Abstract
Violaxanthin de-epoxidase (VDE) is a lumen-localized enzyme that catalyzes the de-epoxidation of violaxanthin in the thylakoid membrane upon formation of a transthylakoid pH gradient. We investigated the developmental expression of VDE in leaves of mature tobacco (Nicotiana tabacum) plants grown under high-light conditions (in the field) and low-light conditions (in a growth chamber). The difference in light conditions was evident by the increased pool size (violaxanthin + antheraxanthin + zeaxanthin, VAZ) throughout leaf development in field-grown plants. VDE activity based on chlorophyll or leaf area was low in the youngest leaves, with the levels increasing with increasing leaf age in both high- and low-light-grown plants. However, in high-light-grown plants, the younger leaves in early leaf expansion showed a more rapid increase in VDE activity and maintained higher levels of VDE transcript in more leaves, indicating that high light may induce greater levels of VDE. VDE transcript levels decreased substantially in leaves of mid-leaf expansion, while the levels of enzyme continued to increase, suggesting that the VDE enzyme does not turn over rapidly. The level of VDE changed in an inverse, nonlinear relationship with respect to the VAZ pool, suggesting that enzyme levels could be indirectly regulated by the VAZ pool.
In the natural environment, the light intensities that plants receive vary over a wide, dynamic range. When the radiant energy exceeds the capacity of the photosynthetic system, this can result in overexcitation and photooxidative damage to the photosynthetic reaction center. A protective mechanism that plants use to deal with excessive radiant energy involves the interconversions between the carotenoids violaxanthin, antheraxanthin, and zeaxanthin (VAZ), otherwise known as the violaxanthin or xanthophyll cycle (Yamamoto et al., 1962). The cycle is catalyzed by two enzymes localized on opposite sides of the thylakoid membrane. Violaxanthin de-epoxidase (VDE) is localized in the lumen of thylakoids and, in the presence of ascorbate and an acidic lumen generated by the proton pump, catalyzes the de-epoxidation half of the cycle (Hager, 1969; Yamamoto et al., 1972). Zeaxanthin epoxidase, which is localized on the stromal side of the thylakoid membrane, catalyzes the regeneration of violaxanthin. Epoxidation proceeds in the dark or under low light and is optimal near pH 7.5 (Hager, 1975; Siefermann and Yamamoto, 1975). The role of zeaxanthin in protecting the photosynthetic apparatus against the adverse effects of excessive light was first proposed by Demmig et al. (1987). Further evidence demonstrates that both antheraxanthin and zeaxanthin, along with the transthylakoid pH gradient, mediate the non-radiative dissipation of excess energy as heat (Gilmore and Yamamoto, 1992, 1993; Gilmore et al., 1995, 1996).
VDE was purified from romaine lettuce to one major polypeptide using conventional purification techniques and a novel lipid-affinity precipitation step using monogalactosyldiacylglycerol (Rockholm and Yamamoto, 1993, 1996). VDE has a molecular mass of 43 kD and a pI of 5.4, as determined by two-dimensional IEF/SDS-PAGE. VDE was also purified from spinach and identified as a 43-kD protein (Arvidsson et al., 1996). The N-terminal region of the spinach VDE protein was sequenced and found to be 90% identical to the lettuce VDE N terminus (Arvidsson et al., 1996; Bugos and Yamamoto, 1996).
The lettuce VDE cDNA was cloned, and verification of the cDNA was accomplished by expression of an active protein in Escherichia coli (Bugos and Yamamoto, 1996). The expressed enzyme also showed the characteristic inhibition by DTT (Yamamoto and Kamite, 1972). The lettuce VDE cDNA encodes a polypeptide of 473 amino acids, with a calculated molecular mass of 54.4 kD (Bugos and Yamamoto, 1996). This preprotein contains a 125-amino acid bipartite transit peptide for transport into the chloroplast and thylakoid lumen. VDE cDNAs from tobacco (Nicotiana tabacum) and Arabidopsis were also isolated using the lettuce VDE cDNA, and their deduced amino acid sequences are highly homologous; only nine amino acid positions in the mature proteins have different amino acids in the three proteins (Bugos et al., 1998). All three proteins also have 13 invariant Cys residues, 11 of which are localized in a Cys-rich domain (Bugos and Yamamoto, 1996) in which DTT most likely has its effect on the reduction of disulfide bridges. Niyogi et al. (1998) found that a single base-pair substitution that changed the last Cys in the Cys-rich domain to a Tyr abolished all VDE activity in Arabidopsis.
The isolation of the cDNA encoding VDE has provided the opportunity to study the regulation of the gene. In this report we analyzed the levels of VDE activity, protein, and mRNA in leaves of mature tobacco plants to observe changes during leaf development. We analyzed plants receiving nonsaturating light conditions (in a growth chamber) and saturating light conditions (in the field). In addition, pigment levels were quantified to determine if there was any correlation between the VAZ pool size and the levels of VDE. Our results provide a benchmark for comparison with transgenic tobacco plants having altered levels of VDE.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Tobacco (Nicotiana tabacum cv Xanthi) plants were grown in plastic pots with potting mix (Supersoil, Rod McLellan, San Francisco, CA). The plants were watered daily and fertilized periodically with a commercial liquid fertilizer and a pellet-type fertilizer in which the fertilizer ingredients are coupled to an organic base.
The plants were grown under two different environments. One set of plants was grown in a growth chamber under light provided by fluorescent and incandescent lamps. The top of the plant received a photon flux density (PFD) of about 300 μmol m−2 s−1 for a 16-h light/8-h dark photoperiod, with day/night temperatures of 27°C/22°C. The other set of plants was grown outdoors during the spring on the university campus under full sunlight in a non-shaded area. The plants received a maximum PFD of 2,400 μmol m−2 s−1 with an approximately 13-h/11-h light/dark photoperiod. The air temperature during the day reached as high as 29°C, while the night temperature reached as low as 20°C.
All plants were grown for 6 to 8 weeks and harvested just prior to appearance of flower buds. Plants were dark adapted for 7 h prior to analysis. The plants typically had between 26 and 28 leaves. Leaves were numbered starting from the top of the plant, and leaf no. 5 was defined as the first leaf to have a midrib length of 9.5 cm. In most cases, there were four younger leaves preceding leaf no. 5.
Pigment Analysis
Discs (13 mm in diameter) cored from tobacco leaves near the tip were ground in a tissue homogenizer with acetone and 10 to 15 mg of CaCO3. Pigments and chlorophylls in 90% (v/v) acetone extracts were separated and quantitated by HPLC using a Spherisorb ODS-1 column (Alltech) and solvents A-1 and B, as described previously (Gilmore and Yamamoto, 1991).
Thylakoid Isolation and Extraction of VDE
Chloroplasts were isolated from tobacco leaf tissue (1–4 g) essentially as described by Neubauer and Yamamoto (1992). For leaf nos. 5 through 8, leaves were pooled from three plants to obtain sufficient tissue for chloroplast isolation. The leaves were sliced into small pieces and macerated three times for 5 s in a blender at low speed in 75 mL of ice-cold grinding buffer (300 mm sorbitol, 1 mm MgCl2, 0.5 mm KH2PO4, 30 mm KCl, and 50 mm MES, adjusted to pH 6.1 with KOH) that was supplemented with 5 mm ascorbate just prior to use. The homogenate was filtered through a 36-μm-mesh nylon cloth and the filtrate centrifuged at 5,500g for 1 min at 4°C. The chloroplast pellet was resuspended in approximately 30 mL of 20 mm HEPES, pH 7.5, 6 mm MgCl2 by vortexing and the resuspension was incubated on ice for 10 min for chloroplast lysis. Following centrifugation at 20,200g for 5 min at 4°C, the pellet containing thylakoids was resuspended in a small volume (0.3–1.0 mL) of 20 mm HEPES, pH 7.5, and 6 mm MgCl2. Thylakoids were lysed with five freeze-thaw cycles using liquid N2 as described previously (Hager and Holocher, 1994). The suspension was centrifuged at 12,000g for 10 min at 4°C, and the supernatant was assayed for VDE activity.
VDE Assay
VDE activity in 5 to 40 μL of extract was tested using the in vitro assay at pH 5.1 and the change in A502−540 as described previously (Yamamoto, 1985). Fatty acid-free BSA (Sigma) was added to the assay at a final concentration of 0.1%. Monogalactosyldiacylglycerol from spinach was obtained from a commercial source (Lipid Products, South Nutfield, UK) and dissolved in methanol to a final concentration of 270 μm. Violaxanthin was isolated from a saponified methanol extract from spinach leaves by TLC. Pigments from the saponified methanol extract were washed into diethyl ether, dried using a rotary evaporator, and dissolved in a small volume of acetone. An aliquot of this acetone extract was applied to a silica-gel plate (Fisher Scientific) and chromatographed using a solvent system of 30% acetone in hexane. The second major band from the bottom of the plate, violaxanthin, was scraped from the plate and eluted with methanol. Purity was assessed by HPLC (Gilmore and Yamamoto, 1991) and spectrophotometric analysis (Yamamoto, 1985). A 10 μm violaxanthin solution in methanol had a A441 of 1.5.
One unit of VDE activity is defined as 1 nmol of violaxanthin de-epoxidized per minute. Activity was calculated from the initial rate of absorbance change using the difference extinction coefficient of 63 mm−1 cm−1 (Yamamoto, 1985).
Protein Determination
Protein was determined using a protein assay reagent (Bio-Rad) and bovine γ-globulin as the standard.
Western-Blot Analysis
Protein (20 μg/lane) extracted from thylakoids by freeze-thaw was resolved on a 6% SDS-polyacrylamide gel as described by Laemmli (1970) and electrophoretically transferred to a PVDF membrane (Millipore). The blots were blocked for 1 h with 5% (w/v) nonfat dry milk in TBST (10 mm Tris-Cl, pH 8.0, 150 mm NaCl, and 0.05% [w/v] Tween 20), and probed for 1 h with a rabbit polyclonal antibody (0.5 μg/mL of blocking solution) prepared against a synthetic peptide from the N terminus of lettuce VDE (VDALKTCACLLK) (Bugos and Yamamoto, 1996; Rockholm and Yamamoto, 1996). Blots were developed by immunodetection using alkaline phosphatase-conjugated secondary antibodies. The relative levels of the VDE immunoreactive bands were determined using image-analysis software (SigmaScan, SPSS, Chicago).
Northern Hybridization Analysis
Total RNA was isolated from 500 mg of leaf tissue using a RNA extraction reagent (TRI reagent, Sigma). The RNA samples had A260/280 ratios of 2.00 ± 0.08. RNA (15 μg/lane) was resolved by electrophoresis in a 0.66 m formaldehyde/1% (w/v) agarose gel (Fourney et al., 1988), transferred by downward capillary blotting to a nylon membrane (Schleicher & Schuell), and fixed by UV cross-linking (Stratagene). The full-length tobacco VDE cDNA (Bugos et al., 1998) was used to synthesize 32P-labeled DNA probes by the random-primed DNA-labeling method (Feinberg and Vogelstein, 1983). Blots were hybridized with the probes at 65°C as described by Church and Gilbert (1984) and washed at the same temperature. Hybridization was detected by autoradiography (Biomax MS film, Kodak). The relative levels of the hybridizing bands were determined using image-analysis software (SigmaScan).
RESULTS AND DISCUSSION
Tobacco plants were grown under two different environments, in the field and in the growth chamber, to analyze the levels of pigments, chlorophyll, VDE protein, and mRNA in leaves at various stages of development. The main difference between the two growth environments was that the growth-chamber-grown plants were exposed to nonsaturating light conditions, whereas the field-grown plants were exposed to saturating light conditions for a large portion of the day. PFD in the growth chamber was adjusted to approximately 300 μmol m−2 s−1 at the top of the plant, with the bottom leaves receiving significantly less light due to the distance from the light source and shading. The field-grown plants were exposed to variable light conditions produced by sunlight, in which the PFD reached a maximum of 2,400 μmol m−2 s−1 around midday.
Although not grown under the same environmental conditions, growth conditions were similar in that both sets of plants received the same fertilizer treatment, and the growth chamber was adjusted so that the temperature and photoperiod were similar to conditions in the field (see Methods). Therefore, the leaves of the plants were analyzed not to only examine the changes that occurred in leaf development, but also to observe if there were differences in the levels of pigments and VDE when plants were grown under saturating and nonsaturating light conditions.
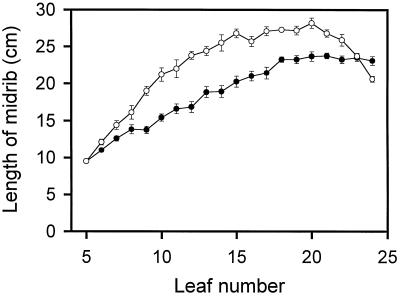
Leaf development in tobacco was followed by measuring the length of the midrib from the youngest leaf (leaf 5) to the oldest leaf (leaf 24) examined in mature tobacco plants (Fig. 1). Even though leaf expansion was more rapid in field-grown plants, leaves from both sets of plants reached a maximum midrib length at leaf no. 20. In contrast, the older leaves (leaves 21–24) of the field-grown plants showed a sequential reduction in the midrib length compared with growth-chamber-grown plants, in which the midrib length was essentially unchanged. This trend in expansion of the leaf midrib is very similar to that reported for leaf development (as measured by leaf area) in 3-month-old greenhouse-grown tobacco plants (Schindler et al., 1994).
Figure 1.
Leaf development in wild-type tobacco as measured by leaf midrib length. Plants were grown under nonsaturating light conditions in the growth chamber (•) or under saturating light conditions in the field (○). Leaf no. 5 was defined as the first leaf at the top of the plant to have a midrib length of 9.5 cm. Error bars represent se values (n = 6 individual plants). For points lacking error bars, the se was smaller than the symbol size.
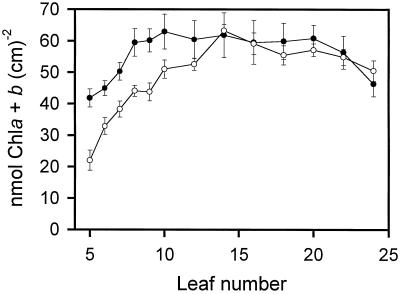
Chlorophyll content in tobacco leaves varied depending on the stage of leaf development. The major difference between the field- and growth-chamber-grown plants was the reduced levels of chlorophyll in the youngest leaves of the field-grown plants (Fig. 2). For example, the youngest leaf (leaf no. 5) from the field-grown plants had almost half the chlorophyll content of the growth-chamber-grown plants. Chlorophyll content in the younger leaves of field-grown plants increased rapidly and reached a peak at leaf no. 14, after which there was a slow decline. Chlorophyll in the younger leaves of the growth-chamber-grown plants also increased rapidly with chlorophyll content, reaching a maximum earlier (at leaf no. 10). The chlorophyll content remained essentially constant from leaf no. 10 to leaf no. 20, after which the chlorophyll content decreased. Figure 2 shows that the chlorophyll levels in both sets of plants were similar from leaf no. 14 to leaf no. 24. The loss in chlorophyll in the oldest leaves most likely indicates that these leaves were starting to undergo senescence. The chlorophyll a/b ratios were similar for both sets of plants, with the ratios continuously declining: from 3.67 in leaf no. 5 to 3.09 in leaf no. 24 of the growth-chamber-grown plants, and from 3.96 in leaf no. 5 to 3.05 in leaf no. 24 of the field-grown plants. A similar trend in chlorophyll content per leaf area was reported previously in leaves from 3-month-old tobacco plants grown in a greenhouse (Schindler et al., 1994).
Figure 2.
Development of total chlorophyll (Chl) content in wild-type tobacco plants grown under nonsaturating light conditions in the growth chamber (•) or under saturating light conditions in the field (○). Error bars represent se values (n = 5 individual plants).
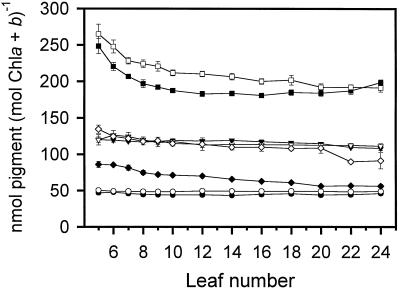
Pigments in tobacco leaves were quantitated by HPLC to observe how the levels change during leaf development. Leaf discs from dark-adapted plants were cored near the leaf tip, since this part of the leaf would likely have the most exposure to light. Neoxanthin and β-carotene were found in nearly identical levels in field- and growth-chamber-grown plants when based on the total chlorophyll content (Fig. 3). The levels of these pigments remained essentially consistent throughout leaf development, suggesting that there was a very tight regulation of the levels of these two pigments with respect to total chlorophyll. The level of lutein, however, was higher in field-grown than in growth-chamber-grown plants throughout most of leaf development. In both sets of plants, the level was somewhat greater in the youngest leaves up to leaf no. 10, after which the level stayed fairly constant.
Figure 3.
Pigment levels and VAZ pool of wild-type tobacco leaves based on total chlorophyll (Chl) determined by HPLC. Error bars represent se values (n = 5 individual plants). For points lacking error bars, the se was smaller than the symbol size. •, Neoxanthin, growth chamber; ○, neoxanthin, field; ▾, β-carotene, growth chamber; ▿, β-carotene, field; ▪, lutein, growth chamber; □, lutein, field; ♦, VAZ, growth chamber; ⋄, VAZ, field.
Demmig-Adams et al. (1996) reported that the levels of lutein and β-carotene in Smilax australis in sun and shade leaves on a per-unit chlorophyll basis were increased by 40% to 60% in the sun leaves, whereas neoxanthin levels remained unchanged. The shade and sun leaves received a peak PFD of 30 and 1,500 μmol m−2 s−1 at midday, respectively. In another study analyzing sun and shade leaves from various plant species, the levels of β-carotene were higher, the levels of neoxanthin remained unchanged, and there was no consistent general difference in lutein content when these pigments were expressed on a per-chlorophyll basis (Demmig-Adams and Adams, 1992). Even though, except for the unchanged β-carotene levels, our data are in agreement with these earlier analyses, the similarity must be viewed cautiously. Although there was nearly an 8-fold difference in the high- and low-light growth conditions, a PFD of 300 μmol m−2 s−1 cannot be considered shade conditions. Nevertheless, the trends between high- and low-light conditions paralleled those of light and shade plants, respectively.
Unlike the other pigments, the VAZ pool differed significantly between the two sets of plants (Fig. 3). The levels of VAZ on a chlorophyll basis remained higher throughout development in the field-grown plants. VAZ levels (nmol mol−1 chlorophyll a + b) in the field-grown plants ranged from 134.49 ± 5.76 in leaf no. 5 to 91.31 ± 11.11 in leaf no. 24, whereas the levels ranged from 85.88 ± 4.17 in leaf no. 5 to 56.12 ± 2.47 in leaf no. 24 of the growth-chamber-grown plants. It is well documented that the size of the xanthophyll cycle pool on a per-unit chlorophyll basis is lower in shade-adapted plants than in sun-adapted plants (Thayer and Björkman, 1990; Demmig-Adams and Adams, 1992; Demmig-Adams et al., 1995, 1996). In our study, both types of plants also showed a gradient in the VAZ pool that decreased from the top to bottom leaves. This effect may have been due to leaf aging, but an effect of shading is also possible. The VAZ pool had a sudden drop in leaf nos. 22 and 24 of the field-grown plants (Fig. 3). These leaves on average were shorter than the leaves directly above (Fig. 1), so the level of shading of the leaf tip was even greater.
The gradual vertical decrease in the VAZ pool was a direct consequence of samples being collected from the same area of the leaf. This sampling method cannot consider the variation in the VAZ pool that can occur within a leaf. For example, when the VAZ pool size was analyzed in two leaves of one of the field-grown plants, it differed greatly. In leaf no. 18, a canopy-shaded leaf, the pool was 105.13 nmol VAZ mol−1 chlorophyll a + b at the leaf tip and 55.06 nmol VAZ mol−1 chlorophyll a + b near the leaf petiole. This indicates that, in this particular leaf, the VAZ pool size can differ across the leaf by almost 50%. In contrast, leaf no. 8 from the same plant, a virtually non-shaded leaf, had a pool size of 118.62 nmol VAZ mol−1 chlorophyll a + b at the leaf tip and 122.26 nmol VAZ mol−1 chlorophyll a + b near the leaf petiole.
Prior to performing the analysis of VDE activity in leaves at various stages of leaf development, we observed that some component in the freeze-thaw extracts was inhibitory to VDE activity. When VDE activity was assayed in an extract from freeze-thawed intact chloroplasts (including both stromal and lumen components) from leaf no. 8 of a growth-chamber-grown plant, the specific activity of VDE decreased about 360% as the amount of extract was increased from 10 to 40 μL in the assay. The results were much less dramatic when VDE was extracted from thylakoids using the freeze-thaw method (stromal components were washed away prior to freeze-thaw). A moderate inhibition of VDE activity occurred only when more than 40 μL of extract was used in the assay. This suggests that the inhibitory component was mainly from the stroma.
All assays in this study used either 5 or 10 μL of extract from freeze-thawed thylakoids, except for leaf nos. 5 and 6, which used 40 μL of extract. In an earlier report, zeaxanthin epoxidase activity in chloroplasts from lettuce was stimulated by the addition of BSA to the reaction assay (Siefermann and Yamamoto, 1975). This was attributed to BSA presumably binding fatty acids that were inhibitory to the zeaxanthin epoxidase. To test the effect on the in vitro VDE assay, fatty acid-free BSA was added to the assay at concentrations ranging from 0.05% to 0.20%. The addition of 0.1% (w/v) BSA produced the optimal result, enhancing VDE activity by approximately 45% in two separate experiments using enzyme extracted from leaf no. 8 of a growth-chamber-grown plant. Therefore, 0.1% BSA was added to all VDE assays in this study.
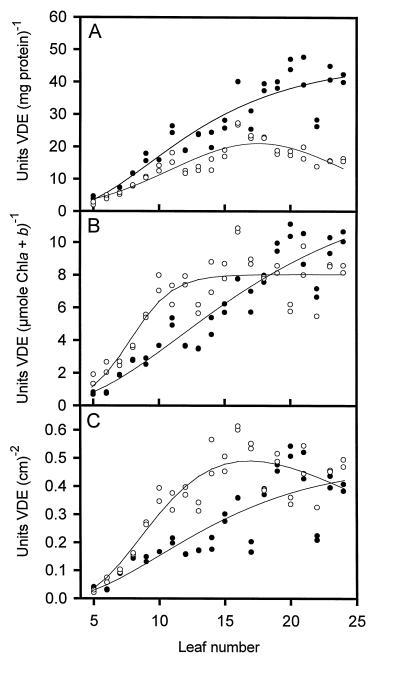
VDE activity was measured throughout leaf development in one field-grown and one growth-chamber-grown plant. When activity was based on total protein extracted from thylakoids by the freeze-thaw method, the specific activity in both plants was very low in the youngest leaves (Fig. 4A). The activity in the growth-chamber-grown plant continued to increase, whereas activity in the field-grown plant reached a maximum at leaf no. 16 and then gradually decreased in the older leaves. The data show that there was a large divergence in VDE activity in leaf nos. 16 through 24. However, the data are somewhat misleading because of the difference in the amount of protein extracted by the freeze-thaw method. In the growth-chamber-grown plant, the extractable protein per gram of fresh weight tissue reached a maximum at leaf no. 15 and then gradually decreased (data not shown). In the field-grown plant, the amount of extractable protein continued to increase to leaf no. 24. In fact, the levels of extractable protein were between 77% and 83% greater in leaf nos. 22 through 24 in the field-grown plant. If the VDE levels were normalized for the amount of protein extracted, they would be similar for the two plants. The levels of VDE protein in the freeze-thaw extracts were also analyzed by western blotting using a polyclonal antibody prepared against a synthetic peptide from the N terminus of lettuce VDE (Bugos and Yamamoto, 1996; Rockholm and Yamamoto, 1996). VDE activity (in units per milligram of protein) correlated very well with the relative levels of VDE protein as determined from western blots for both plants (data not shown).
Figure 4.
Violaxanthin de-epoxidase activity in wild-type tobacco leaves based on total protein (A), total chlorophyll (Chl) (B), and leaf area (C). Enzyme assays were performed in duplicate from enzyme extracted from a single tobacco plant, except for leaf nos. 5 through 8, which were a pooled sample from three tobacco plants. Regression curves were calculated using scientific graphing software (SigmaPlot, SPSS) ○, Field-grown plants; •, growth-chamber-grown plants.
When the specific activity of VDE was expressed on a total chlorophyll or leaf area basis, a different trend was observed. Again, in both plants VDE activities were very low in the youngest leaves (Fig. 4, B and C). However, there was a more rapid increase in the level of VDE in the field-grown plant until leaf no. 18, after which the activities were similar between the two plants. The specific activity in the growth-chamber-grown plant shows that the VDE levels increased in a linear manner. The rapid increase of VDE in the field-grown plant suggests that the increased intensity of light has an effect on inducing higher levels of VDE.
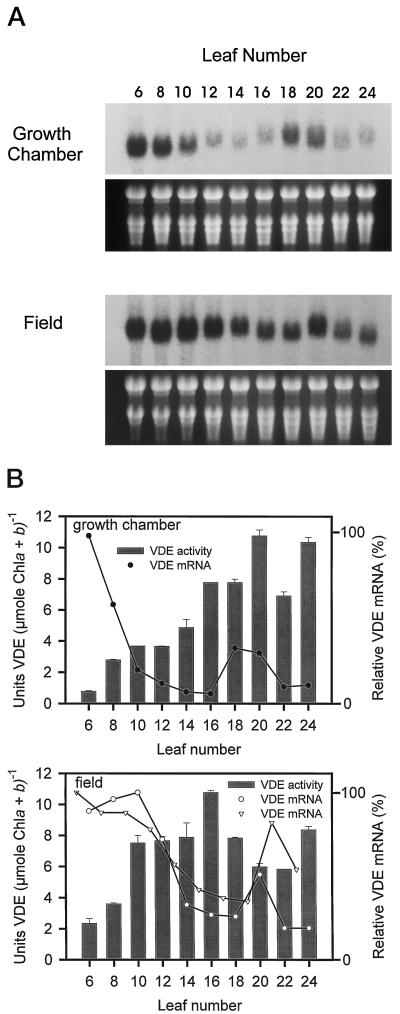
The expression patterns of VDE transcripts were determined by northern-blot analysis from the same leaf tissue used for determining VDE activity. The RNA blots of both plants indicated a biphasic pattern of expression, with maximal expression in the youngest leaves followed by a substantial decrease in VDE transcript level and a second peak of VDE transcript at leaf no. 18 or leaf no. 20 (Fig. 5A). The second peak in the biphasic pattern of expression was not as strong as the VDE transcript levels in the youngest leaves. The 1.7-kb hybridizing band shown in Figure 5A was the only signal detected on the northern blot following autoradiography. Overall, the VDE transcript levels were higher in leaves of the field-grown plant (the blots were hybridized with the same probe and exposed to the same piece of film), with greater levels of VDE transcript expressed for a longer duration in the youngest leaves of the field-grown plant (leaf nos. 6–12) compared with the growth-chamber-grown plant (leaf nos. 6 and 8) (Fig. 5).
Figure 5.
Developmental pattern of VDE transcript and activity in wild-type tobacco. A, RNA blots of total RNA (15 μg/lane) isolated from leaves of a growth-chamber-grown and a field-grown plant. RNA samples are from same leaf tissue used for determining VDE specific activity. The blots were probed with the full-length tobacco VDE cDNA. The hybridizing signals are approximately 1.7 kb. Ethidium bromide staining of rRNA is shown below each blot. B, VDE specific activity based on total chlorophyll (Chl) as reported in Figure 4B. The error bars represent se values (n = two assays). The relative VDE mRNA levels were determined from the RNA blots. The plot labeled with the open inverted triangles represents the relative VDE mRNA levels determined from another field-grown plant in which the odd-numbered leaves were analyzed to show the reproducibility of the RNA-blot analysis. The blots were digitized and the signals quantified according to area and average intensity using image analysis software. A value of 100% on the ordinate represents the maximum steady-state mRNA detected within the experiment.
The increased duration of greater VDE transcript level in the youngest leaves of the field-grown plant correlates with the rapidly increased levels of VDE specific activity based on chlorophyll or leaf area measured in the same leaves (Figs. 4, B and C, and 5B). This suggests that the increased light intensity in the field-grown plant may induce increased levels of VDE transcript and protein. However, the relative level of VDE transcripts seem to peak earlier than the levels of VDE specific activity. The reason for this pattern of expression is not entirely understood. Since the VDE activity levels remain high in the middle to later stages of leaf development, when transcript levels are very low, it appears that the VDE protein either does not turn over rapidly or does not turn over at a single rate during development.
It was recently demonstrated that when spinach plants were shifted from low light (100–250 μmol m−2 s−1) to high light (950 μmol m−2 s−1), VDE activity decreased 15% to 30% on the day following the shift to high light (Eskling and Åkerlund, 1998). The reduced activity was due to a decrease in the level of VDE protein as determined by western-blot analysis. The level of VDE protein was 15% to 30% less in spinach plants after exposure to 5, 6, and 7 d of high light. In contrast, the VAZ pool size based on total chlorophyll doubled on the d 3 of exposure to high light. This may suggest that the level of VDE changes in an inverse relationship with respect to the VAZ pool.
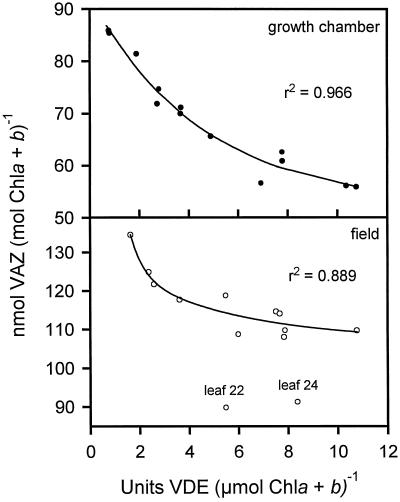
Our results also seem to suggest that the younger tobacco leaves had a larger VAZ pool and lower VDE levels, whereas the older leaves had a lower VAZ pool and higher VDE levels. In fact, plotting the VDE activity based on total chlorophyll (data from Fig. 4B) for the various leaves versus the VAZ pool based on total chlorophyll (data from Fig. 3) shows an inverse nonlinear relationship for both the growth-chamber-grown and field-grown plants (Fig. 6). All of the data fit the inverse trend well except two points representing the values for leaf nos. 22 and 24 of the field-grown plant. These data were not included in the nonlinear regression analysis because the sudden drop in the VAZ pool in these particular leaves caused these points to digress from the trend (Fig. 3). Further research is needed to focus more clearly on whether the VDE promoter is regulated by light and if VDE levels are regulated indirectly by the VAZ pool.
Figure 6.
Relationship between VDE specific activity based on total chlorophyll (Chl) (data from Fig. 4B) versus the VAZ pool based on total chlorophyll (data from Fig. 3) in leaves of both field-grown and growth-chamber-grown plants. Regression curves were calculated using scientific graphing software (SigmaPlot).
ACKNOWLEDGMENTS
We thank Tarana J. Mohamed for technical assistance and Amy S. Verhoeven for reviewing the manuscript.
Footnotes
This work was supported by the U.S. Department of Energy, Division of Energy Biosciences (grant no. DE–FG03–92ER20078).
LITERATURE CITED
- Arvidsson P-O, Bratt CE, Carlsson M, Åkerlund H-E. Purification and identification of the violaxanthin deepoxidase as a 43 kDa protein. Photosynth Res. 1996;49:119–129. doi: 10.1007/BF00117662. [DOI] [PubMed] [Google Scholar]
- Bugos RC, Hieber AD, Yamamoto HY. Xanthophyll cycle enzymes are members of the lipocalin family, the first identified from plants. J Biol Chem. 1998;273:15321–15324. doi: 10.1074/jbc.273.25.15321. [DOI] [PubMed] [Google Scholar]
- Bugos RC, Yamamoto HY. Molecular cloning of violaxanthin de-epoxidase from romaine lettuce and expression in Escherichia coli. Proc Natl Acad Sci USA. 1996;93:6320–6325. doi: 10.1073/pnas.93.13.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B, Winter K, Krüger A, Czygan F-C. Photoinhibition and zeaxanthin formation in intact leaves: a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987;84:218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW., III Carotenoid composition in sun and shade leaves of plants with different life forms. Plant Cell Environ. 1992;15:411–419. [Google Scholar]
- Demmig-Adams B, Adams WW, III, Logan BA, Verhoeven AS. Xanthophyll cycle-dependent energy dissipation and flexible photosystem II efficiency in plants acclimated to light stress. Aust J Plant Physiol. 1995;22:249–260. [Google Scholar]
- Demmig-Adams B, Gilmore AM, Adams WW., III In vivo functions of carotenoids in higher plants. FASEB J. 1996;10:403–412. doi: 10.1096/fasebj.10.4.8647339. [DOI] [PubMed] [Google Scholar]
- Eskling M, Åkerlund H-E. Changes in the quantities of violaxanthin de-epoxidase, xanthophylls and ascorbate in spinach upon shift from low to high light. Photosynth Res. 1998;57:41–50. [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fourney RM, Miyakoshi J, Day RS, III, Paterson MC. Northern blotting: efficient RNA staining and transfer. Focus. 1988;10:5–7. [Google Scholar]
- Gilmore AM, Hazlett TL, Debrunner PG, Govindjee Photosystem II chlorophyll a fluorescence lifetimes and intensity are independent of the antenna size differences between barley wild-type and chlorina mutants: photochemical quenching and xanthophyll cycle-dependent nonphotochemical quenching of fluorescence. Photosynth Res. 1996;48:171–187. doi: 10.1007/BF00041007. [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Hazlett TL, Govindjee Xanthophyll cycle-dependent quenching of photosystem II chlorophyll a fluorescence: formation of a quenching complex with a short fluorescence lifetime. Proc Natl Acad Sci USA. 1995;92:2273–2277. doi: 10.1073/pnas.92.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AM, Yamamoto HY. Resolution of lutein and zeaxanthin using a non-endcapped, lightly carbon-loaded C18 high-performance liquid chromatographic column. J Chromatogr. 1991;543:137–145. [Google Scholar]
- Gilmore AM, Yamamoto HY. Dark induction of zeaxanthin-dependent nonphotochemical fluorescence quenching mediated by ATP. Proc Natl Acad Sci USA. 1992;89:1899–1903. doi: 10.1073/pnas.89.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AM, Yamamoto HY. Linear models relating xanthophylls and lumen acidity to non-photochemical fluorescence quenching: evidence that antheraxanthin explains zeaxanthin-independent quenching. Photosynth Res. 1993;35:67–78. doi: 10.1007/BF02185412. [DOI] [PubMed] [Google Scholar]
- Hager A. Lichtbedingte pH-Erniedrigung in einem Chloroplasten-Kompartiment als Ursache der enzymatischen Violaxanthin- → Zeaxanthin-Umwandlung; Beziehungen zur Photophosphorylierung. Planta. 1969;89:224–243. doi: 10.1007/BF00385028. [DOI] [PubMed] [Google Scholar]
- Hager A. Die reversiblen, lichtabhängigen Xanthophyllumwandlungen im Chloroplasten. Ber Dtsch Bot Ges Bd. 1975;88:27–44. [Google Scholar]
- Hager A, Holocher K. Localization of the xanthophyll-cycle enzyme violaxanthin de-epoxidase within the thylakoid lumen and abolition of its mobility by a (light-dependent) pH decrease. Planta. 1994;192:581–589. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neubauer C, Yamamoto HY. Mehler-peroxidase reaction mediates zeaxanthin formation and zeaxanthin-related fluorescence quenching in intact chloroplasts. Plant Physiol. 1992;99:1354–1361. doi: 10.1104/pp.99.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell. 1998;10:1121–1134. doi: 10.1105/tpc.10.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockholm DC, Yamamoto HY (1993) Purification of violaxanthin de-epoxidase by lipid affinity precipitation. In HY Yamamoto, CM Smith, eds, Photosynthetic Responses to the Environment. American Society of Plant Physiologists, Rockville, MD, p 237
- Rockholm DC, Yamamoto HY. Violaxanthin de-epoxidase: purification of a 43-kilodalton lumenal protein from lettuce by lipid-affinity precipitation with monogalactosyldiacylglyceride. Plant Physiol. 1996;110:697–703. doi: 10.1104/pp.110.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C, Reith P, Lichtenthaler HK. Differential levels of carotenoids and decrease of zeaxanthin cycle performance during leaf development in a green and an aurea variety of tobacco. J Plant Physiol. 1994;143:500–507. [Google Scholar]
- Siefermann D, Yamamoto HY. Properties of NADPH and oxygen-dependent zeaxanthin epoxidation in isolated chloroplasts: a transmembrane model for the violaxanthin cycle. Arch Biochem Biophys. 1975;171:70–77. doi: 10.1016/0003-9861(75)90008-9. [DOI] [PubMed] [Google Scholar]
- Thayer SS, Björkman O. Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res. 1990;23:331–343. doi: 10.1007/BF00034864. [DOI] [PubMed] [Google Scholar]
- Yamamoto HY. Xanthophyll cycles. Methods Enzymol. 1985;110:303–312. [Google Scholar]
- Yamamoto HY, Kamite L. The effects of dithiothreitol on violaxanthin de-epoxidation and absorbance changes in the 500 nm region. Biochim Biophys Acta. 1972;267:538–543. doi: 10.1016/0005-2728(72)90182-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto HY, Kamite L, Wang Y-Y. An ascorbate-induced absorbance change in chloroplasts from violaxanthin de-epoxidation. Plant Physiol. 1972;49:224–228. doi: 10.1104/pp.49.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto HY, Nakayama TOM, Chichester CO. Studies on the light and dark interconversions of leaf xanthophylls. Arch Biochem Biophys. 1962;97:168–173. doi: 10.1016/0003-9861(62)90060-7. [DOI] [PubMed] [Google Scholar]