Abstract
The present study was performed to investigate the effect of quercetin on nasopharyngeal carcinoma (NPC) angiogenesis. The real-time RT-PCR and enzyme-linked immunosorbent assays (ELISA) were performed to analyze the expression levels of vascular endothelial growth factor (VEGF) in nasopharyngeal carcinoma cell lines prior to and after the quercetin treatment. Effect of quercetin on the rate of cell proliferation was measured by MTT assay. It was observed that quercetin treatment at a concentration of 10 mg/mL reduced the rate of NPC039 cell viability to 36% compared to control after 24 h. The expression of VEGF and activity of NF-κB was also markedly reduced. The ability of tube formation in HUVECs was inhibited significantly on exposure to quercetin compared to the untreated cells. Therefore, quercetin plays an important role in the inhibition of NPC039 nasopharyngeal carcinoma and can be of therapeutic importance.
Keywords: Nasopharyngeal carcinoma, Quercetin, VEGF, Viability, Inhibition
1. Introduction
Nasopharyngeal carcinoma (NPC) one of the most commonly detected head and neck malignancies has high rate of incidence in southern China (Albeck et al., 1993, Liu et al., 2014). It has been established that NPC differs significantly from other types of head and neck carcinomas (Wei and Sham, 2005). Metastasis of NPC to lymph nodes in neck in more than 70% patients renders the prognosis ineffective (Chua et al., 2001). In addition, metastasis of NPC to lungs, liver and bones also contributes significantly to the very poor prognosis rate of the treatment (Cheng et al., 1998). Metastasis of tumor to distant organs such as lungs, liver and bones is mediated by various factors including angiogenesis (Matsuo et al., 2004, Matsuo et al., 2009, Tong et al., 2008). Among the various factors involved in angiogenesis, vascular endothelial growth factor (VEGF) is of potential importance. It is well known that VEGF significantly contributes to enhanced rate of proliferation in endothelial cells, formation of new blood vessels and tendency of carcinoma metastasis (Carmeliet, 2003, Ma et al., 2008). Compared to normal people the expression of VEGF in serum of cancer patients is found to be markedly higher (Kikuchi et al., 2011, Karayiannakis et al., 2002). The survival rate of cancer patients is also believed to be related to the expression level of VEGF (Maeda et al., 1996, Maeda et al., 1999).
At present the application of medicinal plants plays an important role in curing the life threatening diseases (Antonisamy et al., 2015, Balamurugan, 2015, Rathi et al., 2015, Nandhini and Stella Bai, 2015, Kalaiselvi et al., 2016, Neelamkavil and Thoppil, 2016). Among various flavonols, quercetin (Fig. 1) alone constitutes more than 70% of the total flavonol intake by the body (Bouktaib, 2002, Valsan and Raphael, 2016, Noorudheen and Chandrasekharan, 2016, Santhosh et al., 2016). Quercetin exhibits the promising tendency to inhibit free radical induced tissue damage in various types of disorders including cancer and chronic inflammation (Hollman and Katan, 1997, Murota and Terao, 2003, Sreeshma et al., 2016, Puthur, 2016, Serasanambati and Chilakapati, 2016). Its mechanism of action involves proton mediated quenching of the free radicals and the resulting parent radical gets resonance stabilized (Mariani et al., 2008). The present study was performed to investigate the role of quercetin in the inhibition of cell proliferation and angiogenesis in NPC.
Figure 1.
Structure of quercetin.
2. Materials and methods
2.1. Cell line and culture
Nasopharyngeal carcinoma cell line, NPC-039 was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in Minimum Essential Medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) and 100 U/mL antibiotics (Peprotech, Rock Hill, NJ, USA). The cells were cultured in a humidified atmosphere of 5% CO2 and 95% air at 37 °C.
2.2. Reagents
Quercetin and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (St. Louis, MO, USA). The other common chemicals were purchased from Merck (Darmstadt, Germany).
2.3. Cell proliferation assays
NPC-039 human NPC cells were cultured in DMEM supplemented with 10% FBS at 37 °C in 5% CO2. MTT [12 μl, 5 mg/mL in phosphate-buffered saline (PBS)] assay was used for the determination of NPC-039 NPC cell viability. Briefly, the cells distributed at a density of 2.5 × 105 cells per well into 96-well plates were treated with quercetin or DMSO as control. The cells were incubated for 48 h followed by the addition of MTT reagent (Sigma-Aldrich) to each well. After incubation for 4 h, dimethyl sulfoxide (150 μl) was added to each well to dissolve the formazan crystals formed. Absorbance was measured for each of the well at a wavelength of 465 nm using an ELISA reader (BD Biosciences, Franklin Lake, NJ, USA).
2.4. Real-time reverse transcription polymerase chain reaction (RT-PCR)
NPC-039 cells were distributed at a density of 2 × 106 cells per plate on to 100 mm diameter tissue culture plates. The cells were treated with quercetin (10 mg/mL) for the indicated periods of time. Following incubation, total RNAs were isolated from the cells using Qiagen mini kit (Qiagen, Valencia, CA). RT-Plus PCR kit (5-PRIME, Minneapolis, MN) was used for the synthesis of first strand cDNA and performing PCR reactions. Nano Drop ND-1000 spectrophotometer was used to measure the concentration of RNA and Taq Man Micro RNA Reverse Transcription kit was used to perform the reverse transcription reactions. Taq Man Micro RNA Assay kits were used to perform Stem-loop real-time PCR on ABI 7500 real time PCR system for 40 cycles. The PCR reactions were carried out in triplicates and gene expression was normalized to GAPDH. For the analysis of the obtained data 7500 system SDS software (ABI) was used. The Prism 4.03 software (Graph Pad, Inc., San Diego, CA) was used for the statistical analyses.
2.5. Enzyme-linked immunosorbent assay (ELISA)
NPC-039 cells at a density of 2 × 105 cells per plate in 100 mm dishes were incubated for 24 h with quercetin. After incubation, the culture supernatants were collected and centrifuged for 10 min at 1, 200 rpm. The supernatants were then tested for VEGF expression using ELISA according to the manufacturer’s instructions (R&D Systems, MN, USA). The plates were coated with anti-mouse VEGF antibody and blocked with bovine serum albumin-containing buffer. The cells were washed and treated with standardized VEGF solution followed by addition of cell culture supernatants. The plates after incubation for 2 h were washed three times. Each of the plate was then treated with biotin-labeled detection antibody and avidin-horseradish peroxidase (HRP). After incubation for 1 h the plates were washed and treated with substrate solution followed by measurement of absorbance at 465 nm.
2.6. Determination of NF-κB (p65) transcription factor activity
NPC-039 cells at a density of 2 × 105 cells per plate in 100 mm were treated with quercetin for 12 h. After incubation, NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, IL, USA) were used to isolate the nuclear proteins. For the determination of protein concentration Pierce BCA Protein Assay kit (Thermo Scientific) was used according to the manufacturer’s instructions. The proteins were put on to double-stranded DNA sequence coated 96-well plates containing NF-κB. Following incubation with anti-NF-κB primary antibody the plates were washed and treated with HRP conjugated secondary antibodies. For each of the well absorbance was measured at 465 nm.
2.7. Angiogenesis assay
NPC-039 cells at a density of 2 × 105 cells per well in the upper compartment were isolated from the lower compartment using 0.45 μm pore bearing membranes. The normal dermal FBs and HUVECs were cultured in 24 well plates containing basal medium supplemented with 2% FBS. Angiogenesis kit (Kurabo, Japan) was used to perform the angiogenesis assay according to the manual protocol.
2.8. Statistical analysis
All the values expressed are the means ± standard deviations (SD). Student’s t-test was used for the comparison of obtained values and p-value less than 0.05 was accepted as the significant difference.
3. Results
3.1. Effects of quercetin on NPC-039 NPC cell viability
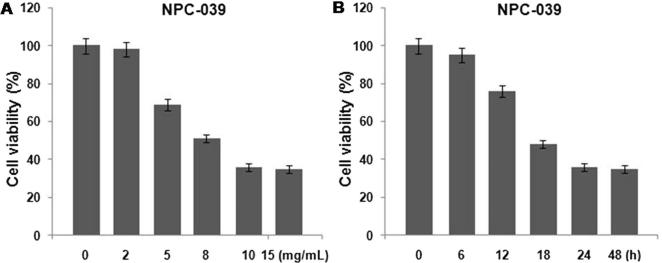
The results from MTT assay revealed a marked reduction in the viability of NPC cells on exposure to quercetin in concentration and time dependent manner. Among the range of quercetin concentrations (2–15 mg/mL) tested the inhibition of cell viability was significant at the concentration of 10 mg/mL after 24 h (P < 0.02). The viability of NPC-039 cells at the concentration of 10 mg/mL was reduced to 36% after 24 h (Fig. 2).
Figure 2.
Inhibition of NPC cell viability by quercetin in (A) concentration and (B) time dependent manner. The cells after quercetin treatment were analyzed by MTT assay for cell viability. All the experiments were performed in triplicates.
3.2. Effect of quercetin on VEGF mRNA expression in NPC-039 NPC cells
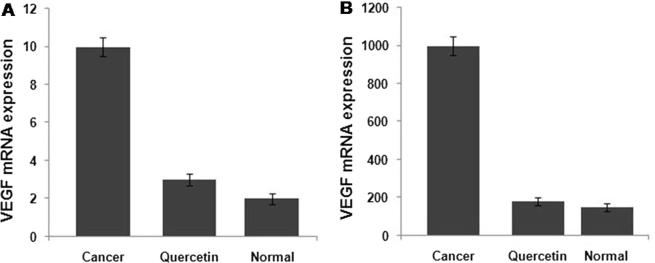
Examination of the expression of VEGF mRNA in NPC-039 cancer cells revealed a significantly higher level compared to the normal cells. However, the treatment of NPC-039 cells with quercetin at a concentration of 10 mg/mL induced a marked reduction in the expression of VEGF mRNA (Fig. 3).
Figure 3.
Quercetin induced changes in the expression of VEGF mRNA. (A) The changes in the VEGF mRNA expression caused by quercetin in NPC-039 cells were analyzed by qPCR. (B) ELISA test was used to determine the changes in VEGF expression from NPC-039 NPC cells by quercetin.
3.3. Effects of quercetin on the activation of NF-κB in NPC-039 NPC cells
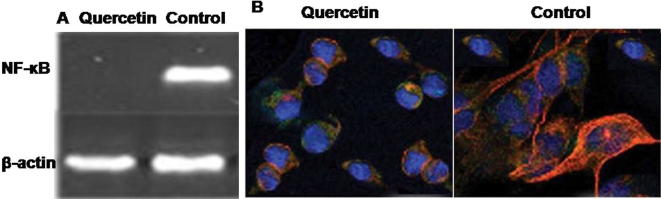
The results from NF-κB (p65) transcription factor assay revealed that quercetin treatment in NPC-039 cells induced a significant decrease in the NF-κB activity. The activity of NF-κB was reduced by around 7-fold in the quercetin treated cells compared to the control cells (Fig. 4).
Figure 4.
Effect of quercetin on NF-κB activity and nuclear proteins in NPC-039 NPC cells. (A) RT-PCR analysis of NF-κB expression level in NPC-039 NPC cells. (B) The protein expression was determined using NF-κB (p65) transcription factor assay.
3.4. Effects of quercetin on HUVEC tube formation
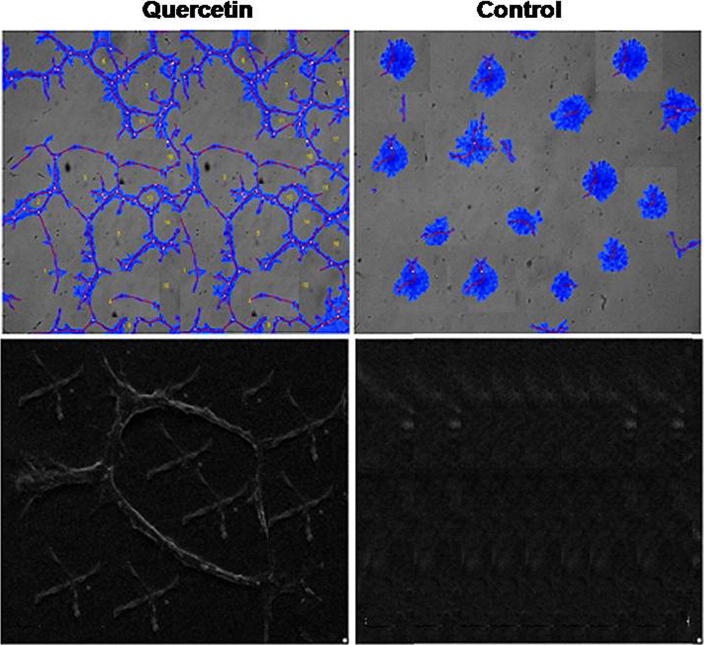
Analysis of the NPC-039 cells cultured with HUVECs revealed higher tube formation ability compared to HUVECs when cultured alone. However, when quercetin was added to the culture of NPC-039 and HUVECs the tube formation ability was inhibited significantly (Fig. 5). These findings suggest that quercetin exhibits inhibitory effect on the angiogenesis in nasopharyngeal carcinoma.
Figure 5.
Effect of quercetin on angiogenesis in nasopharyngeal carcinoma. Human umbilical vein endothelial cells (HUVECs) and NPC-039 cells were cultured in a 24-well plate to analyze the effect of quercetin.
4. Discussion
The present study was designed to investigate the role of quercetin in inhibition of cell viability, NF-κB activity and expression of VEGF in NPC cells. Our results demonstrated that quercetin treatment caused a significant inhibition of cell viability, reduced NF-κB activity and VEGF expression in NPC-039NPC cells. We used MTT assay to investigate the effect of quercetin on the viability of NPC-039NPC cells. Quercetin treatment induced a significant reduction in the viability of NPC-039 cells compared to control cells. Tumor metastasis to adjacent as well as distant organs such as lungs, liver and bones is enhanced by several cellular processes including angiogenesis (Matsuo et al., 2004, Matsuo et al., 2009, Tong et al., 2008). One of the most important factors responsible for tumor angiogenesis is the vascular endothelial growth factor (VEGF). It significantly contributes to enhanced rate of proliferation in endothelial cells, formation of new blood vessels and tendency of carcinoma metastasis (Carmeliet, 2003, Ma et al., 2008). The results from the present study demonstrated that quercetin treatment inhibited the expression of VEGF and consequently the angiogenesis.
NF-κB a member of transcription factor family plays an important role the process of tumorogenesis (Kim and Kim, 2009). It is reported that NF-κB promotes the expression of VEGF and also enhances angiogenesis (Nam et al., 2011). The results from the current study revealed that quercetin treatment inhibited the activity of NF-κB in the NPC cells. Analysis of VEGF expression in NPC cells showed significantly higher level compared to the untreated control cells. Our results also demonstrated that quercetin treatment inhibited the formation of tube by HUVECs and NPC-039 NPC cell cultures.
5. Conclusion
In conclusion, our results indicated that quercetin inhibited both proliferation and angiogenesis in nasopharyngeal cancer through suppression of NF-κB activity. Therefore, quercetin has potential use as a new anti-angiogenic drug for the treatment of gastric cancer.
Acknowledgment
This work was supported by National Nature Science Foundation of China (No. 30700973).
Footnotes
Peer review under responsibility of King Saud University.
References
- Albeck H., Bentzen J., Ockelmann H.H., Nielsen N.H., Bretlau P., Hansen H.S. Familial clusters of nasopharyngeal carcinoma and salivary gland carcinomas in Greenland natives. Cancer. 1993;72:196–200. doi: 10.1002/1097-0142(19930701)72:1<196::aid-cncr2820720135>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Antonisamy P., Duraipandiyan V., Ignacimuthu S., Kim J.-H. Anti-diarrhoeal activity of friedelin isolated from Azima tetracantha lam. in Wistar rats. South Ind. J. Biol. Sci. 2015;1:34–37. [Google Scholar]
- Balamurugan R. Smilax chinensis Linn. (Liliaceae) root attenuates insulin resistance and ameliorate obesity in high diet induced obese rat. South Ind. J. Biol. Sci. 2015;1:47–51. [Google Scholar]
- Bouktaib M.R. Stereoselective synthesis of the major metabolite of quercetin, quercetin-3-O-b-d-glucuronide. Tetrahedron Lett. 2002;43:6263–6266. [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Cheng S.H., Jian J.J., Tsai S.Y. Prognostic features and treatment outcome in locoregionally advanced nasopharyngeal carcinoma following concurrent chemotherapy and radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1998;41:755–762. doi: 10.1016/s0360-3016(98)00092-3. [DOI] [PubMed] [Google Scholar]
- Chua D.T., Sham J.S., Wei W.I., Ho W.K., Au G.K. The predictive value of the 1997 American joint committee on cancer stage classification in determining failure patterns in nasopharyngeal carcinoma. Cancer. 2001;92:2845–2855. doi: 10.1002/1097-0142(20011201)92:11<2845::aid-cncr10133>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Hollman P.C.H., Katan M.B. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed. Pharmacother. 1997;51:305–310. doi: 10.1016/s0753-3322(97)88045-6. [DOI] [PubMed] [Google Scholar]
- Kalaiselvi V., Binu T.V., Radha S.R. Preliminary phytochemical analysis of the various leaf extracts of Mimusops elengi L. South Ind. J. Biol. Sci. 2016;2:24–29. [Google Scholar]
- Karayiannakis A.J., Syrigos K.N., Polychronidis A. Circulating VEGF levels in the serum of gastric cancer patients: correlation with pathological variables, patient survival, and tumor surgery. Ann. Surg. 2002;236:37–42. doi: 10.1097/00000658-200207000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi S., Obata Y., Yagyu K. Reduced serum vascular endothelial growth factor receptor-2 (sVEGFR-2) and sVEGFR-1 levels in gastric cancer patients. Cancer Sci. 2011;102:866–869. doi: 10.1111/j.1349-7006.2011.01860.x. [DOI] [PubMed] [Google Scholar]
- Kim K.K., Kim H.B. Protein interaction network related to Helicobacter pylori infection response. World J. Gastroenterol. 2009;15:4518–4528. doi: 10.3748/wjg.15.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A., Huang W., Zeng G., Ma X., Zhou X., Wang Y., Ouyang C., Cheng A. Expression of the Annexin A1 gene is associated with suppression of growth, invasion and metastasis of nasopharyngeal carcinoma. Mol. Med. 2014;10:3059–3067. doi: 10.3892/mmr.2014.2656. [DOI] [PubMed] [Google Scholar]
- Ma J., Sawai H., Matsuo Y. Interleukin-1alpha enhances angiogenesis and is associated with liver metastatic potential in human gastric cancer cell lines. J. Surg. Res. 2008;148:197–204. doi: 10.1016/j.jss.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Maeda K., Chung Y.S., Ogawa Y. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996;77:858–863. doi: 10.1002/(sici)1097-0142(19960301)77:5<858::aid-cncr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Maeda K., Kang S.M., Onoda N. Vascular endothelial growth factor expression in preoperative biopsy specimens correlates with disease recurrence in patients with early gastric carcinoma. Cancer. 1999;86:566–571. doi: 10.1002/(sici)1097-0142(19990815)86:4<566::aid-cncr4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Mariani C. Flavonoid characterization and in vitro antioxidant activity Aconitum anthor L (Ranunculaceae) Phytochemistry. 2008;69:1220–1226. doi: 10.1016/j.phytochem.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Matsuo Y., Sawai H., Funahashi H. Enhanced angiogenesis due to inflammatory cytokines from pancreatic cancer cell lines and relation to metastatic potential. Pancreas. 2004;28:344–352. doi: 10.1097/00006676-200404000-00025. [DOI] [PubMed] [Google Scholar]
- Matsuo Y., Sawai H., Ochi N. Interleukin-1alpha secreted by pancreatic cancer cells promotes angiogenesis and its therapeutic implications. J. Surg. Res. 2009;153:274–281. doi: 10.1016/j.jss.2008.04.040. [DOI] [PubMed] [Google Scholar]
- Murota K., Terao J. Antioxidative flavonoid quercetin implications of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2003;417:12–17. doi: 10.1016/s0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- Nam S.Y., Ko Y.S., Jung J. A hypoxia-dependent upregulation of hypoxia-inducible factor-1 by nuclear factor-κB promotes gastric tumour growth and angiogenesis. Br. J. Cancer. 2011;104:166–174. doi: 10.1038/sj.bjc.6606020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandhini V.S., Stella Bai G.V. In-vitro phytopharmacological effect and cardio protective activity of Rauvolfia tetraphylla L. South Ind. J. Biol. Sci. 2015;1:97–102. [Google Scholar]
- Neelamkavil S.V., Thoppil J.E. Evaluation of the anticancer potential of the traditional medicinal herb Isodon coetsa. South Ind. J. Biol. Sci. 2016;2:41–45. [Google Scholar]
- Noorudheen N., Chandrasekharan D.K. Effect of ethanolic extract of Phyllanthus emblica on captan induced oxidative stress in vivo. South Ind. J. Biol. Sci. 2016;2:95–102. [Google Scholar]
- Puthur J.T. Antioxidants and cellular antioxidation mechanism in plants. South Ind. J. Biol. Sci. 2016;2:14–17. [Google Scholar]
- Rathi M.A., Meenakshi P., Gopalakrishnan V.K. Hepatoprotective activity of ethanolic extract of Alysicarpus vaginalis against nitrobenzene-induced hepatic damage in rats. South Ind. J. Biol. Sci. 2015;1:60–65. [Google Scholar]
- Santhosh S.K., Venugopal A., Radhakrishnan M.C. Study on the phytochemical, antibacterial and antioxidant activities of Simarouba glauca. South Ind. J. Biol. Sci. 2016;2:119–124. [Google Scholar]
- Serasanambati M., Chilakapati S.R. Function of nuclear factor kappa B (NF-kB) in human diseases - a review. South Ind. J. Biol. Sci. 2016;2:368–387. [Google Scholar]
- Sreeshma P.S., Raphael K.R., Baby A.A. Pharmacognostic studies of leaves of Naravelia zeylanica (Linn) DC. South Ind. J. Biol. Sci. 2016;2:179–182. [Google Scholar]
- Tong Z., Kunnumakkara A.B., Wang H. Neutrophil gelatinase-associated lipocalin: a novel suppressor of invasion and angiogenesis in pancreatic cancer. Cancer Res. 2008;68:6100–6108. doi: 10.1158/0008-5472.CAN-08-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsan A.l., Raphael K.R. Pharmacognostic profile of Averrhoa bilimbi Linn. leaves. South Ind. J. Biol. Sci. 2016;2:75–80. [Google Scholar]
- Wei W.I., Sham J.S. Nasopharyngeal carcinoma. Lancet. 2005;365:2041–2054. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]