Abstract
Background
Acute kidney injury is the most life-threatening complication of rhabdomyolysis. Glycerol is commonly used to induce this injury. The aim was to investigate the renoprotective effects of pioglitazone and the possible advantage of administering the drug for a longer period.
Methods
Twenty-four male Albino Wistar rats were randomly divided into 4 groups (n=6/group): (A) control, (B) glycerol (50%, 10 mL/kg intramuscularly), (C) glycerol+pioglitazone (10 mg/kg orally for 3 days), and (D) glycerol+pioglitazone (for 6 days). Serum urea and creatinine levels were measured to assess the renal function. Reduced glutathione (GSH) levels and histological alterations were also measured. Statistical analysis was performed using Prism (version 6). The numerical data were evaluated by ANOVA, followed by the Tukey tests. The categorical data were evaluated by the Mann–Whitney test and the Fisher exact tests. P<0.05 was considered significant.
Results
In the glycerol-injected rats, the serum urea and creatinine levels were increased (P<0.001), while the GSH levels were decreased (P<0.001) compared to Group A. The nephrotoxicity showed significant tubular (P=0.01) and glomerular (P=0.02) injuries. In the pioglitazone-treated rats, the changes in the serum biomarkers and in the GSH levels were reversed in Group C (P=0.01) and in Group D (P=0.01). The microscopic examinations of the kidneys also showed some improvement. No obvious statistically significant difference was found between these 2 preventive groups in most studied features.
Conclusion
These results indicate that pioglitazone might have nephroprotective effects in this injury model. Pioglitazone succeeded in producing this effect within 3 days. Doubling the drug administration period did not produce any significant superior benefit.
Keywords: Kidney, Glycerol, Rhabdomyolysis, Acute kidney injury, Pioglitazone, Reduced glutathione
What’s Known
Acute renal failure is the most life-threatening complication of rhabdomyolysis.
Glycerol-induced myoglobinuric acute renal failure is the most employed model to study this type of kidney injury.
Pioglitazone has several pleiotropic effects.
To our knowledge, no investigations have been made on its prevention in this nephropathy model.
What’s New
Pioglitazone can protect renal function in rhabdomyolysis-induced acute renal failure.
This protection can probably be mediated by its antioxidant properties.
It has the advantage of rapidly showing its effects, giving the expected effects in 3 days.
Doubling the drug administration period did not produce a significant additional benefit.
Introduction
Acute kidney injury (AKI) is a syndrome characterized by an acute loss of the renal function. This injury might be reversible in some patients, but new and cumulative evidence indicates much less renal recovery. In addition, AKI can lead to chronic kidney disease and even to irreversible end-stage renal disease, necessitating costly permanent renal replacement therapy. Every AKI episode doubles this risk and increases morbidity and mortality rates.1,2
Between 15% and 30% of all AKI cases can be attributed to rhabdomyolysis.3 An estimated 10% to 40% of rhabdomyolysis patients develop AKI. This percentage may reach 42% to 50% in children.4 Rhabdomyolysis is a clinical syndrome caused by damaged skeletal muscles and subsequent release of their intracellular components and breakdown products into the circulation, including myoglobin, creatine kinase, aldolase, lactate dehydrogenase, and electrolytes.3,4 Rhabdomyolysis is caused by direct traumatic injury or by the effects of some drugs.3,4 Glycerol-induced AKI is the most frequently applied model to study this injury.5,6 The pathogenesis is not yet fully understood. It has been suggested that renal vasoconstriction, tubular obstruction, and direct myoglobin-induced cytotoxicity are the main mechanisms involved.3,7 Several studies have shown the role of oxidative stress in the pathogenesis.3,5,8
Pioglitazone, which works on reversing almost all mechanisms involved in the pathogenesis, is the new suggested preventive option in this study.9,10 To the best of our knowledge, no investigations have been made on this prevention, although several previous studies have indicated its renoprotective effects in other nephropathy models.11-13
Pioglitazone is an oral antidiabetic agent and is classified as a peroxisome proliferator-activated receptor-g (PPAR-g) agonist (a member of thiazolidinediones), which binds to a specific site on the DNA helix. It regulates the transcription of numerous target genes and participates in the regulation of several vital processes such as adipocyte differentiation and lipid and carbohydrate metabolism.9,10
The present study aimed to investigate the renoprotective effects of pioglitazone in glycerol-induced AKI during 2 different treatment periods and to evaluate its antioxidant properties by measuring reduced glutathione (GSH) levels in kidney tissues.
GSH is an important tripeptide cofactor involved in the cytoprotection against oxidative and xenobiotic stresses.14,15 It is considered the most important antioxidant in vivo and capable of counteracting various pathological mechanisms underlying this model of AKI.15,16
Materials and Methods
Animals
Twenty-four male Albino Wistar rats (230–300 g) were used. The rats were housed in standard plastic cages and placed in an air-conditioned room at 22±1°C, with a 12-hour light/dark cycle. They were allowed free access to a standard rat diet and water.
Groups
The rats were randomly divided into 4 groups (6 animals/group): Group A: control, Group B: glycerol, Group C: glycerol+pioglitazone for 3 days, and Group D: glycerol+pioglitazone for 6 days.
Chemicals
Glycerol was obtained from Surechem Products Ltd., England, and was diluted in a 0.9% normal saline solution to reach a 50% v/v concentration.
Pioglitazone hydrochloride was obtained from Abhilasha Pharma Pvt., Ltd., Ankleshwar, India. A suspension (1 mg/mL) in carboxymethyl cellulose 0.5% w/v was prepared.
Experimental Protocol
The rats had free access to food but were deprived from drinking water 24 hours before glycerol injection. Kidney injury was induced in the rats by intramuscular injection of 50% glycerol (10 mL/kg) equally divided in both hind limbs17 in Group B, Group C, and Group D. Group A was injected with an equivalent volume of normal saline solution. Pioglitazone (10 mg/kg) was orally administered using an intubation needle,14,18,19 3 days before glycerol injection in Group C and 6 days before glycerol injection in Group D. An equivalent volume of 0.5% carboxymethyl cellulose was administered to Group A and Group B. The animals were sacrificed 24 hours after glycerol injection. Blood samples were collected through heart puncture, and serum was separated for renal function tests (serum urea and creatinine concentrations). The kidneys were excised. One kidney was stored at −70°C for biochemical analysis (tissue GSH levels); the other kidneys were decapsulated and sectioned longitudinally into 2 equally sized pieces before they were fixed in 15% buffered formalin solution for histopathological studies.
Renal Function Tests
Serum Creatinine
A commercially available kit was used (Biosystems). In the sample, creatinine reacts with picrate in alkaline medium, forming a colored complex. The absorbance of the samples and the standard was measured spectrophotometrically at 500 nm, and the concentrations were calculated accordingly.
Serum Urea Concentration
A commercially available kit was employed (Biosystems). Urea in the sample reacts with the kit’s reagent components (urease, nitroprusside, salicylate, and NaClO) and forms a colored complex. The absorbance of the samples and the standard was measured spectrophotometrically at 600 nm, and the concentrations were calculated accordingly.
Determination of Reduced GSH
A commercially available kit was used (Abnova, KA1649). The GSH levels were spectrophotometrically measured according to the improved 5,5’-dithiobis (2-nitro-benzoic acid DTNB) method. DTNB reacts with reduced GSH to form a yellow product. The optical density of the samples and the standard was measured spectrophotometrically at 412 nm, and the concentrations were calculated accordingly.
Histopathological Assessment
The appearance of the kidney sections was examined macroscopically. For microscopic evaluation, the specimens were dehydrated in graded ethanol, cleared in xylene, and then embedded in paraffin wax. Thereafter, 4–5-µm thick serial sections were cut using a microtome and stained with hematoxylin and eosin (H & E). A pathologist, who was unaware of the group assignments, performed a semiquantitative analysis of the kidney sections using a light microscope with a camera connected to a computer for photographic documentation. A minimum of 10 fields for each kidney slide were assessed. The examinations focused on renal tubules for the presence of dilatation, vacuolation, apoptosis, and necrosis. Interstitial edema and medullary congestion were also assessed. The severity of these lesions was determined using scores on a scale of grade 0: negative, grade 1: minimal, grade 2: mild, grade 3: moderate, and grade 4: severe.12
The current study also examined the renal glomerular injury, hemorrhage, inflammation, fibrinoid, and hyaline dystrophies, where the presence of these injuries was given grade 1 and their absence grade 0.
Statistics
The statistical analyses were performed using the Prism (version 6) statistical package. The numerical data are expressed as mean±standard error of the mean (SEM). The data were evaluated by one-way analysis of variance (ANOVA), followed by the Tukey test multiple comparison. The categorical ordinal data were evaluated by the non-parametrical Mann–Whitney U test. The frequency of the categorical binary data was evaluated using the Fisher exact test. Five-percent-error risk P<0.05 were considered statistically significant.
Results
Serum Urea and Creatinine Concentrations
The serum urea and creatinine levels were significantly increased in Group B following glycerol administration (P<0.001). These levels were significantly reduced in the rats treated with pioglitazone (10 mg/kg) for 3 days (Group C) in comparison with Group B (P=0.04). In Group D, when the drug administration period was doubled, these serum biomarkers levels were also significantly reduced when compared with Group B (P=0.01), but no statistically significant difference was found when comparing the 2 preventive groups of C and D (P=0.81). A comparison of the preventive groups of C and D with the controls (Group A) demonstrated that a statistically significant difference still remained (P<0.001). These results are depicted in table 1 and figure 1 (a and b).
Table 1.
Results of serum creatinine, urea, and glutathione assay
| Groups | Serum creatinine (mg/dL) | P | Serum urea (mg/dL) | P | Reduced glutathione levels mg/g | P |
|---|---|---|---|---|---|---|
| A | 0.433 (0.036) | ●●●<0.001, ♦♦♦<0.001 | 34.2 (1.403) | ●●●0.01, ♦♦♦ 0.01 | 6.926 (0.349) | ●●●<0.001, ♦♦♦<0.001 |
| B | 3.447 (0. 350) | ***<0.001 | 146.333 (4.512) | ***<0.001 | 1.404 (0.354) | ***<0.001 |
| C | 2.39 (0.134) | #0.04 | 124.533 (3.325) | #0.04 | 3.492 (0.582) | #0.01 |
| D | 2.072 (0.336) | ##0.01 | 118.133 (8.866) | ##0.01 | 4.052 (0.359) | ##0.01 |
Values are expressed as mean±SEM.
P<0.001 compared with Group A;
P<0.05,
P=0.01 compared with Group B;
P<0.001 compared with Group A;
P<0.001 compared with Group A
Figure 1.
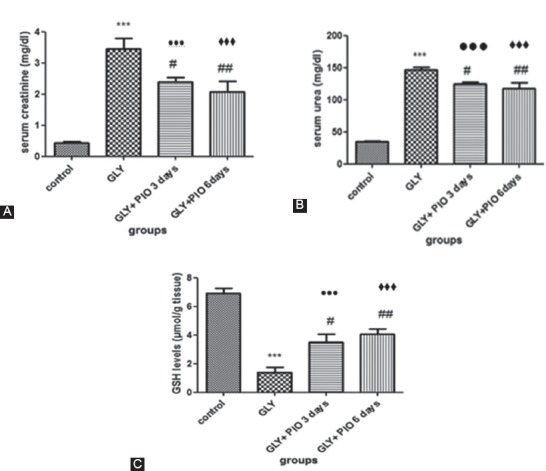
Effects of pioglitazone on serum urea and creatinine levels and on GSH levels in the control group, the glycerol-injected group (GLY), the preventive group treated with pioglitazone for 3 days (GLY+PIO 3days), and the preventive group treated with pioglitazone for 6 days (GLY+PIO 6 days). Values are expressed as mean±SEM. *** P<0.001 compared with the control group; # P<0.05, ## P=0.01 compared with the GLY Group; ●●● P<0.001 compared with Group A; ♦♦♦ P<0.001 compared with Group A; GSH: Glutathione; GLY: Glycerol; PIO: Pioglitazone.
Reduced GSH Levels in the Kidney Tissues
The GSH levels in the kidney tissues were significantly reduced in Group B (P<0.001). They were significantly increased in Group C when compared with Group B (P=0.01). The results also showed a statistically significant difference between Group D and Group B (P=0.01). No statistically significant difference was found when comparing the 2 preventive groups of C and D (P=0.61). A comparison of the preventive groups of C and D with the controls (Group A) showed that a statistically significant difference still remained (P<0.001). These results are illustrated in table 1 and figure 1 (c).
Macroscopic Evaluation
In the controls (Group A), the kidneys had a normal macroscopic appearance. Their surface was smooth and red-brown in color. The sections showed the cortex and medulla, which were different in shade. The blue-red medulla was indented toward the yellowish-red cortex dividing it into renal columns (figure 2a). In the cortex, dots corresponding to the renal glomeruli were visible. The kidneys in the injured Group B were bigger than those in the control group, with a different macroscopic morphology. They showed pale pink cortices with multiple micro abscesses and dark brown medullae with noticeable congestion and edema. The border between the medulla and the cortex was clear (figure 2b). These morphological changes were markedly reversed in the preventive groups of C and D (figures 2c and 2d).
Figure 2.
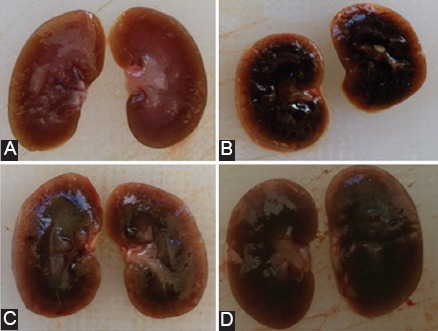
Macroscopic evaluation of the kidney sections in the different groups. (A) Normal macroscopic appearance in the control group A. (B) kidneys are bigger with noticeable medullary congestion and edema and a pale pink cortex (Group B). (C) and (D) Morphological changes are improved in the preventive groups of C and D.
Microscopic Evaluation
Histopathological changes in the kidneys of all the groups were examined and scored. The results are provided in tables 2, 3, and 4. In the controls (Group A), the microscopic picture of the kidneys was normal. The renal tubules were characterized by clearly-visible empty lumens without pathological deposits and shaded with a brush border. The proximal convoluted tubules were regularly arranged, with a 1-layer cubic epithelium. The distal renal tubules had regular round or oval lumens. They were lined with the 1-layer cubic epithelium with poorly-marked margins. The appearance of the glomerular and Bowman’s capsules was normal (figures 3, a1 and a2). Some of them showed minimal changes in the renal tubular histology and medullary congestion. By contrast, moderate to severe lesions were seen in Group B’s kidney tubules injected with glycerol: They showed marked tubular dilatation (P=0.01), vacuolation (P=0.01), and typical apoptotic morphology (P=0.01) including the degeneration of the tubular epithelium, where the tubular epithelial cell walls were flattened, damaged, or completely destroyed. The necrosis was more pronounced in the cortical segments of the proximal tubules. There was also a severe grade of medullary congestion, coagulation, and interstitial edema (P=0.01). The markedly widened lumens of some tubules were filled with degenerate and desquamated epithelial cells. Signs of glomerular injuries were also observed (P=0.02), represented as glomerular necrosis and expansion of the Bowman’s capsules. Fibrinoid and hyaline dystrophies were observed in some kidney samples, but no statistical significant difference was found when compared with the control group (P=0.18). On the other hand, acute inflammation (P=0.01), represented as the interstitial infiltration of the inflammatory cells, was also observed. These histopathological alterations are shown in figure 3 b1 and b2.
Table 2.
Histopathological scores of renal tubules, interstitial edema, and medullary congestion expressed as the frequency of injured animals in each group
| Features groups | Tubular dilatation | Tubular vacuolation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (0) | (1) | (2) | (3) | (4) | P | (0) | (1) | (2) | (3) | (4) | P | |
| A | 0 | 1 | 5 | 0 | 0 | 0.11(compared with Group C, 0.22(compared with Group D) |
2 | 4 | 0 | 0 | 0 | ●●0.01, ♦♦0.01 |
| B | 0 | 0 | 0 | 2 | 4 | ** 0.01 | 0 | 0 | 0 | 6 | 0 | ** 0.01 |
| C | 0 | 0 | 4 | 2 | 0 | ## 0.01 | 0 | 0 | 4 | 2 | 0 | # 0.02 |
| D | 0 | 0 | 5 | 1 | 0 | ## 0.01 | 0 | 0 | 5 | 1 | 0 | ## 0.01 |
| Features groups | Tubular apoptosis | Interstitial edema | ||||||||||
| (0) | (1) | (2) | (3) | (4) | P | (0) | (1) | (2) | (3) | (4) | P | |
| A | 2 | 4 | 0 | 0 | 0 | 0.07(compared with Group D) | 2 | 4 | 0 | 0 | 0 | ●●0.01, ♦♦0.01 |
| B | 0 | 0 | 0 | 0 | 6 | **0.01 | 0 | 0 | 0 | 0 | 6 | ** 0.01 |
| C | 0 | 0 | 0 | 3 | 3 | ●●0.01 | 0 | 0 | 2 | 4 | 0 | ##0.01 |
| D | 0 | 4 | 2 | 0 | 0 | ## 0.01, ▲▲0.01 | 0 | 0 | 3 | 3 | 0 | ##0.01 |
| Features groups | Medullary congestion | |||||||||||
| (0) | (1) | (2) | (3) | (4) | P | |||||||
| A | 3 | 3 | 0 | 0 | 0 | 0.23 (compared with Group D) | ||||||
| B | 0 | 0 | 0 | 0 | 6 | ** 0.01 | ||||||
| C | 0 | 0 | 4 | 0 | 2 | # 0.02, ●●0.01 | ||||||
| D | 2 | 1 | 2 | 0 | 1 | ## 0.01 | ||||||
P=0.01 compared with Group A;
P<0.05,
P=0.01 compared with Group B;
P=0.01 compared with Group C;
p=0.01 compared with Group A;
P=0.01 compared with Group A
Table 3.
Histopathological scores of glomerular injury, fibrinoid dystrophy, and hyaline dystrophy, expressed as the frequency of injured animals in each group
| Features groups | Glomerular injury | Fibrinoid dystrophy | HyalinedDdstrophy | |||
|---|---|---|---|---|---|---|
| Injury | P | Injury | P | Injury | P | |
| A | 0 | 0.99 (compared with Group D) | 0 | 0.99 (compared with Group D) | 0 | 0.99 (compared with Group D), 0.45 (compared with Group C |
| B | 5 | * 0.02 | 3 | 0.18 (compared with Group A) | 3 | 0.18 (compared with Group A) |
| C | 5 | ●●0.01 | 3 | 0.99 (compared with Group B) | 2 | 0.99 (compared with Group B) |
| D | 0 |
# 0.02, ▲ 0.02 |
0 | 0.18 (compared with Group B, C) | 0 | 0.18 (compared with Group B), 0.45(compared with Group C) |
P<0.05 compared with Group A;
P<0.05 compared with Group B;
P<0.05 compared with Group C;
P=0.01 compared with Group A
Table 4.
Histopathological scores on acute inflammation, hemorrhage, and coagulation, expressed as the frequency of injured animals in each group
| Features groups | Coagulation | Hemorrhage | Acute inflammation | |||
|---|---|---|---|---|---|---|
| Injury | P | Injury | P | Injury | P | |
| A | 0 | 0.18 (compared with Group C), 0.99 (compared with Group D) | 0 | 0.99 (compared with Group C and Group D) | 0 | 0.99 (compared with Group C and Group D) |
| B | 6 | **0.01 | 3 | 0.18 (compared with Group A) | 6 | ** 0.01 |
| C | 3 | 0.18 (compared with Group B) | 0 | 0.18 (compared with Group B) | 1 | # 0.02 |
| D | 0 | ## 0.01 | 0 | 0.18 (compared with Group B) | 1 | #0.02 |
P=0.01 compared with Group A;
P<0.05
P=0.01 compared with Group B
Figure 3.
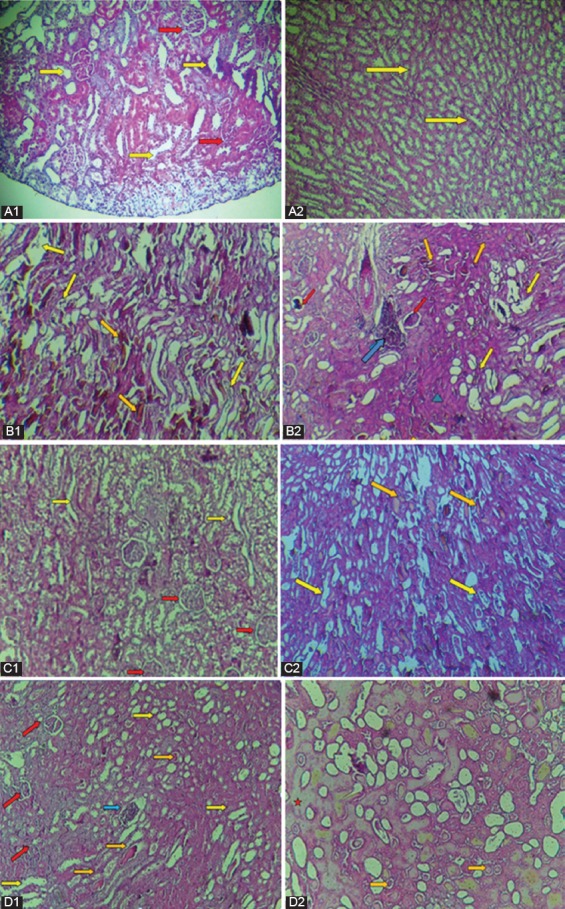
Light microscopic evaluation of the renal tissues stained with hematoxylin and eosin. (a1 ×20) and (a2) Normal histology of the kidney tissues, showing normal glomerulus (red arrow) and normal tubules (yellow arrow) (Group A). (b1) and (b2) Kidney sections of Group B, showing glomerular deformation (red arrow), tubular dilatation, vacuolation, swelling, and degeneration of their lined epithelial cells (yellow arrows), vascular congestion (orange arrow), inflammatory cell infiltration (blue arrow), and fibrinoid dystrophy (green triangle) (×20). (c1, c2) and (d1, d2) Kidney sections of Group C and Group D, respectively, showing the enhancement in tubular and glomerular injuries and other pathologic alterations. The red star represents the hyaline dystrophy (×20).
In the rats treated with pioglitazone (Group C and Group D), the tubular lesions were significantly reduced compared with Group B, concerning dilatation in Group C and Group D (P=0.01), vacuolation in Group C (P=0.02) and in Group D (P=0.01), and apoptosis in Group D (P=0.01). A comparison of these 2 preventive groups yielded no statistically significant difference, except for apoptosis, which was more improved in Group D (P=0.01). Glomerular injury was almost alleviated in Group D (P=0.02), as well as acute inflammation injury (P=0.02). Coagulation injury was significantly reduced in Group D (P=0.01) compared with Group B. The histopathological examinations in Group C are shown in figure 3 c1 and c2 and those in Group D are shown in figure 3 d1 and d2.
Discussion
Rhabdomyolysis is one of the essential causes of nephropathy.3,4 Glycerol is extensively used in studying this type of renal injury.5,6 Its intramuscular injection dissolves striated muscles, releasing the myoglobin and other potentially toxic intracellular components into the systemic circulation in a way similar to what happens in rhabdomyolysis.3 In the current study, nephropathy occurrence was detected by measuring serum urea and creatinine levels, still known to be the most usable and significant indicators in renal function estimation.20
Intramuscular injection of 50% glycerol (10 mL/kg) to the Group B rats caused significant renal injury, represented as elevations in the levels of the above-mentioned serum biomarkers and as histopathological alterations in several kidney parts, mainly in the renal tubules and glomeruli. The development of edema and the enlargement of the kidney also confirmed the renal toxicity. These results conform with those reported by Gu et al.,17 Park et al.,21 and Ustundag et al.,22 who succeeded in inducing nephropathy by using this protocol.
The present study also showed that glycerol administration caused a significant decrease in kidney tissue GSH levels, chiming in with the results reported by several previous studies such as those conducted by Park et al.,21 Manikandan et al.,23 and Liu et al.24
Although the pathogenesis is not yet fully understood, it can nevertheless be mainly attributed to renal vasoconstriction and to the subsequent ischemic injury, caused by a reduced renal blood flow as a result of the excessive leakage of the extracellular fluid into the damaged muscle cells and the secondary activation of the renin–angiotensin–aldosterone system. This effect can also be a result of the nitric oxide scavenging effect of myoglobin.3,7,25 On the other hand, myoglobin exerts direct toxic effects on the renal tubules through 2 principal mechanisms. The 1st one is linked to the release of free iron (Fe+2), which leads to the creation of toxic and reactive hydroxyl radicals (OH) through the Haber–Weiss reaction. The other mechanism is linked to the insertion of myoglobin heme moiety into the redox cycle, causing oxidative injuries such as lipid peroxidation.3,7,25
It was recently noticed that myoglobin itself can exert a peroxidase-like enzyme activity that leads to uncontrolled biomolecules oxidation.26 Myoglobin causes tubular obstruction by interacting with Tamm–Horsfall proteins and forms intratubular casts.3,26 Furthermore, inflammatory lesions caused by cytokines and chemokines released after rhabdomyolysis aggravate glomerular damage.27
The results proved that the rats treated with pioglitazone (10 mg/kg) significantly reversed the effects of glycerol on the serum biomarkers of renal injury, but without reaching normal levels. This protection has been reported earlier in other models of renal impairment induced by cyclosporine,11 cisplatin,12 nephritic syndrome,18 and metabolic syndrome,19 as well as in diabetic nephropathy28 and in ischemiareperfusion injury.29 However, no significant advantage was found in doubling the drug administration period.
It was supposed that if pioglitazone was able to enhance GSH levels in kidney tissues, it would suppress the oxidative stress injury involved in the pathogenesis. The administration of a high-dose GSH was earlier reported to protect the kidney from mercury30 and cisplatin-induced nephrotoxicity.31 N-acetylcysteine, a GSH precursor, was reported to be able to prevent contrast-induced nephropathy.32
Our results showed a significant increase in GSH levels in the rats treated with this drug, in concordance with the very few studies earlier focusing on this measurement.12,28 This result is concordant with other studies suggesting that pioglitazone can reduce oxidative stress by improving some antioxidant enzymes at the kidney level.19,28,29,33
The morphological changes in the rats’ kidneys concerning shape, shade, and weight were improved when pioglitazone was administered. The improvements were also observed microscopically in the renal tubules, in the glomeruli, and in the other studied histologic features. These results support those reported by Reel et al.,29 who indicated that pioglitazone was able to reduce renal histological alterations in ischemia/reperfusion injury, and by Mahmoud et al.,12 who also found that pioglitazone was able to improve microscopic structural changes in cisplatin-induced nephrotoxicity.
The current study showed that using the medicine for 6 consecutive days had more significant effects in alleviating glomerular damage and tubular apoptosis as well as in reducing inflammation than when used for only 3 days.
Pioglitazone has many beneficial effects that may explain its prevention role. Functional PPAR-ɣ receptors have been identified in the renal glomerular and tubular segments and found to be abundant in the inner medulla. The effects of pioglitazone might or might not be directly mediated by PPAR-g activation.34 Pioglitazone can increase renal nitric oxide bioavailability, which has a vasodilating effect on renal arterioles and leads to capillary pressure reduction and renal endothelial function improvement. It has been proved as well that it significantly reduces the potent vasoconstrictor endothelin-1 secretion.8,10,19,34 Several studies have concluded that pioglitazone inhibits the effects of the renin-angiotensin system in the renal vasculature by downregulating angiotensin-1 receptor expression in the vascular smooth muscle cells.10,35 These properties can alleviate ischemic injury resulting from glycerol administration. As has been previously shown, pioglitazone also possesses antifibrotic10,36 and anti-inflammatory effects.10,34 On the other hand, several systemic actions such as reductions in blood glucose and blood pressure levels can be involved in the renoprotective properties of this compound and can reduce glomerular dysfunction.10
There are several limitations regarding our study, first and foremost among which is our relatively small sample size. We only evaluated the traditional markers (i.e., serum urea and creatinine levels) to detect the kidney function, but we did not measure the newer indicators, which would have been more specific in detecting the occurrence of AKI. Another salient drawback is that we did not measure the antioxidant enzymes levels in the kidney tissues, which would have strengthened the impact of our results. However, we clearly showed that pioglitazone was able to reduce renal oxidative stress by measuring reduced GSH levels.
Conclusion
Pioglitazone, which is price wise an acceptable drug and is still widely administrated in the treatment of type 2 diabetic patients, might provide a promising approach in rhabdomyolysis-induced acute renal injury prevention. This protection can probably be mediated, in part, by its antioxidant properties. It has the advantage of rapidly exhibiting its effects, exerting them either systematically or locally at its receptors level, giving the expected effects in 3 days. Doubling the drug administration period did not produce a significant additional benefit. However, the exact mechanisms involved in its antioxidant properties and doses that can be used should be further explored by future clinical trials, especially on diabetic nephropathy patients already treated with this drug.
Acknowledgement
We are grateful to the Faculty of Pharmacy, Damascus University, and the Department of Oral Histopathology in the Faculty of Medicine, Damascus University, Syria, for their valuable cooperation and support.
Conflict of Interest: None declared.
References
- 1.Macedo E, Bouchard J, Mehta RL. Renal recovery following acute kidney injury. Curr Opin Crit Care. 2008;14:660–5. doi: 10.1097/MCC.0b013e328317ee6e. [DOI] [PubMed] [Google Scholar]
- 2.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10:193–207. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 3.Petejova N, Martinek A. Acute kidney injury due to rhabdomyolysis and renal replacement therapy: a critical review. Crit Care. 2014;18:224. doi: 10.1186/cc13897. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres PA, Helmstetter JA, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15:58–69. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 5.Cebi G, Yildiz S, Uzun G, Oztas Y, Sabuncuoglu S, Kutlu A, et al. The effect of hyperbaric oxygen therapy on rhabdomyolysis-induced myoglobinuric acute renal failure in rats. Ren Fail. 2016;38:1554–9. doi: 10.1080/0886022X.2016.1227925. [DOI] [PubMed] [Google Scholar]
- 6.Staff PO. Correction: Penehyclidine Hydrochloride Pretreatment Ameliorates Rhabdomyolysis-Induced AKI by Activating the Nrf2/HO-1 Pathway and Allevi-ating Endoplasmic Reticulum Stress in Rats. PLoS One. 2016;11:e0154138. doi: 10.1371/journal.pone.0154138. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutaud O, Roberts LJ., 2nd Mechanism-based therapeutic approaches to rhabdomyolysis-induced renal failure. Free Radic Biol Med. 2011;51:1062–7. doi: 10.1016/j.freeradbiomed.2010.10.704. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishida K, Watanabe H, Ogaki S, Kodama A, Tanaka R, Imafuku T, et al. Renoprotective effect of long acting thioredoxin by modulating oxidative stress and macrophage migration inhibitory factor against rhabdomyolysis-associated acute kidney injury. Sci Rep. 2015;5:14471. doi: 10.1038/srep14471. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radenkovic M. Pioglitazone and Endothelial Dysfunction: Pleiotropic Effects and Possible Therapeutic Implications. Sci Pharm. 2014;82:709–21. doi: 10.3797/scipharm.1407-16. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarafidis PA, Bakris GL. Protection of the kidney by thiazolidinediones: an assessment from bench to bedside. Kidney Int. 2006;70:1223–33. doi: 10.1038/sj.ki.5001620. [DOI] [PubMed] [Google Scholar]
- 11.Pereira MG, Camara NO, Campaholle G, Cenedeze MA, de Paula Antunes Teixeira V, dos Reis MA, et al. Pioglitazone limits cyclosporine nephrotoxicity in rats. Int Immunopharmacol. 2006;6:1943–51. doi: 10.1016/j.intimp.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Mahmoud MF, El Shazly SM. Pioglitazone protects against cisplatin induced nephrotoxicity in rats and potentiates its anticancer activity against human renal adenocarcinoma cell lines. Food Chem Toxicol. 2013;51:114–22. doi: 10.1016/j.fct.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Helmy MM, Helmy MW, El-Mas MM. Additive Renoprotection by Pioglitazone and Fenofibrate against Inflammatory, Oxidative and Apoptotic Manifestations of Cisplatin Nephrotoxicity: Modulation by PPARs. PLoS One. 2015;10:e0142303. doi: 10.1371/journal.pone.0142303. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nlandu-Khodo S, Dissard R, Hasler U, Schafer M, Pircher H, Jansen-Durr P, et al. NADPH oxidase 4 deficiency increases tubular cell death during acute ischemic reperfusion injury. Sci Rep. 2016;6:38598. doi: 10.1038/srep38598. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saitoh T, Satoh H, Nobuhara M, Machii M, Tanaka T, Ohtani H, et al. Intravenous glutathione prevents renal oxidative stress after coronary angiography more effectively than oral N-acetylcysteine. Heart Vessels. 2011;26:465–72. doi: 10.1007/s00380-010-0078-0. [DOI] [PubMed] [Google Scholar]
- 16.Jin B, Wu BW, Zhang JJ, Luo XP, Shi HM. Preventive effect of reduced glutathione on contrast-induced nephropathy in elderly patients undergoing coronary angiography or intervention: a randomized, controlled trial. Braz J Med Biol Res. 2015;48:839–42. doi: 10.1590/1414-431X20154676. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu H, Yang M, Zhao X, Zhao B, Sun X, Gao X. Pretreatment with hydrogen-rich saline reduces the damage caused by glycerol-induced rhabdomyolysis and acute kidney injury in rats. J Surg Res. 2014;188:243–9. doi: 10.1016/j.jss.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Zuo Y, Yang HC, Potthoff SA, Najafian B, Kon V, Ma LJ, et al. Protective effects of PPARgamma agonist in acute nephrotic syndrome. Nephrol Dial Transplant. 2012;27:174–81. doi: 10.1093/ndt/gfr240. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong X, Zhang DY, Wu HB, Li FX. Losartan and pioglitazone ameliorate nephropathy in experimental metabolic syndrome rats. Biol Pharm Bull. 2011;34:693–9. doi: 10.1248/bpb.34.693. [DOI] [PubMed] [Google Scholar]
- 20.Rouse RL, Stewart SR, Thompson KL, Zhang J. Kidney injury biomarkers in hypertensive, diabetic, and nephropathy rat models treated with contrast media. Toxicol Pathol. 2013;41:662–80. doi: 10.1177/0192623312464122. [DOI] [PubMed] [Google Scholar]
- 21.Park CH, Tanaka T, Cho EJ, Park JC, Shibahara N, Yokozawa T. Glycerol-induced renal damage improved by 7-O-galloyl-D-sedoheptulose treatment through attenuating oxidative stress. Biol Pharm Bull. 2012;35:34–41. doi: 10.1248/bpb.35.34. [DOI] [PubMed] [Google Scholar]
- 22.Ustundag S, Sen S, Yalcin O, Ciftci S, Demirkan B, Ture M. L-Carnitine ameliorates glycerol-induced myoglobinuric acute renal failure in rats. Ren Fail. 2009;31:124–33. doi: 10.1080/08860220802599130. [DOI] [PubMed] [Google Scholar]
- 23.Manikandan R, Beulaja M, Thiagarajan R, Pandi M, Arulvasu C, Prabhu NM, et al. Ameliorative effect of ferulic acid against renal injuries mediated by nuclear factor-kappaB during glycerol-induced nephrotoxicity in Wistar rats. Ren Fail. 2014;36:154–65. doi: 10.3109/0886022X.2013.835223. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Fu X, Gou L, Li S, Lan N, Zheng Y, et al. L-citrulline protects against glycerol-induced acute renal failure in rats. Ren Fail. 2013;35:367–73. doi: 10.3109/0886022X.2012.760408. [DOI] [PubMed] [Google Scholar]
- 25.Cil O, Ertunc M, Gucer KS, Ozaltin F, Iskit AB, Onur R. Endothelial dysfunction and increased responses to renal nerve stimulation in rat kidneys during rhabdomyolysis-induced acute renal failure: role of hydroxyl radical. Ren Fail. 2012;34:211–20. doi: 10.3109/0886022X.2011.643389. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez E, Soler MJ, Rap O, Barrios C, Orfila MA, Pascual J. Risk factors for acute kidney injury in severe rhabdomyolysis. PLoS One. 2013;8:e82992. doi: 10.1371/journal.pone.0082992. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korrapati MC, Shaner BE, Schnellmann RG. Recovery from glycerol-induced acute kidney injury is accelerated by suramin. J Pharmacol Exp Ther. 2012;341:126–36. doi: 10.1124/jpet.111.190249. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuru Karabas M, Ayhan M, Guney E, Serter M, Meteoglu I. The effect of pioglitazone on antioxidant levels and renal histopathology in streptozotocin-induced diabetic rats. ISRN Endocrinol. 2013;2013:858690. doi: 10.1155/2013/858690. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reel B, Guzeloglu M, Bagriyanik A, Atmaca S, Aykut K, Albayrak G, et al. The effects of PPAR-gamma agonist pioglitazone on renal ischemia/reperfusion injury in rats. J Surg Res. 2013;182:176–84. doi: 10.1016/j.jss.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Jan AT, Ali A, Haq Q. Glutathione as an antioxidant in inorganic mercury induced nephrotoxicity. J Postgrad Med. 2011;57:72–7. doi: 10.4103/0022-3859.74298. [DOI] [PubMed] [Google Scholar]
- 31.Jenderny S, Lin H, Garrett T, Tew KD, Townsend DM. Protective effects of a glutathione disulfide mimetic (NOV-002) against cisplatin induced kidney toxicity. Biomed Pharmacother. 2010;64:73–6. doi: 10.1016/j.biopha.2009.09.009. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pezeshgi A, Parsamanesh N, Farhood G, Mahmoodi K. Evaluation of the protective effect of N-acetylcysteine on contrast media nephropathy. J Renal Inj Prev. 2015;4:109–12. doi: 10.12861/jrip.2015.23. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhry J, Ghosh NN, Roy K, Chandra R. Antihyperglycemic effect of a new thiazolidinedione analogue and its role in ameliorating oxidative stress in alloxan-induced diabetic rats. Life Sci. 2007;80:1135–42. doi: 10.1016/j.lfs.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Sarafidis PA, Georgianos PI, Lasaridis AN. PPAR-gamma agonism for cardiovascular and renal protection. Cardiovasc Ther. 2011;29:377–84. doi: 10.1111/j.1755-5922.2010.00222.x. [DOI] [PubMed] [Google Scholar]
- 35.Kong X, Ma MZ, Qin L, Zhang Y, Li XY, Wang GD, et al. Pioglitazone enhances the blood pressure-lowering effect of losartan via synergistic attenuation of angiotensin II-induced vasoconstriction. J Renin Angiotensin Aldosterone Syst. 2014;15:259–70. doi: 10.1177/1470320313489061. [DOI] [PubMed] [Google Scholar]
- 36.Panchapakesan U, Sumual S, Pollock CA, Chen X. PPARgamma agonists exert antifibrotic effects in renal tubular cells exposed to high glucose. Am J Physiol Renal Physiol. 2005;289:F1153–8. doi: 10.1152/ajprenal.00097.2005. [DOI] [PubMed] [Google Scholar]


