Abstract
The present study was aimed to evaluate the influence of olive, sesame and black seed oils on levels of some physiological parameters in male rats exposed to diazinon (DZN). Body weight changes, and levels of serum total protein, albumin, glucose, triglycerides, cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), very low density lipoprotein cholesterol (VLDL-C), atherogenic index (AI), atherogenic coefficient (AC), cardiac risk ratio (CRR), glutathione (GSH), superoxide dismutase (SOD) and malondialdehyde (MAD) were selected as physiological parameters. The experimental animals were distributed into nine groups. Rats group exposed to DZN and fed with normal diet resulted in pronounced severe changes including reduced body weight gain rate, significantly increase in levels of serum albumin, glucose, cholesterol, LDL-C, AI, AC, CRR and MDA while levels of HDL-C, GSH and SOD were decreased. In rats treated with DZN, the supplementation of the olive, sesame and black seed oils showed remarkable lowering influences of physiological alterations. Moreover, the present results confirmed that these oils possess antioxidative effects against DZN toxicity. Finally, the present findings suggest that these oils are safe and promising agents for the treatment of physiological disturbances induced by DZN and may be also by other pollutants, and toxic and pathogenic factors.
Keywords: Diazinon, Olive oil, Sesame oil, Black seed oil, Blood, Rats
1. Introduction
Currently, the environment and people are continually exposed to numerous environmental pollutants. The majority of pollutants are potentially toxic for organisms, some being connected to disease development. In this context, the increase of chronic degenerative disease including cancer in humans, is of considerable concern (Gupta, 2006). Pesticides are a very important group of environmental pollutants used in intensive agriculture for protection against diseases and pests. The estimated annual application is more than 4 million tons, but only 1% of this reaches the target pests (Gavrilescu, 2005). Organophosphorus compounds make up about 70% of the pesticides used worldwide. High-level of exposure to these neurotoxins can result in death, while repeated or prolonged exposure can cause delayed cholinergic toxicity and neurotoxicity (Tuovinen et al., 1994). According to World Health Organization (WHO), 3 million cases of pesticide (mainly organophosphorus compounds) poisoning occur every year, resulting in an excess of 250,000 deaths (Banerjee et al., 2014). Of these, about 1 million are accidental, and 2 million are suicidal poisonings (Joshi et al., 2006). Organophosphate insecticides are widely used for the control of agricultural, industrial and domestic pests. However, the uncontrolled use of insecticides has diverse effects on ecological system and public health (Tuna et al., 2011). Diazinon (DZN) is an organophosphorous insecticide widely used in agriculture and pest control in the environment, which can be highly toxic (Poet et al., 2004, Sarabia et al., 2009). DZN is bioactived by cytochrome P450 enzymes through desulphurization to its corresponding oxon derivative (Sams et al., 2004). Additionally, experimental studies showed that the exposures to DZN induced physiological and histopathological changes in rats, mice and rabbits (Kalender et al., 2006, Al-Attar, 2009, Al-Attar, 2015, Al-Attar and Al-Taisan, 2010, Sarhan and Al-Sahhaf, 2011, Al-Attar and Abu Zeid, 2013, Boroushaki et al., 2013, Abdel-Daim, 2016, Razavi et al., 2016).
Recently, more attention has been paid to the natural antioxidants owing to its protective effects against the toxicity of various pollutants and pathogenic factors, especially whenever reactive oxygen species (ROS) are involved. Currently, there is a strong interest in developing new therapeutic agents from natural products (Fujimori et al., 2010). Olive oil is an integral ingredient in the Mediterranean diet. Olive oil appears to be a functional food with various components such as monounsaturated fatty acids (MUFA) that may have nutritional benefits. It is also a good source of phytochemicals, including polyphenolic compounds (Visioli and Galli, 1998, Lavelli, 2002). The increasing popularity of olive oil is mainly attributed to its antioxidant and antiinflammatory effects which may help prevent disease in humans (Tuck et al., 2001, Tuck and Hayball, 2002, Covas, 2007). Sesame oil has been employed in the food and pharmaceutical industries due to the high lipids and protein content and its distinctive flavor (Abou-Gharbia et al., 1997, Abou-Gharbia et al., 2000). Sesame oil contains sesamin, sesamolin and sesaminol lignan fractions, which are known to play an important role in its oxidative stability and antioxidative activity (Elleuch et al., 2007). Sesame seeds and sesame oil have long been used as health foods and display multiple physiological functions against different pathological factors and symptoms (Matsumura et al., 1995, Namiki, 1995, Sacco et al., 2008, Philip, 2010, Sharif et al., 2013, Hsu et al., 2016). Black seed has been used in many Middle Eastern countries as a natural remedy (Swamy and Tan, 2000). Black seed is most extensively investigated for therapeutic purposes (Aggarwal et al., 2008). Recently, clinical and animal studies have shown that extract of the black seeds have many therapeutic effects (Al-Attar and Al-Taisan, 2010, Boskabady et al., 2011, Parhizkar et al., 2011, Babazadeh et al., 2012, Hamed et al., 2013, Imam et al., 2016). Therefore, the present study was undertaken to evaluate the effectiveness of olive, sesame and black seed oils as protective factors against DZN-induced physiological alterations in male rats.
2. Materials and methods
2.1. Animals
The experiments were done using male rats of the Wistar strain, weighing 92.8–133.3 g. Rats were obtained from the Experimental Animal Unit of King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. The experimental animals were allowed to acclimatize for one week before starting the experimentations. Rats were maintained in controlled temperature (20 ± 1 °C), humidity (65%) and a 12 h dark-light cycle, with balanced food and free access to water. The principles of laboratory animal care were followed throughout the duration of experiment and instruction given by the King Abdul Aziz University ethics committee was followed regarding experimental treatments.
2.2. Experimental protocol
Rats were randomly distributed into nine groups of ten each. The first group was untreated and served as control. The second group was orally treated with 50 mg/kg body weight of DZN in corn oil, daily for 6 weeks. The third group was orally supplemented with olive oil at a dose of 600 mg/kg body weight and after 4 h exposed to DZN at the same dose given to the second group, daily for 6 weeks. The fourth group was orally supplemented with sesame oil at a dose of 600 mg/kg body weight and after 4 h subjected to DZN at the same dose given to the second group, daily for 6 weeks. The fifth group was orally supplemented with black seed oil at a dose of 600 mg/kg body weight and after 4 h treated with DZN at the same dose given to the second group, daily for 6 weeks. The sixth, seventh and eighth groups were orally supplemented with olive, sesame and black seed oils respectively at a dose of 600 mg/kg body weight, daily for 6 weeks. The ninth group was orally supplemented with corn oil at the same dose given to the second group, daily for 6 weeks. The body weights of rats were determined at the start of the experimental period and after six weeks using a digital balance. These weights were measured at the same time during the morning (Al-Attar and Zari, 2010). Moreover, the experimental animals were observed for signs of abnormalities throughout the period of study.
At the end of the experimental period, rats were fasted for 12 h, water was not restricted, and then anaesthetized with diethyl ether. Blood samples were collected from orbital venous plexus in non-heparinized tubes, centrifuged at 2500 rpm for 15 min and blood sera were then collected and stored at −80 °C. The level of serum total protein was measured according to the method of Peters (1968). The level of albumin was estimated using the method of Doumas et al. (1973). The level of glucose was determined using the method of Trinder (1969). To estimate the triglycerides level, Fossati and Prinicip method (1982) was used. The method of Richmond (1973) was used to determine the level of cholesterol. The method of Warnick et al. (1983) was used to measure the level of high density lipoprotein cholesterol (HDL-C). The level of serum low density lipoprotein cholesterol (LDL-C) was estimated according to the equation of Friedewald et al. (1972). Serum very low density lipoprotein cholesterol (VLDL-C) was evaluated using the following equation:
Atherogenic index (AI) values were determined following the method of Pandya et al. (2006).
Atherogenic coefficient (AC) was evaluated according the following equation:
The values of cardiac risk ratio (CRR) were calculated using the following equation:
Levels of serum GSH, SOD and MAD were measured according to the methods of Beutler et al., 1963, Nishikimi et al., 1972 and Ohkawa et al. (1979) respectively.
2.3. Statistical analysis
The calculations and statistical analysis were carried out using the Statistical Package for Social Sciences (SPSS) for Windows version 22.0 software. The obtained data were represented as mean ± standard deviation (S.D.). Data were analyzed using a one-way analysis of variance (ANOVA). Statistical probability of P < 0.05 was considered to be significant.
3. Results
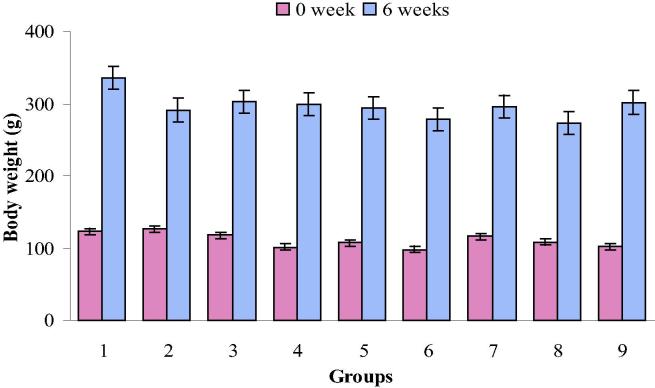
The body weights at the start of the experimental period and after 6 weeks of all experimental groups are represented in Fig. 1. A gradual increase in the body weight of normal control rats (173.2%) and those exposed to DZN plus olive oil (175.8%), DZN plus sesame oil (194.6%), DZN plus black seed oil (173.8%), olive oil (183.8%), sesame oil (153.8%), black seed oil (151.8%) and corn oil (195.4%) was recorded compared with their initial body weights. Significant decreases in the values of body weight gain were observed in rats treated with DZN. The minimum body weight gain was noted in DZN-intoxicated rats (130.2%).
Figure 1.
Changes of body weight at six weeks in control (group 1), DZN (group 2), olive oil plus DZN (group 3), sesame oil plus DZN (group 4), black seed oil plus DZN (group 5), olive oil (group 6), sesame oil (group 7), black seed oil (group 8) and corn oil (group 9) treated rats.
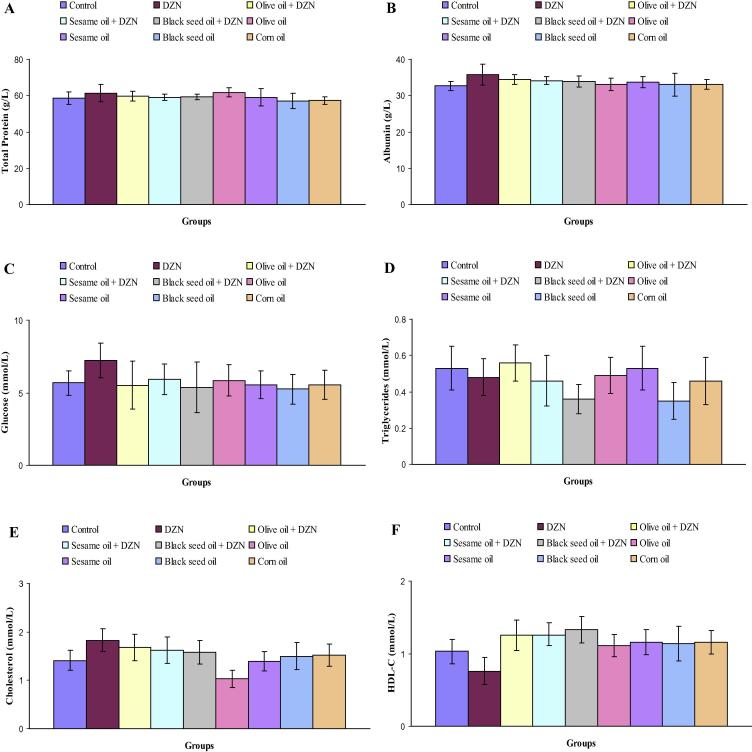
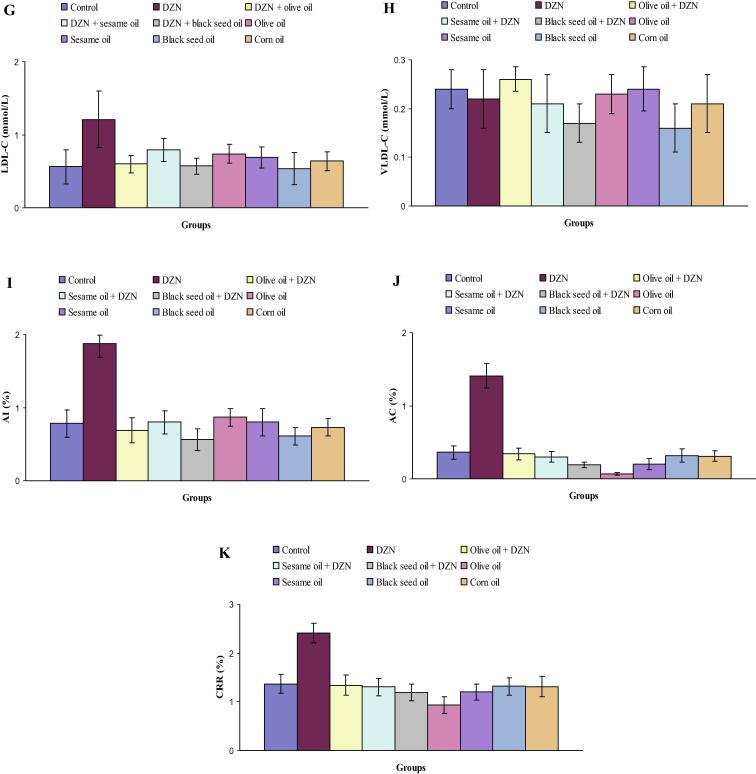
Levels of serum total protein, albumin, glucose, triglycerides, cholesterol, HDL-C, LDL-C, VLDL-C, AI, AC and CRR in control, DZN, olive oil plus DZN, sesame oil plus DZN, black seed oil plus DZN, olive oil, sesame oil, black seed oil and corn oil treated rats are represented in Fig. 2(A–K). Specifically, there were no significant differences in levels of serum total protein in DZN, olive oil plus DZN, sesame oil plus DZN, black seed oil plus DZN, olive oil, sesame oil, black seed oil and corn oil treated rats as compared with control rats (Fig.1A). Notable increases in the level of serum albumin were observed in rats treated with DZN (9.6%), olive oil plus DZN (5.5%) and sesame oil plus DZN (4.5%) compared with control rats (Fig.2B). DZN administration to normal rats significantly increased the level of serum glucose (27.2%) compared with control rats (Fig.2C). Serum triglycerides level was markedly decreased in mice treated black seed oil plus DZN (32.1%) compared to control rats (Fig.2D). In comparison with control rats (Fig.2E), the level of serum cholesterol was significantly elevated in rats treated with DZN (29.8%). Statistically decline in the level of serum cholesterol was detected in rats treated with olive oil (27.0%). As shown in Fig.2F, a significant decrease in the level of serum HDL-C was observed in DZN-intoxicated rats (26.2%) as compared with control rats. Rats treated with black seed oil plus DZN showed a significant increase in the level of serum HDL-C (29.1%) compared with control rats. In comparison with control data (Fig.2G), the level of serum LDL-C was significantly raised in rats treated with DZN (116.1%). Noticeably declines of serum VLDL-C were observed in rats subjected to black seed oil plus DZN (29.2%) and black seed oil (33.3%) compared with control rats (Fig.2H). In comparison with control rats, the administration of DZN alone significantly elevated the level of serum AI (141.0%). Treatment with black seed oil plus DZN induced a significant decrease (39.3%) in the level of AI (Fig.2I). As it is shown in Fig.1J, the level of serum AC was statistically increased in rats exposed to DZN (219.7%). Remarkable declines in the level of serum AC were noted in rats treated with black seed oil plus DZN (47.2%), olive oil (80.8%), sesame oil (44.3%) compared with control rats. The level of serum CRR was statistically enhanced in rats treated with DZN (75.9%). The level of serum CRR was statistically decreased in rats supplemented with olive oil (32.1%) compared with control rats (Fig.2K).
Figure 2.
(A–K) Levels of serum total protein (A), albumin (B), glucose (C), triglycerides (D), cholesterol (E), HDL-C (F), LDL-C (G), VLDL-C (H), AI (I), AC (J) and CRR (K) in control, DZN, olive oil plus DZN, sesame oil plus DZN, black seed oil plus DZN, olive oil, sesame oil, black seed oil and corn oil treated rats.
Levels of serum GSH, SOD and MDA are represented in Table 1. Serum GSH was declined in rats exposed to DZN (43.7%) and sesame oil plus DZN (24.2%) compared with control rats. Levels of serum SOD (Fig.1I) were significantly decreased in rats exposed to DZN (31.1%) and sesame oil plus DZN (9.7%). Additionally, levels of serum MDA were significantly increased in rats treated with DZN (39.4%) and sesame oil plus DZN (21.1%) compared to control rats.
Table 1.
Levels of serum GSH, SOD and MDA of control, DZN, olive oil plus DZN, sesame oil plus DZN, black seed oil plus DZN, olive oil, sesame oil, black seed oil and corn oil treated rats. Percentage changes are included in parentheses.
| Treatments | Parameters |
||
|---|---|---|---|
| GSH (mg/ml) | SOD (U/ml) | MDA (nmol/ml) | |
| Control | 39.94 ± 4.00 | 228.67 ± 18.01 | 135.79 ± 11.15 |
| DZN | 22.49 ± 7.40 (−43.7) | 157.49 ± 23.22 (−31.1) | 189.26 ± 20.1 (+39.4) |
| Olive oil + DZN | 35.94 ± 8.31 (−10.0) | 214.80 ± 13.65 (−6.1) | 132.01 ± 8.00 (−2.8) |
| Sesame oil + DZN | 30.27 ± 5.75 (−24.2) | 206.34 ± 16.06 (−9.8) | 164.37 ± 21.7 (+21.1) |
| Black seed oil + DZN | 37.14 ± 5.91 (−7.0) | 208.71 ± 22.02 (−8.7) | 142.64 ± 18.32 (+5.1) |
| Olive oil | 38.42 ± 4.34 (−3.8) | 221.26 ± 27.24 (−3.2) | 135.64 ± 18.31 (−0.1) |
| Sesame oil | 39.17 ± 4.41 (−1.9) | 220.26 ± 17.23 (−3.7) | 139.30 ± 14.62 (+2.6) |
| Black seed oil | 40.40 ± 7.62 (+1.2) | 238.29 ± 27.18 (+4.2) | 133.66 ± 16.02 (−1.6) |
| Corn oil | 38.83 ± 3.59 (−2.8) | 231.06 ± 30.83 (+1.1) | 137.13 ± 14.31 (+1.0) |
4. Discussion
The exposure to organophosphorus pesticides has been associated with many physiological, biochemical and histopathological alterations. The exposure of pesticides and other toxicants to humans and to a variety of forms of life has become an overall health problem. The present study showed that the maximum increases of body weight were observed in rats treated with corn oil (195.4%) followed by DZN plus sesame oil (194.6%), olive oil (183.8%), DZN plus olive oil (175.8%), DZN plus black seed oil (173.8%), control (173.2%), sesame oil (153.8%) and black seed oil (151.8%) treated rats. The significant increase in body weight in the control rats may be representative of the normal pattern of growth in rodents. Contrary, the minimum increase of body weight was observed in DZN treated rats (130.2%). These observations were also noted in rats exposed to DZN and other pesticides (Mansour et al., 2007, Johari et al., 2010, Mossa et al., 2011, Heikal et al., 2012, Adjrah et al., 2013, El-Sheikh and Galal, 2015). Additionally, the decreased body weight could be due to reduced diet consumption, anorexia, reduced absorption of nutrients from the intestine, increase of catabolic processes, increased degradation of lipids and proteins, and excessive loss of water, salts and proteins as a result of kidney dysfunction.
The present increase in levels of serum albumin, glucose, triglycerides, cholesterol, LDL-C, AI, AC, and CRR with the decrease in the level of serum HDL-C indicate disturbances in protein, carbohydrate and lipid metabolism induced by exposure to DZN. Albumin is synthesized by the liver and as such, it represents a major synthetic protein and is a marker of the ability of the liver to synthesize proteins (Johnston, 1999). The changing levels of serum albumin, thus, provide valuable indices of severity, progress, and prognosis in hepatic disease (Saxena and Saxena, 2010). Hyperalbuminemia is the medical terminology used to describe elevated levels of serum albumin. Chief factors responsible for hyperalbuminemia are severe infections, congenital disorders, severe dehydration, hepatitis, malnourishment, chronic inflammatory diseases, tuberculosis, an overdose of cortisone drugs, excessive synthesis of cortisol by the adrenals, tumor that manufactures cortisol-like substances, congestive cardiac failure, kidney diseases, HIV and cancer. An increase in serum albumin indicates poor liver function or impaired synthesis and it may be either in liver cells damage or diminished protein intake (Kalaiselvi et al., 2015). Blood glucose concentration is known to depend on the ability of the liver to absorb or produce glucose. The liver performs its glucostatic function owing to its ability to synthesize or degrade glycogen according to the needs of the organism, as well as via gluconeogenesis (Ahmed et al., 2006). Several investigations showed that levels of serum glucose were significantly enhanced in rats, mice and rabbits exposed to DZN and other pesticides (Das et al., 2010, Salih, 2010, Zari and Al-Attar, 2011, Al-Attar and Abu Zeid, 2013, Lukaszewicz-Hussain, 2013, Abd Elmonem, 2014). Additionally, Al-Attar (2015) reported that the progressive accumulation of blood glucose revealed that rats became hyperglycemic due to DZN intoxication. This case may be due to the enhancement of the activities of the enzymes involved in gluconeogenesis leading to formation of glucose from non-carbohydrate sources coupled with inhibition of liver glycogenolysis or stimulating glycogenolysis processes to increase the level of blood glucose from the liver as a main source of carbohydrates in the body.
Dyslipidemia, characterized by abnormally elevated plasma triacylglycerol and cholesterol concentrations, is an established risk factor in the development of coronary heart disease (Al-Attar, 2010). Therefore, it is reasonable to expect an abnormal lipid profile in those with severe liver dysfunction (Ghadir et al., 2010). Hyperlipidemia is one of the established major risk factors of coronary heart disease and cerebrovascular disease. The most common risk factors associated with increased risk of atherosclerotic heart disease or stroke are abnormalities of lipids, hypertension, smoking and some coagulation and hemostatic factors (Sarraf-Zadegan et al., 1999). Hypertriglyceridemia could be attributed to the reduction of lipase activity, which could lead to a decrease in triglyceride hydrolysis (Jahn et al., 1985). Hypercholesterolemia could be attributed to damage of hepatic parenchymal cells that lead to disturbance of lipid metabolism in the liver (Havel, 1986). The liver injury of different etiologies is often accompanied by secondary lipoproteinemia, which may lead to the development of atherosclerosis, particularly when associated with hypercholesterolemia characterized by an increase in LDL-C and decrease in HDLC (Miller and Miller, 1975, Steinberg et al., 1989). Atherogenic indices (AI, AC and CRR) have been described as powerful indicators of the risk of cardiovascular disease; the higher the value, the higher the risk of developing the disease and vice versa (Dobiásová, 2004, Rang et al., 2005, Martirosyan et al., 2007, Ikewuchi and Ikewuchi, 2009, Ikewuchi, 2012). Traditionally, the atherogenic lipid profile is made up of increased triglycerides, cholesterol and LDL-C, and decreased HDL-C (Popa et al., 2012). AI indicates the deposition of foam cells or plaque or fatty infiltration or lipids in heart, coronaries, aorta, liver and kidney. The higher the AI, the higher is the risk of above organs for oxidative damage (Basu et al., 2007). However, the present increase of serum AI, AC and CRR indicates that DZN caused the risk of cardiovascular injury. In addition, several studies showed that levels of blood triglycerides, cholesterol, LDL-C and VLDL-C were significantly increased with the decrease in levels of serum HDL-C in experimental animals exposed to DZN and other pesticides (Attia and Nasr, 2009, Rai et al., 2009, Al-Attar and Abu Zeid, 2013, Bhushan et al., 2013, Abd Elmonem, 2014, El-Demerdash and Nasr, 2014, Al-Attar, 2015). The present study showed that the supplementation of olive oil significantly decreased levels of serum cholesterol and CRR in normal rats. Previous studies showed that olive oil reduced levels of serum cholesterol in mice (Rezq et al., 2010), rats (Ayad et al., 2013) and guineapig (Kalita et al., 2014). Olive oil is regarded as healthy dietary oil because of its high concentration of MUFA and phenolic compounds, which have tangible health benefits (Owen et al., 2000, Covas et al., 2006). Olive oil improves the lipid profile because of high concentration of MUFA and phenolic compounds both having a lipid lowering action and prevents LDL-C oxidation. Epidemiological studies also suggest that Mediterranean diet rich in olive oil decreases the risk of cardiovascular disease and improves its major risk factors (Gorinstein et al., 2002, Perez-Jimenez et al., 2005, Cicerale et al., 2010).
In the present study, the administration of DZN induced oxidative stress which was confirmed by the increases of serum GSH and SOD levels and a decrease of MDA level. However, several experimental studies showed similar observations in rats and mice exposed to DZN and other pesticides (Salehi et al., 2012, Abbassy et al., 2014, Mohamed and Ali, 2014, Arafa et al., 2015, Beydilli et al., 2015, Eraslan et al., 2015, Liu et al., 2016). Recently, data indicate that the toxic action of pesticides may include the induction of oxidative stress and accumulation of free radicals in the cell. Many reports showed that the exposures to pesticides caused oxidative damages in humans and animals (Gaikwad et al., 2015). The effects of DZN as inducers of oxidative stress on certain biomarkers in various tissues have been studied (Isika and Celik, 2008). One of the biomarkers for oxidative stress is lipid peroxidation which is claimed to possess predictive significance in quite a number of investigations (Ogutcu et al., 2006, Amirkabirian et al., 2007). Oxidative stress, moreover, is triggered as an absence of balance between antioxidant system and oxidant condition which pesticide toxicity brings about. In fact, reactive oxygen species (ROS) are produced by univalent reduction of dioxygen to superoxide anion (O−2), which in turn is disproportionate to H2O2 and O2 spontaneously or through a reaction catalyzed by SOD. Endogenous H2O2 may be converted to H2O either by catalase or glutathione peroxidase (GSH-Px). Otherwise, it may generate a highly reactive free hydroxyl radical (−OH) via a Fenton reaction, which is responsible for oxidative damage. GSH-Px converts H2O2 or other lipid peroxides to water or hydroxyl lipids, and during this process GSH is converted to oxidized glutathione (Bachowshi et al., 1997). Antioxidants are defense against free radical and oxidative attacks. They act as free radical scavengers and slow down not only radical oxidation but also the accompanying damaging effects in the body (Nice, 1997).
In the present study, the pretreatment of the studied oils inhibited the hematobiochemical alterations induced by DZN toxicity. The effect of these oils was confirmed by an inhibition of oxidative stress which indicated by the enhancement of GSH and SOD levels as well as the decline of MDA level compared to DZN intoxication alone. Scientists have focused on the preventive effects of phenols against degenerative diseases mediated by the ROS. It has been reported that the phenolic compounds are able to interact with the biological systems and act as bioactive molecules. They are particularly important inhibitors of lipid peroxidation, and are believed to be effective through their free radical scavenging and metal-chelating properties (Kandaswami and Middleton, 1994, Salah et al., 1995, Rice-Evans et al., 1996). In experimental studies, olive oil phenolic compounds showed strong antioxidant properties against lipids, DNA and LDL oxidation (Covas et al., 2006). Hydroxytyrosol, one of the phenolic compounds present in olive oil, has been suggested to be a potent antioxidant, thus contributing to the beneficial properties of olive oil (Deiana et al., 1999). Hydroxytyrosol administration has been shown to reduce the consequences of passive smoking-induced oxidative stress, prevent LDL oxidation, platelet aggregation and inhibit leukocyte 5-lipoxygenases (Petroni et al., 1995, Wiseman et al., 1996, De la Puerta et al., 1999, Visioli et al., 2000;). Hydroxytyrosol has shown efficacy in preventing oxidative stress in the liver of rats intoxicated by cadmium (Casalino et al., 2002). In addition, when human hepatoma HepG2 cells were pre-treated with hydroxytyrosol prior to submission to tert-butyl hydroperoxide-induced oxidative stress, cell toxicity was completely prevented, indicating that the antioxidant-treated cells were totally protected against the oxidative insult (Goya et al., 2007). Nakbi et al. (2010) assessed the effects of olive oil, hydrophilic and lipophilic fractions on 2,4-dichlorophenoxyacetic acid-induced oxidative damage in the liver of rats. They showed that olive oil and its extracts protect against oxidative damage of hepatic tissue by preventing excessive lipid peroxidation to increase MUFA composition and by maintaining serum marker enzymes and hepatic antioxidant enzyme activities at near normal concentrations. It is the hydrophilic fraction of olive oil which seems to be the effective one in reducing 2,4-dichlorophenoxyacetic-induced oxidative stress, indicating that hydrophilic extract may exert a direct antioxidant effect on hepatic cells. Abd El-Fattah and Barakat (2013) evaluated the effect of dietary olive oil on liver damage and oxidative stress induced by 2,4-dichlorophenoxyacetic acid in rats. Olive oil administration during 2,4-dichlorophenoxyacetic acid treatment induced an improvement in hepatic antioxidant enzymes, serum transaminase activities and liver free fatty acids levels. Biochemical results were confirmed by the liver histopathological examination. They concluded that olive oil has protective effect against oxidative stress and liver damage induced by 2,4-dichlorophenoxyacetic acid. Amamou et al. (2015) examined the possible protective effect of olive oil consumption against cadmium-induced damage on plasma lipids and stress biochemical parameters of male rats. Co-treatment with olive oil significantly improved the oxidative damage induced by cadmium. The antioxidant potential in the plasma and liver was markedly restored with a significant decline in MDA levels and activity of transaminases. They suggested that olive oil consumption could protect the rat liver against cadmium-induced injury by increasing the activities of antioxidant enzymes and reducing oxidative stress.
Previous studies showed that the sesame oil ameliorated the influences of several chemical factors such as acetaminophen, aminoglycoside, iodinated contrast, cypermethrin, acetic acid, formalin and deoxycorticosterone acetate. Generally, these studies suggested that the protective effect of sesame oil against the influences of chemical toxicities associated with inhibition of the oxidative stress in experimental animals (Chandrasekaran et al., 2010, Hsu et al., 2011, Hussien et al., 2013, Monteiro et al., 2014, Liu et al., 2015, Soliman et al., 2015). Danladi et al. (2013) examined the effect of black seed oil on carbon tetrachloride (CCl4)-induced liver damage in rats. The results showed that the increase and decrease in liver enzymes were almost restored to normal in animals treated with black seed oil and CCl4 has that of the control. CCl4 caused elevated levels of thiobarbituric acid reactive substances (TBARS) and a marked depletion of liver endogenous antioxidant enzymes. Black seed oil treatment positively protects the alterations in these biochemical variables in the CCl4 plus black seed oil-treated rats. Black seed oil markedly reduced elevated TBARS and significantly increased levels of antioxidant enzymes. Black seed oil showed hepatoprotection on liver structures of rats exposed to CCl4. They demonstrated that that black seed oil through its antioxidant activity effectively protects CCl4-induced liver toxicity. Hamed et al. (2013) investigated whether black seed oil could attenuate hepatorenal injury induced by bromobenzene exposure. Treatment with black seed oil alleviated the elevation of GSH, succinate dehydrogenase (SDH), lactate dehydrogenase (LDH), glucose-6-phosphatase, serum protein, nitric oxide, Na-K-adenosine triphosphatase, phospholipids levels and attenuated MDA, SOD, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP). Diminution of collagen content and improvement in liver and kidney architectures were observed. They concluded that black seed oil enhanced the hepatorenal protection mechanism, reduced disease complications and delayed its progression. El-Sheikh et al. (2015) investigated the mechanisms by which thymoquinone, the primary bioactive component of black seed, can prevent methotrexate-induced hepatorenal toxicity in rats. They demonstrated that the thymoquinone reversed oxidative and nitrosative stress, as well as inflammatory and apoptotic signs caused by methotrexate alone. Thus, thymoquinone may be a beneficial adjuvant that confers hepatorenal protection to methotrexate toxicity via antioxidant, antinitrosative, antiinflammatory, and antiapoptotic mechanisms. Samarghandian et al. (2015) studied the protective effect of thymoquinone on gentamicin-induced acute renal failure in rats. They suggested that thymoquinone may ameliorate acute renal failure through modulation of the oxidative stress and inflammatory responses. Collectively, the obtained results provided evidence indicating that the supplementation of the olive, sesame and black seed oils inhibited the physiological alterations induced by DZN intoxication. The influences of these oils were attributed to its ability to reduce the oxidative stress. Therefore, these oils could be beneficial additional therapeutic agents in the treatment of physiological disturbances induced by DZN. Longer duration investigations with different doses of these oils are required to develop potent therapeutic agents against the toxicity of DZN and may be also against other pollutants, and toxic and pathogenic factors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbassy M.A., Marzouk M.A., Mansour S.A., Shaldam H.A., Mossa A.H. Impact of oxidative stress and lipid peroxidation induced by lambdacyhalothrin on p450 in male rats: the ameliorating effect of zinc. J. Environ. Anal. Toxicol. 2014;4:1–5. [Google Scholar]
- Abd El-Fattah H.M., Barakat L.A.A. Hepatoprotective effect of olive and coconut oils against oxidative stress- induced by 2, 4 dichlorophenoxyacetic acid. Indian J. Appl. Res. 2013;3:42–46. [Google Scholar]
- Abd Elmonem H.A. Assessment the effect of pomegranate molasses against diazinon toxicity in male rats. J. Environ. Sci. Toxicol. Food Technol. 2014;8:135–141. [Google Scholar]
- Abdel-Daim M.M. Synergistic protective role of ceftriaxone and ascorbic acid against subacute diazinon-induced nephrotoxicity in rats. Cytotechnology. 2016;68:279–289. doi: 10.1007/s10616-014-9779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Gharbia H.A., Shahidi F., Shehata A.A.Y., Youssef M.M. Effect of processing on oxidative stability of sesame oil extracted from intact and dehulled seed. J. Am. Oil Chem. Soc. 1997;74:215–221. [Google Scholar]
- Abou-Gharbia H.A., Shehata A.A.Y., Shahidi F. Effect of processing on oxidative stability and lipid classes of sesame oil. Food Res. Int. 2000;33:331–340. [Google Scholar]
- Adjrah Y., Karou S.D., Agbonon A., Ameyapoh Y., de Souza C., Gbeassor M. Effect of cypermethrin-treated lettuce (Lactuca sativa) on wistar rat liver. J. Appl. Pharm. Sci. 2013;3:128–132. [Google Scholar]
- Aggarwal B.B., Kunnumakkara A.B., Harikumar K.B., Tharakan S.T., Sung B., Anand P. Potential of spice-derived phytochemicals for cancer prevention. Planta Med. 2008;74:1560–1569. doi: 10.1055/s-2008-1074578. [DOI] [PubMed] [Google Scholar]
- Ahmed O.M., Abdel Hamid H., Bastway M., Hasona N.A. Antihyperglycemic effects of Plantago Ispaghula seeds aqueous extract in diabetic and hypercholesterolemic rats. J. Egypt Ger. Soc. Zool. 2006;51A:371–393. [Google Scholar]
- Al-Attar A.M. The ameliorative role of β-carotene pretreatment on diazinon-induced enzymological and histopathological changes in Wistar male rats. Global J. Pharmacol. 2009;3:171–177. [Google Scholar]
- Al-Attar A.M. Physiological and biochemical alterations in Sprague-Dawley female rats subjected to high fat diet and intermittent fasting. J. Appl. Sci. Res. 2010;6:2096–2104. [Google Scholar]
- Al-Attar A.M. Effect of grapeseed oil on diazinon-induced physiological and histopathological alterations in rats. Saudi J. Biol Sci. 2015;22:284–292. doi: 10.1016/j.sjbs.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Attar A.M., Abu Zeid I.M. Effect of tea (Camellia sinensis) and olive (Olea europaea L.) leaves extracts on male mice exposed to diazinon. BioMed. Res. Int. 2013;2013:1–6. doi: 10.1155/2013/461415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Attar A.M., Al-Taisan W.A. Preventive effects of black seed (Nigella sativa) extract on Sprague Dawley rats exposed to diazinon. Aust. J. Basic Appl. Sci. 2010;4:957–968. [Google Scholar]
- Al-Attar A.M., Zari T.A. Influences of crude extract of tea leaves, camellia sinensis, on streptozotocin diabetic male albino mice. Saudi J. Biol. Sci. 2010;17 doi: 10.1016/j.sjbs.2010.05.007. 295-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amamou F., Nemmiche S., Meziane R.K., Didi A., Yazit S.M., Chabane-Sari D. Protective effect of olive oil and colocynth oil against cadmium-induced oxidative stress in the liver of Wistar rats. Food Chem. Toxicol. 2015;78:177–184. doi: 10.1016/j.fct.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Amirkabirian N., Teimouri F., Esmaily H. Protection by pentoxifylline of diazinon induced toxic stress in rat liver and muscle. Toxicol. Lett. 2007;172:S61–S62. doi: 10.1080/15376510600943783. [DOI] [PubMed] [Google Scholar]
- Arafa M.H., Mohamed D.A., Atteia H.H. Ameliorative effect of N-acetyl cysteine on alpha-cypermethrin-induced pulmonary toxicity in male rats. Environ. Toxicol. 2015;30:26–43. doi: 10.1002/tox.21891. [DOI] [PubMed] [Google Scholar]
- Attia A.A., Nasr H.M. Dimethoate-induced changes in biochemical parameters of experimental rat serum and its neutralization by black seed (Nigella sativa L.) oil. Slovak J. Anim. Sci. 2009;4:87–94. [Google Scholar]
- Ayad A., Merzouk H., Hamed Y.B., Merzouk S.A., Gresti J., Narce M. Beneficial effects of dietary olive and linseed oils on serum and tissue lipids and redox status in the aging obese rat. J. Nat. Prod. Plant Resour. 2013;3:61–71. [Google Scholar]
- Babazadeh B., Sadeghnia H.R., Safarpour Kapurchal E., Parsaee H., Nasri S., Tayarani-Najaran Z. Protective effect of Nigella sativa and thymoquinone on serum/glucose deprivation-induced DNA damage in PC12 cells. Avicenna J. Phytomed. 2012;2:125–132. [PMC free article] [PubMed] [Google Scholar]
- Bachowshi S., Kolaja K.L., Xu Y., Ketcham C.A., Stevenson D.E., Walborg E.F., Jr., Klaunig J.E. Role of oxidative stress in the mechanism of dieldrin’s hepatotoxicity. Ann. Clin. Lab. Sci. 1997;27:196–209. [PubMed] [Google Scholar]
- Banerjee I., Tripathi S.K., Roy A.S. Efficacy of pralidoxime in organophosphorus poisoning: revisiting the controversy in Indian setting. J. Postgrad. Med. 2014;60:27–30. doi: 10.4103/0022-3859.128803. [DOI] [PubMed] [Google Scholar]
- Basu M., Prasad R., Jayamurthy P., Pal K., Arumughan C., Sawhney R.C. Anti-atherogenic effects of seabuckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine. 2007;14:770–777. doi: 10.1016/j.phymed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Beutler E., Duron O., Kelly M.B.J. Improved method for the determination of blood glutathione. Lab Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Beydilli H., Yilmaz N., Cetin E.S., Topal Y., Celik O.I., Sahin C., Topal H., Cigerci I.H., Sozen H. Evaluation of the protective effect of silibinin against diazinon induced hepatotoxicity and free-radical damage in rat liver. Iran. Red. Crescent. Med. J. 2015;17:e25310. doi: 10.5812/ircmj.17(4)2015.25310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B., Pande S., Saxena N., Saxena P.N. Serum biochemical responses under stress of cypermethrin in albino rat. Environ. Exp. Biol. 2013;11:81–89. [Google Scholar]
- Boroushaki M.T., Arshadi D., Jalili-Rasti H., Asadpour E., Hosseini A. Protective effect of pomegranate seed oil against acute toxicity of diazinon in rat kidney. Iran. J. Pharm. Res. 2013;12:821–827. [PMC free article] [PubMed] [Google Scholar]
- Boskabady M.H., Keyhanmanesh R., Khameneh S., Doostdar Y., Khakzad M.R. Potential immunomodulation effect of the extract of Nigella sativa on ovalbumin sensitized guinea pigs. J. Zhejiang Univ. Sci. B. 2011;12:201–209. doi: 10.1631/jzus.B1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalino E., Calzaretti G., Sblano C., Landriscina C. Molecular inhibitory mechanism of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology. 2002;179:37–50. doi: 10.1016/s0300-483x(02)00245-7. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran V.R., Chien S.P., Hsu D.Z., Chang Y.C., Liu M.Y. Effects of sesame oil against after the onset of acetaminophen-induced acute hepatic injury in rats. J. Parenter. Enteral Nutr. 2010;34:567–573. doi: 10.1177/0148607110362584. [DOI] [PubMed] [Google Scholar]
- Cicerale S., Lucas L., Keast R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010;11:458–479. doi: 10.3390/ijms11020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covas M.I. Olive oil and the cardiovascular system. Pharmacol. Res. 2007;55:175–186. doi: 10.1016/j.phrs.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Covas M.I., Ruiz-Gutiérrez V., De La Torre R., Kafatos A., Lamuela-Raventós R.M., Osada J., Owen R.W., Visioli F. Minor components of olive oil: evidence to date of health benefits in humans. Nutr. Rev. 2006;64:S20–S30. [Google Scholar]
- Danladi J., Abdulsalam A., Timbuak J.A., Ahmed S.A., Mairiga A.A., Dahiru A.U. Hepatoprotective effect of black seed (Nigella sativa) oil on carbon tetrachloride (CCl4) induced liver toxicity in adult wistar rats. J. Dent. Med. Sci. 2013;4:56–62. [Google Scholar]
- Das, B., Pervin, K., Roy, A.K., Ferdousi and Z. Saha A.K. 2010. Toxic effects of prolonged endosulfan exposure on some blood parameters in albino rat. J. Life Earth Sci. 5, 29-32.
- De la Puerta R., Ruiz Gutierrez V., Hoult J.R.S. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999;57:445–449. doi: 10.1016/s0006-2952(98)00320-7. [DOI] [PubMed] [Google Scholar]
- Deiana M., Aruoma O.I., Bianchi M.L.P., Spencer J.P., Kaur H., Halliwell B., Aeschbach R., Banni S., Dessi M.A., Corongiu F.P. Inhibition of peroxynitrite dependent DNA base modification and tyrosine nitration by the extra virgin olive oil derived antioxidant hydroxytyrosol. Free Radic. Bio. Med. 1999;26:762–769. doi: 10.1016/s0891-5849(98)00231-7. [DOI] [PubMed] [Google Scholar]
- Dobiásová M. Atherogenic index of plasma [log(triglyceride/HDL-Cholesterol)]: theoretical and practical implications. Clin. Chem. 2004;50:1113–1115. doi: 10.1373/clinchem.2004.033175. [DOI] [PubMed] [Google Scholar]
- Doumas B.T., Perry B.W., Sasse E.A., Straumfjord J.V. Standardization in bilirubin assays: evaluation of selected methods and stability of bilirubin solutions. Clin. Chem. 1973;19:984–993. [PubMed] [Google Scholar]
- El-Demerdash F.M., Nasr H.M. Antioxidant effect of selenium on lipid peroxidation, hyperlipidemia and biochemical parameters in rats exposed to diazinon. J. Trace Elem. Med Biol. 2014;28:89–93. doi: 10.1016/j.jtemb.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Elleuch M., Besbes S., Roiseux O., Blecker C., Attia H. Quality characteristics of sesame seeds and by-products. Food Chem. 2007;103:641–650. [Google Scholar]
- El-Sheikh el-SA, Galal A.A. Toxic effects of sub-chronic exposure of male albino rats to emamectin benzoate and possible ameliorative role of Foeniculum vulgare essential oil. Environ. Toxicol. Pharmacol. 2015;39:1177–1188. doi: 10.1016/j.etap.2015.04.008. [DOI] [PubMed] [Google Scholar]
- El-Sheikh A.A., Morsy M.A., Abdalla A.M., Hamouda A.H., Alhaider I.A. Mechanisms of thymoquinone hepatorenal protection in methotrexate-induced toxicity in rats. Mediators Inflamm. 2015;2015:1–12. doi: 10.1155/2015/859383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraslan, G., Kanbur, M., Siliğ, Y., Karabacak, M., Soyer Sarlca, Z., Şahin, S., 2015. The acute and chronic toxic effect of cypermethrin, propetamphos, and their combinations in rats. Environ. Toxicol. In Press. [DOI] [PubMed]
- Fossati P., Prinicip L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982;28:2077–2080. [PubMed] [Google Scholar]
- Friedewald W.T., Levy R.I., Donald S., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Fujimori S., Gudis K., Sakamoto C. A review of anti-inflammatory drug-induced gastrointestinal injury: focus on prevention of small intestinal injury. Pharmaceuticals. 2010;3:1187–1201. doi: 10.3390/ph3041187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaikwad A.S., Karunamoorthy P., Kondhalkar S.J., Ambikapathy M., Beerappa R. Assessment of hematological, biochemical effects and genotoxicity among pesticide sprayers in grape garden. J. Occup. Med. Toxicol. 2015;10:11–16. doi: 10.1186/s12995-015-0049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilescu M. Fate of pesticides in the environment and its bioremediation. Eng. Life Sci. 2005;5:497–526. [Google Scholar]
- Ghadir M.R., Riahin A.A., Havaspour A., Nooranipour M., Habibinejad A.A. The relationship between lipid profile and severity of liver damage in cirrhotic patients. Hepat. Mon. 2010;10:285–288. [PMC free article] [PubMed] [Google Scholar]
- Gorinstein S., Leontowicz H., Lojek A., Leontowicz M. Olive oils improve lipid metabolism and increase antioxidant potential in rats fed diets containing cholesterol. J. Agric. Food Chem. 2002;50:6102–6108. doi: 10.1021/jf020306k. [DOI] [PubMed] [Google Scholar]
- Goya L., Mateos R., Bravo L. Effect of the olive oil phenol hydroxytyrosol on human hepatoma HepG2 cells. Eur. J. Nutr. 2007;46:70–78. doi: 10.1007/s00394-006-0633-8. [DOI] [PubMed] [Google Scholar]
- Gupta, R.C., 2006. Toxicology of organophosphates and carbamate compounds. 1st Edn. Elsevier Academic Press. ISBN: 9780120885237.
- Hamed M.A., El-Rigal N.S., Ali S.A. Effects of black seed oil on resolution of hepato-renal toxicity induced by bromobenzene in rats. Eur. Rev. Med. Pharmacol. Sci. 2013;17:569–581. [PubMed] [Google Scholar]
- Havel R.J. Functional activities of hepatic lipoproteins receptors. Ann. Rev. Physiol. 1986;48:119–134. doi: 10.1146/annurev.ph.48.030186.001003. [DOI] [PubMed] [Google Scholar]
- Heikal T.M., El-sherbiny M., Hassan S.A., Arafa A., Hassan Z., Ghanem H.Z. Antioxidant effect of selenium on hepatotoxicity induced by chlorpyrifos in male rats. Int. J. Pharm. Pharm. Sci. 2012;4:603–609. [Google Scholar]
- Hsu D.Z., Li Y.H., Chu P.Y., Periasamy S., Liu M.Y. Sesame oil prevents acute kidney injury induced by the synergistic action of aminoglycoside and iodinated contrast in rats. Antimicrob. Agents Chemother. 2011;55:2532–2536. doi: 10.1128/AAC.01597-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu D.Z., Chu P.Y., Jou I.M. Enteral sesame oil therapeutically relieves disease severity in rat experimental osteoarthritis. Food Nutr. Res. 2016;60:29807. doi: 10.3402/fnr.v60.29807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussien H.M., Abdou H.M., Yousef M.I. Cypermethrin induced damage in genomic DNA and histopathological changes in brain and haematotoxicity in rats: the protective effect of sesame oil. Brain Res. Bull. 2013;92:76–83. doi: 10.1016/j.brainresbull.2011.10.020. [DOI] [PubMed] [Google Scholar]
- Ikewuchi C.C. Hypocholesterolemic effect of an aqueous extract of the leaves of Sansevieria senegambica baker on plasma lipid profile and atherogenic indices of rats fed egg yolk supplemented diet. EXCLI. J. 2012;11:318–327. [PMC free article] [PubMed] [Google Scholar]
- Ikewuchi, C.J., Ikewuchi, C.C., 2009. Alteration of plasma lipid profiles and atherogenic indices by Stachytarpheta jamaicensis L. (Vahl) Boikemistri. 21, 71–77.
- Imam A., Ajao M.S., Ajibola M.I., Amin A., Abdulmajeed W.I., Lawal A.Z., Alli-Oluwafuyi A., Akinola O.B., Oyewopo A.O., Olajide O.J., Adana M.Y. Black seed oil ameliorated scopolamine-induced memory dysfunction and cortico-hippocampal neural alterations in male Wistar rats. Bull. Fac. Pharm. Cairo Univ. 2016;54:49–57. [Google Scholar]
- Isika I., Celik I. Acute effects of methyl parathion and diazinon as inducers for oxidative stress on certain biomarkers in various tissues of rainbow trout (Oncorhynchus mykiss) Pest. Biochem. Physiol. 2008;9:38–42. [Google Scholar]
- Jahn C.E., Schaegfetr E.J., Taam L.A., Hoofnagle J.H., Lindgren F.T., Albers J.J., Jones E.A., Brewer H.B., Jr. Lipoprotein abnormalities in primary biliary cirrhosis association with hepatic lipase inhibition as well as altered cholesterol esterification. Gastroenterology. 1985;89:1266–1278. [PubMed] [Google Scholar]
- Johari H., Shariati M., Abbasi S., Sharifi E., Askari H.R. The effects of diazinon on pituitary–gonad axis and ovarian histological changes in rats. Iran. J. Reprod. Med. 2010;8:125–130. [Google Scholar]
- Johnston A. Spices as influencers of body metabolism: an over view of three decades of research. Food Res. Int. 1999;38:77–86. [Google Scholar]
- Joshi S., Biswas B., Malla G. Management of organophosphorus poisoning. Indian J. Pharmacol. 2006;41:69–70. [Google Scholar]
- Kalaiselvi A., Reddy G.A., Ramalingam V. Ameliorating effect of ginger extract (Zingiber officinale Roscoe) on liver marker enzymes, lipid profile in aluminium chloride induced male rats. Int. J. Pharm. Sci. Drug Res. 2015;7:52–58. [Google Scholar]
- Kalender Y., Uzunhisarcikli M., Ogutcu A., Acikgoz F., Kalender S. Effects of diazinon on pseudocholinesterase activity and haematological indices in rats: the protective role of vitamin E. Environ. Toxicol. Pharmacol. 2006;22:46–51. doi: 10.1016/j.etap.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Kalita B., Kusre D., Bhuyan K.C. Effects of sesame oil and olive oil on the plasma total cholesterol, low density lipoprotein and high density lipoprotein cholesterol of guineapig. Int. J, Eng. Sci. Innov. Technol. 2014;3:217–221. [Google Scholar]
- Kandaswami C., Middleton E., Jr. Free radical scavenging and antioxidant activity of plant flavonoids. Adv. Exp. Med. Biol. 1994;366:351–376. doi: 10.1007/978-1-4615-1833-4_25. [DOI] [PubMed] [Google Scholar]
- Lavelli V. Comparison of the antioxidant activities of extra virgin olive oils. J. Agric. Food Chem. 2002;50:7704–7708. doi: 10.1021/jf020749o. [DOI] [PubMed] [Google Scholar]
- Liu C.T., Chien S.P., Hsu D.Z., Periasamy S., Liu M.Y. Curative effect of sesame oil in a rat model of chronic kidney disease. Nephrology. 2015;20:922–930. doi: 10.1111/nep.12524. [DOI] [PubMed] [Google Scholar]
- Liu, H., Ding, Y., Hou, Y., Zhao, G., Lu, Y., Chen, X., Cai, Q., Hong, G., Qiu, Q., Lu, Z. 2016. The protective effect of bone marrow mesenchymal stem cells carrying antioxidant gene superoxide dismutase on paraquat lung injury in mice Zhonghua Lao Dong Wei Sheng. Zhi Ye Bing Za Zhi. 34, 1-7. [DOI] [PubMed]
- Lukaszewicz-Hussain A. Serum glucose concentration in subacute intoxication with chlorpyrifo – organophosphate insecticide. Med. Pr. 2013;64:527–531. doi: 10.13075/mp.5893.2013.0043. [DOI] [PubMed] [Google Scholar]
- Mansour S.A., Mossa A.H., Heikal T.M. Haematoxicity of a new natural insecticide “spinosad” on male albino rats. Int. J. Agri. Biol. 2007;9:342–346. [Google Scholar]
- Martirosyan D.M., Miroshnichenko L.A., Kulokawa S.N., Pogojeva A.V., Zoloedov V.I. Amaranth oil application for heart disease and hypertension. Lipids Health Dis. 2007;6:1. doi: 10.1186/1476-511X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y., Kita S., Morimoto S. Antihypertensive effect of sesamin. I. Protection against deoxycorticosterone acetate-salt-induced hypertension and cardiovascular hypertrophy. Biol. Pharm. Bull. 1995;18:1016–1019. doi: 10.1248/bpb.18.1016. [DOI] [PubMed] [Google Scholar]
- Miller G.J., Miller N.E. Plasma high density lipoprotein concentration and the development of ischemic heart disease. Lancet. 1975;1:16–19. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- Mohamed K., Ali E. Modulatory effect of vitamin E against diazinon induced oxidative stress in lymphoid organs in rats. Int. J. Acad. Res. 2014;6:116–122. [Google Scholar]
- Monteiro, E.M., Chibli, L.A., Yamamoto, C.H., Pereira, M.C., Vilela, F.M., Rodarte, M.P., Pinto, M.A., do Amaral Mda, P., Silvério, M.S., Araújo, A.L., de Araújo Ada, L., Del-Vechio-Vieira, G.,de Sousa, O.V., 2014. Antinociceptive and anti-inflammatory activities of the sesame oil and sesamin. Nutrients 6,1931-1944. [DOI] [PMC free article] [PubMed]
- Mossa A.H., Refaie A.A., Ramadan A. Effect of exposure to mixture of four organophosphate insecticides at no observed adverse effect level dose on rat liver: the protective role of vitamin C. Res. J. Environ. Toxicol. 2011;5:323–335. [Google Scholar]
- Nakbi A., Tayeb W., Grissa A., Issaoui M., Dabbou S., Chargui I., Ellouz M., Miled A., Hammami M. Effects of olive oil and its fractions on oxidative stress and the liver’s fatty acid composition in 2,4-dichlorophenoxyacetic acid-treated rats. Nutr. Metab. (Lond.) 2010;7:80. doi: 10.1186/1743-7075-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki M. The chemistry and physiological function of sesame. Food Rev. Int. 1995;11:281–329. [Google Scholar]
- Nice, D., 1997. Antioxidant based nutraceuticals. In: New Technologies for Healthy Foods and Nutraceuticals, Yalpani, M. (Edn.), Science Publishers, Shrewsbury, pp, 105–123.
- Nishikimi M., Roa N.A., Yogi K. The occurrence of superoxide 728 anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Bioph. Res. Common. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Ogutcu A., Uzunhisarcikli M., Kalender S., Durak D., Bayrakdar F., Kalender Y. The effects of organophosphate insecticide diazinon on malondialdehyde levels and myocardial cells in rat heart tissue and protective role of vitamin E. Pest. Biochem. Physiol. 2006;86:93–98. [Google Scholar]
- Ohkawa, H., Ohishi, W., Yagi, K., 1979. Anal. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction Biochem. 95, 351–358. [DOI] [PubMed]
- Owen R.W., Giacosa A., Hull W.E., Haubner R., Würtele G., Spiegelhalder B., Bartsch H. Olive oil consumption and health: the possible role of antioxidants. Lancet Oncol. 2000;1:107–112. doi: 10.1016/s1470-2045(00)00015-2. [DOI] [PubMed] [Google Scholar]
- Pandya N., Santani D., Jain S. Antioxidant activity of ezetimibe in hypercholesterolemic rats. Indian J. Pharmacol. 2006;38:205–2006. [Google Scholar]
- Parhizkar S., Latiff L., Rahman S., Dollah M.A. Assessing estrogenic activity of Nigella sativa in ovariectomized rats using vaginal cornification assay. Afr. J. Pharm. Pharmacol. 2011;5:137–142. [Google Scholar]
- Perez-Jimenez F., Alvarez de Cienfuegos G., Badimon L., Barja G. International conference on the healthy effect of virgin olive oil. Eur. J. Clin. Invest. 2005;35:421–424. doi: 10.1111/j.1365-2362.2005.01516.x. [DOI] [PubMed] [Google Scholar]
- Peters T. Total protein: Direct Biuret method. Clin. Chem. 1968;14:1147–1159. [PubMed] [Google Scholar]
- Petroni A., Blasevich M., Salami M., Papini N., Montedoro G.F., Galli C. Inhibition of platelet aggregation and eicosanoid production by phenolic components of olive oil. Thromb. Res. 1995;78:151–160. doi: 10.1016/0049-3848(95)00043-7. [DOI] [PubMed] [Google Scholar]
- Philip J.K. Biochemical analysis of black and white sesame seeds from China. Am. J. Biochem. Mol. Biol. 2010;1:145–157. [Google Scholar]
- Poet T.S., Kousba A.A., Dennison S.L., Timchalk C. Physiologically base pharmacokinetic/pharmacodynamic model for the organophosphorus pesticide diazinon. Neurotoxicology. 2004;25:1013–1030. doi: 10.1016/j.neuro.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Popa C.D., Arts E., Fransen J., van Riel P.L.C.M. Atherogenic index and high-density lipoprotein cholesterol as cardiovascular risk determinants in rheumatoid arthritis: the impact of therapy with biologicals. Mediat. Inflamm. 2012;2012:1–9. doi: 10.1155/2012/785946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai K.D., Rai P.K., Gupta A., Watal G., Sharma B. Cartap and carbofuran induced alterations in serum lipid profile of Wistar rats. Indian J. Clin. Biochem. 2009;24:198–201. doi: 10.1007/s12291-009-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang, H.P., Dale, M.M., Ritter, J.M., Moore, P.K., 2005 Pharmacology. Elsevier India, 5th Edn.
- Razavi B.M., Hosseinzadeh H., Abnous K., Khoei A., Imenshahidi M. Protective effect of crocin against apoptosis induced by subchronic exposure of the rat vascular system to diazinon. Toxicol. Ind. Health. 2016;32:1237–1245. doi: 10.1177/0748233714554941. [DOI] [PubMed] [Google Scholar]
- Rezq A.A., Labib F.A., Attia A.M. Effect of some dietary oils and fats on serum lipid profile, calcium absorption and bone mineralization in mice. Pak. J. Nutr. 2010;9:643–650. [Google Scholar]
- Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Bio. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Richmond W. Preparation and properties of a cholesterol oxidase nocardia species and its application to the enzymatic assay of total cholesterol in serum. Clin. Chem. 1973;19:1350–1356. [PubMed] [Google Scholar]
- Sacco S.M., Chen J., Power K.A., Ward W.E., Thompson L.U. Lignan-rich sesame seed negates the tumor-inhibitory effect of tamoxifen but maintains bone health in a postmenopausal athymic mouse model with estrogen-responsive breast tumors. Menopause. 2008;15:171–179. doi: 10.1097/gme.0b013e3180479901. [DOI] [PubMed] [Google Scholar]
- Salah N., Miller N.J., Paganga G., Tijburg L., Bolwell G.P., Rice-Evans C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 1995;322:339–346. doi: 10.1006/abbi.1995.1473. [DOI] [PubMed] [Google Scholar]
- Salehi M., Jafari M., Saleh-Moqadam M., Asgari A. The comparison of the effect of diazinon and paraoxon on biomarkers of oxidative stress in rat serum. Zahedan J. Res. Med. Sci. 2012;14:18–23. [Google Scholar]
- Salih E.M.A. Toxic effect of dimethoate and diazinon on the biochemical and hematological parameters in male rabbits. Jordan J. Biol. Sci. 2010;3:77–82. [Google Scholar]
- Samarghandian S., Azimi-Nezhad M., Mehrad-Majd H., Mirhafez S.R. Thymoquinone ameliorates acute renal failure in gentamicin-treated adult male rats. Pharmacology. 2015;96:112–117. doi: 10.1159/000436975. [DOI] [PubMed] [Google Scholar]
- Sams C., Cocker J., Lennard M.S. Biotransformation of chlorpyrifos and diazinon by human liver microsomes and recombinant human cytochrome P450s (CYP) Xenobiotica. 2004;34:861–873. doi: 10.1080/00498250400017273. [DOI] [PubMed] [Google Scholar]
- Sarabia L., Maurer I., Bustos-Obregón E. Melatonin prevents damage elicited by the organophosphorous pesticide diazinon on the mouse testis. Ecotoxicol. Environ. Saf. 2009;72:938–942. doi: 10.1016/j.ecoenv.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Sarhan O.M.M., Al-Sahhaf Z.Y. Histological and biochemical effects of diazinon on liver and kidney of rabbits. Life Sci. J. 2011;8:1183–1189. [Google Scholar]
- Sarraf-Zadegan N., Boshtam M., Rafiei M. Risk factors for coronary artery disease in Isfahan. Iran. Eur. J. Public Health. 1999;9:20–26. [Google Scholar]
- Saxena P., Saxena A.K. Cypermethrin induced biochemical alterations in the blood of albino rats. Jordan J. Biol. Sci. 2010;3:111–114. [Google Scholar]
- Sharif M.R., Alizargar J., Sharif A. Evaluation of the wound healing activity of sesame oil extract in rats. World J. Med. Sci. 2013;9:74–78. [Google Scholar]
- Soliman M.M., Attia H.F., El-Ella G.A. Genetic and histopathological alterations induced by cypermethrin in rat kidney and liver: protection by sesame oil. Int. J. Immunopathol. Pharmacol. 2015;28:508–520. doi: 10.1177/0394632015575950. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Pathasarathy S., Carew T.E., Khoo J.C., Witztum J.L. Modification of low-density lipoprotein that increase atherogenicity. N. Engl. J. Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Swamy S.M., Tan B.K. Cytotoxic and immunopotentiating effects of ethanolic extract of Nigella sativa L. seeds. J. Ethnopharmacol. 2000;70:1–7. doi: 10.1016/s0378-8741(98)00241-4. [DOI] [PubMed] [Google Scholar]
- Trinder P. Determination of glucose in body fluids. Ann. Clin. Biochem. 1969;6:24. [Google Scholar]
- Tuck K.L., Hayball P.J. Major phenolic compounds in olive oil: metabolism and health effects. J. Nutr. Biochem. 2002;13:636–644. doi: 10.1016/s0955-2863(02)00229-2. [DOI] [PubMed] [Google Scholar]
- Tuck K.L., Freeman M.P., Hayball P.J., Stretch G.L., Stupans L. The in vivo fate of hydroxytyrosol and tyrosol, antioxidant phenolic constituents of olive oil, following intravenous and oral dosing of labeled compounds to rats. J. Nutr. 2001;131:1993–1996. doi: 10.1093/jn/131.7.1993. [DOI] [PubMed] [Google Scholar]
- Tuna G.B., Ozturk N., Comelekoglu U., Yilmaz B.C. Effects of organophosphate insecticides on mechanical properties of rat aorta. Physiol. Res. 2011;60:39–46. doi: 10.33549/physiolres.931941. [DOI] [PubMed] [Google Scholar]
- Tuovinen K., Kalistekorhone E., Raushel F.M., Hanninen O. Phosphotriesterases – a promising candidate for use in detoxification of organophosphates. Fundam. Appl. Toxicol. 1994;23:578–584. doi: 10.1006/faat.1994.1143. [DOI] [PubMed] [Google Scholar]
- Visioli F., Galli C. The effect of minor constituents of olive oil on cardiovascular disease: new findings. Nutr. Rev. 1998;56:142–147. doi: 10.1111/j.1753-4887.1998.tb01739.x. [DOI] [PubMed] [Google Scholar]
- Visioli F., Galli C., Plasmati E., Viappiani S., Hernandez A., Colombo C., Sala A. Olive phenol hydroxytyrosol prevents passive smoking-induced oxidative stress. Circulation. 2000;102:2169–2171. doi: 10.1161/01.cir.102.18.2169. [DOI] [PubMed] [Google Scholar]
- Warnick G.R., Benderson V., Albers N. Selected methods of enzymatic analysis. Lin. Chem. 1983;10:91–99. [Google Scholar]
- Wiseman S.A., Mathot J.N., de Fouw N.J., Tijburg L.B. Dietary non-tocopherol antioxidants present in extra virgin olive oil increase the resistance of low density lipoproteins to oxidation in rabbits. Atherosclerosis. 1996;120:15–23. doi: 10.1016/0021-9150(95)05656-4. [DOI] [PubMed] [Google Scholar]
- Zari T.A., Al-Attar A.M. Therapeutic effects of olive leaves extract on rats treated with a sublethal concentration of carbendazim. Euro. Rev. Med. Pharmacol. Sci. 2011;15:413–426. [PubMed] [Google Scholar]