Abstract
Background
Urtica dioica is known as an anti-hyperglycemic plant. Urtica dioica distillate (UD) is a traditional Iranian drink, locally known as “aragh gazaneh”. In spite of its widespread consumption in Iran, according to traditional Iranian medicine, there is no scientific report on the usefulness of UD for diabetic patients. This survey was designed to evaluate its protective effects for the recovery from diabetes by determining the serum insulin, blood glucose, volume of pancreatic islets, and the number and volume of β-cells in diabetic rats.
Methods
A total of 48 Sprague-Dawley male rats (200-250 g) were randomly distributed into 6 groups (n=8), including non-diabetic plus distilled water (DW), non-diabetic plus UD, diabetic plus DW, diabetic plus UD, diabetic plus insulin, and diabetic plus glibenclamide. DW, UD, and glibenclamide were administered via intragastric gavage and insulin was injected subcutaneously. After four weeks of experiments, blood samples were collected for serum insulin and blood glucose assay. Pancreas was also evaluated using stereological method. The SPSS software was used for statistical analysis. Kruskal-Wallis, repeated measurements, and Mann-Whitney U test were applied for comparisons between the groups.
Results
The treatment of diabetic rats with UD reduced the blood glucose dramatically (P<0.001) and increased serum insulin levels significantly (P=0.03) in comparison to the diabetic plus DW rats. Treatment with UD did not affect the mean β-cell volumes in the diabetic rats when compared to the diabetic plus DW rats, but the islet volumes and β-cell numbers were significantly recovered.
Conclusion
UD treatment in diabetic rats improves hyperglycemia by partially restoring plasma insulin levels. The data suggest that UD prevents islet atrophy and/or regenerate pancreatic β-cells.
Keywords: Diabetes mellitus, Pancreatic beta cell, Urtica dioica distillate, Rats, Streptozotocin
What’s Known
Previous studies regarding the anti-diabetic properties of Urtica dioica were limited to different extracts of this plant.
In spite of high consumption of U. dioica distillate (aragh gazaneh) by diabetic patients in Iran, there is no scientific report about the effectiveness of this drink.
What’s New
Administration of U. dioica distillate in STZ-induced diabetic rats lowers the blood glucose level and recovers the insulin level and pancreatic islet volumes via β-cell regeneration with no significant effect on healthy normal rats in this regard.
Introduction
Diabetes is a widespread endocrine disorder characterized by hyperglycemia resulting from flawed insulin discharge, resistance to insulin action or both.1,2 The statistics show that 30 million people were diagnosed with diabetes worldwide in 1985 and it is predicted by the World Health Organization that, at the current rate, there will be 300 million by the year 2025.3 Despite substantial improvement in the management of diabetes by hypoglycemic drugs, search for newer drugs is continuing because the current anti-diabetic agents have several restrictions such as lack of efficacy and undesirable side effects.4 It is stated that the plant materials derivatives have provided the models for 50% of the Western drugs.5 For example, metformin was developed based on a biguanide compound from the anti-diabetic herb known as French liliac, and is now the first-line drug for type II of diabetes.4 Among medicinal plants, more than 1,200 have been reported as remedies for diabetes.4
According to WHO, more than 80% of the world’s population relies on traditional and herbal medicine for their medical disorders.5 Herbal drug preparations mostly include extraction, fractionation, purification, concentration, fermentation, and distillation.6 The most common is in the form of plant extracts, which are widely studied.7 Herbal and/or plant distillates are currently being used in Egypt, Turkey, and substantially in Iran, where it is locally called “aragh” or “araq”. According to the literature, it is also known as herbal/essential/floral water, hydrolate, and hydrosol. There are few reports about the actual chemical components of the volatile compartment of some plant/herb, including Phoenix dactylifera L. (Tarooneh),8 Operculina turpethum (Turbad),9 and rose water (Golāb).10 They showed that these distillates contained essential oil compounds consisting of organic acids and some other water soluble and volatile plant components.
Urtica dioica has been widely used in folk medicine as an anti-hyperglycemic agent to treat diabetes mellitus.11 Urtica is a genus of flowering plants in the family Urticaceae. Many species have stinging hairs and may be called nettles or stinging nettles, although the latter name is particularly used for Urtica dioica.12 A variety of pharmacological effects have been demonstrated for nettle leaves, including insulin secretagogue13 and insulin mimetics.14 Others also reported that U. dioica extract has proliferation potency15 and alpha-glucosidase inhibitory activities.16 The whole plant is also used in folk medicine to treat allergies, kidney stones, burns, anemia, rashes, and internal bleeding.5,6 Moreover, there are some other studies indicating that the consumption of U dioica hydroalchoholic extract has no hypoglycemic effect and/or β-cell regeneration in diabetic rats.17
Iran is one of the ancient countries in the utilization of medicinal plants for the treatment and prevention of disorders. Because of the wide diversity of climate and geographical conditions of Iran, many different medicinal species are under cultivation.18 In the south-center of Iran, the city of Shiraz, the most popular system used for home remedies and regaining well-being has been the use of plant distillates since ancient times. The distillate obtained from U. dioica is being widely used as a non-alcoholic drink among the Iranian diabetic patients. Therefore, the aim of the current study was to evaluate the anti-diabetic properties of Urtica dioica distillate (UD). With regard to the fact that the size and number of Langerhans islets correlate with pancreatic endocrine function, the serum insulin and blood glucose in streptozotocin (STZ)-induced diabetic rats, as well as the structure of pancreatic islets, were assessed. Briefly, this survey was designed to answer the following questions: How much does the serum insulin and blood glucose change in diabetic rats and if their treatment with UD recovers these changes? How much does the volume of pancreas as a whole, the Langerhans islets, and the number and volume of beta cells change in diabetic rats and if their treatment with UD recovers the changes?
Materials and Methods
Preparation of Urtica Dioica Distillate
Urtica dioica plant was collected from the farms around Sari, a city in the north of Iran, and then authenticated by Sari Agricultural Sciences and Natural Resources University. For the preparation of 1 liter of UD, 3.75 kg fresh plants were harvested, washed, transferred into the upper partition of steam boiler, and added with 1.5 liters of water. The procedure was performed as described previously by Seghatoleslam et al.19 Keeping the heat of the boiling water constant at 65-70 ºC, the steam from U. dioica plant was cooled in the condenser duct and the resulting UD (called aragh gazaneh) was collected in a light-protected bottle and conserved at 4 ºC until use.
Experimental Rats
A total of 48 Sprague-Dawley male rats (4-5 weeks of age, 200-250 g of weight) were obtained from the Animal Center of Shiraz University of Medical Sciences and randomly distributed into 6 groups, each including 8 rats. Distilled water (DW: 12.5 ml/kg/day), U. dioica distillate (UD: 12.5 ml/kg/day), and glibenclamide (0.6 mg/kg/day) were administered via intragastric gavage. Insulin (3 units of protamine insulin, twice a day) was injected subcutaneously. The experiments were performed for 4 weeks. Experimental groups were designed as follows:
Group I: Non-diabetic plus DW
Group II: Non-diabetic plus UD
Group III: Diabetic plus DW
Group IV: Diabetic plus UD
Group V: Diabetic plus insulin
Group VI: Diabetic plus glibenclamide
All protocols of the study were approved by the Institutional Animal Ethics Committee of Shiraz University of Medical Sciences (Shiraz, Iran). The animals were kept in a temperature-controlled condition (24±2 ºC) with a 12-h light/dark alternating cycle and free access to water and standard diet.
Diabetes was induced in overnight-fasting rats by a single intraperitoneal (ip) injection of streptozotocin (STZ, Sigma chemical company, St. Louis, MO, USA) in a dose of 50 mg/kg. STZ was dissolved in citrate buffer as the vehicle (0.1 M, pH 4.5) immediately before use. The rats in groups I and II received only the vehicle. Fasting blood glucose (FBS) levels were determined 72 h after STZ injection with a glucometer in the blood drawn from the tail vein. Diabetes was confirmed in the rats with FBS above 250 mg/dl (13.8 mM).
FBS of the animals was measured once every 9 days (zero, 9th, 18th, and 27th) using blood from the tail. At the end of the experiments, the rats in the fed state were anesthetized by CO2, the blood samples were obtained from the heart, and the rats were then sacrificed. The sera were separated to measure the insulin levels. The pancreas was gently dissected out, cleaned from fat and connective tissues, weighed, and kept in formalin buffer (pH=7.4) until use.
Biochemical Parameters
Fasting blood sugar measurement
FBS was measured as mentioned above by Accu-Chek Glucometer (Accu-Chek® Active, Roche Diagnostics GmbH, Hannheim, Germany) and expressed in terms of mg/dl.
Measurement of serum insulin
Serum insulin levels were measured in the experimental rats except for the groups V and VI. ELISA method was applied using a kit from Mercodia Corporation (Uppsala, Sweden).
Stereological Parameters
Estimation of pancreas volume
Primary volume (Vprimary) of the pancreas was estimated according to Scherle’s immersion method.20,21 The isotropic uniform random (IUR) slabs of the pancreas were obtained with the orientator method.21 Two circular pieces (3 mm diameter) were punched out from two random slabs.21 All the slabs and the circular pieces were molded in a paraffin cube. After tissue sectioning, processing, and staining of the slabs and pieces, the area of the circular pieces was measured. The modified Gomori’s aldehyde-fuchsin, according to Bangle’s method was used to stain the tissue sections.22 Two sections including 4 μm and 25 μm were cut from the slabs. The degree of shrinkage “D(sh)” was estimated using the following formula:
D(sh)=1–(AA/AB)1.5
Where AA and AB are the areas of each circular piece of the pancreas after and before processing, sectioning, and staining, respectively.
Estimation of volume density of the pancreatic islets
The 4 μm thickness microscopic slides were analyzed by a video microscopy system using the point-counting method. The following formula was used to estimate the volume density of the islets:
Vv(islet, pancreas)=P(islet)/P(reference)
Where the respective P (islet) and P (reference) are the numbers of the test-points hitting the islet profile and the reference space.
Then, the final islet volume “V(islets)” was estimated according to the following formula:
V(islets)=Vv(islet, pancreas)×V(primary)
Numerical Density of β-Cells
β-cells were counted according to the optical disector method, an oil immersion objective lens (60×oil, numerical aperture 1.4 at a final magnification of 2000X).23 The following formula was also used to estimate the numerical density of the cells “NV(cells/islets)”:
NV(cells/islets)=[ΣQ/(Σp×(a/f)×h)]×(t/BA)
Where “ΣQ” is the number of the β-cells counted in all the dissectors, “Σp” is the total number of the counted frames, “a/f” is the area of the counting frame (here was 184 μm2), “h” is the height of the optical disector (here was 15 μm), “BA” is the microtome block advance to cut the block (here was 25 μm), and “t” is the mean of final section thickness (24.3 μm on average).23 In order to determine the total number of β-cells “N(cells)”, the following formula was used:
N(cells)=NV(cells/islets)×V(islets)×D(sh)
Estimation of beta cells volume
The number-weighed mean volumes of the cells were estimated using “nucleator”. The cells were tested using disector method. The intercept length (ln) from the center of β-cell nucleus to cell membrane was measured in four directions.24 The following formula was used for the estimation of the nucleus or cell volume:
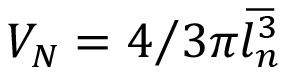
Statistical Analysis
Statistical analysis and graphic design were performed by SPSS (version 23.0) and GraphPad Prism 5 software (San Diego, CA, USA). For comparisons between groups Kruskal–Wallis, repeated measurements and Mann-Whitney U test (P<0.05 was considered as a significant difference) were used. The stereological values were presented as dot plots.
Results
Effect of UD on Blood Glucose Level
FBS levels of the experimental rats during 4-week treatment are presented in figure 1. As shown, the rats in the diabetic group receiving water (group III) displayed a significant increase (P<0.001) in the FBS levels compared to the non-diabetic groups (I and II). Obviously, the treatment of diabetic rats with UD significantly decreased (P<0.001) the blood glucose compared to the control diabetic rats while displaying a significant difference with the normal rats. Insulin injection also reduced FBS significantly (P<0.001) when compared to diabetic rats and normalized it on day 27. Glibenclamide also lowered FBS levels, but not significant when compared to the control diabetic rats. Furthermore, no significant differences were observed for FBS between the normal rats in groups I and II.
Figure 1.
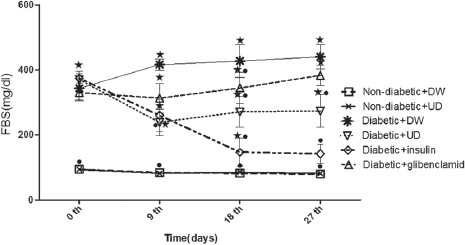
Comparison of fasting blood sugar in 6 groups of experimental rats (n=8) during 27 days. Data represent the average of the results of all rats in each group. *Significant difference between non-diabetic plus DW group vs. all groups; ●-Significant difference between diabetic plus DW group vs. all groups (P<0.05).
Effect of UD on Serum Insulin Levels
As shown, the serum insulin levels decreased significantly (P=0.01) in the diabetic rats on day 27 compared to the non-diabetic rats (figure 2). According to our data, in the diabetic animals, treatment with UD remarkably (P=0.03) increased the serum insulin levels when compared to the diabetic rats, but there was still a significant difference in comparison with non-diabetic rats (P<0.001). No significant changes in the serum insulin levels were observed in the non-diabetic plus UD compared to the non-diabetic plus DW rats (figure 2).
Figure 2.
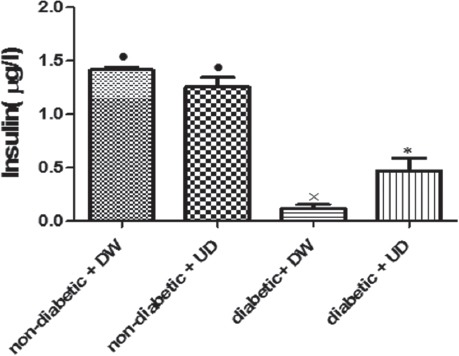
Effect of UD on serum insulin levels of experimental rats (n=8) on day 27. Data are represented as mean±SEM. Kruskal-Wallis was used for comparisons between groups. Bars (•, *, ×) indicate significant (P<0.05) differences between groups.
Effect of UD on Stereological Parameters
The mean islet volume, mean β-cell numbers, and mean β-cell volumes were reduced by 37%, 31%, and 62%, respectively, in the diabetic plus DW animals compared to the non-diabetic plus DW rats (figure 3). The volume of pancreas revealed no significant changes in the experimental rats (figure 3A). In the diabetic rats treated with UD, the volumes of the islets were approximately the same as non-diabetic plus DW rats (figure 3B). Interestingly, there was no difference between the β-cell numbers in diabetic plus UD and non-diabetic plus DW rats, thus the number of β-cells was recovered in diabetic plus UD (figure 3C). However, the mean β-cells volume was not recovered after UD treatment in diabetic rats (figure 3D).
Figure 3.
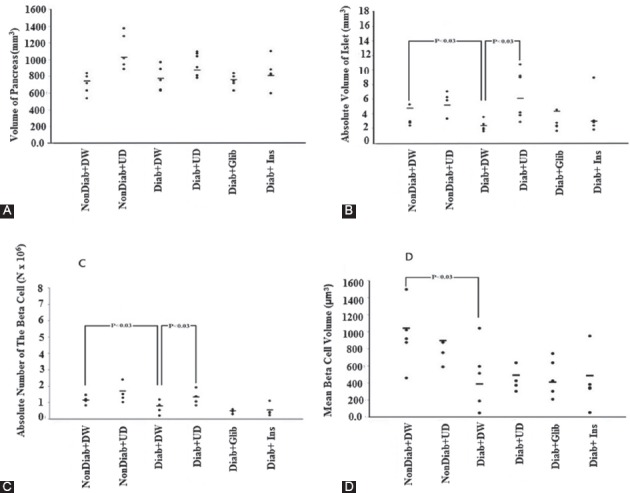
The estimated parameter using stereological methods in 6 groups of experimental rats (n=8). The dot plots show the total volume of the pancreas (A), absolute volume of the islet (B), total number of the β-cells (C), and mean β-cells volume (D). Each dot represents an animal in different groups. The significant differences are indicated in the figures.
Qualitative histological examinations are also presented in figure 4. Lower β-cells number was seen in the diabetic plus DW (C) in comparison with non-diabetic plus UD group (A and B). In the diabetic plus UD (D) group, the number of β-cells was higher than diabetic plus DW (D). There was no increase in the β-cells number of rats in diabetic plus insulin (F) and diabetic plus glibenclamide (E) groups in comparison to the diabetic plus DW group (C).
Figure 4.
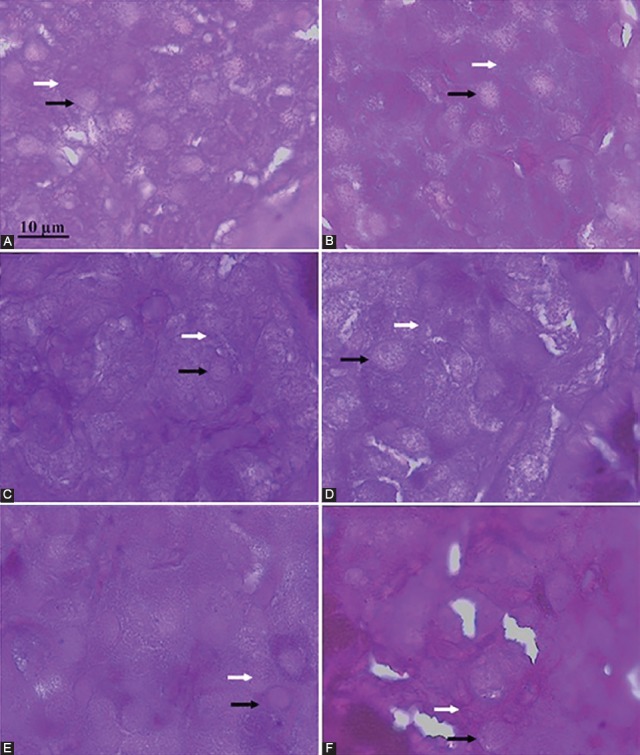
Light microscopic examination of pancreatic islets dissected from the experimental rats. Photomicrographs represent non-diabetic plus DW (A), non-diabetic plus UD (B), diabetic plus DW (C), diabetic plus UD (D), diabetic plus glibenclamide (E), and diabetic plus insulin (F). The β-cells stained with Gomori’s aldehyde fuchsin in which nucleus and cytoplasm (contained granules) are shown with black and white arrows, respectively.
Discussion
Diabetes is a metabolic disorder of the endocrine system. The spread of this disease around the world is a serious threat to human health. The side effects of chemical drugs and population growth, increase the need for effective medication and a change in the pattern of drug use.1-4 Medicines based on medicinal plants are the most common unconventional medical treatments that have been used since the ancient times in traditional Iranian medicine (TIM).9,11 The distillates prepared from different plant species (aragh) are known as common beverages in different parts of Iran. They are widely being used by people to treat health problems. Although a lot of reports emphasize the anti-diabetic effects of nettle extract, the anti-hyperglycemic properties of its hydro-distillate or aragh has not yet been studied.11 To evaluate the current beliefs on the usefulness of UD for the treatment of diabetes in folk medicine, we performed a series of experiments using STZ-induced diabetic rat model.
Streptozotocin (STZ), used in laboratory animals to induce type 1 diabetes, is a toxic glucose analogue which irreversibly destroys pancreatic β-cells. Hyperglycemia is the clear symptom of STZ-induced diabetes caused by destroying pancreatic islets, reduction of β-cells’ mass, and its insulin content.23 The mechanism of action of STZ on rodent β–cell is related to the uptake of STZ by β-cells and consequent DNA strand break causing a lethal depletion of cellular nicotinamide adenine dinucleotide (NAD+) and ATP.25
The results of the present study showed that UD reduces FBS and recovers the insulin levels during 4 weeks of administration in STZ diabetic rats. While the injection of insulin normalized FBS level in diabetic rats, glibenclamide as the inducer of insulin secretion from β–cells26 did not significantly reduce the blood sugar in diabetic animals; indicating that the secreted insulin from the remaining β-cells was not enough for blood sugar normalization. Interestingly, the consumption of UD resulted in a significant reduction of FBS in diabetic rats as a result of increased insulin level. Although UD was unable to normalize the blood sugar, its hypoglycemic effect on STZ-diabetic rats was confirmed using stereological studies. The results of this part indicated that UD could recover the pancreatic damage by regenerating β–cells and increasing the serum insulin level. Our findings are consistent with some other reports about anti-hyperglycemic activity13-15,27 and β-cell regeneration potency of U. dioica extract.18 Ranjbari et al. showed that the administration of STZ-induced diabetic rats by different doses of U. dioica aqueous extracts with swimming activity during the 4 weeks decreased the serum glucose level and insulin resistance, and increased insulin sensitivity significantly. Also, the regeneration of pancreatic β-cells was seen in the treated rats.28 Gaballu et al. showed that Peganum harmala, Rhus coriaria, and U. dioica extracts, especially their combination, has significant anti-diabetic, hypolipidemic, liver, and renal damage recovering effects.29
Some studies showed that U. dioica extract had an insulin secretagouge effect that is related to the proliferation and regeneration activity characteristics.14,15 Some others revealed that antidiabetic activities of this extract was related to the alpha glucosidase inhibitor activity, inhibition of the absorption of glucose from intestine,13,18 and also the improvement of glucose tolerance.30 Golalipour et al. also revealed that U. dioica extract has antioxidant activities and free radical scavenger properties.31 Qujeq et al. suggested the anti-inflammatory effect of U. dioica extract32 and Namazi et al. investigated its effect on insulin sensitivity and anti-inflammatory marker in patients with type 2 diabetes.33 They showed that U. dioica extract decreased some inflammatory factors and suggested a protective role for cardiovascular disease in patient with type 2 diabetes.32
Moore et al. showed that U. dioica extract does not have a potential health risk and suggested that daily consumption of herbal distillates and decoctions at the indicated doses poses no significant health risk to a normal adult.6 Interestingly, in a parallel study from our lab, we found no toxic effects on the rat kidney and liver under experimental conditions (data not shown).
In spite of the existence of many reports indicating anti-diabetic properties of U. dioica, the results of two controversial studies led to different conclusions. One has claimed that U. dioica consumption has no effect on blood glucose and β-cells regeneration in diabetic rats.18 The other study has asserted that the methanolic extract of nettle aerial parts is unable to increase insulin and C-peptide secretion from the culture of rat pancreatic β-cells.34 These inconsistencies could be due to the differences in the cultivation conditions, the climate, and the soil which affect plant’s properties.35
Although many studies have proven the anti-diabetic activities of U. dioica extract, the details of the actual anti-diabetic mechanism is still unknown. One has shown that this activity of the extract is related to the active component of the U. dioica leaves such as flavonoids, peptides, and coxamarins.31 Some others discussed the presence of numerous different phytochemicals of ethanolic, petroleum ether, chloroform, and methanolic extract of U. dioica.7,36
Due to the lack of information about the composition of UD, a parallel study in our laboratory was performed. We examined 23 elements based on their roles in the prevention or progression of diabetes. Our results (unpublished) indicated that the distillate of U. dioica is rich in macronutrients such as Mg, Ca, Si, and K and also contained microelements including Zn, Cu, Fe, Cr, Mn, Mo, and V which are active components exerting anti-diabetic activities.37,38 We also found that this distillate contained numerous (about 89) bioactive compounds belonging to the various classes of terpens, polyphenols, flavonoids, etc. (unpublished). These phytochemicals included secondary metabolites such as limonene, thymol, eugenol, pulegone, and eucalyptol. All these bioactive compounds have antioxidant activity and could protect the cells against oxidative stress. These components have anti-inflammatory, anti-carcinogenic, and anti-diabetic therapeutic effects as well.39 The effect of UD on the other biochemical parameters such as lipid profile and its potential antioxidant activity are under examination in a joint project.
Despite the tremendous use of many herbal distillates as practical therapeutic interventions in Iranian folk medicine, related pharmaceutical research is largely neglected by medical scientists. It must be noted that few evidences exist for the safety problem associated with the use of herbal/plant distillates. One has claimed that herbal ingredients may contain byproducts such as methanol that possess pharmacological effects as the result of boiling and evaporating woody parts of the plants.40 Furthermore, potential complications associated with the industrial preparation, standardization, used dosages, and quality control may make it less applicable in comparison to modern pharmaceuticals. However, there is relatively little known regarding efficacy, stability, and safety issues in herbal distillates use, which is a major concern for consumers. The massive use of herbal distillates makes it increasingly important to monitor the progress of the clinical literature and aid practitioners in advising their patients.
Conclusion
A 28-day oral UD treatment in STZ-diabetic rats improved hyperglycemia by partially restoring the plasma insulin and lowering fasting blood glucose. This action was through proliferative activity of UD in preventing the islets’ atrophy and increasing β-cell numbers. Due to the beneficial effects and less/lack of toxic side effects of UD, it could be recommended as a drug supplement for reducing blood sugar in diabetic patients. However, in those who drink UD and receive anti-diabetic drug simultaneously, physicians should accordingly pay attention to the drug dose in proportion to avoid undesired complications.
Acknowledgement
This work was financially supported by the grant number 93-7234 from Shiraz University of Medical Sciences, Shiraz, Iran. The work was partially performed at the Histomorphometry and Stereology Research Centre, Shiraz University of Medical Sciences, and was a part of the thesis written by Ali Gohari (PhD student of Clinical Biochemistry). We thank Dr. Zahra Khoshdel from the Department of Biochemistry of Shiraz Medical School for providing some information about the composition of UD used in the discussion section.
Conflict of Interest: None declared.
References
- 1.De D, Chatterjee K, Ali KM, Bera TK, Ghosh D. Antidiabetic Potentiality of the Aqueous-Methanolic Extract of Seed of Swietenia mahagoni (L.). Jacq. in Streptozotocin-Induced Diabetic Male Albino Rat: A Correlative and Evidence-Based Approach with Antioxidative and Antihyperlipidemic Activities. Evid Based Complement Alternat Med. 2011;2011:892807. doi: 10.1155/2011/892807. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang T, Gao J, Jin ZY, Xu XM, Chen HQ. Protective effects of polysaccharides from Lilium lancifolium on streptozotocin-induced diabetic mice. Int J Biol Macromol. 2014;65:436–40. doi: 10.1016/j.ijbiomac.2014.01.063. [DOI] [PubMed] [Google Scholar]
- 3.Lal V, Gupta P, Awanish P. Hypoglycemic effect of kyllinga triceps in STZ induced diabetic rats. J Diabetes Metab. 2012;5:1000203. [Google Scholar]
- 4.Chang CL, Lin Y, Bartolome AP, Chen YC, Chiu SC, Yang WC. Herbal therapies for type 2 diabetes mellitus: Chemistry, biology, and potential application of selected plants and compounds. Evid Based Complement Alternat Med. 2013;2013:378657. doi: 10.1155/2013/378657. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dar SA, Ganai FA, Yousuf AR, Balkhi MU, Bhat TM, Sharma P. Pharmacological and toxicological evaluation of Urtica dioica. Pharm Biol. 2013;51:170–80. doi: 10.3109/13880209.2012.715172. [DOI] [PubMed] [Google Scholar]
- 6.Moore F, Akhbarizadeh R, Keshavarzi B, Tavakoli F. Potential Health Risk of Herbal Distillates and Decoctions Consumption in Shiraz, Iran. Biol Trace Elem Res. 2015;167:326–37. doi: 10.1007/s12011-015-0286-7. [DOI] [PubMed] [Google Scholar]
- 7.Al-Tameme HJ, Hadi MY, Hameed IH. Phytochemical analysis of Urtica dioica leaves by fourier-transform infrared spectroscopy and gas chromatography-mass spectrometry. Journal of Pharmacognosy and Phytotherapy. 2015;7:238–52. doi: 10.5897/JPP2015.0361. [DOI] [Google Scholar]
- 8.Hamedi A, Mohagheghzadeh A, Rivaz S. Preliminary pharmacognostic evaluation and volatile constituent analysis of spathe of Phoenix dactylifera L (Tarooneh) Pharmacognosy Journal. 2013;5:83–6. doi: 10.1016/j.phcgj.2013.02.005. [DOI] [Google Scholar]
- 9.Hamedi A, Mohagheghzadeh A, Rivaz S. Hydrodistilled volatile constituents obtained from the roots of Operculina turpethum. Pharmacognosy Journal. 2014;6:36–7. doi: 10.5530/pj.2014.2.6. [DOI] [Google Scholar]
- 10.Moein M, Zarshenas MM, Delnavaz S. Chemical composition analysis of rose water samples from Iran. Pharm Biol. 2014;52:1358–61. doi: 10.3109/13880209.2014.885062. [DOI] [PubMed] [Google Scholar]
- 11.Bnouham M, Merhfour FZ, Ziyyat A, Mekhfi H, Aziz M, Legssyer A. Antihyperglycemic activity of the aqueous extract of Urtica dioica. Fitoterapia. 2003;74:677–81. doi: 10.1016/s0367-326x(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 12.Kavalali G, Tuncel H, Goksel S, Hatemi HH. Hypoglycemic activity of Urtica pilulifera in streptozotocin-diabetic rats. J Ethnopharmacol. 2003;84:241–5. doi: 10.1016/s0378-8741(02)00315-x. [DOI] [PubMed] [Google Scholar]
- 13.Farzami B, Ahmadvand D, Vardasbi S, Majin FJ, Khaghani S. Induction of insulin secretion by a component of Urtica dioica leave extract in perifused Islets of Langerhans and its in vivo effects in normal and streptozotocin diabetic rats. J Ethnopharmacol. 2003;89:47–53. doi: 10.1016/s0378-8741(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 14.Domola MS, Vu V, Robson-Doucette CA, Sweeney G, Wheeler MB. Insulin mimetics in Urtica dioica: Structural and computational analyses of Urtica dioica extracts. Phytother Res. 2010;24:S175–82. doi: 10.1002/ptr.3062. [DOI] [PubMed] [Google Scholar]
- 15.Golalipour MJ, Ghafari S, Kouri V, Kestkar AA. Proliferation of the ß-Cells of Pancreas in Diabetic Rats Treated with Urtica Dioica. Int J Morphol. 2010;28:399–404. [Google Scholar]
- 16.Onal S, Timur S, Okutucu B, Zihnioglu F. Inhibition of alpha-glucosidase by aqueous extracts of some potent antidiabetic medicinal herbs. Prep Biochem Biotechnol. 2005;35:29–36. doi: 10.1081/PB-200041438. [DOI] [PubMed] [Google Scholar]
- 17.Golalipour MJ, Khori V, Ghafari S, Gharravi AM. Chronic effect of the hydroalcholic extract of Urtica dioica leaves on regeneration of β-cells of hyperglycemic rats. Pak J Biol Sci. 2006;9:1482–5. [Google Scholar]
- 18.Ghorbani M. Iranian traditional medicine for treatment of type II diabetes, anxiety and hypertension with introduction of zebrafish model system for their screening. International Journal of Herbal Medicine. 2014;2:13–9. [Google Scholar]
- 19.Seghatoleslam A, Namavari M, Azarmehr B, Nejabat M. The Potential Effects of Herbal Distillates with Hot and Cold Temperament on Cell Metabolic Activity and Growth: A Preliminary in Vitro Study. J Pharm Biomed Sci. 2014;4:532–5. [Google Scholar]
- 20.Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970;26:57–60. [PubMed] [Google Scholar]
- 21.Noorafshan A, Hoseini L, Karbalay-Doust S, Nadimi E. A simple stereological method for estimating the number and the volume of the pancreatic beta cells. JOP. 2012;13:427–32. doi: 10.6092/1590-8577/802. [DOI] [PubMed] [Google Scholar]
- 22.Bangle R., Jr Factors influencing the staining of beta-cell granules in pancreatic islets with various basic dyes, including paraldehyde-fuchsin. Am J Pathol. 1956;32:349–62. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 23.Pirmoradi L, Noorafshan A, Safaee A, Dehghani GA. Quantitative Assessment of Proliferative Effects of Oral Vanadium on Pancreatic Islet Volumes and Beta Cell Numbers of Diabetic Rats. Iran Biomed J. 2016;20:18–25. doi: 10.7508/ibj.2016.01.003. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebrahimpoor-Mashhadi MR, Khaksar Z, Noorafshan A, Mogheisi B. Stereological study of the effects of orally administrated Otostegia persica extract on pancreatic beta cells in male diabetic rats. Comp Clin Path. 2014;23:761–7. [Google Scholar]
- 25.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–46. [PubMed] [Google Scholar]
- 26.Proks P, Reimann F, Green N, Gribble F, Ashcroft F. Sulfonylurea stimulation of insulin secretion. Diabetes. 2002;51:S368–76. doi: 10.2337/diabetes.51.2007.S368. [DOI] [PubMed] [Google Scholar]
- 27.Das M, Sarma B, Rokeya B, Parial R, Nahar N, Mosihuzzaman M, et al. Antihyperglycemic and antihyperlipidemic activity of Urtica dioica on type 2 diabetic model rats. J Diabetol. 2012;2:1–6. [Google Scholar]
- 28.Ranjbari A, Azarbayjani MA, Yusof A, Halim Mokhtar A, Akbarzadeh S, Ibrahim MY, et al. In vivo and in vitro evaluation of the effects of Urtica dioica and swimming activity on diabetic factors and pancreatic beta cells. BMC Complement Altern Med. 2016;16:101. doi: 10.1186/s12906-016-1064-6. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abedi Gaballu F, Abedi Gaballu Y, Moazenzade Khyavy O, Mardomi A, Ghahremanzadeh K, Shokouhi B, et al. Effects of a triplex mixture of Peganum harmala, Rhus coriaria, and Urtica dioica aqueous extracts on metabolic and histological parameters in diabetic rats. Pharm Biol. 2015;53:1104–9. doi: 10.3109/13880209.2014.960943. [DOI] [PubMed] [Google Scholar]
- 30.Akmali M, Ahmadi R, Vessal M. Pre- and post-treatment of streptozocin administered rats with melatonin: Effects on some hepatic enzymes of carbohydrate metabolism. Arch Iran Med. 2010;13:105–10. [PubMed] [Google Scholar]
- 31.Golalipour MJ, Khori V. The protective activity of Urtica dioica leaves on blood glucose concentration and beta-cells in streptozotocin-diabetic rats. Pak J Biol Sci. 2007;10:1200–4. doi: 10.3923/pjbs.2007.1200.1204. [DOI] [PubMed] [Google Scholar]
- 32.Qujeq D, Tatar M, Feizi F, Parsian H, Sohan Faraji A, Halalkhor S. Effect of Urtica dioica Leaf Alcoholic and Aqueous Extracts on the Number and the Diameter of the Islets in Diabetic Rats. Int J Mol Cell Med. 2013;2:21–6. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 33.Namazi N, Esfanjani AT, Heshmati J, Bahrami A. The effect of hydro alcoholic Nettle (Urtica dioica) extracts on insulin sensitivity and some inflammatory indicators in patients with type 2 diabetes: A randomized double-blind control trial. Pak J Biol Sci. 2011;14:775–9. doi: 10.3923/pjbs.2011.775.779. [DOI] [PubMed] [Google Scholar]
- 34.Mobaseri M, Aliasgarzadeh A, Bahrami A, Zargami N, Tabrizi A, Ave G. Efficacy of the total extract of urtica dioica on the glucose utilization by the human muscle cells. J Clin Diagn Res. 2012;6:437–40. [Google Scholar]
- 35.Balabanova B, Stafilov T, Baceva K. Bioavailability and bioaccumulation characterization of essential and heavy metals contents in R. acetosa, S. oleracea and U. dioica from copper polluted and referent areas. J Environ Health Sci Eng. 2015;13:2. doi: 10.1186/s40201-015-0159-1. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahigi SH, Amini K, Moradi P, Asaadi K. Investigating the chemical composition of different parts extracts of bipod nettle Urtica dioica L. in Tonekabon region. Physiology. 2011;2:337–40. [Google Scholar]
- 37.Zheng Y, Li XK, Wang Y, Cai L. The role of zinc, copper and iron in the pathogenesis of diabetes and diabetic complications: Therapeutic effects by chelators. Hemoglobin. 2008;32:135–45. doi: 10.1080/03630260701727077. [DOI] [PubMed] [Google Scholar]
- 38.Kazi TG, Afridi HI, Kazi N, Jamali MK, Arain MB, Jalbani N, et al. Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol Trace Elem Res. 2008;122:1–18. doi: 10.1007/s12011-007-8062-y. [DOI] [PubMed] [Google Scholar]
- 39.More TA, Kulkarni BR, Nalawade ML, Arvindekar AU. Antidiabetic activity of linalool and limonene in streptozotocin-induced diabetic rat: A combinatorial therapy approach. Int J Pharm Pharm Sci. 2014;6:159–63. [Google Scholar]
- 40.Karimi G, Hassanzadeh M, Shahidi N, Samie Z. Quantitative determination of methanol in plant water produced in Mashhad by spectrophotometry method. Journal of Medicinal Plants. 2008;1:56–9. [Google Scholar]


