Abstract
The majority of the textile dyes are harmful to the environment and potentially carcinogenic. Among strategies for their exclusion, the treatment of dye contaminated wastewater with fungal extract, containing lignin peroxidase (LiP), may be useful. Two fungi isolates, Pleurotus ostreatus (PLO9) and Ganoderma lucidum (GRM117), produced the enzymatic extract by fermentation in the lignocellulosic residue, Jatropha curcas seed cake. The extracts from PLO9 and GRM117 were immobilized on carbon nanotubes and showed an increase of 18 and 27-fold of LiP specific activity compared to the free enzyme. Also, LiP from both fungi extracts showed higher Vmax and lower Km values. Only the immobilized extracts could be efficiently reused in the dye decolourization, contrary, the carbon nanotubes became saturated and they should be discarded over time. This device may offer a final biocatalyst with higher catalytic efficiency and capability to be reused in the dye decolourization process.
Keywords: Lignocellulosic residue, Solid state fermentation, Immobilization, Fungi
1. Introduction
Dyes are an important class of macromolecules which are an integral part of our lives and used by, textile, paint and plastic, solar cells, optics, metal extraction and sensor industries, among others (Rauf and Ashraf, 2012). Due to the extensive use, they have become a part of industrial effluent. It is estimated that up to 50% of dye contents are lost after dyeing textiles and about 10–15% are discarded in effluents (Papadoulou et al., 2013). Further to esthetic pollution, the colored effluent promotes the inhibition of light penetration along the depth of water bodies, affecting biological cycles, particularly the photosynthesis processes. Most of these dyes are harmful and potentially carcinogenic in nature and their exclusion from wastewaters is a major environmental challenge (Chequer et al., 2011).
The use of enzymes for environmental remediation purposes has increased due to peculiar properties of this class of proteins. These molecules operate in a wide range of contaminant concentrations, pH, temperature and salinity (Rao et al., 2014). The ligninolytic enzyme system (lignin peroxidase, manganese peroxidase and laccase) produced by the white rot fungi is of particular interest because it has a low substrate specificity, no steric selectivity and strong oxidative abilities (Ashger et al., 2014). Besides, due to the high cost of biotechnology processes, the current challenge is to increase the production of these enzymes, using low-cost substrates. Therefore the use of lignocellulosic residues, such as the Jatropha curcas seed cake, as substrate for the synthesis of enzymes is an alternative for cost reduction and at the same time solves an environmental problem regarding the disposal of these agro-industrial wastes. Among those enzymes, lignin peroxidase (LiP) stands out as a relatively nonspecific enzyme, able to mineralize various recalcitrant aromatic and halogenated phenolic compounds, with high redox potential compared with the classical peroxidases, therefore being a stronger oxidant (Wong, 2009). Regarding the biotechnological potential, this enzyme has been successfully applied in food and pharmaceutical industries, wastewater treatment of pulp and paper and textile industry, bioremediation and biomass delignification (Wong, 2009, Ashger et al., 2014).
Although the natural catalysts are sustainable, selective and efficient, they are often not perfectly adapted for industrial applications. To promote the use of enzymes in industrial processes, its stability and reuse ability should be considered (Gardossi et al., 2010). Hence enzyme immobilization emerges as a key enabling technology (Liese and Hilterhaus, 2013). Enzyme immobilization on various solid supports offers a number of advantages such as easy handling and a possible increase in the thermal stability and pH range supported by the protein. An additional benefit is greater stability under storage and operating conditions, for example, to heat denaturation, organic solvents or autolysis (Gardossi et al., 2010).
Recent interest in nanotechnology has provided a wide variety of materials that may potentially be used as supports for immobilization. Among the various nanostructures, carbon nanotubes (CNTs) have played a key role in the nanotechnology revolution. Due to the large specific surface area of CNTs, these carbon nanoparticles have been preconized as a good biomolecules carrier, being promising scaffolds for peptides, proteins and enzymes (Feng and Ji, 2011, Marchesan and Prato, 2015). Furthermore, carbon nanotubes exhibit extraordinary mechanical properties, electrical, thermal and chemical stability (Zhang and Henthorn, 2010); hence this nanoparticle has been applied in diverse fields such as materials, electronics and nanomedicine (Oliveira et al., 2015). One of the primary fields to benefit from nanoparticle-enzyme conjugates is biocatalysis, which is becoming one of the most powerful tools in biotechnology, having a profound impact on environmental protection (Illanes et al., 2012).
The main objective of this study is the production of fungal enzymatic extract, containing LiP, and its immobilization on carbon nanotubes. We also evaluated the LiP catalytic efficiency, stability and the capability of reuse in dye decolourization. The results obtained from this study can contribute to developing a biocatalyst that can be applied in diverse environmental applications in the future, especially in the treatment of textile effluents.
2. Materials and methods
2.1. Strain and chemicals
The fungi, Pleurotus ostreatus (PLO9) and Ganoderma lucidum (GRM117) were obtained from Mycorrhizal Associations Laboratory at Universidade Federal de Viçosa (Minas Gerais, Brazil). They were stored on potato dextrose agar (PDA) slants at 4 °C and multiplied, as needed, in the same medium.
All analyses were made with analytical reagent grade. Veratryl alcohol (VA) and Remazol brilliant blue R (RBBR) dye were purchased from Sigma–Aldrich (St. Louis, MO – USA) and the commercial lignin peroxidase (LiPc) was purchased from Santa Cruz Biotechnology, Inc (Dallas, TX – USA).
2.2. Enzymatic extract production: solid state fermentation (SSF)
Fungal cultures were multiplied in PDA plates at 25 °C for up to 7 days for inoculum production. Four mycelial disks, containing 1 cm agar-disk, were grown in substrate containing 20 g of J. curcas L. seed cake, previously humidified (70% of water) and autoclaved for one hour at 121 °C. Substrates were supplemented with 2 mL of Kirk’s medium (Tien and Kirk, 1983) and 1 mL of exogenous inductor Tween 80 (0.3 mmol l−1), and incubated at 25 °C (room temperature). After 7 days of inoculation, the crude enzymatic extract (CEE) was obtained by adding 100 mL sodium tartrate buffer (100 mmol l−1, pH 3.5) supplemented with EDTA (5 mmol l−1), then centrifuged at 4000×g for 30 min and filtrated (Whatman No. 1 filter paper).
This procedure was done for SSF of both fungal isolates in triplicates, including the control (without inoculum) and the one flask inoculated but without exogenous inductor.
2.3. Partial purification of enzymatic extract
The crude enzymatic extract was centrifuged at 4000×g for 15 min. The supernatant was firstly brought to 40% saturation by the gradual addition of solid crystals of ammonium sulfate and kept overnight at 4 °C. The precipitate was collected by centrifugation (4000×g) for 15 min at 4 °C and to the supernatant more crystals of ammonium sulfate were added to achieve 80% saturation. It was again kept overnight at 4 °C and centrifuged as described previously. After centrifugation, the sediments were dissolved in sodium tartrate buffer (100 mM l−1, pH 3.5) and dialyzed against the same buffer. The dialysate was applied to DEAE cellulose (Sigma–Aldrich, Brazil) ion-exchange column (3 × 14 cm) which had been equilibrated with sodium tartrate buffer (100 mmol l−1, pH 5.5). The column was washed stepwise with 50, 100, 150, 200, 300 and 500 mmol l−1 de sodium chloride solution (1 mol l−1). The fractions were collected with a flow rate of 1 mL min−1 and the LiP activity and protein contents were determined for each fraction. The active fractions were then pooled and utilized as the partial purified enzymatic extract (PPE) after lyophilization.
2.4. LiP activity and protein content
The lignin peroxidase activity was determined by spectrophotometry based on change in absorbance at 310 nm at 30 °C (Multiskan™ FC Microplate Photometer). The enzyme assay contained 100 μL of sodium tartrate buffer (100 mmol l−1, pH 3.5), 100 μL of veratryl alcohol (4 mmol l−1), 50 μL hydrogen peroxide (0.2 mol l−1) and 10 μL of PPE.
LiP activity was expressed in units (U) per mL. One unit (U) LiP was defined as the amount of enzyme required to oxidize 1 μmol of veratryl alcohol in 1 min, at 30 °C and pH 3.5. The activity of reaction mixture was measured against reagent blank at 310 nm (ɛ310 = 9300).
To determine the protein contents of the crude and purified enzyme extracts, the Bradford micro assay (Bradford, 1976) using bovine serum albumin as standard was used.
2.5. PPE immobilization on carbon nanotubes
Carboxylated carbon nanotubes with HNO3 and H2SO4 were used. Carbon nanotubes were produced by the chemical vapor deposition method using iron as a catalyst. The CNT produced had a diameter of 20–90 nm with a purity of 83% and a length between 10 and 30 μm.
Carbon nanotubes, 5 mg, were suspended in 1.5 mL of sodium phosphate buffer (0.2 mol l−1, pH 7.7) and stirred for 30 min in a sonicator (Thornton – INPEC Electronic S/A). Different amounts of the lyophilized enzymatic extract (5, 25 and 50 mg) were suspended in 2 mL of the same buffer. The whole volume was added to nanotube solution and this mixture was sonicated for 3 h. After that, enzymes not linked to the nanotubes were removed by repeated washing (up to 4×) with 2 mL of phosphate buffer (0.2 mol l−1, pH 7.7).
Immobilized LiP activity was determined, as described in the previous section for the free enzyme.
Supernatant, just after immobilization, and from washing solutions was analyzed to quantify the protein by the Bradford method.
The immobilized protein concentration ([LiP]immob) was determined by the difference between the initial protein concentration ([LiP]i) and the concentration of proteins present in the supernatant after immobilization ([LiP]s). In turn, the protein concentration that remained bound to the carbon nanotube ([LiP]b) was determined by the difference between ([LiP]immob) and the sum of the protein concentration present in washing solutions (Σ [LiP]w). The immobilization efficiency was determined by the equation (i).
Immob. Efficiency = {([LiP]b)/([LiP]i)} × 100. (i)
where: [LiP]b = Enzyme bound to the Carbon nanotube
[LiP]i = Initial protein concentration
2.6. Effect of pH and temperature on LiP activity
The effect of varying pH (pH 3–9) on the activities of free and immobilized LiP was investigated using sodium tartrate buffers in pH 3.0, 5.0, 7.0 or 9.0. The free and immobilized LiPs were incubated at room temperature (±25 °C) for 15 min.
Effect of varying temperatures on LiP activity was also studied. Both the free and immobilized LiPs were incubated with sodium tartrate buffer pH 3.5 for 15 min, at temperatures of 15, 30, 45 or 60 °C.
2.7. Effect of substrate concentration: determination of Km and Vmax
The Michalis–Menten kinetic constants (Km) and maximum velocity (Vmax) for free and immobilized LiP were determined using varying concentrations of veratryl alcohol (25, 50, 75, 100, 150, 200, 250, 300, 500, 700 and 900 μmol l−1) as substrate. To determine the kinetic constants free and immobilized LiPs were incubated for 15 min at room temperature (25 °C) in the same conditions previously described for enzyme assay.
The Km and Vmax for free and immobilized LiPs were calculated with at least seven initial substrate concentrations using the program SigmaPlot V. 12.0 (Systat Software, San Jose, CA).
2.8. Dye decolourization
Decolourization experiment of the liquid RBBR dye by free PPEs (100 mg mL−1) and immobilized (25 mg ml−1) was carried out in tubes containing sodium tartrate buffer (100 mM L−1, pH 3.5), hydrogen peroxide (0.2 mol l−1) and RBBR concentrations of 10 and 30 mg l-. The decolourization was monitored spectrophotometrically at 595 nm after 24 h of incubation at 25 °C.
The free and immobilized PPEs were reused once in RBBR decolourization of 10 mg l−1 and again in RBBR decolourization at the concentration of 30 mg l−1.
The decolourization rate was determined by the difference between the initial absorbance and absorption observed divided by the initial absorbance. In the calculation, the effect of time of incubation as well as the effect of the sample composition was discounted through the control sample which contained the carbon nanotubes and PPE from the solid fermentation control sample.
In order to evaluate the capability of the free and immobilized commercial lignin peroxidase (LiPc) and just carbon nanotubes (without enzymes) in decolorizing the RBBR dye, a second decolourization experiment was performed.
The LiPc was immobilized according to the methodology previously described. In this assay, the absorbance decrease caused by dyes adsorbed on carbon nanotubes was not negligible. The assay was carried out in tubes containing sodium tartrate buffer (100 mmol l−1, pH 3.5), hydrogen peroxide (0.2 mol l−1) and RBBR concentrations of 10, 30 and 50 mg l−1.
The decolourization was also monitored spectrophotometrically at 595 nm after 1, 3, 11 and 14 days of incubation at room temperature. Just the immobilized LiPc was reused 3 times subsequently, 2, 8, and 3 days after the first, second and third measurement, respectively. In this case, the free LiPc was diluted in the solution, so they could not be reused.
3. Results and discussion
3.1. Enzymatic extract production: solid state fermentation (SSF)
Both fungi tested in solid fermentation condition in J. curcas seed cake plus exogenous inductor Tween 80 showed a high potential for LiP production (Table 1). We have obtained similar volumes of crude enzymatic extract from both isolates, but the LiP specific activity from GRM117 was higher than from PLO9 extract (Table 1).
Table 1.
Characteristics of enzymatic extract containing lignin peroxidase (LiP) from Pleurotus ostreatus PLO9 and Ganoderma lucidum GRM117.
| Purification steps | Total volume (mL) | Total LiP activity (U mL−1) | Total protein content (mg mL−1) | LiP specific activity (U mg−1) | Purification fold | Yield (%) |
|---|---|---|---|---|---|---|
| Pleurotus ostreatus PLO9 | ||||||
| Crude enzymatic extract | 123 | 10602.68 | 0.34 | 30623.93 | 1 | 100 |
| (NH4)2SO4 precipitation | 37 | 10556.27 | 0.25 | 41283.92 | 1.3 | 100 |
| DEAE-cellulose | 25 | 4247.3118 | 0.063 | 66686.76 | 1.6 | 40 |
| Ganoderma lucidum GRM117 | ||||||
| Crude enzymatic extract | 120 | 9419.35 | 0.13 | 69814.04 | 1 | 100 |
| (NH4)2SO4 precipitation | 21 | 7715.05 | 0.13 | 56836.99 | 0.8 | 82 |
| DEAE-cellulose | 25 | 1442.65 | 0.019 | 75424.97 | 1.3 | 15 |
The high potential of P. ostreatus PLO9 and G. lucidum GRM-117 in producing ligninolytic enzyme was expected, since both are basidiomycetes, belonging to the white rot fungus group, known to include generally robust microorganisms that produce extracellular enzymes such as lignin peroxidase, manganese peroxidase, and laccase (Da Luz et al., 2014).
Further to the fungal species used, the growth medium also favored the production of ligninolytic enzymes, in particular the lignin peroxidase, once J. curcas seed cake has considerable lignin contents (Da Luz et al., 2014). In Brazil, J. curcas has been widely used for biodiesel production, after extracting oil, a solid residue, called seed cake, is produced (Da Luz et al., 2014). The fermentation medium, in addition to the lignocellulosic residue, was supplemented with Tween 80. Several authors have demonstrated an increase in the secretion of enzymes in the presence of Tween 80 in cultures of the fungus Phanerochaete chrysosporium. It is believed that the surfactant transforms the structure of the cell membrane and promotes the permeability of lignin peroxidase from fungal cells to the extracellular medium (Asther et al., 1988). Moreover, supplementation of culture medium Kirk also favors for greater LiP production, since this medium is known to induce the production, in particular, of the lignin peroxidase enzyme (Tien and Kirk, 1983). In our experiments, we did not detect LiP activity in the extracts produced without Tween 80, suggesting that LiP is an inducible enzyme for our isolates.
3.2. Partial purification of enzymatic extract
We observed an increase in specific activity of PLO9 in the two steps of purification with 40% of yield after DEAE-cellulose (Table 1). In turn, for the GRM117 fungal extract, the yield after this later step of purification was 2.66 times smaller than the yield of PLO9 (Table 1).
The partially purified enzyme extract of these fungi presented high total and specific activities of LiP (Table 1), considering the values reported in the literature (Asgher et al., 2007, Asgher et al., 2012, Iqbal et al., 2011). Although the purification steps present lower efficiency compared to that described in other studies (Table 1), the partial purity obtained in our extracts was adequate to our final purpose. Thus, once the purification is the most expensive step in the whole process of enzyme attainment, the crude or partially purified enzymatic extract can be used, as low-cost alternative, in some environmental applications, for example, in the dye decolourization.
3.3. PPEs immobilization on carbon nanotubes
After the immobilization, the lignin peroxidase present in both the partially purified enzymatic extract showed a higher specific activity (Table 2). The PPE from PLO9, at concentrations of 12.5 and 25 mg mL−1, showed an increase of 18 and 9-fold in LiP specific activity, respectively, compared to the free enzyme (Table 2). For the PPE from GRM-117, only at the concentration of 25 mg mL−1, it was possible to calculate the specific activity after the immobilization that was 27 times higher than the free enzyme (Table 2). These results are significant since, for example, Asgher et al. (2012) verified an increase in the specific activity of only 1.27 of LiP partially purified, after the immobilization by trapping in the xerogel.
Table 2.
Immobilization of partial purified enzymatic extract (PPE) containing lignin peroxidase (LiP) from Pleurotus ostreatus PLO9 and Ganoderma lucidum GRM117 on carbon nanotubes (CNT).
| CNT (mg) | PPE (mg)⁎ | PPE (mg mL−1) | Free LiP |
Immobilized LiP |
Immobilization efficiency (%) | ||
|---|---|---|---|---|---|---|---|
| Total activity (U mL−1) | Specific activity (U mg−1) | Total activity (U mL−1) | Specific activity (U mg−1) | ||||
| Pleurotus ostreatus PLO9 | |||||||
| 5.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5.0 | 5.0 | 2.5 | ND | ND | 586.02 | ND | ND |
| 5.0 | 25.0 | 12.5 | 39.42 | 4877 | 489.24 | 89522 | 67.6 |
| 5.0 | 50.0 | 25 | 170.25 | 13053 | 577.06 | 128,100 | 34.5 |
| Ganoderma lucidum GRM117 | |||||||
| 5.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5.0 | 5.0 | 2.5 | ND | ND | 446.23 | ND | ND |
| 5.0 | 25.0 | 12.5 | ND | ND | 557.34 | ND | ND |
| 5.0 | 50.0 | 25 | 44.80 | 10,826 | 507.16 | 302,572 | 40.5 |
Data referent to the enzymatic extract that was partially purified and lyophilized.
The high LiP specific activities, after the immobilization process, may be explained by the carrier used, once the catalytic behavior of immobilized enzymes strongly depends on the properties of their carriers, such as material types, structures, and compositions. Carbon nanotubes have been shown one of the best supports for immobilization as compared to traditional carriers such as zirconia, silica and epoxy (Saifuddin et al., 2013). Furthermore, they are more stable in extreme conditions, presenting the capacity to carry a larger amount of enzyme and increase up the catalytic activity by two times more than the other supports and by ten times more than the free enzyme (Feng and Ji, 2011, Saifuddin et al., 2013). Xu et al. (2015) showed that the introduction of carbon nanotubes (MWNTs) to the membrane improved the properties of immobilized laccase, such as enzyme loading, activity retention, pH stability, thermal stability, operational stability, and storage stability. These improvements can be attributed to the enhanced electrical conductivity and biocompatible microenvironment provided by the carbon nanotubes.
3.4. Effect of pH and temperature on LiP activity
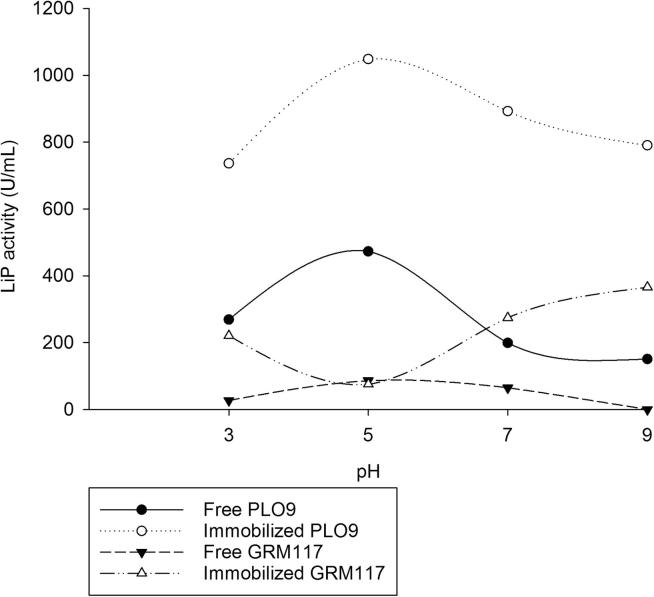
For the PPE from PLO9, the immobilization did not cause changes, maintaining optimum activity around pH 5 (Fig. 1), and the extract from GRM117, showed higher LiP activity at neutral to basic conditions after the immobilization (Fig. 1).
Figure 1.
Effect of pH on LiP activity. LiP from partially purified enzymatic extract (PPE) of Pleurotus ostreatus PLO9 and Ganoderma lucidum GRM117. (Free PPE = 10 mg mL−1; immobilized PPE = 25 mg mL−1).
The LiP activities were higher in PPEs immobilized on carbon nanotubes than free PPEs. Despite being unknown mechanisms, the immobilization protects the enzyme of pH effects and this is important, for example, to apply the immobilized LiP in the treatment of textile effluents which present a wide range of pH.
Previous studies report that the higher activities for LiPs from white rot fungi are generally observed between pH 2 and 5 and it is usually verified, after the immobilization procedure, a slight change in pH of LiP toward more acidic conditions (Asgher et al., 2007, Asgher et al., 2012). However, LiP of GRM117 has optimal pH in basic conditions after immobilization (Fig. 1). It is difficult to predict the enzyme behavior when interacting with the support, there are many possible conformations and thus it is variable how these respond to changing environmental conditions.
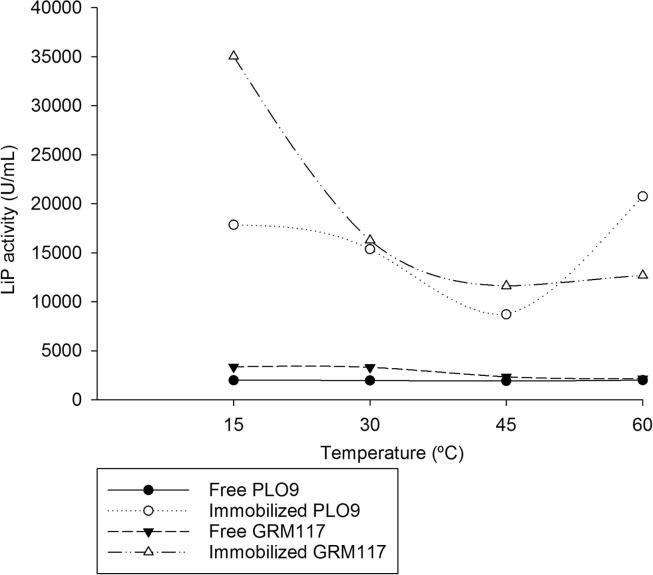
Free enzymes were thermostable between 15 and 60 °C (Fig. 2). Generally, the optimum temperature of different LiPs depends on fungal species. For example, LiP of Loweporus lividus MTCC-1178 showed optimum activity around 24 °C (Yadav et al., 2009a), in contrast, LiP of P. chrysosporium showed higher stability at 55 °C (Alam et al., 2009). After immobilization, the LiP from PLO9 showed the highest activity at 60 °C (Fig. 2), similar results were reported by Qiu et al. (2009), however, the immobilized LiP from GRM117 showed the highest activity at 15 °C. Generally the temperature profile of enzymes has been extended after attachment to material surfaces (Talbert and Goddard, 2012). This expansion of the temperature profile enables the enzyme to be used for applications that require the protein to be subjected to conditions not suitable for the free enzyme. In this case, these different enzymatic extracts can be used together or in sequence in order to extend the temperature range that these enzymes can be applied, keeping its activities.
Figure 2.
Effect of temperature on LiP activity. LiP from PPEs of Pleurotus ostreatus PLO9 and Ganoderma lucidum GRM117 (Free PPE from PLO9 = 100 mg mL−1; free PPE from GRM117 = 200 mg mL−1; immobilized PEE = 25 mg mL−1).
Although the immobilized enzymes become more susceptible to temperature changes, their activities of immobilized LiP were higher compared to free enzymes (Fig. 2). Higher activities and a relative thermal stability are attractive and desirable characteristics for industrial applicability of enzymes.
3.5. Effect of substrate concentration: determination of Km and Vmax
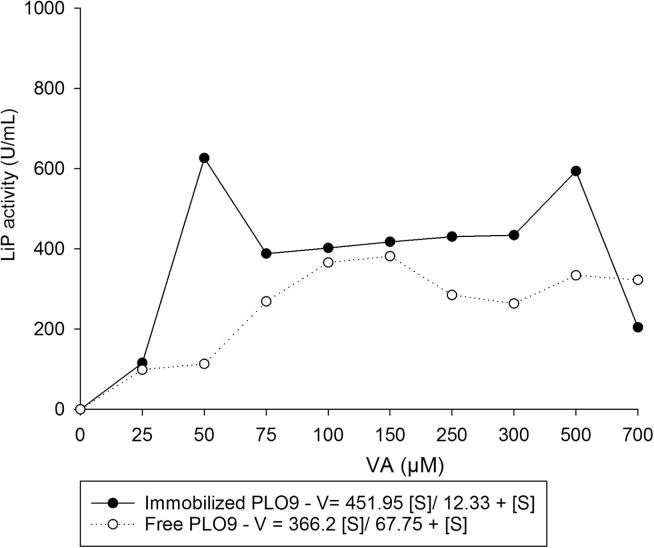
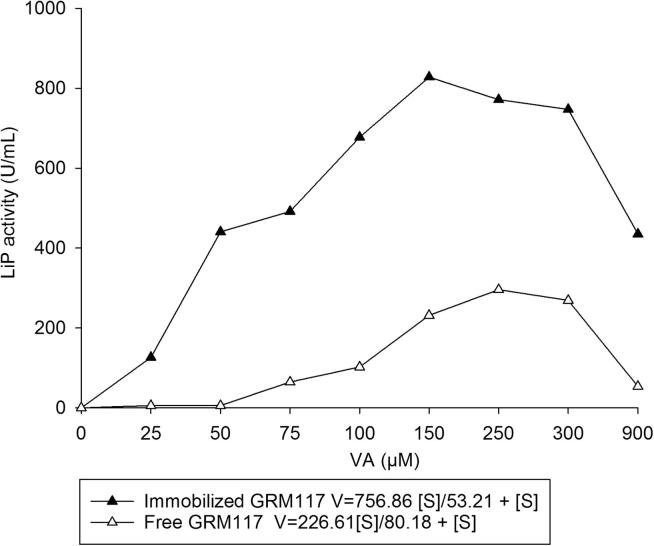
After immobilization, LiP in the PPE from both fungi showed the Vmax higher than 400 U mL−1and low values of Km, 12 μM for PLO9 and 53 μM for GRM117 (Figure 3, Figure 4) which were lower values than those observed in free PPEs (Figure 3, Figure 4). The lower Km and higher Vmax indicate that immobilization favored for higher affinity to the substrate and improved the enzyme catalytic efficiency.
Figure 3.
Michalis–Menten kinetic constants (Km) and maximum velocity determination of free and immobilized LiP from PPE of Pleurotus ostreatus PLO9. (Free PPE = 10 mg mL−1, Vmax = 366.2, Km = 67.75 μM; immobilized PPE = 25 mg mL−1, Vmax = 451.9, Km = 12.3 μM). VA = Veratryl alcohol.
Figure 4.
Michalis–Menten kinetic constants (Km) and maximum velocity determination of free and immobilized LiP from the PPE Ganoderma lucidum GRM117. (Free PPE = 10 mg mL−1, Vmax = 226.61, Km = 80.18 μM; immobilized PEE = 25 mg mL−1, Vmax = 756.86, Km = 53.21 μM).
We observed two points of higher activities in PPE from PLO9 that can be due to isoenzyme activities (Fig. 3). Ollikka et al. (1993) showed application of three LIP isoenzymes in decolourization of recalcitrant dyes. In relation to the PPE from GRM117 only one higher activity was observed between 150 and 300 μM (Fig. 4). These values were different compared to other studies. For LiP from Loweporus lividus MTCC-1178, the Km was 58 mM and 83 mM respectively for veratryl alcohol and hydrogen peroxide substrates (Yadav et al., 2009a, Yadav et al., 2009b). While Trametes versicolor LiP showed Km of 56 μM after immobilization in xerogel (Asgher et al., 2012). In turn, the Vmax values (Figure 3, Figure 4) were higher compared to those found by Yadav et al., 2009a, Yadav et al., 2009b, Asgher et al., 2012.
Immobilizing enzymes onto surfaces can limit their performance due to multiple factors including the distortion of native protein configuration, mass transfer limitations and enzyme orientation (Talbert and Goddard, 2012, Ding et al., 2015). However, the immobilization of enzymatic extract on carbon nanotubes increases the LiP catalytic activity. The increase in activity could be attributed to changes in the collision frequency between the enzyme-immobilized nanoparticles and the substrate. According to the collision theory equations, the apparent Km can be reduced with a decreased size of the carrier, resulting in increased velocity of product formation (Wu et al., 2011). Furthermore, since carbon nanotubes maintain higher radii of curvature due to their smaller diameters, these materials can also promote increased activity of an immobilized enzyme due to decrease of the limiting unfavorable protein-to-protein interactions (Ding et al., 2015).
3.6. Dye decolourization
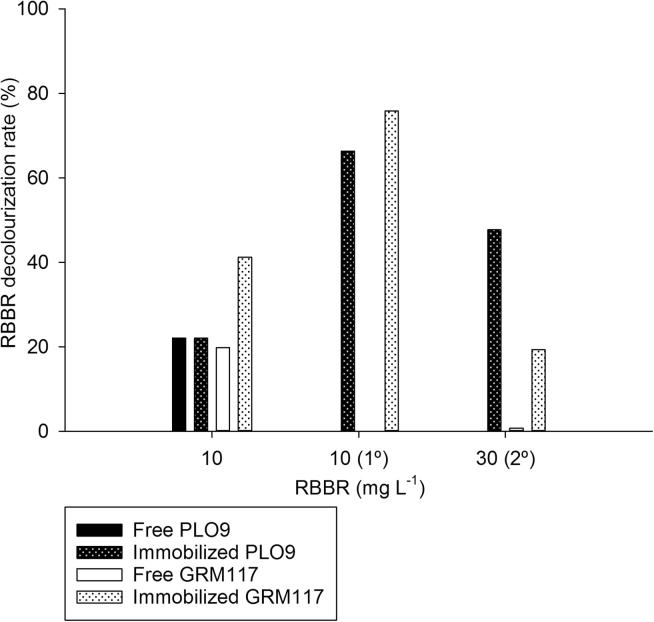
Both PPEs, free and immobilized, were able to decolorize the liquid dye RBBR at the concentrations of 10 mg l−1, the first time that they were used (Fig. 5). The free extracts did not have the ability to decolorize the RBBR at concentrations of 10 mg l−1, in the first reuse round, and 30 mg l−1 in second reuse (Fig. 5). Only the immobilized EEP continued to discoloring the dye on reuses. These reuse rounds should be considered since enzymes were kept active over time and hence could contribute to decreasing the costs of effluent treatment. In addition, the decolourization rate was higher than those observed for free enzymes (Fig. 5).
Figure 5.
RBBR decolourization rate by free and immobilized PPEs from Pleurotus ostreatus PLO9 and Genoderma lucidum GRM117. (1○) = first reuse round. (2○) = second reuse round of the same PPE.
The improvement in catalytic efficiency of LiP reflected the ability of immobilized PPEs in decolorizing the RBBR dye (Fig. 5). The immobilization procedure favored, especially, the enzymatic extract from PLO9, regarding the effects of different pH values and temperatures and the catalytic efficiency (Figure 1, Figure 2, Figure 3, Figure 4). This was evidenced by the decolourization in which the immobilized PPE from PLO9 had a higher percentage of decolourization (over 40%) in the highest concentration analyzed of the dye (Fig. 5). It is well established that fungi show the capacity to decolorize dyes (Zhuo et al., 2011), including the P. ostreatus species, which has already reported that its extracellular peroxidases may decolorize the RBBR dye and other groups structurally different, including azos and polymeric dyes (Kim et al., 1996). Furthermore, the immobilized LiP on different supports also showed a higher capability of decolorizing different dyes (Qiu et al., 2009, Hu et al., 2012).
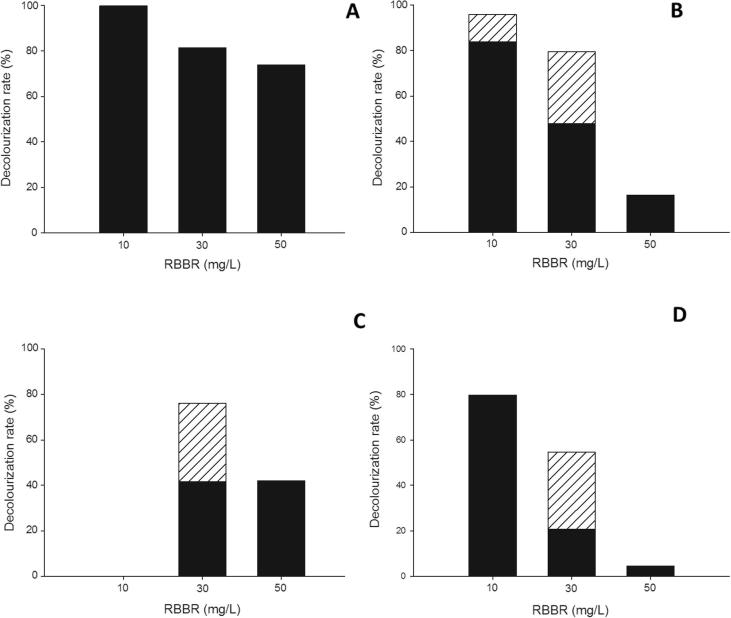
In the second decolourization experiment, just the commercial lignin peroxidase (LiPc) immobilized on carbon nanotubes could be reused (Fig. 6). In the first measurement, after 1 day of incubation, just the carbon nanotubes were responsible by the color removal (Fig.6a), once the carbon nanotubes are able to adsorb dyes after determined time of incubation with the dyes (Kuo et al., 2008). In the first reused round, after 2 days of incubation, LiPc immobilized got started to participate in the decolourization at the concentration of 10 and 30 mg l−1 (Fig.6b). In the second reused round, after 8 days of incubation, the immobilized LiPc at the dye concentration of 30 mg l−1 showed the similar contribution with carbon nanotubes in the color removal process (Fig.6c). In the third reused round, after 14 days of incubation, the immobilized LiPc at the concentration of 30 mg l−1 showed the decolourization percentage higher than carbon nanotubes (Fig.6d), indicating that the dye decolourization was mainly caused by the catalytic reaction of the enzyme. This sample evidences that the carbon nanotubes may be saturated by dyes over time, and it could not more be reused and should be discarded contrary, the enzyme keeps the capacity of dye decolourization, it not being spent in the reaction and besides showed a high stability after 14 days of the run experiment.
Figure 6.
RBBR decolourization rate by commercial lignin peroxidase (LiPc) free and immobilized on carbon nanotubes (hatched columns) and by carbon nanotubes (black columns). (A) First measurement; (B) first reused round; (C) second reused round; (D) third reused round.
The free LiPc at the concentration of 30 mg l−1 showed higher decolourization percentage than the immobilized LiPc (data no shown). This was expected, once this sample of immobilized LiPc retained just 30% of the free LiP activity (data no shown). However, the free LiP cannot be reused. This case proves why it is promising to use the enzyme conjugated with the carbon nanotube than use each component separately, even if the enzyme has a decrease in its activity after the immobilization process.
4. Conclusion
The fungal isolates and the fermentation conditions studied showed great potential for the ligninolytic enzyme production, especially LiP. Furthermore, the immobilization process of the partially purified enzymatic extracts on carbon nanotubes promoted an increase of LiP catalytic efficiency. Considering the environmental applications as the final goal, the enzymatic extract also can be used without the need for complete purification.
The immobilized enzymatic extract on carbon nanotubes also showed a great potential for the dye decolourization, with a higher catalytic efficiency, stability and capability to be reused in the dye decolourization process. Therefore, these preliminary results may contribute for developing a final biocatalyst that can be applied in diverse environmental applications in the future, especially in the treatment of textile effluents.
Acknowledgments
The authors are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do estado de Minas Gerais (FAPEMIG) for financial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alam M.Z., Mansor M.F., Jalal K.C.A. Optimization of lignin peroxidase production and stability by Phanerochaete chrysosporium using sewage treatment-plant sludge as substrate in a stirred-tank bioreactor. J. Ind. Microbiol. Biotechnol. 2009;36:757–764. doi: 10.1007/s10295-009-0548-5. [DOI] [PubMed] [Google Scholar]
- Asgher M., Asad M.J., Bhatti H.N., Legge R.L. Hyperactivation and thermostablization of Phanerochaete chrysosporium lignin peroxidase by immobilization in xerogels. World J. Microbiol. Biotechnol. 2007;23:525–531. [Google Scholar]
- Asgher M., Iqbal H.M.N., Irshad M. Characterization of purified and Xerogel immobilized novel lignin peroxidase produced from Trametes versicolor IBL-04 using solid state medium of Corncobs. BMC Biotechnol. 2012;12:46. doi: 10.1186/1472-6750-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashger M., Shahid M., Kamal S., Iqbal H.M.N. Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial biotechnology. J. Mol. Catal. B: Enzym. 2014;101:56–66. [Google Scholar]
- Asther M., Lesage L., Drapon R., Corrieu G., Odier E. Phospholipid and fatty acid enrichment of Phanerochaete chrysosporium INA-12 in relation to ligninase production. Appl. Environ. Microbiol. 1988;27:393–398. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chequer F.M.D., Lizier T.M., de Felício R., Zanon M.V., Debonsi H.M., Lopes N.P., Marcos R., de Oliveira D.P. Analyses of the genotoxic and mutagenic potential of the products formed after the biotransformation of the azo dye Disperse Red 1. Toxicol. in Vitro. 2011;25:2054–2063. doi: 10.1016/j.tiv.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Da Luz J.M.R., Nunes M.D., Paes S.A., Torres D.P., Kasuya M.C.M. Biodetoxification of Jatropha curcas seed cake by Pleurotus ostreatus. Afr. J. Microbiol. Res. 2014;1148 1156-1156. [Google Scholar]
- Ding S., Cargill A.A., Medintz I.L., Claussen J.C. Increasing the activity of immobilized enzymes with nanoparticle conjugation. Curr. Opin. Biotechnol. 2015;34:242–250. doi: 10.1016/j.copbio.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Gardossi L., Poulsen P.B., Ballesteros A., Hult K., Švedas V.K., Vasić-Rački D., Carrea G., Magnusson A., Schmid A., Wohlgemuth R., Halling P.J. Guidelines for reporting of biocatalytic reactions. Trends Biotechnol. 2010;28:171–180. doi: 10.1016/j.tibtech.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Feng W., Ji P. Enzymes immobilized on carbon nanotubes. Biotechnol. Adv. 2011;29:889–895. doi: 10.1016/j.biotechadv.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Hu Z., Xu L., Wen X. Mesoporous silicas synthesis and application for lignin peroxidase immobilization by covalent binding method. J. Environ. Sci. 2012;25:181–187. doi: 10.1016/s1001-0742(12)60008-4. [DOI] [PubMed] [Google Scholar]
- Illanes A., Cauerhffb A., Wilsona L., Castro G.R. Recent trends in biocatalysis engineering. Bioresour. Technol. 2012;115:48–57. doi: 10.1016/j.biortech.2011.12.050. [DOI] [PubMed] [Google Scholar]
- Iqbal H.M.N., Asgher M., Bhatti H.N. Optimization of physical and nutritional factors for synthesis of lignin degrading enzymes by a novel strain of Trametes versicolor. Bioresources. 2011;6:1273–1278. [Google Scholar]
- Kim B., Ryu S., Shin K. Effect of culture parameters on the decolorization of remazol brilliant blue R by Pleurotus ostreatus. J. Microbiol. 1996;34:101–104. [Google Scholar]
- Kuo C., Wu C., Wu J. Adsorption of direct dyes from aqueous solutions by carbon nanotubes: Determination of equilibrium, kinetics and thermodynamics parameters. J. Colloid Interface Sci. 2008;328:308–315. doi: 10.1016/j.jcis.2008.08.038. [DOI] [PubMed] [Google Scholar]
- Liese A., Hilterhaus L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013;42:6236–6249. doi: 10.1039/c3cs35511j. [DOI] [PubMed] [Google Scholar]
- Marchesan S., Prato M. Under the lens: carbon nanotube and protein interaction at the nanoscale. Chem. Commun. (Camb.) 2015;51:4347–4359. doi: 10.1039/c4cc09173f. [DOI] [PubMed] [Google Scholar]
- Oliveira S.F., Bisker G., Bakh N.A., Gibbs S.L., Landry M.P., Strano M.S. Protein functionalized carbon nanomaterials for biomedical applications. Carbon. 2015;95:767–779. [Google Scholar]
- Ollikka P., Alhonmaki K., Leppanen V.-M., Glumoff T., Raijola T., Suominen I. Decolorization of azo, triphenyl methane, heterocyclic, and polymeric dyes by Lignin peroxidase isoenzymes from Phanerochaete chrysosporium. Appl. Environ. Microb. 1993;59:4010–4016. doi: 10.1128/aem.59.12.4010-4016.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadoulou K., Kalagona I.M., Philippoussis A., Rigas F. Optimization of fungal decolorization of azo and anthraquinone dyes via Box-Behnken design. Int. Biodeterior. Biodegrad. 2013;77:31–38. [Google Scholar]
- Qiu H., Li Y., Ji G., Zhou G., Huang X., Qu Y., Gao P. Immobilization of lignin peroxidase on nanoporous gold: enzymatic properties and in situ release of H2O2 by co-immobilized glucose oxidase. Bioresour. Technol. 2009;100:3837–3842. doi: 10.1016/j.biortech.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Rao M.A., Scelza R., Acevedo F., Diez M.C., Gianfreda L. Enzymes as useful tools for environmental purposes. Chemosphere. 2014;107:145–162. doi: 10.1016/j.chemosphere.2013.12.059. [DOI] [PubMed] [Google Scholar]
- Rauf M.A., Ashraf S.S. Survey of recent trends in biochemically assisted degradation of dyes. Chem. Eng. J. 2012;209:520–530. [Google Scholar]
- Saifuddin N., Raziah A.Z., Junizah A.R. Carbon nanotubes: a review on structure and their interaction with proteins. J. Chem. 2013;2013:1–18. ID 676815. [Google Scholar]
- Talbert J.N., Goddard J.M. Enzymes on material surfaces. Colloids Surf. B Biointerfaces. 2012;93:8–19. doi: 10.1016/j.colsurfb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T.K. Lignin-degrading enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983;221:661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Wong D.W.S. Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 2009;157:174–209. doi: 10.1007/s12010-008-8279-z. [DOI] [PubMed] [Google Scholar]
- Wu C., Lee C., Wu C., Yang Y., Ko F. Size-modulated catalytic activity of enzyme-nanoparticle conjugates: a combined kinetic and theoretical study. Chem. Commun. 2011;47:7446–7448. doi: 10.1039/c1cc11020a. [DOI] [PubMed] [Google Scholar]
- Xu R., Tang R., Zhou Q., Li F., Zhang B. Enhancement of catalytic activity of immobilized laccase for diclofenac biodegradation by carbon nanotubes. Chem. Eng. J. 2015;262:88–95. [Google Scholar]
- Yadav M., Yadav P., Yadav K.D.S. Purification and characterization of lignin peroxidase from Loweporus lividus MTCC-1178. Eng. Life Sci. 2009;9:124–129. [Google Scholar]
- Yadav M., Singh S.K., Yadav K.D.S. Purification and characterisation of lignin peroxidase from Pleurotus sajor-caju MTCC-141. J. Wood Chem. Technol. 2009;29:1–15. [Google Scholar]
- Zhang P., Henthorn D.B. Synthesis of PEGylated single wall carbon nanotubes by a photoinitiated graft from polymerization. AIChE J. 2010;56:1610–1615. [Google Scholar]
- Zhuo R., Ma L., Fan F., Gong Y., Wan X., Jiang M., Zhang X., Yang Y. Decolorization of different dyes by a newly isolated white-rot fungi strain Ganoderma sp.En3 and cloning and functional analysis of its laccase gene. J. Hazard. Mater. 2011;192:855–873. doi: 10.1016/j.jhazmat.2011.05.106. [DOI] [PubMed] [Google Scholar]