Abstract
Cardiac energy demands during early embryonic periods are sufficiently met through glycolysis, but as development proceeds, the oxidative phosphorylation in mitochondria becomes increasingly vital. Adrenergic hormones are known to stimulate metabolism in adult mammals and are essential for embryonic development, but relatively little is known about their effects on metabolism in the embryonic heart. Here, we show that embryos lacking adrenergic stimulation have ∼10-fold less cardiac ATP compared with littermate controls. Despite this deficit in steady-state ATP, neither the rates of ATP formation nor degradation was affected in adrenergic hormone-deficient hearts, suggesting that ATP synthesis and hydrolysis mechanisms were fully operational. We thus hypothesized that adrenergic hormones stimulate metabolism of glucose to provide chemical substrates for oxidation in mitochondria. To test this hypothesis, we employed a metabolomics-based approach using LC/MS. Our results showed glucose 1-phosphate and glucose 6-phosphate concentrations were not significantly altered, but several downstream metabolites in both glycolytic and pentose–phosphate pathways were significantly lower compared with controls. Furthermore, we identified glyceraldehyde-3-phosphate dehydrogenase and glucose-6-phosphate dehydrogenase as key enzymes in those respective metabolic pathways whose activity was significantly (p < 0.05) and substantially (80 and 40%, respectively) lower in adrenergic hormone-deficient hearts. Addition of pyruvate and to a lesser extent ribose led to significant recovery of steady-state ATP concentrations. These results demonstrate that without adrenergic stimulation, glucose metabolism in the embryonic heart is severely impaired in multiple pathways, ultimately leading to insufficient metabolic substrate availability for successful transition to aerobic respiration needed for survival.
Keywords: metabolomics, heart development, heart failure, glycolysis, pentose–phosphate pathway (PPP), ATP, dehydrogenase
Introduction
The developing heart experiences a number of physiological and structural changes while transitioning through developmental stages. Metabolic shifts occur during embryonic heart development as cardiac energy demands increase to promote oxidative phosphorylation of ADP to ATP in mitochondria. Early embryonic heart development is characterized by a glycolytic metabolism with carbohydrates as the primary energy source (1–4). However, as energy demands increase, cardiac metabolism undergoes an “embryonic shift” in metabolism from anaerobic to aerobic (5). The mechanisms underlying the embryonic shift initiating mitochondrial metabolism during heart development are not well-understood. The adrenergic hormones, epinephrine (EPI)2 and norepinephrine (NE), are regulators of stress and the sympathetic nervous system in adult mammals. Adrenergic hormones, produced in the heart as early as embryonic day 8.5 (E8.5), are also active and essential during embryonic heart development (5–7). Targeted disruption of the essential dopamine β-hydroxylase (Dbh) gene prevents NE and EPI biosynthesis and leads to embryonic lethality due to heart failure in mice (6, 8). Subsequent disruption of the phenylethanolamine N-methyltransferase (Pnmt) gene leads to loss of EPI, but it does not impede prenatal development (9, 10). Thus, NE is crucial for embryonic heart development and survival, but the regulatory roles and physiological targets of adrenergic stimulation during embryonic heart development remain unknown.
Adrenergic hormone-deficient (Dbh−/−) embryos appear to develop and function normally through approximately embryonic day 9.5 (E9.5), but they begin to show cardiac distress between E10.5 and E12 (8). These adrenergic hormone-deficient (Dbh−/−) embryos undergo steady deterioration that ultimately ends in death within 24 h of initial symptoms. To gain insight into transcriptional changes between Dbh+/+ and Dbh−/−, a genome-wide expression screen showed that the largest set of differentially expressed genes are involved in metabolism (11). Further evidence demonstrated that Dbh−/− embryos are energy-depleted with >95% ATP/ADP reduction by E11.5 (12). Oxygen consumption rates (OCR), an indicator of mitochondrial respiration, are established in E10.5 hearts; however, Dbh−/− hearts lag behind by ∼24 h later compared with age-matched littermate controls. These results established that NE and EPI influence embryonic heart energy metabolism; however, the mechanism(s) and metabolic targets regulated by adrenergic hormones remain unknown.
Aerobic metabolism in mitochondria becomes increasingly important as the heart transitions from embryonic to fetal stages. Several studies using genetic mutations to disrupt aerobic respiration and/or mitochondrial structure/function have resulted in embryonic lethality due to heart failure (5, 13–16). Similarly, lack of adrenergic hormones leads to delayed transition to aerobic metabolism in mitochondria and is associated with embryonic lethality due to apparent heart failure. We thus hypothesized that adrenergic hormones facilitate the embryonic shift in metabolism from anaerobic glycolysis toward aerobic oxidative phosphorylation in mitochondria. This study focuses primarily on carbohydrate metabolism because lipid metabolism is not fully established until late fetal and neonatal stages (17–19). We tested our hypothesis using metabolomics analysis and measurements of key enzymatic reaction rates to evaluate metabolic changes in response to adrenergic deficiency in embryonic mouse hearts. In addition, we were able to show that metabolic blockade could be overcome, in part, by addition of downstream metabolites, leading to significant recovery of steady-state ATP in adrenergic hormone-deficient embryonic hearts.
Results
Influence of adrenergic hormones on embryonic heart metabolism
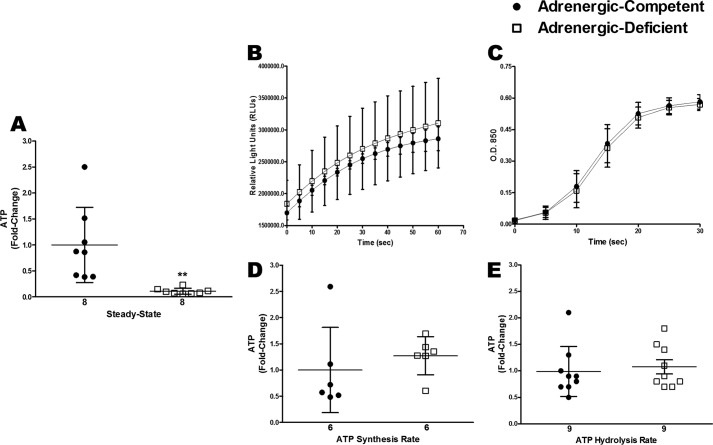
To determine whether cardiac energy levels were impaired in adrenergic hormone-deficient embryonic hearts, we first isolated E11.5 mouse hearts to measure steady-state ATP concentrations. Our results show a dramatic and significant 10-fold decrease (p < 0.01) in steady-state ATP concentrations in adrenergic hormone-deficient hearts compared with adrenergic hormone-competent littermate controls (Fig. 1A). To determine whether adrenergic deficiency slows ATP production or increases ATP utilization/degradation as a potential cause of energy depletion, we measured rates of ATP synthesis and hydrolysis in adrenergic hormone-competent and -deficient embryonic hearts (Fig. 1, B–E). Embryonic heart lysates were incubated with pyruvate and malate prior to measurements to provide substrates for ATP synthesis. We observed no significant difference in ATP synthesis rates in adrenergic hormone-competent and -deficient hearts, suggesting that ATP synthesis rates were not impaired when the hearts were provided with necessary substrates and conditions. Similarly, no significant differences were observed in ATP hydrolysis rates in adrenergic hormone-competent and -deficient hearts, suggesting that the observed depletion of steady-state ATP concentrations may not necessarily be the result of increased rates of ATP hydrolysis. These data suggest ATP synthesis and hydrolysis rates were not impaired in adrenergic hormone-deficient embryonic hearts when supplied with sufficient substrates.
Figure 1.
Steady-state ATP concentrations compared with rates of ATP synthesis and hydrolysis in E11.5 isolated adrenergic hormone-deficient hearts compared with controls. A, steady-state ATP concentrations in adrenergic hormone-competent (●) and adrenergic hormone-deficient (□) embryonic hearts at embryonic day 11.5. B and D, ATP synthesis rate in adrenergic hormone-competent (●) and adrenergic hormone-deficient (□) embryonic hearts, expressed as relative light units (RLUs) produced/s and fold-change, respectively. C and E, ATP hydrolysis rates in adrenergic hormone-competent (●) and adrenergic hormone-deficient (□) embryonic hearts expressed as absorbance/s and fold-change, respectively. Numerical values below the x axes refer to the number (n) of samples analyzed. Data are presented as fold-change compared with competent control littermates. Student's t test was used to compare means between competent and deficient groups. **, p < 0.01.
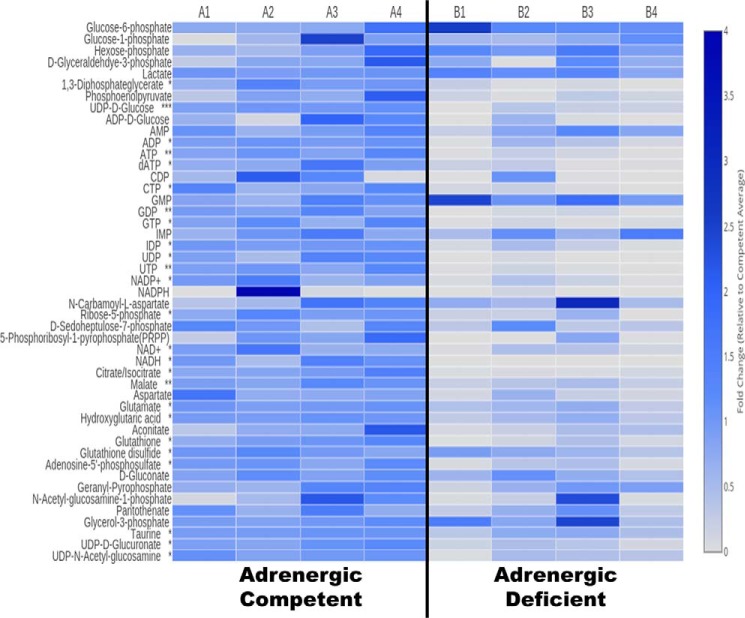
To test whether metabolic substrates were limited in adrenergic hormone-deficient embryonic hearts, we utilized LC/MS to generate metabolomic profiles. The results are shown in the Fig. 2 heat map depicting relative concentrations of 47 key metabolites involved in carbohydrate, amino acid, and nucleotide metabolism. Other metabolites in these pathways, such as acetyl-CoA, were also evaluated but are not included here because we did not obtain reliable measurements for them due to limiting concentrations in both adrenergic hormone-competent control and adrenergic hormone-deficient hearts at this embryonic stage of development (E11.5). Initial analysis of the heat map data clearly reveals that many but not all of the measured metabolites were substantially decreased in adrenergic hormone-deficient hearts relative to littermate controls (Fig. 2). Remarkably, adrenergic hormone-deficient hearts show significant decreases in several metabolites from pentose–phosphate pathway (PPP), glycolysis, and the TCA cycle, suggesting that glucose metabolism was severely compromised (Fig. 2). Despite this, adrenergic hormone-deficient hearts had comparable glucose 6-phosphate, glucose 1-phosphate, and glyceraldehyde 3-phosphate concentrations compared with adrenergic hormone-competent controls, indicating that there was sufficient starting substrate (glucose). Nevertheless, there were ∼10-fold decreases in downstream glycolytic metabolites such as 1,3-diphosphoglycerate (p < 0.05) and phosphoenolpyruvate, indicating the “pay-off” phase of glycolysis was likely impaired. In contrast, lactate concentrations did not differ between adrenergic hormone-deficient and -competent hearts suggesting that some glycolytic activity remains or that lactate levels may be stabilized from embryonic/maternal circulation.
Figure 2.
LC-MS metabolomics analysis of isolated adrenergic hormone-deficient embryonic hearts. Columns A1–A4 represent the adrenergic hormone-competent controls with three embryonic hearts analyzed per group. Columns B1–B4 represent the adrenergic hormone-deficient group with three embryonic hearts analyzed per group. Data are presented as fold-change compared with competent control littermates. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Another pathway that was clearly abrogated in adrenergic hormone-deficient hearts was the PPP as evidenced by the 5-fold decreases in critically important intermediate metabolites, including ribose 5-phosphate (p < 0.05) and phosphoribosyl pyrophosphate (PRPP) as well as 2-fold decreases in sedoheptulose 7-phosphate. Consistent with these results, we also found major decreases in cardiac concentrations of many nucleotides, which rely in large part on the PPP for crucial substrates for their biosynthesis.
Furthermore, adrenergic hormone-deficient hearts had significantly lower concentrations of several tricarboxylic acid (TCA) cycles and related intermediates, including citrate/isocitrate, aconitate, malate, glutamate, and aspartate, that likely contributed to impaired mitochondrial metabolism (Fig. 2). In addition, adrenergic hormone-deficient hearts had 2-fold lower pantothenate concentrations (not significant) compared with adrenergic hormone-competent controls suggesting that CoA biosynthesis, an essential precursor for acetyl-CoA and succinyl-CoA, may also be compromised (20). These results demonstrate that entry and phosphorylation of glucose are functional; however, further utilization to produce downstream metabolic intermediates for glycolysis/glucose oxidation and PPP may be compromised in adrenergic hormone-deficient hearts.
In addition, several metabolites involved in oxidation/reduction were significantly decreased in adrenergic hormone-deficient hearts (Fig. 2). Notably, adrenergic hormone-deficient hearts had a significant 10-fold decrease in GSH (p < 0.05), 1.5-fold decrease in GSH disulfide (p < 0.05), and 2-fold decrease in taurine (p < 0.05) suggesting that these hearts may be experiencing some oxidative stress due to limited antioxidants (21–23). In addition, NAD+ and NADH concentrations were significantly decreased (5-fold, p < 0.05 and 500-fold, p < 0.05, respectively) in adrenergic hormone-deficient hearts. NADP+ and NADPH concentrations were also significantly decreased (10-fold, p < 0.05 and greater than 30-fold, respectively) in adrenergic hormone-deficient hearts. These data thus suggest that there is increased oxidative and decreased reducing capacity in adrenergic hormone-deficient hearts, indicating that metabolic pathways with redox enzymes, such as dehydrogenases, may have limited activities due to limitations in essential NAD+/NADH and NADP+/NADPH cofactors (24).
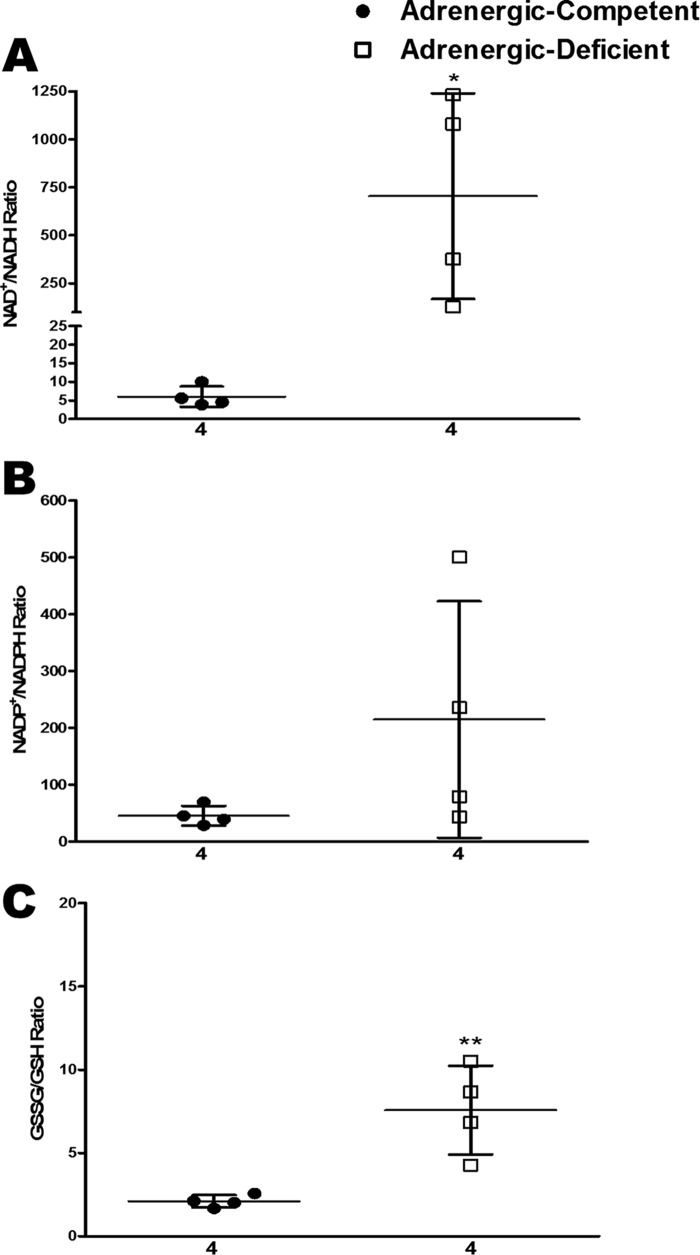
Based on these metabolomic data, the redox state of the embryonic heart appeared to be substantially less reduced in the absence of adrenergic stimulation. To quantify this, we calculated the ratios of NAD+/NADH, NADP+/NADPH, and GSSG/GSH from the LC/MS data. Remarkably, all three ratios were clearly elevated in adrenergic hormone-deficient hearts compared with competent controls (Fig. 3). It may be important to also note that in each of these examples, the oxidized forms (i.e. NAD+, NADP+, and GSSG) were also decreased in adrenergic hormone-deficient hearts compared with controls (Fig. 2), and as seen from the ratiometric displays in Fig. 3, the reduced forms were decreased even further, resulting in a shift to a more oxidized state that is less favorable for energy production. These results indicate that an adrenergic hormone-deficient state leads to an overall shift toward increased oxidized and corresponding decreased reduced forms of critical enzymatic co-factors necessary for metabolic oxidation–reduction reactions.
Figure 3.
Estimation of oxidative stress in adrenergic hormone-deficient embryonic hearts compared with controls. A–C, steady-state NAD+/NADH, NADP+/NADPH, and GSSG/GSH at E11.5 in adrenergic hormone-competent (●) and adrenergic hormone-deficient littermates (□). Numerical values below the x axes refer to the number (n) of samples analyzed. Student's t test was used to compare means between competent and deficient groups. *, p < 0.05; **, p < 0.01.
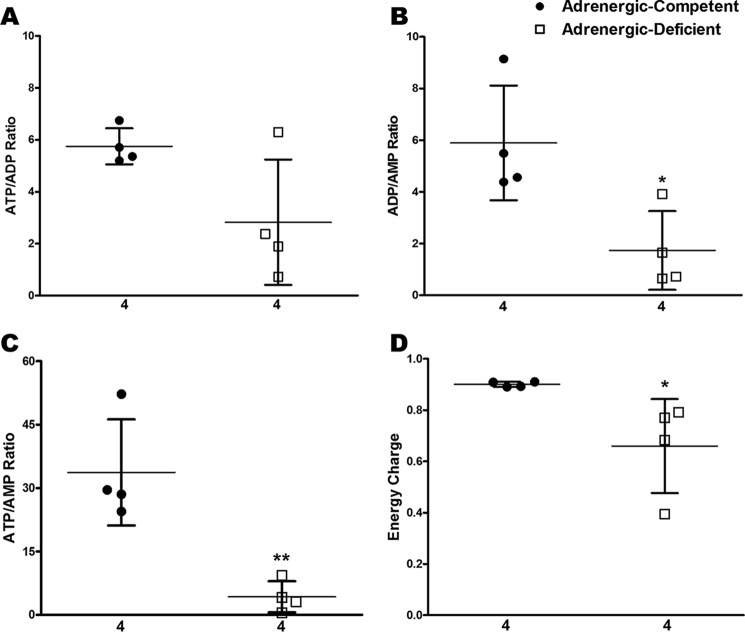
Consistent with these findings, we found that the ratios of ATP/ADP, ADP/AMP, and ATP/AMP were all decreased in adrenergic hormone-deficient E11.5 hearts relative to competent controls (Fig. 4, A–C, respectively). Furthermore, the overall “Energy Charge” or “EC” (EC = ([ATP] + 0.5[ADP])/([ATP] + [ADP] + [AMP])) (25, 26) in E11.5 mouse hearts showed that adrenergic hormone-competent control hearts had an EC of 0.90 ± 0.005, consistent with normal, healthy tissues. In contrast, the EC of adrenergic hormone-deficient hearts was significantly lower (0.66 ± 0.091, p < 0.05; Fig. 4D). It is important to note that the redox and energy ratios in Figs. 3 and 4 are not based on absolute values, but instead they are relative comparisons calculated from the metabolite pools measured by LC/MS. These results nevertheless indicate that adrenergic hormone-deficient hearts had significantly diminished energy status by E11.5 compared with adrenergic hormone-competent controls.
Figure 4.
Estimation of energy charge in adrenergic hormone-deficient embryonic hearts compared with controls. A–D, steady-state ATP/ADP, ADP/AMP, ATP/AMP, and EC = ([ATP] + 0.5 [ADP])/([ATP] + [ADP] + [AMP]) at E11.5 in adrenergic hormone-competent (●) and adrenergic hormone-deficient littermates (□). Numerical values below the x axes refer to the number (n) of samples analyzed. Student's t test was used to compare means between competent and deficient groups. *, p < 0.05; **, p < 0.01.
AMPK activation compromised in absence of adrenergic hormones
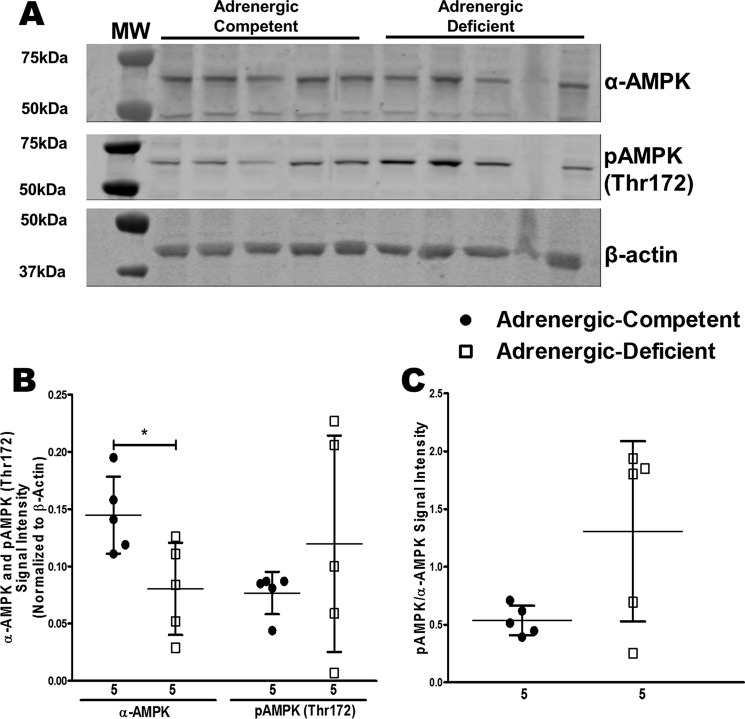
Significant decreases in ATP/AMP and EC in adrenergic hormone-deficient hearts suggested potential involvement of AMPK (27–29). We measured protein expression of the catalytic subunit, α-AMPK, and its phosphorylated form, pAMPK (Thr-172) in adrenergic hormone-deficient embryonic trunks containing the heart to assess AMPK activity. Adrenergic hormone-deficient embryos had significantly decreased α-AMPK protein concentrations compared with adrenergic hormone-competent controls. Interestingly, pAMPK protein concentrations were not significantly affected in adrenergic hormone-deficient embryos (Fig. 5, A and B). pAMPK normalized to total α-AMPK also indicated that adrenergic hormone-deficient embryos have slightly more activated AMPK compared with adrenergic hormone-competent embryos (Fig. 5C, not significant). These data suggest that AMPK activation is intact in adrenergic hormone-deficient embryos; however, this signaling is not sufficient for survival, possibly due to lower overall α-AMPK protein.
Figure 5.
Effects of adrenergic deficiency on (p)AMPK protein concentrations. A and B, α-AMPK (62 kDa) and pAMPK (62 kDa) protein concentrations in E11.5 adrenergic hormone-competent (●) and adrenergic hormone-deficient (□) embryonic trunks containing the heart (one embryo per lane) normalized to β-actin. C, pAMPK/α-AMPK in adrenergic hormone-competent (●) and adrenergic hormone-deficient embryonic trunks. Numerical values below the x axes refer to the number (n) of samples analyzed. Student's t test was used to compare means between competent and deficient groups. *, p < 0.05.
Glycolysis and the pentose–phosphate pathway are impaired in the absence of adrenergic stimulation
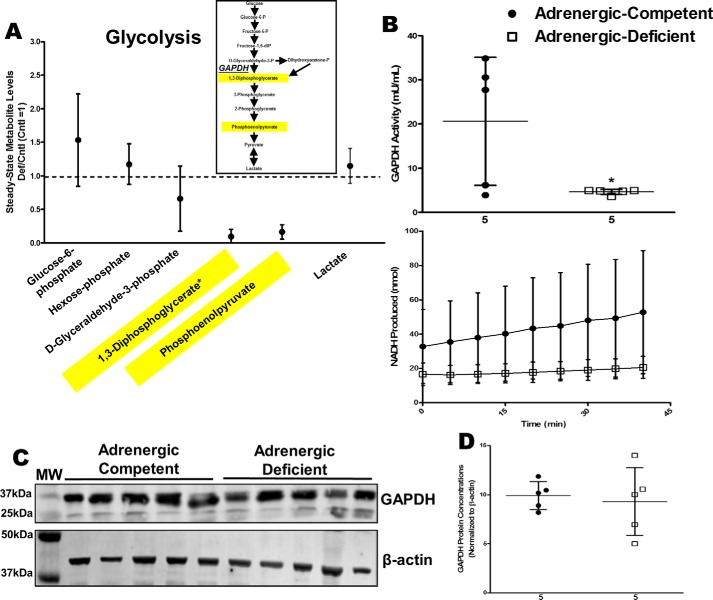
Using the metabolomics data, we examined relative amounts of reactants (substrates) and products for known metabolic reactions to identify other potential targets of adrenergic stimulation. Adrenergic hormone-deficient hearts had comparable glyceraldehyde-3-phosphate concentrations but decreased 1,3-diphosphoglycerate concentrations compared with adrenergic hormone-competent controls suggesting impairment of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Fig. 6A, see yellow highlights). To determine whether the lack of adrenergic stimulation affected GAPDH activity, we measured GAPDH rate kinetics. Our results showed that adrenergic hormone-deficient hearts had significantly decreased GAPDH activity (80% reduction, p < 0.05) compared with adrenergic hormone-competent controls (Fig. 6B). GAPDH protein concentrations were similar in adrenergic hormone-deficient and -competent embryos, suggesting adrenergic control of GAPDH likely occurs through post-translational mechanisms (Fig. 6, C and D). These results identify glycolysis and GAPDH as a metabolic pathway and checkpoint, respectively, impaired by adrenergic deficiency.
Figure 6.
Effects of adrenergic deficiency on GAPDH activity and protein concentrations. A, steady-state metabolites involved in glycolysis in E11.5 adrenergic hormone-deficient hearts compared with controls (dotted line). Schematic representation of glycolysis pathway provided as reference. B, GAPDH activity in adrenergic hormone-competent (●) and adrenergic hormone-deficient (□) embryonic hearts, expressed as milliunits of enzyme per ml of sample and NADH produced per min, respectively. Milliunits/ml are calculated as ((nmol of NADH)/(min)(ml of sample)). C and D, GAPDH protein concentrations (36 kDa) in adrenergic hormone-deficient embryonic trunks (□) compared with controls (●), normalized to β-actin. The β-actin panel is re-used as a representative image of six replicated experiments probing for GAPDH, G-6-PDH, and PDH. Numerical values below the x axes refer to the number (n) of samples analyzed. Student's t test was used to compare means between competent and deficient groups. *, p < 0.05.
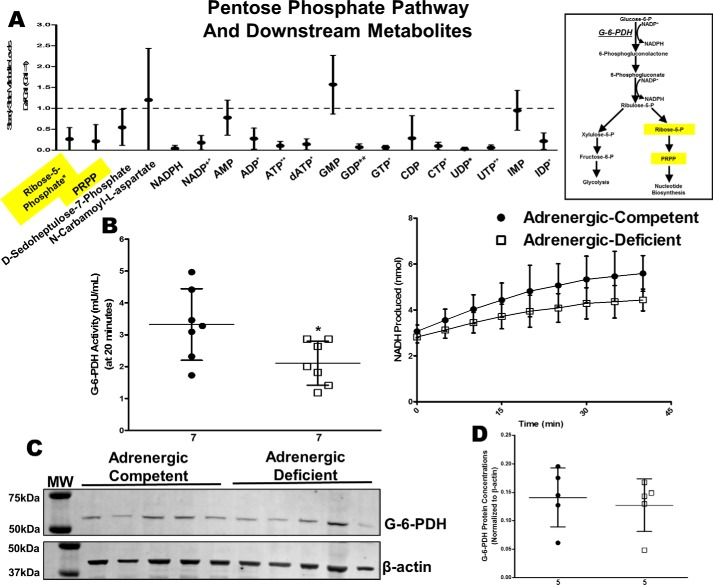
Adrenergic hormone-deficient hearts also had significantly lower concentrations of ribose -phosphate and PRPP but comparable levels of glucose 6-phosphate in adrenergic hormone-deficient hearts suggesting that glucose-6-phosphate dehydrogenase (G-6-PDH), a key rate-limiting enzymatic reaction in the PPP, may also be impaired (Fig. 7A, see yellow highlights). G-6-PDH activity was significantly lower (40% reduction, p < 0.05) in adrenergic hormone-deficient hearts (Fig. 7B);however, G-6-PDH protein concentrations did not differ compared with adrenergic hormone-competent controls (Fig. 7, C and D). Consequently, this result suggests G-6-PDH protein concentrations are not limiting, but rather that G-6-PDH activity may be lower due to lack of post-translational regulation in the absence of adrenergic hormone stimulation. Many nucleotides, including GTP, UTP, CTP, and dATP, were also significantly decreased in adrenergic hormone-deficient hearts suggesting that impaired G-6-PDH activity may also impact nucleotide biosynthesis. Thus, G-6-PDH was another major metabolic checkpoint affected by adrenergic deficiency.
Figure 7.
Effects of adrenergic deficiency on G-6-PDH activity and protein concentrations. A, steady-state metabolites involved in pentose–phosphate pathway and nucleotide biosynthesis metabolism in E11.5 adrenergic hormone-deficient hearts compared with controls (dotted line). Schematic representation of pentose–phosphate pathway and related pathways provided as reference. B, G-6-PDH activity in adrenergic hormone-competent (●) and adrenergic hormone-deficient (□) embryonic hearts, expressed as milliunits of enzyme per ml of sample and NADH produced per min, respectively. The milliunits/ml are calculated as ((nmol of NADH)/(min)(ml of sample)). C and D, G-6-PDH protein concentrations (∼50–60 kDa) in adrenergic hormone-deficient embryonic trunks (□) compared with controls (●), normalized to β-actin. The β-actin panel is re-used as a representative image of six replicated experiments probing for GAPDH, G-6-PDH, and PDH. Numerical values below the x axes refer to the number (n) of samples analyzed. Student's t test was used to compare means between competent and deficient groups. *, p < 0.05; **, p < 0.01.
Adrenergic deficiency changes phosphorylation status of GAPDH and G-6-PDH
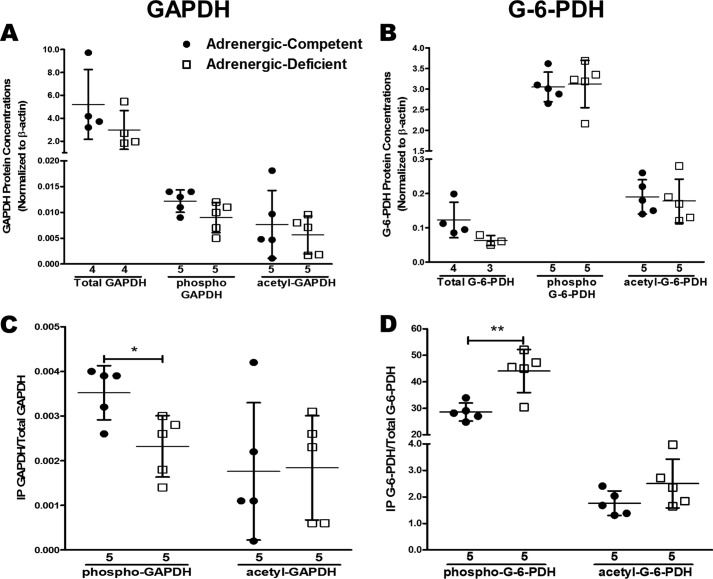
Significant decreases in GAPDH and G-6-PDH activity but similar protein concentrations in adrenergic hormone-deficient embryos compared with adrenergic hormone-competent controls suggested that adrenergic hormones regulate GAPDH and G-6-PDH activity via post-translational mechanisms. To identify post-translational changes in adrenergic hormone-deficient embryos, we immunoprecipitated phosphoserine and acetyl-lysine proteins, respectively, and phosphorylated/acetylated GAPDH and G-6-PDH were detected using Western blotting (Fig. 8). When comparing the total amounts of GAPDH, phospho-GAPDH, and acetylated GAPDH for adrenergic hormone-deficient and -competent controls, no significant differences were observed (Fig. 8A). The same was true for G-6-PDH when similar analysis was performed (Fig. 8B). In contrast, the ratio of pGAPDH relative to total GAPDH protein was found to be significantly decreased in adrenergic hormone-deficient embryos compared with adrenergic hormone-competent controls, although there was no change in the ratio of acetylated GAPDH to total GAPDH protein in these groups (Fig. 8C). There was a similar lack of significant change in the ratio of acetylated G-6-PDH to total G-6-PDH, although a slight tendency toward increased acetylated G-6-PDH was apparent in the adrenergic hormone-deficient group (Fig. 8D). Moreover, there was a significant increase in the ratio of phosphorylated G-6-PDH to total G-6-PDH protein in the adrenergic hormone-deficient group relative to adrenergic hormone-competent controls (p < 0.01; Fig. 8D). These results show that lack of adrenergic stimulation alters the phosphorylation status of GAPDH and G-6-PDH during embryonic development, thereby providing a potential mechanistic explanation for how adrenergic hormones may regulate the activity of these enzymes during embryonic development.
Figure 8.
Effects of adrenergic deficiency on phosphorylation and acetylation of GAPDH and G-6-PDH. A and B, total, phospho-, and acetyl-GAPDH and G-6-PDH protein concentrations in E11.5 adrenergic hormone-competent (●) and adrenergic hormone-deficient (□) embryonic trunks, normalized β-actin. C and D, relative phospho- and acetyl-GAPDH and G-6-PDH protein concentrations in adrenergic hormone-competent (●) and adrenergic hormone-deficient (□) embryonic trunks, normalized to total GAPDH protein or G-6-PDH protein. Numerical values below the x axes refer to the number (n) of samples analyzed. Student's t test was used to compare means between competent and deficient groups. *, p < 0.05; **, p < 0.01.
Lack of adrenergic stimulation does not affect pyruvate dehydrogenase
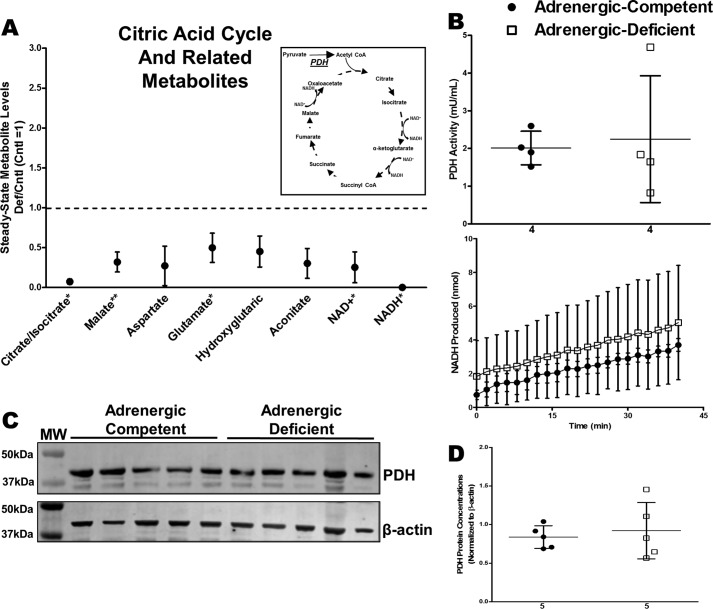
TCA cycle intermediates, citrate/isocitrate, aconitate, and malate, were significantly decreased in adrenergic hormone-deficient hearts implicating impaired mitochondrial metabolism (Fig. 9A). We measured pyruvate dehydrogenase (PDH) activity to determine whether entry into the TCA cycle was impaired in adrenergic hormone-deficient hearts. PDH activity did not differ in adrenergic hormone-deficient and -competent hearts (Fig. 9B). Adrenergic hormone-deficient hearts appeared to have slightly increased PDH activity (not significant) compared with controls, indicating that PDH clearly is not impaired by lack of adrenergic stimulation. PDH concentrations were also not affected between adrenergic hormone-deficient and -competent hearts (Fig. 9, C and D). These results indicate that adrenergic deficiency had little influence on PDH activity, thereby suggesting that addition of pyruvate substrate may aid in rescuing the observed metabolic deficiency.
Figure 9.
Effects of adrenergic deficiency on PDH activity and protein concentrations. A, steady-state metabolites involved in TCA cycle in E11.5 adrenergic hormone-deficient hearts compared with controls (dotted line). Schematic representation of TCA cycle and related pathways provided as reference. B, PDH activity in adrenergic hormone-competent (●) and adrenergic hormone-deficient (□) embryonic hearts, expressed as milliunits of enzyme per ml of sample and NADH produced per min, respectively. Milliunits/ml are calculated as ((nmol of NADH)/(min)(ml of sample)). C and D, PDH protein concentrations (39 kDa) in adrenergic hormone-deficient embryonic trunks (□) compared with controls (●), normalized to β-actin. The β-actin panel is re-used as a representative image of six replicated experiments probing for GAPDH, G-6-PDH, and PDH. Numerical values below the x axes refer to the number (n) of samples analyzed. Student's t test was used to compare means between competent and deficient groups. *, p < 0.05; **, p < 0.01.
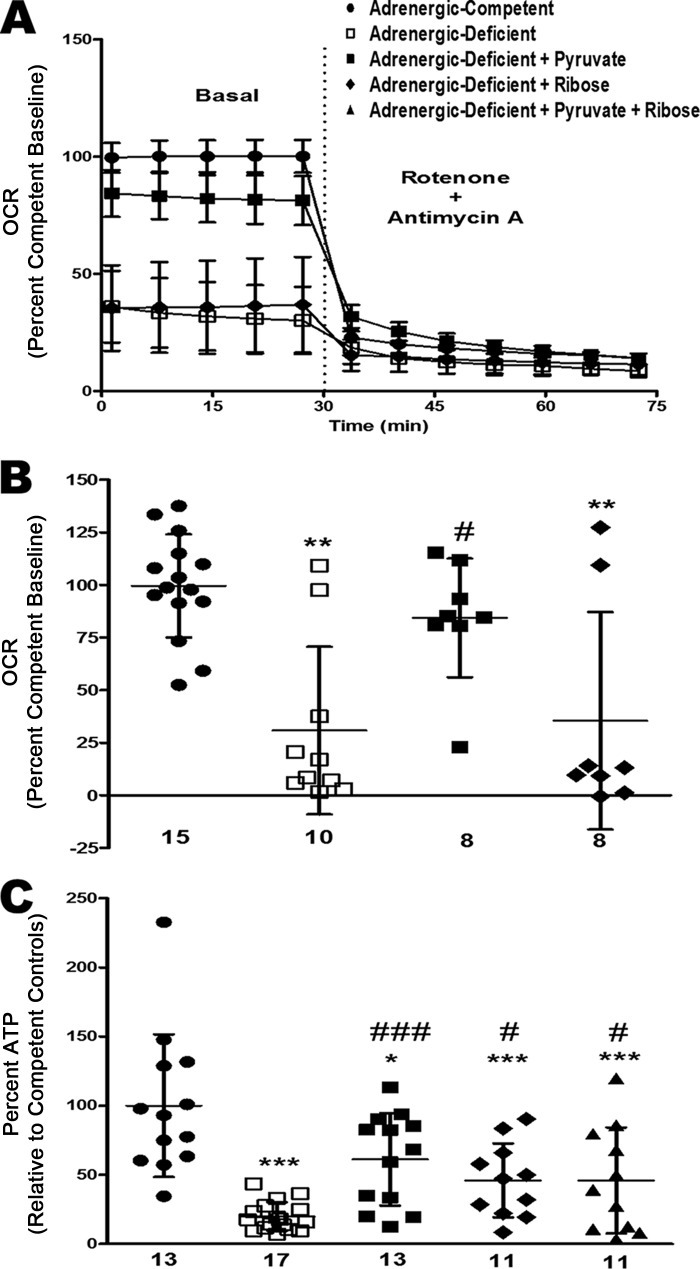
Pyruvate rescues adrenergic hormone-deficient OCR and steady-state ATP
By E11.5, adrenergic hormone-deficient embryonic hearts had significantly lower amounts of metabolites needed to produce ATP and NADH, indicating that at this developmental stage, these embryos may be metabolically starved relative to adrenergic hormone-competent controls. We thus attempted to rescue cardiac energy levels by providing essential metabolites that were limiting, such as pyruvate (for TCA cycle) and ribose (for PPP) to embryonic hearts ex vivo. Pyruvate significantly rescued OCR (∼50% increase, p < 0.05) and steady-state ATP concentrations (∼40% increase, p < 0.001) compared with untreated adrenergic hormone-deficient controls. Ribose had no effect on OCR in adrenergic hormone-deficient hearts, but it significantly rescued steady-state ATP concentrations (∼25% increase, p < 0.05; Fig. 10, A–C). Addition of pyruvate and ribose did not have an additive effect on ATP concentrations in adrenergic hormone-deficient hearts (Fig. 10C) suggesting that energy deficiency is not further rescued with both pyruvate and ribose. These results support the hypothesis that adrenergic hormone-deficient embryonic hearts are unable to produce sufficient chemical substrates (e.g. pyruvate and ribose) for glucose oxidation and nucleotide synthesis, respectively.
Figure 10.
Rescue of OCR and steady-state ATP in adrenergic hormone-deficient embryonic hearts with pyruvate or ribose. A and B, oxygen consumption rates in E11.5 adrenergic hormone-competent (●) and adrenergic hormone-deficient (□) embryonic hearts after addition of pyruvate (■), ribose (♦), and rotenone/antimycin A treatment, expressed as percentage compared with adrenergic hormone-competent control baseline. C, steady-state ATP concentrations in adrenergic hormone-competent and adrenergic hormone-deficient embryonic hearts after addition of pyruvate and ribose. Numerical values below the x axes refer to the number (n) of samples analyzed. Data are represented as a percentage compared with competent controls. One-way analysis of variance was performed with Dunnett's multiple comparison test used to evaluate differences between treated groups and control groups. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with adrenergic hormone-competent controls. #, p < 0.05; ###, p < 0.001 compared with adrenergic hormone-deficient controls.
Discussion
The aim of this study was to identify metabolic targets of adrenergic stimulation necessary for the embryonic shift from anaerobic to aerobic metabolism during a critical period of cardiac development. Recent evidence demonstrated that the electron transport chain is activated beginning at E11.5 via closing of the mitochondrial transition pore (30). Therefore, impaired flux from glycolysis to the TCA cycle would disrupt essential ATP production from the mitochondria that normally would occur during this critical transition period in cardiac development (5). Our metabolomics analysis revealed adrenergic hormone-deficient embryonic hearts have a severely compromised glucose metabolism impeding ATP production. We also demonstrated that adrenergic hormone-deficient hearts appear to harbor a more oxidized intracellular environment compared with adrenergic hormone-competent controls and thus may have impairments in anaplerotic processes, including nucleotide, amino acid, and other biosynthesis pathways. Our results also indicated an increased trend of AMPK activation via phosphorylation in adrenergic hormone-deficient embryos; however, energy status is not sufficiently restored likely due to decreased total α-AMPK. Furthermore, we have identified two key enzymes impaired as a consequence of adrenergic deficiency that impact two major avenues of ATP production: (i) GAPDH of glycolysis/aerobic respiration and (ii) G-6-PDH of PPP.
Redox shift during embryonic development
Normal developmental processes, such as differentiation and organ formation, require gradual increases in cellular oxidation and redox signaling. However, excessive increases in oxidation can lead to abnormal development and embryonic lethality (31–34). NADPH, GSH, and other antioxidants have essential roles preventing excessive oxidized levels during development (35–37). GSSG is formed as GSH peroxidase reduces reactive oxygen species to a more stable species, H2O. NADPH produced from G-6-PDH is required to regenerate GSH from GSSG via GSH reductase thus maintaining low GSSG levels (33, 38). Relative ratios of NAD(H) also serve as an indicator of the cellular redox and metabolic states (33, 39). Our results show significant increases in NAD+/NADH and GSSG/GSH ratios as well as nonsignificant increases in NADP+/NADPH ratios suggesting that the redox state in adrenergic hormone-deficient hearts is shifted to a more oxidized state with less reducing potential needed to drive metabolic reactions, including glycolysis, PPP, and TCA cycle pathways.
GAPDH
GAPDH, an essential enzyme of glycolysis that converts glyceraldehyde 3-phosphate to 1,3-diphosphoglycerate, was identified as an important target affected by the absence of adrenergic hormone influence. Decreased GAPDH activity in adrenergic hormone-deficient hearts was accompanied by decreased phosphorylation of GAPDH and decreased 1,3-diphosphoglycerate and phosphoenolpyruvate concentrations implicating adrenergic regulation of GAPDH. One potential regulatory mechanism following adrenergic stimulation is phosphorylation of GAPDH to increase activity (40, 41). Studies have shown several kinases regulated by adrenergic stimulation, including Akt/PKB, CaMKIIβ, PKC, and AMPK, phosphorylate GAPDH to enhance activity (42–45). Thus, the observed decrease in the ratio of phosphorylated to total GAPDH protein in adrenergic hormone-deficient embryos is consistent with the decreased enzymatic activity observed for GAPDH in these embryos. Although activation of AMPK appears to be intact in adrenergic hormone-deficient embryos, decreased α-AMPK protein concentrations may be preventing sufficient GAPDH phosphorylation and activation leading to disruption of the glycolysis pathway. Evidence also suggests that GAPDH activity is highly sensitive to oxidative stress (37, 46–50), and hence, the limitations in important NAD+/NADH co-factors likely also contributed to the observed decreases in GAPDH activity in adrenergic hormone-deficient hearts.
Interestingly, however, cardiac lactate concentrations were not significantly affected by adrenergic deficiency. Possible explanations for this finding could be minimal GAPDH activity is compensated by extra-cardiac (including maternal circulation) lactate or other pyruvate sources, such as alanine, glycine, and/or glycerol 3-phosphate (which was not diminished in concentration in adrenergic hormone-deficient hearts, see Fig. 2, heat map), that help maintain lactate concentrations under anaerobic conditions (51–53). This would indicate that adrenergic hormones are necessary to shift the fate of glucose from anaerobic lactate formation to glucose oxidation and aerobic respiration during this phase of embryonic development (embryonic shift) (5). Another explanation is lactate produced from glucose feeds into the TCA cycle (54). Because the TCA cycle is compromised in adrenergic hormone-deficient embryonic hearts, this may also contribute to the relatively unaffected lactate concentrations in these hearts compared with those found in adrenergic hormone-competent controls.
G-6-PDH
Our metabolomic results also identified G-6-PDH, the rate-limiting enzyme in PPP that converts glucose 6-phosphate to 6-phosphogluconolactone, as another target of adrenergic stimulation. We show that adrenergic hormone-deficient embryos have decreased G-6-PDH activity, increased ratios of phosphorylated to total G-6-PDH protein, and decreased ribose 5-phosphate, PRPP, and nucleotide concentrations indicating G-6-PDH and PPP are effectively regulated by adrenergic stimulation during embryonic development. Other studies have also shown adrenergic regulation of PPP in working rat hearts exposed to NE and other adrenergic agonists with increased G-6-PDH activity, PRPP concentrations, and adenine nucleotide biosynthesis (59–61). During development, G-6-PDH has been shown to be protective against oxidative stress resulting from placental circulation and nutrient/respiratory exchange (31, 38). Additionally, phosphorylation of G-6-PDH has been shown to decrease activity leading to increased oxidative stress (62, 63). The decreased G-6-PDH activity and increased NAD+/NADH and NADP+/NADPH indicate adrenergic hormone-deficient embryos have shifted to a more oxidized status with consequently less reducing power that could contribute to oxidative stress. Therefore, our results suggest that adrenergic stimulation may regulate G-6-PDH by reducing phosphorylation status, which in turn leads to increased enzymatic activity thereby allowing for protection against oxidative stresses in the embryonic heart.
Furthermore, observed deficits in ribose 5-phosphate concentrations in adrenergic hormone-deficient hearts suggested that addition of d-ribose may also be an effective rescue strategy to bypass the G-6-PDH reaction and improve energy status. Consistent with this hypothesis, we showed that addition of ribose significantly improved ATP concentrations in adrenergic hormone-deficient hearts, although it was interestingly neither additive nor synergistic when applied in conjunction with pyruvate, possibly reflecting the overall increase in oxidized status under conditions when adrenergic hormones are not present.
Metabolic rescue of energy metabolism
Our observations of comparable glucose 6-phosphate, but decreased citrate/isocitrate and malate, suggested that pyruvate oxidation and PDH activity may have been impaired in adrenergic hormone-deficient hearts. Intriguingly, however, PDH activity was not affected in adrenergic hormone-deficient hearts, indicating that not all metabolic dehydrogenase enzymes were equally affected by the absence of adrenergic stimulation possibly due to differing sensitivities to oxidation (55, 56). For example, GAPDH and G-6-PDH have been shown to be sensitive to oxidative stress, whereas oxidative environments have lesser effects on PDH (57, 58).
Because PDH rates were similar to adrenergic hormone-competent controls, addition of the metabolic substrate pyruvate to adrenergic hormone-deficient hearts should provide effective rescue that could facilitate restoration of cellular energy levels. Ex vivo delivery of pyruvate to adrenergic hormone-deficient embryonic hearts substantially improved OCR and steady-state ATP levels, demonstrating that apparent metabolic starvation could be overcome by addition of the appropriate substrate(s). We previously showed that adrenergic hormone-deficient embryos have larger and more elongated mitochondria compared with controls, consistent with a “starvation” phenotype (12, 64, 65). Taken together, these results suggest that mitochondrial metabolism is capable of functioning in adrenergic hormone-deficient embryos, but impaired GAPDH activity may limit the amount of pyruvate substrate entering mitochondria to fuel aerobic respiration.
Summary
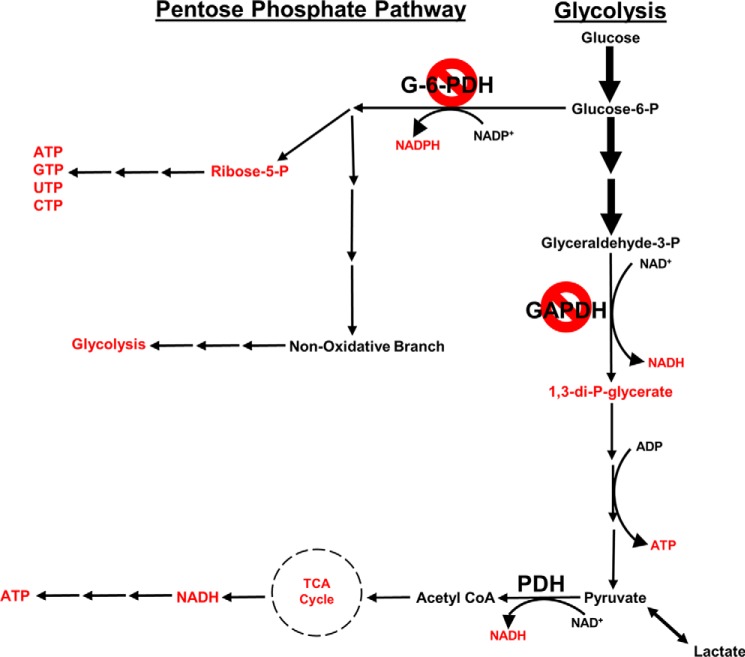
Adrenergic hormone-deficient embryos have severely compromised glucose metabolism, specifically involving glycolysis, PPP, and the TCA cycle (Fig. 11). We have shown that the absence of adrenergic hormones selectively affects GAPDH and G-6-PDH during embryonic cardiac development, suggesting that adrenergic stimulation is necessary to enable GAPDH and G-6-PDH to produce sufficient metabolic fuel and anaplerotic substrates, respectively, for successful transition from anaerobic to aerobic metabolism during a crucial phase of embryonic development.
Figure 11.
Summary of metabolic pathways and targets affected by adrenergic deficiency during embryonic heart development. Shown is a schematic representation of metabolites and enzymes of PPP, glycolysis, and TCA cycle. Metabolites and enzymes are significantly lower in adrenergic hormone-deficient embryos compared with competent controls as indicated in red or by the red symbol, respectively.
Experimental procedures
Mice
Female (2–6 months) and male (2–12 months) C57BL/6J mice mated in the present studies were housed in a 12-h light/12-h dark cycle and given ad libitum access to food and water. All procedures and handling of mice were conducted in accordance with and approved by the University of Central Florida Institutional Animal Care and Use Committee and the guidelines established by the National Institutes of Health for vertebrate animal research (66, 67). The Dbh mouse colony was kindly provided by the Palmiter laboratory (University of Washington, Seattle) (8) and maintained as described previously (11, 12, 68). Timed pregnancies were determined by the presence of a vaginal plug at E0.5. Pregnancies were confirmed by high-resolution ultrasound at E8.5 using a Visual Sonics Vevo3100 instrument with an MX250 transducer.
Embryonic tissue collections
All embryos appearing healthy in color, size (crown–rump length = 6–7 mm), texture, morphology, heartbeat, and blood circulation during microscopic examination were used for this study. All unhealthy and dead embryos were discarded. No differences were seen upon gross examination of adrenergic hormone-deficient (Dbh−/−) and adrenergic hormone-competent control (Dbh+/+ and Dbh+/−) embryonic hearts at time of isolation. Embryonic heads were isolated for genotyping. For LC-MS metabolomics analysis, enzyme activity assays, and immunoblotting E11.5 hearts or trunks containing the heart were flash-frozen in liquid nitrogen and stored at −80 °C for further analysis.
Ex vivo embryonic heart culture
E11.5 hearts were isolated under aseptic conditions and cultured overnight in Dulbecco's modified Eagle's medium (DMEM) (no glucose, no glutamine, no phenol red) (Thermo Fisher Scientific) containing 5% fetal bovine serum (Hyclone Laboratories) that was charcoal-stripped of catecholamines and steroid hormones. The medium was additionally supplemented with penicillin G (100,000 units/liter) and streptomycin (100 mg/liter). Cultures were incubated overnight prior to next day steady-state ATP measurements.
Reagents
Chemical reagents were purchased from Sigma, except where noted otherwise.
Steady-state ATP measurements
Isolated E11.5 embryonic hearts were flash-frozen in liquid nitrogen and stored at −80 °C. Samples were homogenized with 6% TCA. Ex vivo embryonic heart cultures were placed in DMEM supplemented with pyruvate (11 mm), ribose (6.6 mm), or control (no supplement) following overnight incubation. Cultures were incubated for 2 h, then collected and sonicated in 50 μl of media and 10 μl of 6% TCA.
All samples were centrifuged at 6000 × g for 5 min at 4 °C. Supernatant was collected and neutralized with 1× Tris acetate, as described previously (12, 69). ATP measurements were performed with the ATPlite Bioluminescence Assay (PerkinElmer Life Sciences) according to the manufacturer's protocol. Standard curves were generated with known concentrations of ATP. Luminescence was measured with Envision Multilabel plate reader, and ATP measurements were normalized to total protein concentrations determined using Thermo Fisher Scientific NanoDrop 8000 spectrophotometer.
ATP synthesis/hydrolysis assays
Isolated E11.5 embryonic hearts were flash-frozen in liquid nitrogen and stored at −80 °C. Samples were homogenized for 30 s in a buffer containing 25 mm Tris-HCl, 40 mm KCl, 2 mm EGTA, 5 mm MgCl2, 0.8 m sucrose, and 200 μm phenylmethylsulfonyl fluoride. Samples went through two freeze/thaw cycles and vortexing before centrifugation at 600 × g for 10 min at 4 °C. Supernatant was collected and solubilized in 50 μg/μl digitonin.
ATP synthesis standards were generated with known concentrations of ATP. Synthesis activity was measured in 96-well white wall plates in 100-μl reactions containing 1 mm pyruvate, 1 mm malate, 30 μm P1,P5-di(adenosine 5′)-pentaphosphate (Ap5A, adenylate kinase inhibitor), 0.5 μg/ml luciferase, 40 μm luciferin, and 100 μm ADP (70, 71). Luminescence was measured from 5-μl sample extracts in 5-s intervals for 1 min.
ATP hydrolysis standards were generated with known concentrations of monopotassium phosphate. Hydrolysis activity was measured in 1-ml reactions containing 1 mm ATP, 80 mm sulfuric acid, 10 mm ascorbic acid, 50 μm tartrate, and 700 μm ammonium molybdate (72). Absorbance was measured at 850 nm in 2-μl extracts in quartz cuvettes in 5-s intervals for 30 s.
Liquid chromatography-mass spectrometry
Isolated E11.5 embryonic hearts were flash-frozen in liquid nitrogen and stored at −80 °C. Samples were prepared as previously described (73). Briefly, metabolites were serially extracted from tissue powder of each heart in 80% methanol (1 then 0.5 ml) and extracts were dried under nitrogen gas and stored at −80 °C. Samples were suspended in 120 μl of 50% methanol and, for amino acid detection, were derivatized with 2% (v/v) triethylamine and benzyl chloroformate. Metabolites were separated by reverse phase HPLC (Shimadzu) and identified by single reaction monitoring on a triple-quadrupole mass spectrometer (Thermo Fisher Scientific QuantumUltra). Data were analyzed using a publicly available MzRockmachine learning tool kit (http://code.google.com/p/mzrock/), which automated metabolite identification based on retention time, whole-molecule mass, collision energy, and fragment mass. Selected metabolites were also analyzed using XCalibur Qual Browser (Thermo Fisher Scientific). We attempted to measure pyruvate and acetyl-CoA by LC/MS and other biochemical techniques; however, detection was limited in both adrenergic hormone-competent as well as deficient controls, possibly due to rapid flux from pyruvate to acetyl-CoA to citrate (74, 75).
GAPDH, G-6-PDH, and PDH activities
GAPDH activity assay kit (catalogue number ab204732) was purchased from Abcam. G-6-PDH and PDH activity assay kits (catalogue numbers MAK105 and MAK183) were purchased from Sigma. Standard curves were generated for these activity assays with known concentrations of NADH. Flash-frozen embryonic hearts were sonicated for 5 s in cold PBS or buffer specified in manufacturers' protocols.
For the GAPDH assay, extracts were incubated on ice for 10 min and centrifuged at 10,000 × g for 5 min at 4 °C, and supernatant was collected. Absorbance was measured at 450 nm in 25-μl extracts in a clear 96-well plate in 5-min intervals for 40 min, according to the manufacturer's protocol.
For the G-6-PDH assay, extracts were centrifuged at 15,000 × g for 10-min at 4 °C, and the supernatant was collected. Absorbance was measured at 450 nm in 25-μl extracts in a clear 96-well plate in 5-min intervals for 40 min, according to the manufacturer's protocol.
For the PDH assay, extracts were incubated on ice for 10 min and centrifuged at 10,000 × g for 5 min at 4 °C, and the supernatant was collected. Absorbance was measured at 450 nm in 25-μl extracts in a clear 96-well plate in 2-min intervals for 40 min, according to the manufacturer's protocol.
Western blotting
Flash-frozen embryonic trunks were sonicated in 1× RIPA Buffer supplemented with 100× HaltTM Protease Inhibitor Mixture and 100× HaltTM Phosphatase Inhibitor Single Use Mixture (ThermoFisher Scientific). Protein was quantified using a Bradford assay (Sigma). Protein lysates (50 μg/lane) were separated on an 4–12% BisTris Precast gel (Thermo Fisher Scientific) followed by transfer to PVDF membrane (Thermo Fisher Scientific). Membranes were incubated in TBS blocking buffer for 1 h at 4 °C (LICOR Odyssey). Membranes were then incubated overnight at 4 °C in primary antibodies. Antibodies used included the following: phospho-AMPK (Thr-172) (rabbit polyclonal IgG, 1:1000, Cell Signaling Technology (CST), catalogue number 2531S, lot number 15); α-AMPK (rabbit polyclonal IgG, 1:1000, CST, catalogue number 2532S, lot number 19); GAPDH (rabbit polyclonal IgG, 1:2500, Thermo Fisher Scientific, catalogue number PA1–987, lot number RF233990); G-6-PDH (rabbit polyclonal IgG, 1:1000, (CST), catalogue number 8866, lot number 3); and PDH (rabbit polyclonal IgG, 1:500, CST, catalogue number 2784, lot number 2). β-Actin (monoclonal mouse IgG2b, 1:5000, Thermo Fisher Scientific catalogue number MA5-15739, lot number RH236746) was used as a loading control. Following three TBS-T washes, membranes were incubated for 1 h at room temperature in secondary antibodies (LICOR Odyssey goat anti-rabbit or anti-mouse IRDye 680 or 800, catalogue number 926-32210, lot number C40528-02, and catalogue number 926-68021, lot number C40415-04). This procedure was replicated by probing six individual blots for GAPDH, G-6-PDH, and β-actin, then stripping each blot with Restore Fluorescent Western blotting Stripping Buffer (Thermo Fisher Scientific), and re-probing for PDH. pAMPK and β-actin were also probed together, then stripped and re-probed for AMPK. Blots were visualized using a LICOR Odyssey IR imaging system. All blots were analyzed using ImageStudio software and normalized to β-actin.
Immunoprecipitations
Flash-frozen embryonic trunks were sonicated in 1× Tris-HCl supplemented with 100× Protease Inhibitor Mixture, 100× Phosphatase Inhibitor Mixture, and 100× sodium butyrate. Protein was quantified using a Bradford assay. Antibodies were incubated with 100 μl of Pierce protein A-agarose (Thermo Fisher Scientific) for 1 h at room temperature. Antibodies used to precipitate phosphoproteins and acetylated proteins were anti-phosphoserine (pSer) (rabbit polyclonal IgG, 20 μg/mg lysate, Abcam, catalogue number ab9332, lot number GR3179704-4) and anti-acetylated lysine (rabbit polyclonal IgG, 1:100, CST, catalogue number 9441S, lot number 13). Embryonic trunk lysates (50 μg) were incubated with antibody/agarose conjugates overnight at 4 °C. Bound protein was washed three times in 1× Tris-HCl and eluted with 1:1 1× Tris-HCl and 2× Laemmli sample buffer (4% SDS, 10% DTT, 20% glycerol, 0.004% bromphenol blue, and 0.125 m Tris-HCl, pH 6.8) at 95 °C for 5 min. Lysates were analyzed for phosphorylation and acetylation of G-6-PDH and GAPDH by Western blotting.
OCR measurements
Ex vivo E11.5 mouse hearts were isolated as mentioned above and incubated overnight in a Seahorse Biosciences XF24 Islet Capture Microplate with mesh grids placed on top of the specimen to prevent floating and probe interference. The next day, hearts were incubated in Seahorse XF Base Medium Minimal DMEM (0 mm glucose) supplemented with pyruvate (1 mg/ml), ribose (1 mg/ml), or control (no supplement) for 2 h. Basal OCR was measured at 6-min intervals over 72 min using a Seahorse XFe Biosciences system. Rotenone (5 μm) and antimycin A (20 μm) were added simultaneously to block mitochondrial electron transport (76).
Statistics
Data are expressed as means ± S.D. Student's t tests were performed, unless otherwise noted, to compare means between adrenergic hormone-competent and adrenergic hormone-deficient groups, with p < 0.05 required to reject the null hypothesis.
Author contributions
J. N. P., P. S. B., G. A. P., and S. N. E. conceptualization; J. N. P. data curation; J. N. P., T. M., Q. L., G. A. P., and S. N. E. formal analysis; J. N. P. validation; J. N. P., T. M., Q. L., S. M. N., P. S. B., G. A. P., V. L. D., and S. N. E. investigation; J. N. P., T. M., Q. L., S. M. N., P. S. B., and V. L. D. methodology; J. N. P. writing-original draft; J. N. P., T. M., Q. L., P. S. B., G. A. P., V. L. D., and S. N. E. writing-review and editing; P. S. B., V. L. D., and S. N. E. supervision; G. A. P. and S. N. E. resources; S. N. E. funding acquisition; S. N. E. project administration.
This work was supported by University of Central Florida College of Medicine (to S. N. E.), National Institutes of Health Grant R01-HL-071158 (to P. S. B.), and American Heart Association Founder's Affiliate Grant 12GRNT12060233 (to G. A. P.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- EPI
- epinephrine
- AMPK
- AMP-activated protein kinase
- Dbh
- dopamine β-hydroxylase
- EC
- energy charge
- G-6-PDH
- glucose-6-phosphate dehydrogenase
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- NE
- norepinephrine
- OCR
- oxygen consumption rate
- PDH
- pyruvate dehydrogenase
- PKC
- protein kinase C
- PPP
- pentose–phosphate pathway
- PRPP
- phosphoribosyl pyrophosphate
- TCA
- tricarboxylic acid cycle
- DMEM
- Dulbecco's modified Eagle's medium
- Ap5A
- P1,P5-di(adenosine 5′)-pentaphosphate
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
References
- 1. Houghton F. D., Thompson J. G., Kennedy C. J., and Leese H. J. (1996) Oxygen consumption and energy metabolism of the early mouse embryo. Mol. Reprod. Dev. 44, 476–485 10.1002/(SICI)1098-2795(199608)44:4%3C476::AID-MRD7%3E3.0.CO%3B2-I [DOI] [PubMed] [Google Scholar]
- 2. Taegtmeyer H., Sen S., and Vela D. (2010) Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann. N.Y. Acad. Sci. 1188, 191–198 10.1111/j.1749-6632.2009.05100.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cox S. J., and Gunberg D. L. (1972) Energy metabolism in isolated rat embryo hearts: effect of metabolic inhibitors. J. Embryol. Exp. Morphol. 28, 591–599 [PubMed] [Google Scholar]
- 4. Cox S. J., and Gunberg D. L. (1972) Metabolite utilization by isolated embryonic rat hearts in vitro. J. Embryol. Exp. Morphol. 28, 235–245 [PubMed] [Google Scholar]
- 5. Baker C. N., and Ebert S. N. (2013) Development of aerobic metabolism in utero: requirement for mitochondrial function during embryonic and foetal periods. OA Biotech. 2, 16 [Google Scholar]
- 6. Zhou Q. Y., Quaife C. J., and Palmiter R. D. (1995) Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse development. Nature 374, 640–643 10.1038/374640a0 [DOI] [PubMed] [Google Scholar]
- 7. Porter G. A., and Rivkees S. A. (2001) Ontogeny of humoral heart rate regulation in the embryonic mouse. Am. J. Physiol. Regul. Integ. Comp. Physiol. 281, R401–R407 10.1152/ajpregu.2001.281.2.R401 [DOI] [PubMed] [Google Scholar]
- 8. Thomas S. A., Matsumoto A. M., and Palmiter R. D. (1995) Noradrenaline is essential for mouse fetal development. Nature 374, 643–646 10.1038/374643a0 [DOI] [PubMed] [Google Scholar]
- 9. Ebert S. N., Rong Q., Boe S., and Pfeifer K. (2008) Catecholamine-synthesizing cells in the embryonic mouse hearts. Ann. N.Y. Acad. Sci. 1148, 317–324 10.1196/annals.1410.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xia J., Varudkar N., Baker C. N., Abukenda I., Martinez C., Natarajan A., Grinberg A., Pfeifer K., and Ebert S. N. (2013) Targeting of the enhanced green fluorescent protein reporter at adrenergic cells in mice. Mol. Biotechnol. 54, 350–360 10.1007/s12033-012-9570-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Osuala K., Baker C. N., Nguyen H. L., Martinez C., Weinshenker D., and Ebert S. N. (2012) Physiological and genomic consequences of adrenergic deficiency during embryonic/fetal development in mice: impact on retinoic acid metabolism. Physiol. Genomics 44, 934–947 10.1152/physiolgenomics.00180.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baker C. N., Gidus S. A., Price G. F., Peoples J. N., and Ebert S. N. (2015) Impaired cardiac energy metabolism in embryos lacking adrenergic stimulation. Am. J. Physiol. Endocrinol. Metab. 308, E402–E413 10.1152/ajpendo.00267.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Larsson N. G., Wang J., Wilhelmsson H., Oldfors A., Rustin P., Lewandoski M., Barsh G. S., and Clayton D. A. (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 18, 231–236 10.1038/ng0398-231 [DOI] [PubMed] [Google Scholar]
- 14. Chen H., Detmer S. A., Ewald A. J., Griffin E. E., Fraser S. E., and Chan D. C. (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 160, 189–200 10.1083/jcb.200211046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishihara N., Nomura M., Jofuku A., Kato H., Suzuki S. O., Masuda K., Otera H., Nakanishi Y., Nonaka I., Goto Y., Taguchi N., Morinaga H., Maeda M., Takayanagi R., Yokota S., and Mihara K. (2009) Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat. Cell Biol. 11, 958–966 10.1038/ncb1907 [DOI] [PubMed] [Google Scholar]
- 16. Rahn J. J., Stackley K. D., and Chan S. S. (2013) Opa1 is required for proper mitochondrial metabolism in early development. PLoS ONE 8, e59218 10.1371/journal.pone.0059218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Warshaw J. B., and Terry M. L. (1970) Cellular energy metabolism during fetal development II. Fatty acids oxidation by the developing heart. J. Cell Biol. 44, 354–360 10.1083/jcb.44.2.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herrera E., and Amusquivar E. (2000) Lipid metabolism in the fetus and newborn. Diabetes Metab. Res. Rev. 16, 202–210 10.1002/1520-7560(200005/06)16:3%3C202::AID-DMRR116%3E3.0.CO%3B2-# [DOI] [PubMed] [Google Scholar]
- 19. Bartelds B., Takens J., Smid G. B., Zammit V. A., Prip-Buus C., Kuipers J. R., and van der Leij F. R. (2004) Myocardial carnitine palmitoyltransferase I expression and long-chain fatty acid oxidation in fetal and newborn lambs. Am. J. Physiol. Heart Circ. Physiol. 286, H2243–H2248 10.1152/ajpheart.00864.2003 [DOI] [PubMed] [Google Scholar]
- 20. Robishaw J. D., and Neely J. R. (1984) Pantothenate kinase and control of CoA synthesis in heart. Am. J. Physiol. 246, H532–H541 [DOI] [PubMed] [Google Scholar]
- 21. Hill M. F., and Singal P. K. (1997) Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circulation 96, 2414–2420 10.1161/01.CIR.96.7.2414 [DOI] [PubMed] [Google Scholar]
- 22. Jong C. J., Azuma J., and Schaffer S. (2012) Mechanism underlying the antioxidant activity of taurine: prevent of mitochondrial oxidant production. Amino Acids 42, 2223–2232 10.1007/s00726-011-0962-7 [DOI] [PubMed] [Google Scholar]
- 23. Nandhini A. T., Thirunavukkarasu V., Ravichandran M. K., and Anuradha C. V. (2005) Effect of taurine on biomarkers of oxidative stress in tissues of fructose-fed insulin-resistant rats. Singapore Med. J. 46, 82–87 [PubMed] [Google Scholar]
- 24. Ying W. (2008) NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid. Redox Signal. 10, 179–206 10.1089/ars.2007.1672 [DOI] [PubMed] [Google Scholar]
- 25. Atkinson D. E. (1968) The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 7, 4030–4034 10.1021/bi00851a033 [DOI] [PubMed] [Google Scholar]
- 26. Chapman A. G., and Atkinson D. E. (1973) Stabilization of adenylate energy charge by the adenylate deaminase reaction. J. Biol. Chem. 248, 8309–8312 [PubMed] [Google Scholar]
- 27. Kahn B. B., Alquier T., Carling D., and Hardie D. G. (2005) AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 1, 15–25 10.1016/j.cmet.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 28. Kemp B. D., Stapleton D., Campbell D. J., Chen Z. P., Murthy S., Walter M., Gupta A., Adams J. J., Katsis F., van Denderen B., Jennings I. G., Iseli T., Michell B. J., and Witters L. A. (2003) AMP-activated protein kinase, super metabolic regulator. Biochem. Soc. Trans. 31, 162–168 10.1042/bst0310162 [DOI] [PubMed] [Google Scholar]
- 29. Zaha V. G., and Young L. H. (2012) AMP-activated protein kinase regulation and biological actions in the heart. Circ. Res. 111, 800–814 10.1161/CIRCRESAHA.111.255505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beutner G., Eliseev R. A., and Porter G. A. (2014) Initiation of electron transport chain activity in the embryonic heart coincides with the activation of mitochondrial complex I and formation of supercomplexes. PLoS ONE 9, e113330 10.1371/journal.pone.0113330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dennery P. A. (2007) Effects of oxidative stress on embryonic development. Birth Defects Res. C Embryo Today 81, 155–162 10.1002/bdrc.20098 [DOI] [PubMed] [Google Scholar]
- 32. Matsui M., Oshima M., Oshima H., Takaku K., Maruyama T., Yodoi J., and Taketo M. M. (1996) Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev. Biol. 178, 179–185 10.1006/dbio.1996.0208 [DOI] [PubMed] [Google Scholar]
- 33. Harvey A. J., Kind K. L., and Thompson J. G. (2002) REDOX regulation of early embryo development. Reproduction 123, 479–486 10.1530/rep.0.1230479 [DOI] [PubMed] [Google Scholar]
- 34. Land S. C., and Wilson S. M. (2005) Redox regulation of lung development and perinatal lung epithelial function. Antioxid. Redox Signal. 7, 92–107 10.1089/ars.2005.7.92 [DOI] [PubMed] [Google Scholar]
- 35. Schafer F. Q., and Buettner G. R. (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 30, 1191–1212 10.1016/S0891-5849(01)00480-4 [DOI] [PubMed] [Google Scholar]
- 36. Hansen J. M., Choe H. S., Carney E. W., and Harris C. (2001) Differential antioxidant enzyme activities and glutathione content between rat and rabbit conceptuses. Free Radic. Biol. Med. 30, 1078–1088 10.1016/S0891-5849(01)00502-0 [DOI] [PubMed] [Google Scholar]
- 37. Grant C. M. (2008) Metabolic reconfiguration is a regulation response to oxidative stress. J. Biol. 7, 1 10.1186/jbiol63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nicol C. J., Zielenski J., Tsui L., and Wells P. G. (2000) An embryo protective role for glucose-6-phosphate dehydrogenase in developmental oxidative stress and chemical teratogenesis. FASEB J. 14, 111–127 10.1096/fasebj.14.1.111 [DOI] [PubMed] [Google Scholar]
- 39. Barron J. T., Gu L., and Parrillo J. E. (1998) Malate-aspartate shuttle, cytoplasmic NADH redox potential and energetics in vascular smooth muscle. J. Mol. Cell. Cardiol. 30, 1571–1579 10.1006/jmcc.1998.0722 [DOI] [PubMed] [Google Scholar]
- 40. Nicholls C., Li H., and Liu J. P. (2012) GAPDH: a common enzyme with uncommon functions. Clin. Exp. Pharmacol. Physiol. 39, 674–679 10.1111/j.1440-1681.2011.05599.x [DOI] [PubMed] [Google Scholar]
- 41. Sergienko E. A., Kharitonenkov A. I., Bulargina T. V., Muronetz V. V., and Nagradova N. K. (1992) d-Glyceraldehyde-3-phosphate dehydrogenase purified from rabbit muscle contains phosphotyrosine. FEBS Lett. 304, 21–23 10.1016/0014-5793(92)80580-A [DOI] [PubMed] [Google Scholar]
- 42. Baba T., Kobayashi H., Kawasaki H., Mineki R., Naito H., and Ohmori D. (2010) Glyceraldehyde-3-phosphate dehydrogenase interacts with phosphorylated Akt resulting from increased blood glucose in rat cardiac muscle. FEBS Lett. 584, 2796–2800 10.1016/j.febslet.2010.05.015 [DOI] [PubMed] [Google Scholar]
- 43. Tisdale E. J. (2002) Glyceraldehyde-3-phosphate dehydrogenase is phosphorylated by protein kinase C and plays a role in microtubule dynamics in the early secretory pathway. J. Biol. Chem. 277, 3334–3341 10.1074/jbc.M109744200 [DOI] [PubMed] [Google Scholar]
- 44. Singh P., Salih M., Leddy J. J., and Tuana B. S. (2004) The muscle-specific calmodulin-dependent protein kinase assembles with the glycolytic enzyme at the sarcoplasmic reticulum and modulates the activity of glyceraldehyde-3-phosphate dehydrogenase in a Ca2+/calmodulin-dependent manner. J. Biol. Chem. 279, 35176–35182 10.1074/jbc.M402282200 [DOI] [PubMed] [Google Scholar]
- 45. Chang C., Su H., Zhang D., Wang Y., Shen Q., Liu B., Huang R., Zhou T., Peng C., Wong C. C., Shen H. M., Lippincott-Schwartz J., and Liu W. (2015) AMPK-dependent phosphorylation of GAPDH triggers Sirt1 activation and is necessary for autophagy upon glucose starvation. Mol. Cell 60, 930–940 10.1016/j.molcel.2015.10.037 [DOI] [PubMed] [Google Scholar]
- 46. Schmalhausen E. V., Pleten' A. P., and Muronetz V. I. (2003) Ascorbate-induced oxidation of glyceraldehyde-3-phosphate dehydrogenase. Biochem. Biophys. Res. Commun. 308, 492–496 10.1016/S0006-291X(03)01421-9 [DOI] [PubMed] [Google Scholar]
- 47. Arutyunova E. I., Danshina P. V., Domnina L. V., Pleten A. P., and Muronetz V. I. (2003) Oxidation of glyceraldehyde-3-phosphate dehydrogenase enhances its binding to nucleic acids. Biochem. Biophys. Res. Commun. 307, 547–552 10.1016/S0006-291X(03)01222-1 [DOI] [PubMed] [Google Scholar]
- 48. Pierce A., Mirzaei H., Muller F., De Waal E., Taylor A. B., Leonard S., Van Remmen H., Regnier F., Richardson A., and Chaudhuri A. (2008) GAPDH is conformationally and functionally altered in association with oxidative stress in mouse models of amyotrophic lateral sclerosis. J. Mol. Biol. 382, 1195–1210 10.1016/j.jmb.2008.07.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tristan C., Shahani N., Sedlak T. W., and Sawa A. (2011) The diverse functions of GAPDH: views from different subcellular compartments. Cell. Signal. 23, 317–323 10.1016/j.cellsig.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eaton P., Wright N., Hearse D. J., and Shattock M. J. (2002) Glyceraldehyde phosphate dehydrogenase oxidation during cardiac ischemia and reperfusion. J. Mol. Cell. Cardiol. 34, 1549–1560 10.1006/jmcc.2002.2108 [DOI] [PubMed] [Google Scholar]
- 51. Ruderman N. B. (1975) Muscle amino acid metabolism and gluconeogenesis. Annu. Rev. Med. 26, 245–258 10.1146/annurev.me.26.020175.001333 [DOI] [PubMed] [Google Scholar]
- 52. Owen O. E., Kalhan S. C., and Hanson R. W. (2002) The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 277, 30409–30412 10.1074/jbc.R200006200 [DOI] [PubMed] [Google Scholar]
- 53. Carstensen M. H., Leichtweiss H. P., and Schröder H. (1983) Lactate carriers in the artificially perfused human term placenta. Placenta 4, 165–174 10.1016/S0143-4004(83)80029-0 [DOI] [PubMed] [Google Scholar]
- 54. Hui S., Ghergurovich J. M., Morscher R. J., Jang C., Teng X., Lu W., Esparza L. A., Reya T., Le Zhan, Yanxiang Guo J., White E., and Rabinowitz J. D. (2017) Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 10.1038/nature24057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Abdel-Wahab M. H., El-Mahdy M. A., Abd-Ellah M. F., Helal G. K., Khalifa F., and Hamada F. M. (2003) Influence of p-Coumaric acid on doxorubicin-induced oxidative stress in rat's heart. Pharmacol. Res. 48, 461–465 10.1016/S1043-6618(03)00214-7 [DOI] [PubMed] [Google Scholar]
- 56. Saravanan R., and Pugalendi V. (2006) Impact of ursolic acid on chronic ethanol-induced oxidative stress in the rat heart. Pharmacol. Rep. 58, 41–47 [PubMed] [Google Scholar]
- 57. Jain M., Brenner D. A., Cui L., Lim C. C., Wang B., Pimentel D. R., Koh S., Sawyer D. B., Leopold J. A., Handy D. E., Loscalzo J., Apstein C. S., and Liao R. (2003) Glucose-6-phosphate dehydrogenase modulates cytosolic redox status and contractile phenotype in adult cardiomyocytes. Circ. Res. 93, e9–e16 10.1161/01.RES.0000083489.83704.76 [DOI] [PubMed] [Google Scholar]
- 58. Janero D. R., Hreniuk D., and Sharif H. M. (1994) Hydroperoxide-induced oxidative stress impairs heart muscle cell carbohydrate metabolism. Am. J. Physiol. 266, C179–C188 10.1152/ajpcell.1994.266.1.C179 [DOI] [PubMed] [Google Scholar]
- 59. Zimmer H. G., Ibel H., and Suchner U. (1990) β-Adrenergic agonists stimulate the oxidative pentose–phosphate pathway in the rat heart. Circ. Res. 67, 1525–1534 10.1161/01.RES.67.6.1525 [DOI] [PubMed] [Google Scholar]
- 60. Zimmer H. G., Lankat-Buttgereit B., Kolbeck-Rühmkorff C., Nagano T., and Zierhut W. (1992) Effects of norepinephrine on the oxidative pentose–phosphate pathway in the rat heart. Circ. Res. 71, 451–459 10.1161/01.RES.71.2.451 [DOI] [PubMed] [Google Scholar]
- 61. Zimmer H. G., and Ibel H. (1979) Studies on the mechanism for the isoproterenol-induced stimulation of cardiac glucose-6-phosphate dehydrogenase. FEBS Lett. 106 335–337 [DOI] [PubMed] [Google Scholar]
- 62. Zhang Z., Apse K., Pang J., and Stanton R. C. (2000) High glucose inhibits glucose-6-phosphate dehydrogenase viz cAMP in aortic endothelial cells. J. Biol. Chem. 275, 40042–40047 10.1074/jbc.M007505200 [DOI] [PubMed] [Google Scholar]
- 63. Xu Y., Osborne B. W., and Stanton R. C. (2005) Diabetes causes inhibition of glucose-6-phosphate dehydrogenase via activation of PKA, which contributes to oxidative stress in rat kidney cortex. Am. J. Physiol. Renal Physiol. 289, F1040–F1047 10.1152/ajprenal.00076.2005 [DOI] [PubMed] [Google Scholar]
- 64. Youle R. J., and van der Bliek A. M. (2012) Mitochondrial fission, fusion, and stress. Science 337, 1062–1065 10.1126/science.1219855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rambold A. S., Kostelecky B., Elia N., and Lippincott-Schwartz J. (2011) Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc. Natl. Acad. Sci. U.S.A. 108, 10190–10195 10.1073/pnas.1107402108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Office of Animal Welfare (2017) Office of Research and Commercialization, National Institutes of Health, Bethesda: http://www.research.ucf.edu/Research/OfficeOfAnimalWelfare.html [Google Scholar]
- 67. Office of Laboratory Animal Welfare (2017) Vertebrate Animals Section. Office of Extramural Research, National Institutes of Health, Bethesda: https://grants.nih.gov/grants/olaw/vertebrate_animal_section.htm [Google Scholar]
- 68. Baker C., Taylor D. G., Osuala K., Natarajan A., Molnar P. J., Hickman J., Alam S., Moscato B., Weinshenker D., and Ebert S. N. (2012) Adrenergic deficiency leads to impaired electrical conduction and increased arrythmic potential in the embryonic mouse heart. Biochem. Biophys. Res. Commun. 423, 536–541 10.1016/j.bbrc.2012.05.163 [DOI] [PubMed] [Google Scholar]
- 69. Cosen-Binker L. I., Binker M. G., Cosen R., Negri G., and Tiscornia O. (2006) Relaxin prevents the development of severe acute pancreatitis. World J. Gastroenterol. 12, 1558–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Manfredi G., Yang L., Gajewski C. D., and Mattiazzi M. (2002) Measurements of ATP in mammalian cells. Methods 26, 317–326 10.1016/S1046-2023(02)00037-3 [DOI] [PubMed] [Google Scholar]
- 71. Fujikawa M., and Yoshida M. (2010) A sensitive, simple assay of mitochondrial ATP synthesis of cultured mammalian cells suitable for high-throughput analysis. Biochem. Biophys. Res. Commun. 401, 538–543 10.1016/j.bbrc.2010.09.089 [DOI] [PubMed] [Google Scholar]
- 72. Bartolommei G., Moncelli M. R., and Tadini-Buoninsegni F. (2013) A method to measure hydrolytic activity of adenosinetriphosphatases (ATPases). PloS ONE 8, e58615 10.1371/journal.pone.0058615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nadtochiy S. M., Urciuoli W., Zhang J., Schafer X., Munger J., and Brookes P. S. (2015) Metabolomic profiling of the heart during acute ischemic preconditioning reveals a role for SIRT1 in rapid cardioprotective metabolic adaptation. J. Mol. Cell. Cardiol. 88, 64–72 10.1016/j.yjmcc.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Munger J., Bennett B. D., Parikh A., Feng X. J., McArdle J., Rabitz H. A., Shenk T., and Rabinowitz J. D. (2008) Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 26, 1179–1186 10.1038/nbt.1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schroeder M. A., Cochlin L. E., Heather L. C., Clarke K., Radda G. K., and Tyler D. J. (2008) In vivo assessment of pyruvate dehydrogenase flux in the heart using hyperpolarized carbon-13 magnetic resonance. Proc. Natl. Acad. Sci. U.S.A. 105, 12051–12056 10.1073/pnas.0805953105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang J., Nuebel E., Wisidagama D. R., Setoguchi K., Hong J. S., Van Horn C. M., Imam S. S., Vergnes L., Malone C. S., Koehler C. M., and Teitell M. A. (2012) Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat. Protoc. 7, 1068–1085 10.1038/nprot.2012.048 [DOI] [PMC free article] [PubMed] [Google Scholar]