-Graiwer J., Korsgren M., Figueroa A. L., Fredrickson J., Rubin B., Hoffmann U., Truong Q. A., Min J. K., et al. (2015) Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc. Imaging 8, 121–130 10.1016/j.jcmg.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wigren M., Kolbus D., Dunér P., Ljungcrantz I., Söderberg I., Björkbacka H., Fredrikson G. N., and Nilsson J. (2011) Evidence for a role of regulatory T cells in mediating the atheroprotective effect of apolipoprotein B peptide vaccine. J. Intern. Med. 269, 546–556 10.1111/j.1365-2796.2010.02311.x [DOI] [PubMed] [Google Scholar]
- 43. Engelbertsen D., Foks A. C., Alberts-Grill N., Kuperwaser F., Chen T., Lederer J. A., Jarolim P., Grabie N., and Lichtman A. H. (2015) Expansion of CD25+ innate lymphoid cells reduces atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 35, 2526–2535 10.1161/ATVBAHA.115.306048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Newland S. A., Mohanta S., Clément M., Taleb S., Walker J. A., Nus M., Sage A. P., Yin C., Hu D., Kitt L. L., Finigan A. J., Rodewald H. R., Binder C. J., McKenzie A. N. J., Habenicht A. J., and Mallat Z. (2017) Type-2 innate lymphoid cells control the development of atherosclerosis in mice. Nat. Commun. 8, 15781 10.1038/ncomms15781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oliphant C. J., Hwang Y. Y., Walker J. A., Salimi M., Wong S. H., Brewer J. M., Englezakis A., Barlow J. L., Hams E., Scanlon S. T., Ogg G. S., Fallon P. G., and McKenzie A. N. (2014) MHCII-mediated dialog between group 2 innate lymphoid cells and CD4+ T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 41, 283–295 10.1016/j.immuni.2014.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fallon P. G., Ballantyne S. J., Mangan N. E., Barlow J. L., Dasvarma A., Hewett D. R., McIlgorm A., Jolin H. E., and McKenzie A. N. (2006) Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203, 1105–1116 10.1084/jem.20051615 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.