Abstract
There are three key medicinal components (phellodendrine, berberine and palmatine) in the extracts of Phellodendron bark, as one of the fundamental herbs of traditional Chinese medicine. Different extraction methods and solvent combinations were investigated to obtain the optimal technologies for high-efficient extraction of these medicinal components. Results: The results showed that combined solvents have higher extracting effect of phellodendrine, berberine and palmatine than single solvent, and the effect of ultrasonic extraction is distinctly better than those of distillation and soxhlet extraction. Conclusion: The hydrochloric acid/methanol-ultrasonic extraction has the best effect for three medicinal components of fresh Phellodendron bark, providing an extraction yield of 103.12 mg/g berberine, 24.41 mg/g phellodendrine, 1.25 mg/g palmatine.
Keywords: Phellodendron, Cortex phellodendri, Extraction methods, Medicinal components
1. Introduction
Phellodendron bark has been widely known as one of the fundamental herbs of traditional Chinese medicine. Phellodendron bark is also called cortex phellodendri, which is the bark of two Phellodendron trees: P. amurense and P. chinense. Phellodendron bark is characterized by humorism, as bitter and cold, affecting the kidney, urinary bladder and large intestine meridians in a traditional Chinese medicine counterpart (Zhang et al., 2016). Phellodendron bark is also medically used to clear heat, reduce fire, dry dampness and release toxins (Li et al., 2014). Modern pharmacological researches indicated that Phellodendron bark has the functions of anti-pathogenic microorganism, anti-ulcer, antihypertensive and anti-arrhythmic, etc. Phellodendron bark can reduced blood uric acid levels in mice with hyperuricemia by inhibiting xanthine oxidase activity (Yang et al., 2015).
Many scientific findings had revealed the biomedical activities of extracts from Phellodendron bark. The further researches indicated that there are three key bioactive components (phellodendrine, berberine and palmatine) in the extracts of Phellodendron bark. Ethanol extracts of Phellodendron bark displayed antidiarrheal activity by attenuating ion transport by intestinal epithelium (Tsai et al., 2004, Guo et al., 2017). Medicinal extracts of Phellodendron bark reduced the rate of growth of Candida, which has been ascribed to berberine and palmatine content (Park et al., 1999). Different extracts of Phellodendron bark showed multiple functions, especially including: protect against airway inflammation in response to lipopolysaccharide treatment of mice (Mao et al., 2010, Gao et al., 2017), reduce blood glucose levels and slow the development of diabetic nephropathy in mice treated with streptozocin to induce diabetes (Kim et al., 2008, Muhammad et al., 2017a), reduce contractions of smooth muscle of isolated rat prostate glands (Xu and Ventura, 2010), and reduce cell replication of tumors in mice (Park et al., 2004).
Therefore, the efficient extraction of medicinal components from Phellodendron bark was focused by researches (Boost et al., 2016). The widely used extraction methods were soxhlet extraction, distillation and ultrasonic extraction, and the extraction solvents were water, organic solvents and their combinations (Wang et al., 2015, Chen et al., 2014). To obtain the optimal technologies for high-efficient extraction of principal medicinal components (phellodendrine, berberine and palmatine) from fresh Phellodendron bark, the three extraction methods and several solvent combinations were systematically analyzed.
2. Methodology
2.1. Preparation of Phellodendron barks
The fresh Phellodendron barks were collected from the average 15a trees in Phellodendron plantation in Cili country, Hunan province, China (Choi et al., 2014, Xian et al., 2011). The barks were treated by drying at 50 °C for 10 h, then meshed sieved by 60 meshes. The bark powders obtained were stored 4 °C.
2.2. Preparation of standard samples
Three standard samples (phellodendrine hydrochloride, berberine and palmatine hydrochloride) were dissolved in solution of acetonitrile/0.1% phosphoric acid to form sample solutions of gradient concentration (Hu et al., 2010, Garazd et al., 2017).
2.3. Distillation extraction
Accurately weigh 5.0 g powders of Phellodendron barks and put them into the round bottom flask, then add 40 times of ultrapure water (200 mL), followed by refluxing extraction in 100 °C. The extracts were collected finally by centrifugation (1000r/min) for 1 min (Jung et al., 2009).
2.4. Ultrasonic extraction
Weigh 0.50 g powders (by 20 mesh sieve) for 3 copies, then put them in the conical flask of 25 mL and add different extraction solvents: hydrochloric acid/methanol (1:100 v/v), hydrochloric acid/ethanol (1:100 v/v) and hydrochloric acid/water (1:100 v/v) in 50 mL (Kim et al., 2008). Powders were extracted with ultrasonic 20 min under the condition of 40 kHz and 250 W. The extracts were collected by centrifugation (1000 r/min) 1 min.
2.5. Soxhlet extraction
Ethanol extraction. Weigh 10.0 g powders, wrapped with filter paper and bounded with string, then put them into the drawer tube, then add 20 times amount of 70% ethanol (Chan et al., 2007). The extraction condition: extraction temperature 80 °C, 3 siphoning treatment (each for 1 h). Finally, combine the 3 extracting solutions to form extracts.
Acid-water extraction. Weigh 5.0 g powders, rapped with filter paper and bounded with string, then soaked in 2% hydrochloric acid for 1 h. Put them in the drawer tube and add 10 times amount of water (Li et al., 2006). The extraction condition: extraction temperature 100 °C, 3 siphoning treatment (each for 1 h). Finally, combine the 3 extracting solutions to form extracts (Muhammad et al., 2017b).
Double solvents extraction. Weigh 5.0 g powders of Phellodendron barks for 3 copies, wrapped with filter paper and bounded with string, then put them into the drawer tube (Liu et al., 1993, Wu et al., 2017). Then add 200 ml double solvents: hydrochloric acid/methanol (1:100 v/v), hydrochloric acid/ethanol (1:100 v/v) and hydrochloric acid/water (1:100 v/v). The extraction condition: extraction temperature 90 °C, 3 siphoning treatment (each for 1 h). Finally, combine the 3 extracting solutions to form extracts.
2.6. HPLC condition
Phellodendrine and berberine were gradient eluted with Kromasil RP-C18 chromatographic column (4.6 mm RP-C18), and palmatine is gradient eluted with Waters symmetry C18 chromatographic column (4.6 ∗ 250 mm, 5 μm).
Mobile phase for berberine: Acetonitrile/0.1% phosphoric acid (50:50 v/v) (plus 0.1 g sodium dodecyl sulfonate per 100 mL), and the detection wavelength is 265 nm.
Mobile phase for Phellodendrine: Acetonitrile/0.1% phosphoric acid (36:64 v/v) (plus 0.2 g sodium dodecyl sulfonate per 100 mL), and the detection wavelength is 284 nm.
Mobile phase for Palmatine gradient eluting: 0.05% TFA ddH2O (A)/0.05% TFA acetonitrile (B), and the detection wavelength is 270 nm.
3. Results & discussion
3.1. Component identification of Phellodendron barks extracts by HPLC
According to the peak area integral (mAU) and concentrations (µg/mL) of three standard samples (phellodendrine hydrochloride, berberine and palmatine hydrochloride), their regression equations were established (Fig. 1).
Fig. 1.
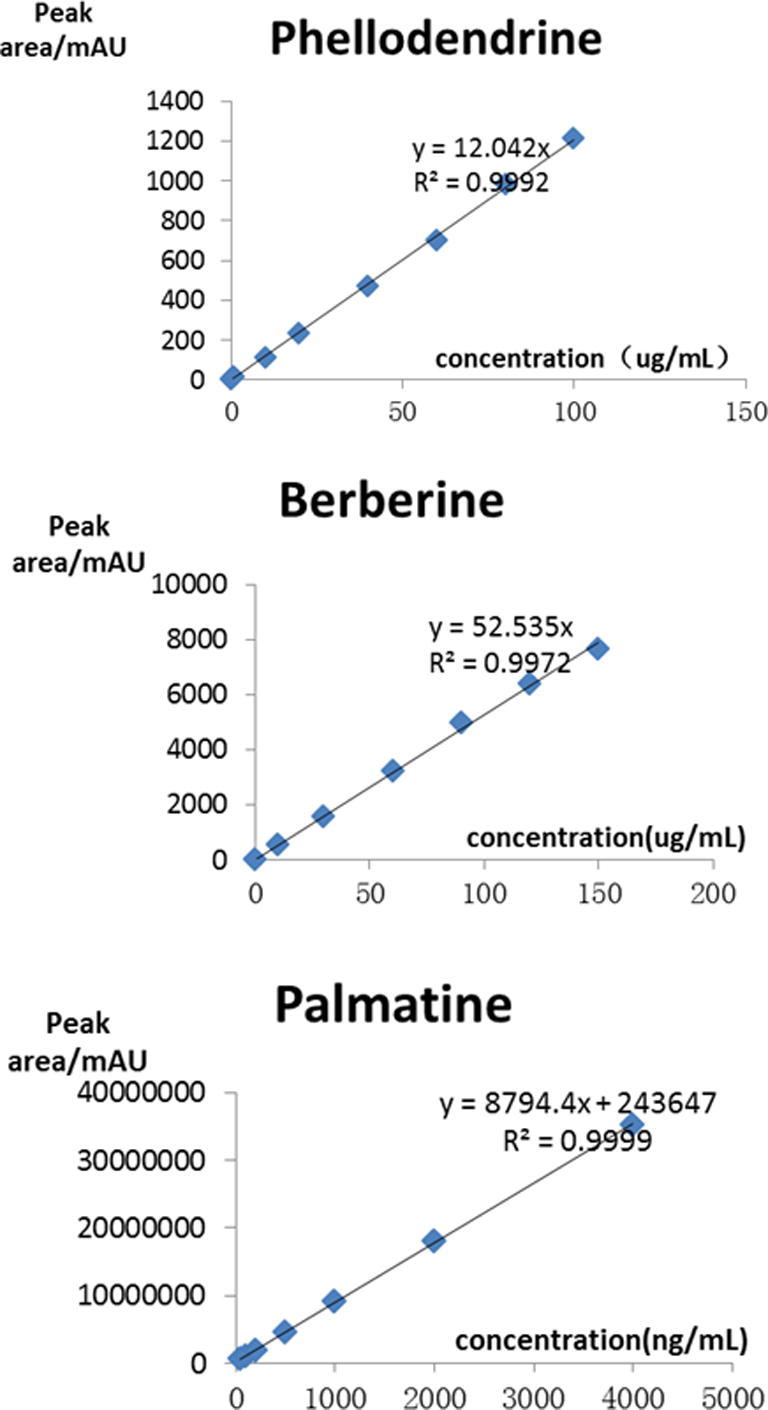
Regression equations of phellodendrine hydrochloride, berberine and palmatine hydrochloride.
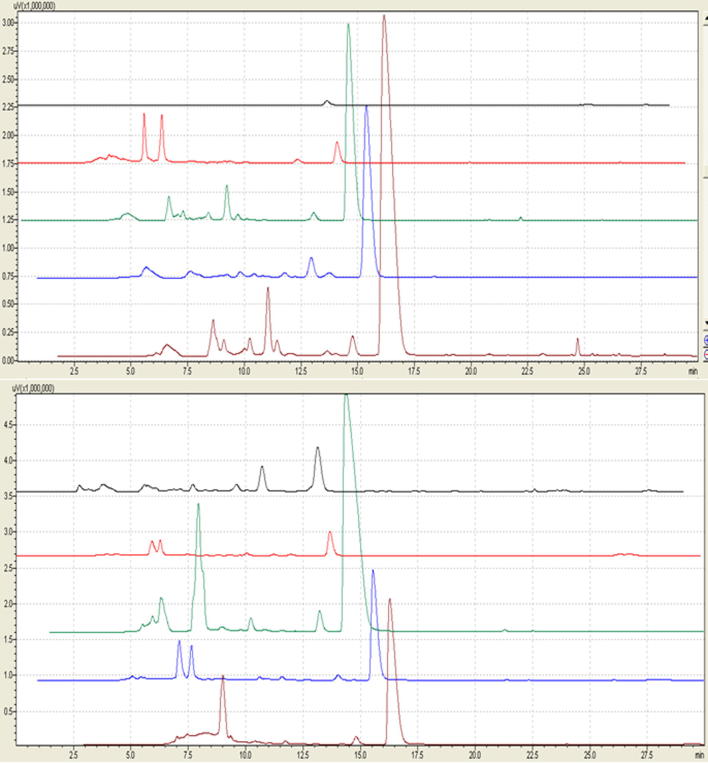
Nine combinations of three extraction methods and solvent combinations were used to extract Phellodendron barks, and their principal medicinal components were identified by HPLC (High Performance Liquid Chromatography) and verified by three different standard samples (Fig. 2).
Fig. 2.
HPLC chromatograms of Palmatine in nine extracts of Phellodendron barks.
3.2. Extraction yield of medicinal components from Phellodendron barks
The extraction effects of principal medicinal components in fresh Phellodendron barks were investigated using five extracting solvents (distilled water, methanol, ethanol, hydrochloric acid/methanol, hydrochloric acid/ethanol, hydrochloric acid/distilled water) and three extracting methods (soxhlet extraction, distillation and ultrasonic extraction).
The analytical result showed that the extraction effects of hydrochloric acid/methanol, hydrochloric acid/ethanol, hydrochloric acid/distilled water are better than those of hydrochloric acid, methanol, ethanol and distilled water, suggesting that combined solvents have higher extracting effect of phellodendrine, berberine and palmatine than single solvent (Mustafa et al., 2017). Further, the hydrochloric acid/methanol (1:100 v/v) display the highest extracting efficiency.
The three-good extraction yield of phellodendrine were achieved by hydrochloric acid/methanol-ultrasonic, hydrochloric acid/distilled water-ultrasonic and hydrochloric acid/ethanol-ultrasonic. The three-good extraction yield of berberine were achieved by hydrochloric acid/methanol-ultrasonic, hydrochloric acid/distilled water-ultrasonic and hydrochloric acid/ethanol-ultrasonic. The three-good extraction yield of palmatine were achieved hydrochloric acid/ethanol-ultrasonic, hydrochloric acid/methanol-ultrasonic and hydrochloric acid/methanol-Soxhlet (Nawaz et al., 2017).
Among the three extraction methods, the effect of ultrasonic extraction is distinctly better than those of distillation and soxhlet extraction.
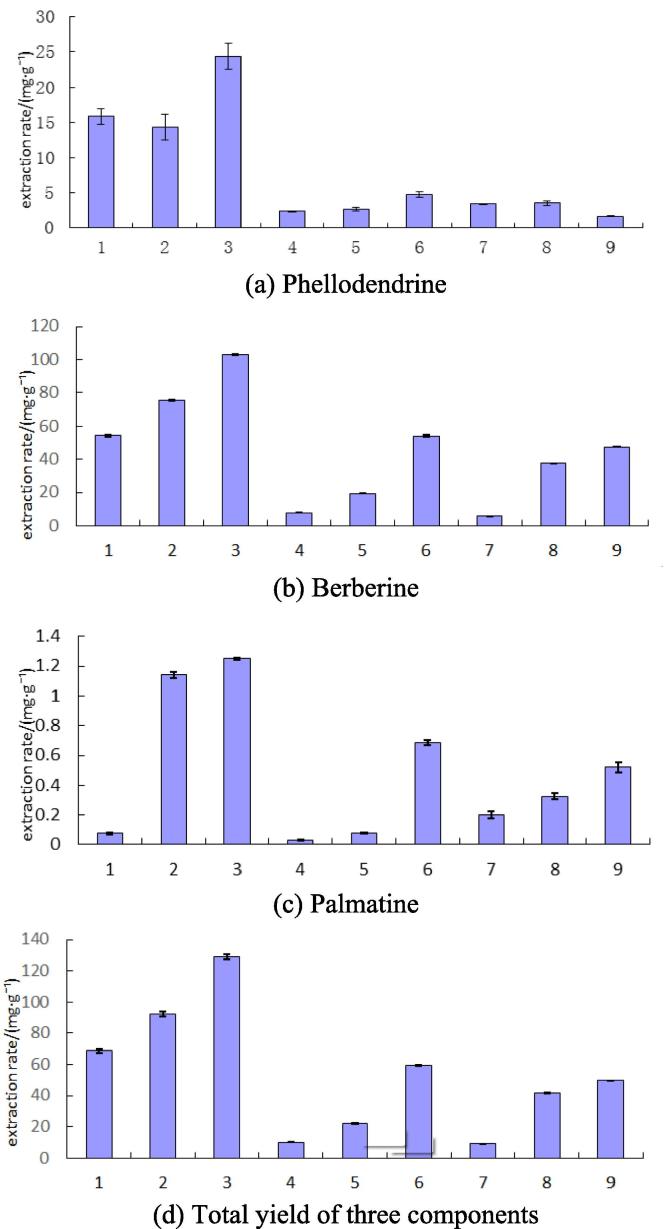
According to the analytical result, it is confirmed that the hydrochloric acid/methanol-ultrasonic extraction has the best effect, providing an extraction yield of 103.12 mg/g berberine, 24.41 mg/g phellodendrine, 1.25 mg/g palmatine (see Fig. 3).
Fig. 3.
Extraction yields of principal medicinal components by different solvents and extraction methods (mg/g).
It is reported in the literatures that the results of the experiment are different because of raw material origin, variety, pre-treatment methods and data analysis methods. The findings here focused on the exploration of the best extraction technology of medicinal components from fresh Phellodendron barks, which maybe have high quality than those of long-term stored barks (Sarfraz et al., 2017).
The phellodendrine, berberine and palmatine both are quaternary ammonium type alkaloid with good water solubility, and are generally insoluble in lipophilic organic solvent but soluble in water, alcohol and acid water. Free alkaloids and their salts are soluble in methanol and ethanol. Therefore, methanol and ethanol were used as the extraction solvents.
The optimal extraction method of three medicinal components is hydrochloric acid/methanol-ultrasonic. The extraction yield of berberine reaches to 16.59 mg/g, and the drying degree of raw materials may affect extraction. The highest extraction yield of phellodendrine is 24.41 mg/g, which is close to the Pharmacopoeia provided. The highest extraction yield of palmatine is 1.25 mg/g, which is higher than reported in the literatures.
Three extraction methods including distillation, ultrasonic extraction and soxhlet extraction were used here. The effect of ultrasonic extraction is significantly higher than those of distillation and soxhlet extraction. The ultrasonic extraction method has advantages of short extraction time, less material consumption, simple operation and low cost of materials. Therefore, it is priority to use the ultrasonic extraction as the best extraction method for medicinal components from fresh Phellodendron barks.
4. Conclusion
Combined solvents have higher extracting effect of than single solvent for the extraction of medicinal components from fresh Phellodendron barks, and the effect of ultrasonic extraction is distinctly better than those of distillation and soxhlet extraction. The hydrochloric acid/methanol-ultrasonic extraction has the best effect for three medicinal components of fresh Phellodendron bark, and the extraction yield reaches to 103.12 mg/g berberine, 24.41 mg/g phellodendrine, 1.25 mg/g palmatine.
Acknowledgements
This research was financially supported by Science and Technology project of Hunan Province (Grant No. 2016NK2154).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Dangquan Zhang, Email: zhangdangquan@163.com.
Wanxi Peng, Email: pengwanxi@163.com.
References
- Boost M., Yau P., Yap M., Cho P. Determination of cytotoxicity of traditional Chinese medicine herbs, Rhizoma coptidis, Radix scutellariae, and Cortex phellodendri, by three methods. Cont. Lens. Anterior. Eye. 2016;39(2):128–132. doi: 10.1016/j.clae.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Chan C.O., Chu C.C., Mok D.K., Chau F.T. Analysis of berberine and total alkaloid content in cortex phellodendri by near infrared spectroscopy (NIRS) compared with high-performance liquid chromatography coupled with uible spectrometric detection. Anal. Chim. Acta. 2007;592(2):121–131. doi: 10.1016/j.aca.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Chen G., Li K.K., Fung C.H. Er-Miao-San, a traditional herbal formula containing Rhizoma Atractylodis and Cortex Phellodendri inhibits inflammatory mediators in LPS-stimulated RAW264.7 macrophages through inhibition of NF-κB pathway and MAPKs activation. J. Ethnopharmacol. 2014;154(3):711–718. doi: 10.1016/j.jep.2014.04.042. [DOI] [PubMed] [Google Scholar]
- Choi Y.Y., Kim M.H., Han J.M. The anti-inflammatory potential of Cortex Phellodendron in vivo and in vitro: down-regulation of NO and iNOS through suppression of NF-κB and MAPK activation. Int. Immunopharmacol. 2014;19(2):214–220. doi: 10.1016/j.intimp.2014.01.020. [DOI] [PubMed] [Google Scholar]
- Gao W. The first multiplication atom-bond connectivity index of molecular structures in drugs. Saudi Pharm. J. 2017;25(4):548–555. doi: 10.1016/j.jsps.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garazd Y., Garazd M., Lesyk R. Synthesis and evaluation of anticancer activity of 6-pyrazolinylcoumarin derivatives. Saudi Pharm. J. 2017;25(2):214–223. doi: 10.1016/j.jsps.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J.J., Han J. An ethnobotanical survey of medicinal plants used for the ailment of diabetes mellitus in Changzhi city of Shanxi province, China. Biomed. Res. – India. 2017;28(3):1370–1377. [Google Scholar]
- Hu Y.M., Su G.H., Sze S.C., Ye W., Tong Y. Quality assessment of Cortex Phellodendri by high-performance liquid chromatography coupled with electrospray ionization mass spectrometry. Biomed. Chromatogr. 2010;24(4):438–453. doi: 10.1002/bmc.1311. [DOI] [PubMed] [Google Scholar]
- Jung H.W., Jin G.Z., Kim S.Y., Kim Y.S., Park Y.K. Neuroprotective effect of methanol extract of Phellodendri Cortex against 1-methyl-4-phenylpyridinium (MPP+)-induced apoptosis in PC-12 cells. Cell. Biol. Int. 2009;33(9):957–963. doi: 10.1016/j.cellbi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Kong M.K., Kim Y.C. Beneficial effects of Phellodendri Cortex extract on hyperglycemia and diabetic nephropathy in streptozotocin-induced diabetic rats. BMB Rep. 2008;41(10):710–715. doi: 10.5483/bmbrep.2008.41.10.710. [DOI] [PubMed] [Google Scholar]
- Li C.Y., Lu H.J., Lin C.H., Wu T.S. A rapid and simple determination of protoberberine alkaloids in cortex phellodendri by 1H NMR and its application for quality control of commercial traditional Chinese medicine prescriptions. J. Pharm. Biomed. Anal. 2006;40(1):173–178. doi: 10.1016/j.jpba.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Li S., Liu C., Guo L. Ultrafiltration liquid chromatography combined with high-speed countercurrent chromatography for screening and isolating potential α-glucosidase and xanthine oxidase inhibitors from Cortex Phellodendri. J. Sep. Sci. 2014;37(18):2504–2512. doi: 10.1002/jssc.201400475. [DOI] [PubMed] [Google Scholar]
- Liu Y.M., Sheu S.J., Chiou S.H., Chang H.C., Chen Y.P. A comparative study on commercial samples of phellodendri cortex. Planta Med. 1993;59(6):557–561. doi: 10.1055/s-2006-959761. [DOI] [PubMed] [Google Scholar]
- Mao Y.F., Li Y.Q., Zong L., You X.M., Lin F.Q., Jiang L. Methanol extract of Phellodendri cortex alleviates lipopolysaccharide-induced acute airway inflammation in mice. Immunopharm. immunotoxic. 2010;32(1):110–115. doi: 10.3109/08923970903193325. [DOI] [PubMed] [Google Scholar]
- Muhammad G., Rashid I., Firyal S. Practical aspects of treatment of organophosphate and carbamate insecticide poisoning in animals. Matrix Sci. Pharma. 2017;1(1):10–11. [Google Scholar]
- Muhammad G., Rashid I., Firya S., Saqib M. Successful treatment of idiopathic generalized subcutaneous emphysema in kajli a ram by large bore injection needle. Matrix Sci. Med. 2017;1(1):01–02. [Google Scholar]
- Mustafa G., Arif R., Atta A., Sharif S., Jamil A. Bioactive compounds from medicinal plants and their importance in drug discovery in Pakistan. Matrix Sci. Pharma. 2017;1(1):17–26. [Google Scholar]
- Nawaz S., Shareef M., Shahid H., Mushtaq M., Sajid S., Sarfraz M. Lipid lowering effect of synthetic phenolic compound in a high-fat diet (HFD) induced hyperlipidemic mice. Matrix Sci. Pharma. 2017;1(1):12–16. [Google Scholar]
- Park K.S., Kang K.C., Kim J.H., Adams D.J., Johng T.N., Paik Y.K. Differential inhibitory effects of protoberberines on sterol and chitin biosyntheses in Candida albicans. J. Antimicrob. Chemother. 1999;43(5):667–674. doi: 10.1093/jac/43.5.667. [DOI] [PubMed] [Google Scholar]
- Park S.D., Lai Y.S., Kim C.H. Immunopontentiating and antitumor activities of the purified polysaccharides from Phellodendron chinese SCHNEID. Life Sci. 2004;75(22):2621–2632. doi: 10.1016/j.lfs.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Sarfraz M., Ashraf Y., Ashraf S. A Review: Prevalence and antimicrobial susceptibility profile of listeria species in milk products. Matrix Sci. Med. 2017;1(1):03–09. [Google Scholar]
- Tsai J.C., Tsai S., Chang W.C. Comparison of two Chinese medical herbs, Huangbai and Qianniuzi, on influence of short circuit current across the rat intestinal epithelia. J. Ethnopharm. 2004;93(1):21–25. doi: 10.1016/j.jep.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Wang L., Yan G., Zhang A., Shi H., Sun H., Wang X. Fingerprinting and simultaneous determination of alkaloids and limonins in Phellodendri amurensis cortex from different locations by high-performance liquid chromatography with diode array detection. J. Chromatogr. Sci. 2015;53(1):161–166. doi: 10.1093/chromsci/bmu034. [DOI] [PubMed] [Google Scholar]
- Wu J., Qu Y. Molecular docking studies of kirenol a traditional Chinese medicinal compound against rheumatoid arthritis cytokine drug targets (TNF-alpha, IL-1 and IL-6) Biomed. Res. – India. 2017;28(5):1992–1995. [Google Scholar]
- Xian Y.F., Mao Q.Q., Ip S.P., Lin Z.X., Che C.T. Comparison on the anti-inflammatory effect of Cortex Phellodendri Chinensis and Cortex Phellodendri Amurensis in 12-O-tetradecanoyl-phorbol-13-acetate-induced ear edema in mice. J. Ethnopharmacol. 2011;137(3):1425–1430. doi: 10.1016/j.jep.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Xu Y., Ventura S. Extracts of bark from the traditional Chinese herb Phellodendron amurense inhibit contractility of the isolated rat prostate gland. J. Ethnopharmac. 2010;127(1):196–199. doi: 10.1016/j.jep.2009.09.047. [DOI] [PubMed] [Google Scholar]
- Yang C., Zhu J.X., Wang Y., Wen Y.L., Kong L.D. Effects of processing Phellodendron amurense with salt on anti-gout. China J. Chinese Mater. Med. 2015;30(2):145–148. [PubMed] [Google Scholar]
- Zhang Z., Zhang Y., Zhang Z. Comparative analysis of DNA barcoding and HPLC fingerprint to trace species of Phellodendri Cortex, an important traditional Chinese medicine from multiple sources. Biol. Pharm. Bull. 2016;39(8):1325–1330. doi: 10.1248/bpb.b16-00210. [DOI] [PubMed] [Google Scholar]