Abstract
Introduction
Chronic low back pain (LBP) is a major health problem worldwide. Multidisciplinary rehabilitation and exercise is recommended for the management of chronic LBP. However, there is a need to investigate effective exercise interventions that is available in clinics and as home-based training on a large scale. This article presents the design and rationale of the first randomized clinical trial investigating the effects of progressive resistance training with elastic bands in addition to multidisciplinary rehabilitation for patients with moderate to severe chronic LBP.
Methods and analysis
We aim to enroll 100 patients with chronic LBP referred to a specialized outpatient hospital clinic in Norway. Participants will be randomized equally to either; a) 3 tion including whole-body progressive resistance training using elastic bands – followed by home-based progressive resistance training for 9 weeks, or b) 3 weeks of multidisciplinary rehabilitation including general physical exercise – followed by home-based general physical exercise for 9 weeks. Questionnaires and strength tests will be collected at baseline, weeks 3 and 12, and at 6 and 12 months. The primary outcome is between-group changes in pain-related disability at week 12 assessed by the Oswestry disability index. Secondary outcomes include pain, work ability, work status, mental health, health-related quality of life, global rating of change, general health, and muscular strength and pain-related disability up to 12 months of follow-up.
Discussion
This study will provide valuable information for clinicians working with patients with chronic LBP.
Trial registration
ClinicalTrials.gov, number NCT02420236.
Keywords: Musculoskeletal disorders, Disability evaluation, Low back pain, Chronic pain, Resistance training, Exercise
1. Introduction
Low back pain (LBP) is a leading cause of reduced quality of life and disability worldwide [1], [2]. Current guidelines advocate physical exercise in the management of chronic LBP, without recommending any particular exercise modality [3]. Some recent studies indicate that progressive resistance training (PRT) may have a particularly positive effect on pain and disability in patients with chronic LBP [4], [5], [6], [7] and other types of musculoskeletal pain [7], [8].
Preferably, a training intervention should be easy to implement in clinical practice and as home-based training. Elastic resistance bands are relatively inexpensive, safe, easy to use, portable and require little space, and could therefore represent an attractive and feasible alternative to free weights and training machines for PRT interventions. Studies have shown that the muscular activation level is similar for several resistance training exercises using elastic bands compared to conventional resistance training equipment [9]. However, we are not aware of any randomized clinical trial (RCT) investigating the effect of PRT using elastic bands for patients with chronic LBP.
Chronic LBP often entails a bio-psycho-social symptom picture, i.e., widespread pain, work disability, reduced quality of life, fear-avoidance beliefs, mental symptoms and social withdrawal [10], [11]. Thus, multidisciplinary rehabilitation (MDR) approaches are commonly used for dealing with this disorder [12], [13], [14], [15]. However, studies seeking to identify effective exercise components of MDR are lacking.
This paper comprises the study protocol for an RCT investigating effects of PRT in addition to MDR for patients with moderate to severe chronic LBP. Our hypothesis is that PRT combined with MDR reduces pain-related disability more than MDR with general physical exercise (GPE).
2. Methods and design
2.1. Project context
The study is carried out in a specialized outpatient hospital clinic. The MDR program is provided by the Department of Physical Medicine and Rehabilitation, St. Olavs Hospital, Trondheim University Hospital, Norway. Patients will be recruited from the clinic.
2.2. Design
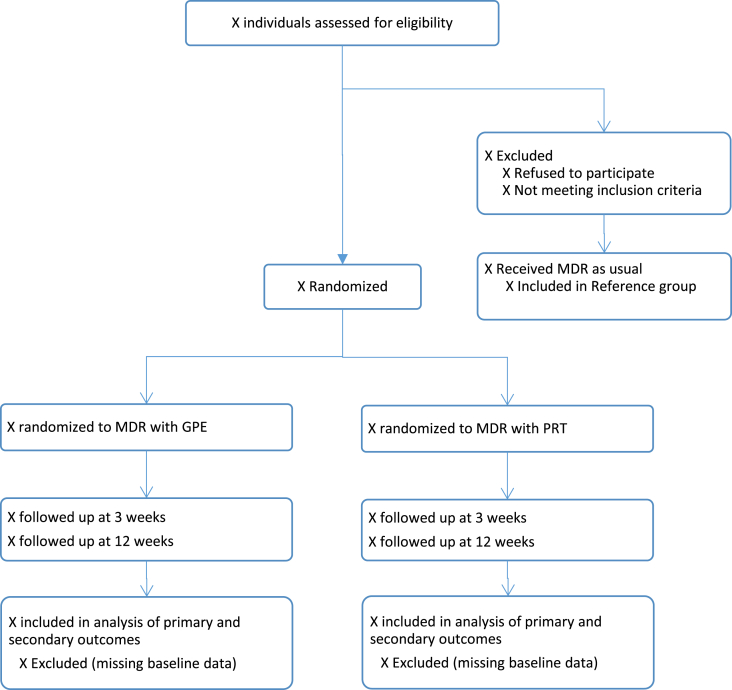
The study is a single-blinded RCT (researchers), involving patients with chronic LBP. Participants will be randomized to participate in i) 3 weeks MDR including PRT at the clinic and 9 weeks home-based PRT, or to ii) 3 weeks MDR including GPE at the clinic and 9 weeks home-based GPE. Three supervised booster sessions will be offered to all participants during the home-period. The study design is presented in Fig. 1. The study protocol adheres to the SPIRIT 2013 checklist [16], and results from the RCT will be published in accordance with the CONSORT statements [17].
Fig. 1.
Patient flow. Included patients are randomized to multidisciplinary rehabilitation (MDR) with progressive resistance training (PRT), or MDR with general physical exercise (GPE). Outcomes will be assessed at baseline, after 3 and 12 weeks, and 6 and 12 months.
2.3. Inclusion and exclusion criteria
Inclusion criteria: 1) Referred to the clinic for LBP, 2) Chronic (≥3 months) or recurrent (≥2 periods with duration ≥ 4 weeks in the past year) non-specific LBP, 3) Strongest LBP in the last two weeks ≥ 4 on numerical rating scale (0–10), and 4) 16–70 years of age. Exclusion criteria: 1) Severe somatic condition (e.g., cancer, inflammatory rheumatic disease, severe osteoporosis), 2) Psychiatric condition that severely impairs group functioning, 3) Insufficient comprehension of Norwegian language to participate in group sessions and fill out questionnaires, 4) Awaiting surgery of lumbar spine, 5) Alcohol or drug abuse, 6) Ongoing compensation claim or applying for disability pension due to LBP, 7) Engaged in high-intensity resistance training on a regular basis for the last six months, and 8) Contra-indications for high-intensity resistance training (e.g., shoulder complications severely limiting the ability to conduct the training program or where existing shoulder training protocols are advised).
2.4. Recruitment of participants
Patients referred to the outpatient clinic due to LBP will first undergo an ordinary routine clinical examination by a physician. In addition to the formal inclusion and exclusion criteria of the study, physicians performing the clinical examination will also consider whether the participants will benefit from MDR based on the clinical history and motivation of the patient, and whether sufficient treatment has been attempted in primary care. Recruitment and interventions will take place in the period 12/2014 to 01/2017.
-
1.
The physician informs eligible participants about the study and hands out an envelope containing written information about the study and the consent form. Participants can call or e-mail the project leader for supplementary information.
-
2.
Patients get a minimum of three days to consider the invitation before they receive a call from a secretary at the clinic and are asked if he/she would like to participate. Those who want to participate provide oral consent.
-
3.
Patients providing oral consent are consecutively included and randomized.
-
4.
Participants are required to fill in a written consent form before baseline testing.
The MDR groups include up to 10 patients, and are organized so that every other group is an intervention and a comparative group. Patients scheduled to participate in the MDR program at the clinic, but are excluded from or are unwilling to participate in the study, will be asked to complete a baseline questionnaire during their first day at the clinic. Patients willing to participate in the reference group are required to fill in a written consent form.
2.5. MDR at the clinic
The MDR program will be carried out as usual within the study period - five full rehabilitation days in week one and three. There are no sessions in week two, but during this week, the patients are encouraged to apply what they have learned in week one. The teams delivering the MDR program consist of physiotherapists, physicians, social workers, and psychologists.
The MDR programs include individual consultations with physicians and social workers in addition to lectures, patient reflections and discussions. Themes covered are back anatomy, understanding of pain, coping with stress, exercise, psychosocial aspects related to living with pain, making plans and setting goals for work participation, and leisure time, etc. Additionally, there are sessions with physical activity which consist of GPE in the comparative group, and primarily PRE in the intervention group.
2.6. General physical exercise (comparative group)
Participants in the comparative group receive four sessions of GPE in week one and five sessions in week three. The sessions include circle-training, low-intensity resistance exercises, endurance training, ball games, body awareness, stretching, and relaxation techniques. Patients are encouraged to continue to stay physically active at home and are provided a home-training program upon completion of MDR. The program contains exercises and recommendations based upon the patient's interests and individual needs, along with the physiotherapist's recommendations. Participants will be summoned to participate in three supervised booster sessions at two, five, and seven weeks after completion of the MDR. These sessions will be used to adjust the individual program and to motivate the patients to stay physically active.
2.7. Progressive resistance training (intervention group)
In the intervention group, three of the regular weekly physical activity sessions at the clinic are replaced with PRT. These sessions aim to familiarize patients with using elastic resistance bands and to learn proper execution of the different resistance exercises. After completing the MDR, patients will continue with PRT at home three times per week for nine weeks (12 weeks in total). Three supervised booster sessions will be held at similar time intervals as in the comparative group. Improving technique, adjusting resistance and maintaining adherence and compliance will be the main objective of these sessions.
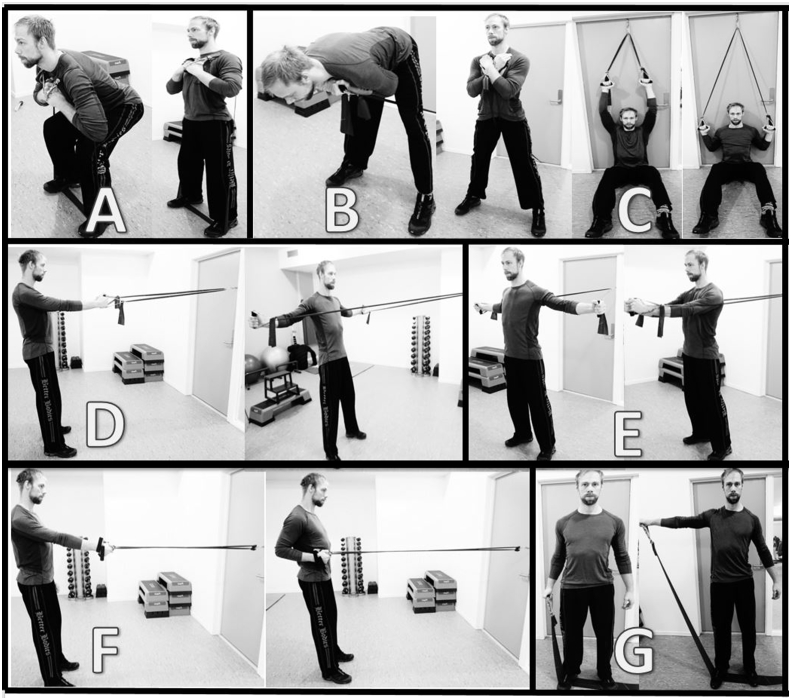
The PRT program will be carried out in accordance with the guidelines described by the American College of Sports Medicine [18], and recommendations for introducing resistance training for persons with musculoskeletal pain [7]. The program is sequenced into four periods (weeks 1–2, 3–5, 6–8, and 9–12). The training load will be increased progressively so that 15–20 repetitions is performed in the first period and 8–10 repetitions in the last period, corresponding to about 50% and 75–85% of one repetition maximum– i.e. the maximum weight that can be lifted in one repetition with proper execution of the exercise. The training load will be increased when the person is able to do 1–2 repetitions more than prescribed. External resistance will be applied using Theraband® Elastic bands (colours: yellow-gold). Bands can be combined in order to increase resistance. Since many LBP patients experience pain from several other sites [19], [20], and often have low general muscular fitness [7], [21], [22], the program consist of exercises for the whole body in order to improve general muscle strength and physical functioning – i.e., squats, stiff-legged deadlifts, flies, unilateral rows, reversed flies, unilateral shoulder abduction and lateral pulldown (see Fig. 2). Similar resistance training exercises have previously been used in studies showing positive results for chronic musculoskeletal pain [4], [5], [6], [23], [24], [25]. Physiotherapists at the clinic were involved in the development of the training programs.
Fig. 2.
Illustration of the strength exercises. Squats (A), stiff-legged deadlifts (B), lateral pulldown (C), reversed flies (D), flies (E), unilateral rows (F), lateral raise (G).
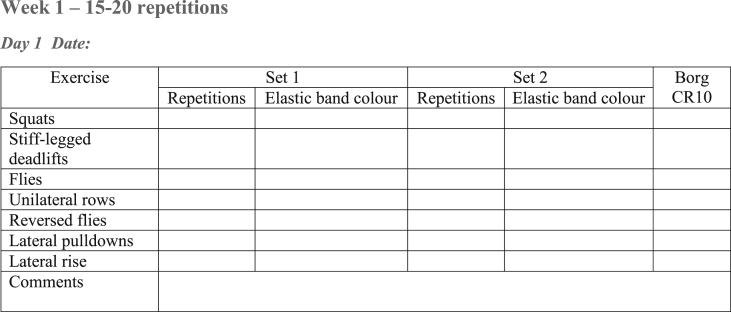
Participants are requested to record all PRT sessions in a training diary (Fig. 3). The Borg CR-10 scale [26] is used to record perceived exertion, as it provides an adequate reflection of resistance training effort [26].
Fig. 3.
Example of the training diary. Participants are instructed to fill in number of repetitions performed, colour of the elastic bands used, and the Borg CR10 rating immediately after finishing the last set of an exercise.
2.8. Training adjustments
In case of acute worsening of symptoms during a specific exercise, the participants will be instructed to: (i) reduce load in the specific exercise, (ii) reduce movement velocity, (iii) reduce range of motion, and (iv) avoid the specific exercise for at least three sessions [27], [28]. Participants can contact a physiotherapist at the clinic if they have questions about symptom progression and/or the training schedule. All deviation from the prescribed training schedule is to be recorded in the training diary.
2.9. Outcomes
Outcomes will be measured at baseline, weeks 3 and 12, and at 6 and 12 months.
2.9.1. Primary outcome
2.9.2. Secondary outcomes
-
○
Oswestry disability index (at three weeks and 6 and 12 months)
-
○
Intensity of LBP at each test session, last two weeks, and last four weeks is assessed by the 11-point Numerical pain rating scale [31].
-
○
Pain sites in the last month is assessed using pain drawings [32].
-
○
Workability is assessed with the single item “current workability compared with the lifetime best” from the Workability index [33].
-
○
Anxiety and depressive symptoms is assessed using Hopkins symptom checklist [34].
-
○
Health-related quality of life is assessed using EQ-5D-5L [35].
-
○
Fear-avoidance beliefs using the fear-avoidance beliefs questionnaire [36].
-
○
Functional capacity will be assessed using the patient specific function scale [37], [38].
-
○
Patient-rated efficacy of the treatment is assessed using the Global rating of change scale [39] – a seven point scale ranging from “feeling very much improved” to “feeling very much worse”.
-
○
Grip strength and low back strength (see strength tests)
2.9.3. Additional measures
-
○
Sex (male/female).
-
○
Marital status (married/live-in partner, single, divorced).
-
○
Height (measured to the nearest 0.1 cm using a wall mounted stadiometer).
-
○
Body weight (measured to the nearest 0.1 kg, using the Bosch personal scale PPW33000)).
-
○
Education level (primary school/middle school, high school, higher education).
-
○
Level of leisure time physical activity is assessed by three questions [40]: 1)” How frequently do you exercise?” (never, less than once a week, once a week, 2–3 times per week, almost every day), 2) “How long does each session last?” (less than 15 min, 16–30 min, 30 min to an hour, more than 1 h), and 3) “If you do such exercise as frequently as once or more times a week: How hard do you push yourself?” (I take it easy without breaking into sweat or losing my breath, I push myself so hard that I lose my breath and break into sweat, I push myself to near exhaustion.) Index score
-
○
Work status and social security benefits status with response options: Employment status (full-time employee, part time employee [stated in percent employed], unemployed, retired, student, other, line of work [specify]), Social security benefits (reported sick [stated in percent and duration], work assessment allowance, disability pension [stated in percent and duration], other, not relevant).
-
○
Patient-rated health status is assessed through the question “How is your health at the moment?”, with response options poor, not so good, good, very good [41].
-
○
Use of analgesics assessed by the two questions “Have you used analgesics for your back pain the last week” (yes/no), and “Have you used any other medications during the last week” (yes/no), “If yes, which kind of analgesics do you use”.
-
○
Duration of current LBP: <3months, 3–6 months, 1–2 years, and >2 years.
-
○
Previous history of LBP is assessed by two items “When did you first experience pain of the same character as you have today in the back, which lasted more than a week” (Never experienced pain like this before, less than a year ago, 1–5 years ago, 6–10 years ago, >10 years ago) and “LBP recurrence” (Never experienced pain like this before, ≤once per year, two to three times per year,> three times per year).
-
○
Description of work is assessed by the question “If you have had paid or unpaid employment, how would you describe your job?”, with response options: work that mostly involves sitting (ex: desk work, assembly worker); Work that requires much walking (e.g.: clerk, light industry worker, teacher); Work that requires much walking and lifting (e.g.: mail carrier, nurse, construction worker); heavy physical labor (e.g.: forester, farmer, heavy construction worker).
2.10. Strength tests
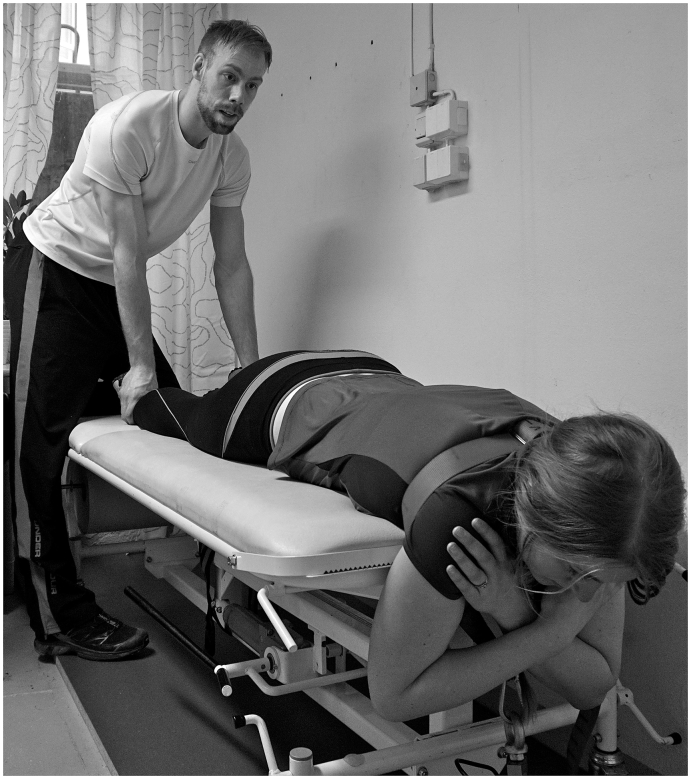
Low back strength (Fig. 4) is assessed through maximal voluntary isometric contractions (MVCs) of the back extensors. Participants are instructed to lie on their stomach on a bench with their arms hanging over the edge, and the armpits pressed against the end of the bench. A rigid strap is fastened around the participants' torso from armpit to armpit. The strap is attached to a force transducer on the platform. Four back extensor MVCs, with 1-min rest intervals will be performed. Force is increased in a gradual manner in the first two contractions and in an explosive manner in the two last contractions. Maximal force is to be held for 3 s in all trials. The test leader is pressing the participant's legs down during the test. Maximal force (N) and rate of force development (N/s) are measured by a force transducer and analyzed using Musclelab software (version 10.3.26.0, Ergotest Technology AS, Langesund, Norway). The highest maximal force value of the four tests, and the highest rate of force development score for the two explosive tests will be used in the analysis.
Fig. 4.
Setup for maximal voluntary contraction of the back extensors.
Handgrip strength (Fig. 5) of the dominant hand is assessed using a hand dynamometer (JAMAR hydraulic hand dynamometer, model J00105). The second narrowest handle position will be used [42]. During testing, subjects sit on a stool with their back against the wall, the upper arm hanging down alongside the body and a 90° flexion in the elbow. Subjects are instructed to squeeze the dynamometer as hard as possible and continue to squeeze until the force starts to decline. Two tests are performed with 1-min rest intervals. A third test is performed if the second test is <10% different from the first test. The highest value will be used in the analysis.
Fig. 5.
Setup for the grip strength test.
2.11. Compensation and lotteries
Participants will be compensated for travel expenses, and a lottery with prizes will be included to stimulate participation and compliance.
2.12. Sample size
The sample size calculation is done for the mixed linear model described in the Statistical analysis section. With 80 participants (40 in each arm) we will have 80% power to detect a difference of 5 points (0–100) between the groups (alpha level = 0.05), assuming that the marginal standard deviation for ODI is 9 points (based on previous studies [43], [44] and unpublished data from the present study population) and that the correlation between baseline and the 12 weeks test, within participants, is 0.5. A ∼20–25% dropout rate was expected based on a previous study on patients in the present clinic [45], and studies employing resistance training interventions for chronic LBP patients [4], [46]. Thus, we aim to enroll 100 participants.
2.13. Randomization and blinding
Block randomization with unknown block sizes is performed using a web-based program delivered by the Unit for Applied Clinical Research, Norwegian University of Science and Technology. Due to the nature of the study, it is impossible to blind the participants, and the health personnel at the clinic. However, test leaders and researchers conducting the analysis will be unaware of group allocation.
2.14. Data management
All data acquired from objective tests and questionnaires are coded with an identification code, and plotted in excel files stored on a secure network station. Identifying information about the participants, including the signed consent form, is stored in locked filing cabinets kept behind locked doors throughout the study period. All authors have access to the data.
2.15. Statistical analysis
Effect analysis will be performed in accordance with the intention-to-treat principle and per protocol. Effect-differences between groups for the primary outcome will be assessed with mixed linear models. The effect of time and treatment will be included as a fixed effect with levels ‘baseline’, ’12 weeks PRT′ and ’12 weeks GPE’. Due to randomization, there will be no systematic difference between the groups at baseline. Participant ID will be included as a random effect to account for repeated measurements. Effect differences for secondary outcomes will be assessed with mixed linear models or with multilevel, mixed logistic regression, as appropriate. Per-protocol analysis of the primary outcome will be performed for participants completing more than 60% of the planned PRT sessions.
3. Discussion
Current guidelines recommend physical exercise in the management of chronic LBP, without emphasizing any particular exercise modality [3] This will be the first RCT to assess the effects of resistance training in addition to MDR for patients with chronic LBP.
A limitation is that study participants and therapists could not be blinded. Another limitation is that the same physiotherapists are group leaders of both the PRT and GPE groups, thus a carry-over effect could occur (e.g. by introducing aspects of high-intensity resistance training to the comparative group). However, clear procedures for management of the groups have been made. Since it is voluntary to participate in the study, it is possible that study participants could differ from the ones who do not wish to participate. However, patients included in MDR, but who refuse to participate or are excluded from the study are asked to fill in a questionnaire to assess the generalizability of the results.
A challenge with home-based training interventions is to ensure high compliance and adherence to the prescribed training program [47], [48]. In order to increase the likelihood of sufficient adherence and compliance we have included an intensive introduction phase, regular follow-ups to reinforce motivation, and use of training diaries to increase commitment, as recommended [48], [49].
In summary, this RCT will provide important knowledge which can improve the future treatment of patients with moderate to severe chronic LBP. The strength training intervention is low-cost, safe, portable, and easy to implement in rehabilitation facilities and as home-training on a large scale. The results will be published in international peer-reviewed journals and presented at national and international conferences.
Authors' contributions
VMI, MSF, OV and PJM conceived the initial idea of the study. All authors contributed in developing the study design and/or training program. VMI wrote the first draft of the article and coordinated the writing of the article. ØS participated in the power calculations and planning of statistical analyses. All authors critically reviewed the manuscript, and approved the article.
Funding
These studies are funded by KLP – Kommunal Landspensjonskasse, and Norwegian Research Council through the FYSIOPRIM project– a research program on physiotherapy in primary care. Performance Health (Akron, OH, USA) provided Theraband® elastic bands for this study. None of the funders had/have any saying in developing the study design, data collection and analysis, decision to publish or preparation of this manuscript or future manuscripts.
Conflict of interest
None declared.
Ethical approval
The studies were approved by the Regional Committee for Medical and Health Research Ethics in Central Norway (no.: 2014/1157).
Acknowledgements
Thanks to employees at St. Olavs Hospital – Department of Physical Medicine and Rehabilitation, Trondheim, Norway, for their enthusiasm and effort in the project. Special thanks to Bitten Kihl Olsen for assistance with recruitment and randomization, Lars Vikan Rise for managing booster sessions, Synnøve Fossheim Karlsen and Atle Melleby Kongsvold for assistance with testings, and to all patients participating in the project.
References
- 1.Murray C.J., Vos T., Lozano R., Naghavi M., Flaxman A.D., Michaud C. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Airaksinen O., Brox J.I., Cedraschi C., Hildebrandt J., Klaber-Moffett J., Kovacs F. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur. Spine J. 2006;15(Suppl 2):S192–S300. doi: 10.1007/s00586-006-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kell R.T., Asmundson G.J. A comparison of two forms of periodized exercise rehabilitation programs in the management of chronic nonspecific low-back pain. J. Strength Cond. Res. 2009;23:513–523. doi: 10.1519/JSC.0b013e3181918a6e. [DOI] [PubMed] [Google Scholar]
- 5.Kell R.T., Risi A.D., Barden J.M. The response of persons with chronic nonspecific low back pain to three different volumes of periodized musculoskeletal rehabilitation. J. Strength Cond. Res. 2011;25:1052–1064. doi: 10.1519/JSC.0b013e3181d09df7. [DOI] [PubMed] [Google Scholar]
- 6.Jackson J.K., Shepherd T.R., Kell R.T. The influence of periodized resistance training on recreationally active males with chronic nonspecific low back pain. J. Strength Cond. Res. 2011;25:242–251. doi: 10.1519/JSC.0b013e3181b2c83d. [DOI] [PubMed] [Google Scholar]
- 7.Kristensen J., Franklyn-miller A. Resistance training in musculoskeletal rehabilitation: a systematic review. Br. J. Sports Med. 2012:719–726. doi: 10.1136/bjsm.2010.079376. [DOI] [PubMed] [Google Scholar]
- 8.Van Eerd D., Munhall C., Irvin E., Rempel D., Brewer S., van der Beek A.J. Effectiveness of workplace interventions in the prevention of upper extremity musculoskeletal disorders and symptoms: an update of the evidence. Occup. Environ. Med. 2015;73:62–70. doi: 10.1136/oemed-2015-102992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aboodarda S.J., Page P.A., Behm D.G. Muscle activation comparisons between elastic and isoinertial resistance: a meta-analysis. Clin. Biomech. (Bristol, Avon) 2016;39:52–61. doi: 10.1016/j.clinbiomech.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Froud R., Patterson S., Eldridge S., Seale C., Pincus T., Rajendran D. A systematic review and meta-synthesis of the impact of low back pain on people's lives. BMC Musculoskelet. Disord. 2014;15:50. doi: 10.1186/1471-2474-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamé I.E., Peters M.L., Vlaeyen J.W.S., Kleef M.V., Patijn J. Quality of life in chronic pain is more associated with beliefs about pain, than with pain intensity. Eur. J. Pain. 2005;9:15–24. doi: 10.1016/j.ejpain.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Hancock M.J., Maher C.G., Laslett M., Hay E., Koes B. Discussion paper: what happened to the 'bio' in the bio-psycho-social model of low back pain? Eur. Spine J. 2011;20:2105–2110. doi: 10.1007/s00586-011-1886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pincus T., Kent P., Bronfort G., Loisel P., Pransky G., Hartvigsen J. Twenty-five years with the biopsychosocial model of low back pain-is it time to celebrate? A report from the twelfth international forum for primary care research on low back pain. Spine. 2013;38:2118–2123. doi: 10.1097/BRS.0b013e3182a8c5d6. [DOI] [PubMed] [Google Scholar]
- 14.Smeets R.J., Vlaeyen J.W., Hidding A., Kester A.D., van der Heijden G.J., van Geel A.C. Active rehabilitation for chronic low back pain: cognitive-behavioral, physical, or both? First direct post-treatment results from a randomized controlled trial [ISRCTN22714229] BMC Musculoskelet. Disord. 2006;7:5. doi: 10.1186/1471-2474-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Middelkoop M., Rubinstein S.M., Kuijpers T., Verhagen A.P., Ostelo R., Koes B.W. A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non-specific low back pain. Eur. Spine J. 2011;20:19–39. doi: 10.1007/s00586-010-1518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan A.W., Tetzlaff J.M., Gotzsche P.C., Altman D.G., Mann H., Berlin J.A. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ Clin. Res. Ed. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garber C.E., Blissmer B., Deschenes M.R., Franklin B.A., Lamonte M.J., Lee I.M. American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 19.Kamaleri Y., Natvig B., Ihlebaek C.M., Bruusgaard D. Localized or widespread musculoskeletal pain: does it matter? Pain. 2008;138:41–46. doi: 10.1016/j.pain.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Hagen E.M., Svensen E., Eriksen H.R., Ihlebaek C.M., Ursin H. Comorbid subjective health complaints in low back pain. Spine. 2006;31:1491–1495. doi: 10.1097/01.brs.0000219947.71168.08. [DOI] [PubMed] [Google Scholar]
- 21.van Wilgen C.P., Akkerman L., Wieringa J., Dijkstra P.U. Muscle strength in patients with chronic pain. Clin. Rehabil. 2003;17:885–889. doi: 10.1191/0269215503cr693oa. [DOI] [PubMed] [Google Scholar]
- 22.Heneweer H., Picavet H.S.J., Staes F., Kiers H., Vanhees L. Physical fitness, rather than self-reported physical activities, is more strongly associated with low back pain: evidence from a working population. Eur. Spine J. 2012;21:1265–1272. doi: 10.1007/s00586-011-2097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen L.L., Kjaer M., Sogaard K., Hansen L., Kryger A.I., Sjogaard G. Effect of two contrasting types of physical exercise on chronic neck muscle pain. Arthritis Rheum. 2008;59:84–91. doi: 10.1002/art.23256. [DOI] [PubMed] [Google Scholar]
- 24.Andersen L.L., Kjaer M., Andersen C.H., Hansen P.B., Zebis M.K., Hansen K. Muscle activation during selected strength exercises in women with chronic neck muscle pain. Phys. Ther. 2008;88:703–711. doi: 10.2522/ptj.20070304. [DOI] [PubMed] [Google Scholar]
- 25.Salo P., Ylonen-Kayra N., Hakkinen A., Kautiainen H., Malkia E., Ylinen J. Effects of long-term home-based exercise on health-related quality of life in patients with chronic neck pain: a randomized study with a 1-year follow-up. Disabil. Rehab. 2012;34:1971–1977. doi: 10.3109/09638288.2012.665128. [DOI] [PubMed] [Google Scholar]
- 26.Shinya Yamauchi S.M. Rating of perceived exertion for quantification of the intensity of resistance exercise. Int. J. Phys. Med. Rehabil. 2013:01. [Google Scholar]
- 27.Sundstrup E., Jakobsen M.D., Andersen C.H., Jay K., Persson R., Aagaard P. Participatory ergonomic intervention versus strength training on chronic pain and work disability in slaughterhouse workers: study protocol for a single-blind, randomized controlled trial. BMC Musculoskelet. Disord. 2013;14:67. doi: 10.1186/1471-2474-14-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen L.L., Zebis M.K., Pedersen M.T., Roessler K.K., Andersen C.H., Pedersen M.M. Protocol for work place adjusted intelligent physical exercise reducing musculoskeletal pain in shoulder and neck (VIMS): a cluster randomized controlled trial. BMC Musculoskelet. Disord. 2010;11:173. doi: 10.1186/1471-2474-11-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roland M., Fairbank J. The roland-morris disability questionnaire and the Oswestry disability questionnaire. Spine. 2000;25:3115–3124. doi: 10.1097/00007632-200012150-00006. [DOI] [PubMed] [Google Scholar]
- 30.Tonosu J., Takeshita K., Hara N., Matsudaira K., Kato S., Masuda K. The normative score and the cut-off value of the Oswestry Disability Index (ODI) Eur. Spine J. 2012;21:1596–1602. doi: 10.1007/s00586-012-2173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Childs J.D., Piva S.R., Fritz J.M. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine. 2005;30:1331–1334. doi: 10.1097/01.brs.0000164099.92112.29. [DOI] [PubMed] [Google Scholar]
- 32.Margolis R.B., Tait R.C., Krause S.J. A rating system for use with patient pain drawings. Pain. 1986;24:57–65. doi: 10.1016/0304-3959(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 33.Ahlstro L., Grimby-Ekman A., Hagberg M., Delve L. The work ability index and single-item question: associations with sick leave, symptoms, and health – a prospective study of women on long-term sick leave. Scand. J. Work Environ. Health. 2010;36:404–412. doi: 10.5271/sjweh.2917. [DOI] [PubMed] [Google Scholar]
- 34.Derogatis L.R., Lipman R.S., Rickels K., Uhlenhuth E.H., Covi L. The Hopkins symptom checklist (HSCL): a self report symptom inventory. Behav. Sci. 1974;19:1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- 35.Agborsangaya C.B., Lahtinen M., Cooke T., Johnson J.A. Comparing the EQ-5D 3L and 5L: measurement properties and association with chronic conditions and multimorbidity in the general population. Health Qual. life outcomes. 2014;12:74. doi: 10.1186/1477-7525-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waddell G., Newton M., Henderson I., Somerville D., Main C.J. A fear-avoidance beliefs questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52:157–168. doi: 10.1016/0304-3959(93)90127-B. [DOI] [PubMed] [Google Scholar]
- 37.Stratford P., Gill C., Westaway M., Binkley J. Assessing disability and change on individual patients: a report of a patient specific measure. Physiother. Can. 1995;47:258–263. [Google Scholar]
- 38.Moseng T., Tveter A.T., Holm I., Dagfinrud H. Et nyttig verktøy for fysioterapeuter i primærhelsetjenesten : Pasient-Spesifikk Funksjons Skala. Fysioterapeuten. 2013:20–26. [Google Scholar]
- 39.Kamper S.J., Maher C.G., Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J. Man. Manip. Ther. 2009;17:163–170. doi: 10.1179/jmt.2009.17.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurtze N., Rangul V., Hustvedt B.E., Flanders D.W. Reliability and validity of self-reported physical activity in the Nord-Trøndelag Health Study - HUNT 1. Scand. J. Public Health. 2008;36:52–61. doi: 10.1177/1403494807085373. [DOI] [PubMed] [Google Scholar]
- 41.The HUNT 3 Study, Norway.
- 42.Tveter A.T., Dagfinrud H., Moseng T., Holm I. Health-related physical fitness measures: reference values and reference equations for use in clinical practice. Arch. Phys. Med. Rehab. 2014;95:1366–1373. doi: 10.1016/j.apmr.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 43.Unsgaard-Tondel M., Fladmark A.M., Salvesen O., Vasseljen O. Motor control exercises, sling exercises, and general exercises for patients with chronic low back pain: a randomized controlled trial with 1-year follow-up. Phys. Ther. 2010;90:1426–1440. doi: 10.2522/ptj.20090421. [DOI] [PubMed] [Google Scholar]
- 44.Unsgaard-Tondel M., Nilsen T.I., Magnussen J., Vasseljen O. Are fear avoidance beliefs associated with abdominal muscle activation outcome for patients with low back pain? Physiother. Res. Int. J. Res. Clin. Phys. Ther. 2013;18:131–139. doi: 10.1002/pri.1539. [DOI] [PubMed] [Google Scholar]
- 45.Borke B. Norwegian University of Science and Technology; Janne-Birgitte: 2009. Maximal Strength Training in Hack Squat Influences Pain, Function and Disability in Individuals with Chronic Low Back Pain. [Google Scholar]
- 46.Steele J., Bruce-Low S., Smith D., Jessop D., Osborne N. A randomized controlled trial of limited range of motion lumbar extension exercise in chronic low back pain. Spine. 2013;38:1245–1252. doi: 10.1097/BRS.0b013e318291b526. [DOI] [PubMed] [Google Scholar]
- 47.Karlsson L., Takala E., Gerdle B., Larsson B. Evaluation of pain and function after two home exercise programs in a clinical trial on women with chronic neck pain - with special emphasises on completers and responders. BMC Musculoskelet. Disord. 2014;15 doi: 10.1186/1471-2474-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rolving N., Christiansen D.H., Andersen L.L., Skotte J., Ylinen J., Jensen O.K. Effect of strength training in addition to general exercise in patients on sick leave due to non-specific neck pain. A randomized clinical trial. Eur. J. Phys. Rehab. Med. 2014;50(6):617–626. [PubMed] [Google Scholar]
- 49.Ghafouri N., Ghafouri B., Fowler C.J., Larsson B., Turkina M.V., Karlsson L. Effects of two different specific neck exercise interventions on palmitoylethanolamide and stearoylethanolamide concentrations in the interstitium of the trapezius muscle in women with chronic neck shoulder pain. Pain Med. (Malden, Mass) 2014;15(8):1379–1389. doi: 10.1111/pme.12486. [DOI] [PubMed] [Google Scholar]