Abstract
To characterize hepatitis C virus (HCV) epidemiology in Pakistan and estimate the pooled mean HCV antibody prevalence in different risk populations, we systematically reviewed all available records of HCV incidence and/or prevalence from 1989 to 2016, as informed by the Cochrane Collaboration Handbook. This systematic review was reported following the PRISMA guidelines. Populations were classified into six categories based on the risk of exposure to HCV infection. Meta-analyses were performed using DerSimonian and Laird random-effects models with inverse variance weighting. The search identified one HCV incidence study and 341 prevalence measures/strata. Meta-analyses estimated the pooled mean HCV prevalence at 6.2% among the general population, 34.5% among high-risk clinical populations, 12.8% among populations at intermediate risk, 16.9% among special clinical populations, 55.9% among populations with liver-related conditions and 53.6% among people who inject drugs. Most reported risk factors in analytical epidemiologic studies related to healthcare procedures. Pakistan is enduring an HCV epidemic of historical proportions—one in every 20 Pakistanis is infected. HCV plays a major role in liver disease burden in this country, and HCV prevalence is high in all-risk populations. Most transmission appears to be driven by healthcare procedures. HCV treatment and prevention must become a national priority.
Keywords: Hepatitis C virus, epidemiology, prevalence, incidence, Middle East and North Africa
1. Introduction
Hepatitis C virus (HCV) is a blood-borne pathogen and a significant global health concern [1]. Following the acquisition of the virus, acute HCV infection can progress to chronic infection [2], which is associated with several morbidities, such as liver cirrhosis and cancer [3–5]. HCV-related morbidity strains healthcare systems worldwide, with approximately 71 million people chronically infected globally [6]. Direct-acting antivirals (DAAs), a highly efficacious HCV treatment, can clear HCV infection and may substantially reduce HCV disease burden and onward transmission [7]. As such, global targets have been set by the World Health Organization (WHO) to eliminate HCV infection by 2030 [8,9].
The Middle East and North Africa (MENA) region is the most affected region by HCV infection, with approximately 15 million individuals chronically infected [6]. HCV is highly endemic in Pakistan, where a national survey, conducted in 2007–2008, estimated HCV prevalence at 4.8% [10]. Ongoing transmission appears to be widespread, occurring in both healthcare and community settings [10]. Understanding HCV epidemiology in Pakistan is critical in developing and targeting cost-effective prevention and treatment interventions against HCV, in order to meet the global target of HCV elimination.
The objective of this systematic review is to characterize HCV epidemiology in Pakistan by: (i) systematically reviewing and synthesizing available published data of HCV incidence and prevalence in six population categories defined according to risk of exposure and (ii) pooling available HCV prevalence measures in each of the six pre-defined risk population categories to estimate population-specific pooled mean HCV prevalence.
This work was conducted as part of the MENA HCV Epidemiology Synthesis Project, which aims to characterize HCV epidemiology in MENA to inform key public health research, policy, programming and resource allocation priorities [11–24].
2. Methods
The methodology used in this study follows that used in previous systematic reviews of the MENA HCV Epidemiology Synthesis Project [11–17]. The subsequent subsections summarize this methodology. Further details are available in previous publications [11–17].
2.1. Data sources and search strategy
All available records reporting HCV incidence and/or prevalence measures in Pakistan were systematically reviewed, as informed by the Cochrane Collaboration Handbook [25]. Results were reported using the Preferred Reporting Items for Systematic and Meta-analyses (PRISMA) guidelines (electronic supplementary material, table S1) [26]. Our main data sources included PubMed and Embase databases. Broad search criteria (electronic supplementary material, figure S1) were used to retrieve articles and abstracts on PubMed and Embase, from 1989 (the year in which HCV was first identified [27,28]) up to 19 April 2016, with no language restrictions.
2.2. Study selection
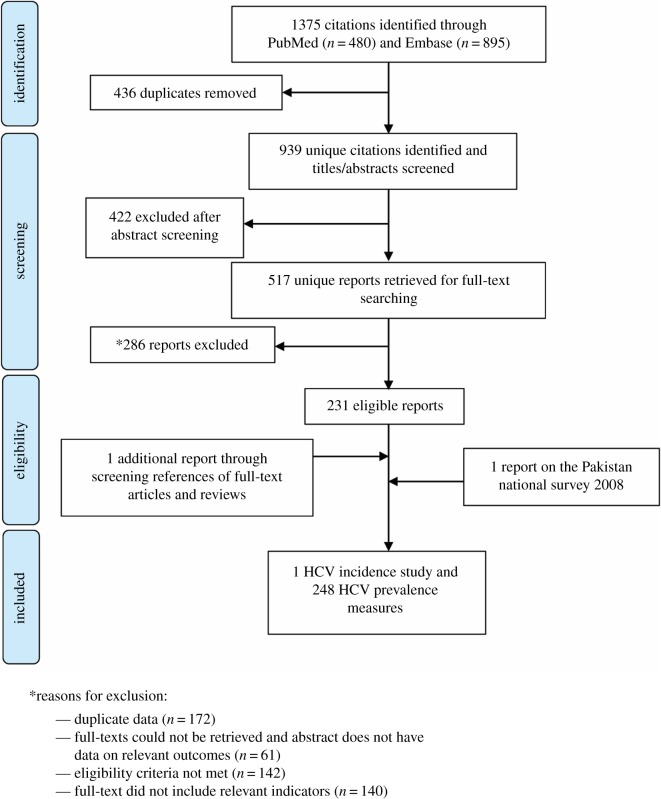
Similar to our previous systematic reviews [11–17], all records identified through our search were imported into the reference manager Endnote, where duplicate publications were identified and excluded. The remaining unique reports were subjected to a two-stage screening process, performed by Z.A.K. and S.P.K. In the first stage, titles and abstracts were screened for relevance. Records marked as relevant or potentially relevant proceeded to the second stage of screening, in which full-texts were obtained and assessed for eligibility based on predetermined inclusion/exclusion criteria. Eligible reports were included in this study, and ineligible reports were excluded with reasons specified in figure 1. Additional records were identified by screening references in full-text articles and the literature reviews, as well as a country-level report.
Figure 1.
Flow chart of article selection for the systematic review of hepatitis C virus (HCV) incidence and prevalence in Pakistan, adapted from the PRISMA 2009 guideline [26].
2.3. Inclusion and exclusion criteria
The inclusion and exclusion criteria used in this study were adapted from our previous systematic reviews [11–17]. Briefly, any article reporting HCV antibody incidence and/or antibody prevalence, based on primary data, qualified for inclusion in this review. An article was excluded if it was a case report, case series, editorial, letter to editor(s), commentary, review, referred to HCV as non-A non-B hepatitis, contained duplicate information, reported HCV prevalence based on self-reporting, and if the study population was Pakistani nationals residing outside Pakistan.
In this work, for clarity, a ‘report’ refers to a document (article, conference abstract, country-level report and others) including one or several outcome measures of those included in our systematic review, while a ‘study’ refers to any one specific single outcome measure. One report may contribute multiple studies (say several prevalence measures in different populations), and multiple reports of the same outcome measure (say same prevalence measure in the same specific sample) were identified as duplicates and deemed as one study.
2.4. Data extraction and data synthesis
Data from relevant reports were extracted by Z.A.K., of which 20% were double extracted by S.P.K. to ensure consistency. Nature of extracted data followed our previous systematic reviews [11–17]. HCV prevalence measures were extracted and reported as per original reports. These measures were rounded to one decimal place except for measures below 0.1%, which were rounded to two decimal places.
Risk factors that were found to be significantly associated with HCV infection through multivariable regression analyses were extracted. HCV ribonucleic acid (RNA) prevalence among HCV antibody-positive individuals (that is HCV viraemic rate [20]) was extracted whenever available in reports including an HCV prevalence.
The extracted data were synthesized by risk population in six distinct categories defined according to the risk of exposure to HCV infection as follows:
General population (populations at low risk): these included blood donors, pregnant women, children, refugees, household-based survey participants and national army recruits, among others.
High-risk clinical populations: these included populations exposed to frequent medical injections and/or blood transfusions, such as haemodialysis, thalassaemia, haemophilia and multi-transfused patients, among others.
Populations at intermediate risk: these included populations whose risk of exposure is higher than the general population but lower than populations at high risk, such as healthcare workers (HCWs), household contacts of HCV-infected patients, patients with diabetes and prisoners, among others.
Special clinical populations: these included clinical populations whose risk of exposure to HCV infection is difficult to ascertain, such as patients with non-liver-related malignancies, dermatological manifestations and rheumatological disorders, among others.
Populations with liver-related conditions: these included patients with liver-related conditions of an epidemiological significance to HCV infection such as patients with chronic liver disease, acute viral hepatitis, hepatocellular carcinoma and liver cirrhosis, among others.
People who inject drugs (PWID).
2.5. Quantitative analysis
The quantitative analysis approach was similar to that in our previous HCV systematic reviews [11–17]. HCV prevalence measures were presented by risk population in reports with a sample size greater than or equal to 50 in tables 1–3; electronic supplementary material, S2–S4. If no explicit HCV prevalence measure was reported, it was calculated based on the sample size and number of events reported, if available. HCV prevalence for the total sample was replaced with stratified measures, whenever the sample size was greater than or equal to 25 participants for each stratum. Stratified data were included using a pre-defined order that prioritizes stratifications by population followed by sex, year, region and age. Meta-analyses were conducted for studies/strata with a minimum sample size of 25 participants. Only one final stratification per study was included in the meta-analyses.
Table 1.
Studies reporting hepatitis C virus (HCV) prevalence among the general population (populations at low risk) in Pakistan. Prev, prevalence; CC, case-control; CS, cross-sectional; Conv, convenience; MsRS, multi-stage random sampling, RCS, random cluster sampling; SRS, simple random sampling; SsCS, single-stage cluster sampling; NWFP, North West Frontier Province; NHL, non-Hodgkin's lymphoma.
| author (citation) | year(s) of data collection | province or city | study site | study design | study sampling procedure | population | sample sizea | HCV prevb (%) |
|---|---|---|---|---|---|---|---|---|
| Agboatwalla [29] | 1990–1991 | — | community | CS | Conv | healthy children | 226 | 0.4 |
| Kakepoto [30] | 1989–1994 | Karachi and Hyderabad | blood donation camps | CS | Conv | blood donors | 16 705 | 1.2 |
| Luby [31] | 1993 | Hafizabad | community | CS | RCS | general population | 309 | 6.5 |
| Parker [32] | — | Lahore | hospital | CS | Conv | pregnant women | 417 | 6.7 |
| Parker [32] | — | Lahore | hospital | CS | Conv | children | 538 | 1.3 |
| Mujeeb [33] | 1996–1997 | Karachi | medical centre | CS | Conv | blood donors from students' community | 612 | 0.5 |
| Khan [34] | 1995 | Darsano Channo Karachi | general clinic | CS | Conv | outpatients from health clinics | 135 | 44.0 |
| Mujeeb [35] | 1997–1998 | Karachi | medical centre | CS | Conv | replacement blood donors | 7047 | 2.4 |
| Aslam [36] | 2000 | Lahore | community | CS | Conv | general population | 488 | 15.9 |
| Aslam [36] | 2000 | Gujranwala | community | CS | Conv | general population | 1922 | 23.8 |
| Khattak [37] | 1996–2000 | Rawalpindi | community | CS | Conv | healthy blood donors | 103 858 | 4.1 |
| Qureshi [38] | 1996–1999 | Karachi | blood bank in a hospital | CS | Conv | blood donors | 401 | 4.5 |
| Mumtaz [39] | 2001–2002 | Rawalpindi | hospital | CS | Conv | healthy blood donors | 563 | 6.2 |
| Asif [40] | 2002–2003 | Northern Pakistan | blood transfusion unit | CS | Conv | replacement blood donors | 3187 | 5.1 |
| Asif [40] | 2002–2003 | Northern Pakistan | blood transfusion unit | CS | Conv | voluntary blood donors | 243 | 2.5 |
| Khokhar [41] | 2001–2002 | Islamabad | hospital | CS | Conv | pregnant women | 503 | 4.8 |
| Aslam [42] | — | Lahore | community | CS | Conv | general population | 523 | 14.9 |
| Jaffery [43] | 2001–2002 | Islamabad | hospital | CC | Conv | pregnant women | 947 | 3.3 |
| Muhammad [44] | 1998–2002 | Buner, NWFP | hospital | CS | Conv | outpatients | 16 400 | 4.6 |
| Jafri [45] | 2003–2004 | Karachi | community | CS | Conv | healthy children | 3533 | 1.6 |
| Mujeeb [46] | 2000 | Karachi | medical centre | CS | Conv | first time replacement blood donors | 7325 | 3.6 |
| Rifat-uz [47] | 2004 | Bahawalpur | community | CS | Conv | general population | 6815 | 4.4 |
| Ahmad [48] | 2004 | Faisalabad | hospital | CS | SRS | blood donors and general population | 300 | 20.6 |
| Bhatti [49] | 2003–2005 | Rawalpindi | blood transfusion unit | CS | Conv | blood donors | 94 177 | 4.2 |
| Bhatti [49] | 2004 | Rawalpindi | blood transfusion unit | CS | Conv | blood donors | 966 | 3.8 |
| Sultan [50] | 1996–2005 | Lahore | tertiary care centre | CS | Conv | replacement blood donors | 41 498 | 3.7 |
| Abbas [51] | — | Sukkar | community | CS | SCS | general population | 873 | 33.7 |
| Butt [52] | 2004–2005 | — | hospital | CS | Conv | army recruits | 5707 | 1.7 |
| Hakim [53] | 2002–2006 | Karachi | university | CS | Conv | female university students | 4000 | 5.2 |
| Idrees [54] | 1999–2007 | Punjab | community | CS | Conv | general population | 6817 | 15.1 |
| Khattak [55] | — | Peshawar | blood banks in hospitals | CS | Conv | male blood donors | 1131 | 4.1 |
| Mujeeb [56] | 2004–2007 | Sindh | blood bank in a medical centre | CS | Conv | blood donors | 5345 | 7.5 |
| Abbas [57] | 2005–2008 | — | liver clinic | CS | Conv | blood donors | 804 | 14.0 |
| Ali [58] | 2003–2005 | Multan | community | CS | SRS | general population | 116 | 6.7 |
| Bangash [59] | 2007 | Parachinar and Sadda | blood transfusion unit | CS | Conv | blood donors | 1300 | 1.6 |
| Bangash [60] | 2007–2008 | Parachinar | blood bank in a hospital | CS | Conv | blood donors | 10 343 | 0.4 |
| Gul [61] | 2006–2007 | Abbottabad | hospital | CS | Conv | pregnant women | 500 | 8.9 |
| Jalbani [62] | — | Khairpur Nathan Shah and Shahdadkot | community | CS | Conv | general population | 406 | 30.3 |
| Junejo [63] | 2007–2008 | Hyderabad | hospital | CS | Conv | outpatients | 931 | 17.2 |
| Hussain [64] | 2008 | Karachi | blood bank | CS | Conv | blood donors | 98 012 | 6.0 |
| Sami [65] | 2005 | Karachi | medical centre | CS | Conv | pregnant women | 5902 | 1.8 |
| Shaikh [66] | 2006–2007 | Larkana city | general clinic | CS | Conv | general population | 450 | 6.6 |
| Sheikh [67] | 2006 | Karachi | Hospital | CS | Conv | pregnant women | 2592 | 0.7 |
| Abbas [68] | — | Karachi | community | CS | Conv | general population | 504 | 3.2 |
| Ali [69] | 2009–2010 | Khyber Pakhtunkhwa | community | CS | SRS | healthy inhabitants of District Mansehra | 400 | 7.0 |
| Aziz [70] | 2007–2008 | Sindh | community | CS | Conv | general population from peri-urban area | 129 | 3.9 |
| Aziz [70] | 2007–2008 | Sindh | community | CS | Conv | general population from rural area | 388 | 28.6 |
| Hashmi [71] | 2006 | Islamabad | community | CS | MsRS | female inhabitants: 15–50 years old | 252 | 24.6 |
| Hyder [72] | 2007–2009 | Punjab | community | CS | Conv | healthy men: 16–59 years old | 58 680 | 6.9 |
| Jadoon [73] | 2008 | — | blood bank in a hospital | CS | Conv | healthy blood donors | 550 | 8.2 |
| Jadoon [74] | — | Multan | hospital | CC | Conv | blood donors | 10 000 | 4.9 |
| Jamil [75] | 2010 | Tehsil Oghi | community | CS | Conv | general population | 648 | 10.3 |
| Janjua [76] | 2005 | Karachi | community | CS | SCS | general population | 1997 | 23.8 |
| Qureshi [10] | 2007–2008 | All regions of Pakistan | national | CS | MsRS | household survey members | 47 043 | 4.8 |
| Shah [77] | 2007–2008 | Karachi | hospital | CS | Conv | blood donors | 32 042 | 1.6 |
| Taseer [78] | 2006–2007 | Multan | hospital | CS | Conv | pregnant women | 500 | 7.0 |
| Aziz [79] | 2005–2009 | Karachi | hospital | CS | Conv | pregnant women: 18–45 years old | 18 000 | 5.8 |
| Borhany [80] | 2007–2009 | Karachi | specialized clinic | CS | Conv | blood donors | 5717 | 1.9 |
| Iqbal [81] | — | Gadap Town, Karachi | community | CS | Conv | previously unscreened adults: more than 10 years old | 600 | 5.0 |
| Khan [82] | 2009 | Khyber Pakhtunkhwa | blood bank | CS | Conv | voluntary blood donors | 7148 | 1.9 |
| Rauf [83] | 2009 | NWFP | refugee camp | CS | Conv | refugees in Baghicha Dheri camps | 590 | 8.8 |
| Safi [84] | 2008–2009 | NWFP | blood bank in a hospital | CS | Conv | blood donors | 62 251 | 2.6 |
| Saleem [85] | 2008 | Azad Kashmir | hospital | CS | Conv | outpatients | 9564 | 6.4 |
| Yousaf [86] | — | all regions of Pakistan | laboratory | CS | SRS | general population | 120 | 29.2 |
| Ahmed [87] | 2007–2009 | Balochistan | community | CS | MsRS | general population | 2000 | 5.5 |
| Ansari [88] | 2010 | Karachi | specialized clinic | CS | Conv | blood donors | 5517 | 1.9 |
| Attaullah [89] | 2008–2011 | Khyber Pakhtunkhwa | blood bank in a hospital | CS | Conv | blood donors | 127 828 | 2.5 |
| Bhutta [90] | 2010 | Sargodha | hospital | CS | Conv | replacement blood donors | 100 | 12.0 |
| Hafeez-ud [91] | 2010 | Punjab | community | CS | Conv | healthy adult males | 14 027 | 3.1 |
| Ijaz [92] | 2011–2012 | Lahore | hospital | CS | Conv | blood donors | 3652 | 12.4 |
| Khan [93] | 2008 | Lakki Marwat | hospital | CS | Conv | outpatients | 1443 | 4.4 |
| Khan [94] | 2009–2012 | Peshawar | hospital | CS | Conv | blood donors | 6513 | 1.1 |
| Memon [95] | 2007–2008 | Karachi | private security company | CS | Conv | security personnel | 457 | 9.0 |
| Muhammad [96] | 2009–2010 | Sindh | medical centre | CC | Conv | family members of NHL patients (controls) | 584 | 7.7 |
| Nawaz [97] | 2011 | — | hospital | CS | Conv | general population | 435 | 12.2 |
| Waheed [98] | 2010 | Islamabad | hospital | CS | Conv | blood donors | 10 145 | 8.3 |
| Abbas [99] | — | Balochistan | community | CS | Conv | general population | 2800 | 7.0 |
| Butt [100] | 2013 | Lahore | blood bank in a hospital | CS | Conv | male blood donors | 833 | 1.9 |
| Irfan [101] | 2004–2010 | Karachi | hospital | CS | Conv | blood donors | 108 598 | 2.7 |
| Khan [102] | 2011 | Quetta, Balochistan | hospital | CS | Conv | male blood donors | 356 | 20.8 |
| Khan [103] | 2010–2011 | Karachi | community | CS | SRS | household survey members | 679 | 8.0 |
| Qadeer [104] | 2007–2012 | Punjab | community | CS | Conv | blood donors from students’ community | 5000 | 4.1 |
| Rauf [105] | 2011 | Karachi | community | CS | Conv | male garbage scavengers | 117 | 8.5 |
| Seema [106] | 2010 | Hyderabad Sindh | hospital | CS | Conv | pregnant women | 3078 | 4.7 |
| Zaffar [107] | — | — | transfusion unit | CS | Conv | blood donors | 246 611 | 2.9 |
| Ali [108] | — | Mardan | hospitals and clinics | CS | Conv | general population | 1419 | 11.7 |
| Ilyas [109] | 2013–2014 | Peshawar | community | CS | Conv | general population | 982 | 13.4 |
| Moiz [110] | 2011–2012 | Southern Pakistan | hospital | CS | Conv | healthy noncommercial blood donors | 42 830 | 1.7 |
| Parveen [111] | 2013 | Multan | hospital | CS | Conv | potential employees sent for HCV screening | 10 666 | 2.9 |
| Kumari [112] | 2012 | Karachi | hospital | CS | Conv | pregnant women | 300 | 13.3 |
| Niazi [113] | 2012–2013 | Rawalpindi | transfusion unit | CS | Conv | blood donors | 56 772 | 1.8 |
| Sheikh [114] | — | Gwadar Port | disaster management camp | CS | Conv | blood donors in rural areas | 300 | 4.3 |
| Donchuk [115] | 2015–2016 | Karachi | community | CS | Conv | outpatients | 4589 | 27.0 |
| Karim [116] | 2015 | Mardan | hospitals | CS | Conv | blood donors | 5318 | 1.1 |
aThe table reports only studies whose sample size is greater than or equal to 50 participants. For space considerations, the table shows the overall HCV measure of each study rather than stratifications within population subgroups.
bThe decimal places of the prevalence figures are as reported in the original report, but prevalence figures with more than one decimal place were rounded to one decimal place, with the exception of those below 0.1%.
Table 3.
Studies reporting hepatitis C virus prevalence among people who inject drugs (PWID) in Pakistan.
| author (citation) | year(s) of data collection | province or city | study site | study design | study sampling procedure | population | sample sizea | HCV prevb (%) |
|---|---|---|---|---|---|---|---|---|
| Kuo [131] | 2003 | Lahore and Quetta | outpatient centres | CS | Conv | PWID | 351 | 88.0 |
| Achakzai [132] | 2004 | Quetta | community | CS | Conv | PWID | 50 | 60.0 |
| Altaf [133] | 2003 | Karachi | rehabilitation centre | CS | Conv | PWID | 161 | 94.3 |
| Abbasi [134] | 2003 | Quetta | community | CS | Conv | PWID | 300 | 44.7 |
| Platt [135] | 2007 | Rawalpindi | community | CS | RDS | PWID | 302 | 17.3 |
| Platt [135] | 2007 | Abbottabad | community | CS | RDS | PWID | 102 | 8.0 |
| Rehan [136] | 2004 | Karachi | community | CS | SRS | PWID | 399 | 87.0 |
| Rehan [136] | 2004 | Lahore | community | CS | SRS | PWID | 380 | 91.8 |
| Rehman [137] | — | Khyber Pakhtunkhwa | community | CS | Conv | PWID | 200 | 31.5 |
| Memon [95] | 2007–2008 | Karachi | laboratory | CS | Conv | PWID | 407 | 68.3 |
| Daud [125] | 2008–2012 | Islamabad | HIV care centre | CS | Conv | HIV patients who inject drugs | 81 | 77.8 |
aThe table reports only studies whose sample size is greater than or equal to 50 participants. For space considerations, the table shows the overall HCV measure of each study rather than stratifications within population subgroups.
bThe decimal places of the prevalence figures are as reported in the original report, but prevalence figures with more than one decimal were rounded to one decimal place, with the exception of those below 0.1%. Prev, prevalence; CS, cross-sectional; Conv, convenience; RDS, respondent-driven sampling; SRS, simple random sampling.
The variance of the prevalence measures was stabilized using the Freeman–Tukey type arcsine square-root transformation [138]. Estimates for HCV prevalence were weighted by the inverse variance and pooled using a DerSimonian–Laird random-effects model. This model accounts for sampling variation (random chance) and expected heterogeneity in effect size across studies [139]. Heterogeneity was assessed and characterized using several statistical measures.
With a recently identified potential issue with the Freeman–Tukey type arcsine square-root transformation [140], we conducted sensitivity analyses by performing meta-analyses using the generalized linear mixed models (GLMM) method to confirm validity of our results.
Meta-analysis of RNA HCV prevalence measures among HCV antibody-positive individuals (that is HCV viraemic rate) was also conducted to estimate the pooled mean of this prevalence measure.
A sensitivity analysis was further performed to examine whether the advent of more specific and sensitive diagnostic tools (third or fourth generation assays) could have affected the prevalence estimates in the general population. Meta-analyses were performed on the general population prior to and after 2005, since after this year the vast majority of studies were likely to have been conducted using third of fourth generation assays. The results of the meta-analyses were assessed to determine whether the estimated pooled mean HCV prevalence was significantly different prior to 2005.
Meta-analyses were conducted in R v. 3.1.2. [141], using the package meta [142].
2.6. Quality assessment
The quality of HCV prevalence measures was assessed for each study as informed by the risk of bias (ROB) Cochrane approach [143], as well as by examining the precision of each reported measure. The ROB assessment was based on three domains: type of HCV ascertainment (biological assays versus unclear), the sampling methodology (probability-based versus convenience sampling) and the response rate (greater than or equal to 80% versus less than or equal to 80% of the target sample size).
Studies were considered as having high precision if the number of HCV tested individuals was at least 100 participants, as informed by previous studies [11–17].
3. Results
3.1. Search results
Figure 1 describes the process of study selection, adapted from the PRISMA flow diagram [26]. A total of 1375 citations were identified: 480 through PubMed and 895 through Embase. A total of 517 reports were identified as relevant or potentially relevant after removing duplicates and screening the titles and abstracts. Out of these, 285 reports were excluded for various reasons as summarized in figure 1. An additional report was identified through screening of articles' references, and 11 HCV prevalence measures/strata were obtained from the Pakistan National Survey [10]. Finally, 233 eligible reports were included in this systematic review, yielding one incidence study and 248 prevalence measures. The 248 prevalence measures contributed 341 prevalence measures/strata. Though no language restrictions were imposed, all identified studies were in English.
3.2. HCV incidence overview
Our search identified one HCV incidence study, which reported seroconversion risk. This study included (as its baseline) HCV-negative HCWs who reported a needle stick injury from documented HCV-positive patients. After six weeks follow-up, investigators reported a seroconversion risk of 4.8% [144].
3.3. HCV prevalence overview
3.3.1. General population
Among the general population (table 1), our search identified 148 prevalence measures/strata, ranging from 0.4 to 44.0%, with a median of 5.3%. Among blood donors (number of studies; n = 57), HCV prevalence ranged from 0.4 to 20.8%, with a median of 3.5%. Among pregnant women (n = 12), HCV prevalence ranged from 0.7 to 20.7%, with a median of 6.0%. Among outpatients (n = 9), HCV prevalence ranged from 4.4 to 51.0%, with a median of 9.0%. Among other general populations (n = 65), HCV prevalence ranged from 0.4 to 35.9%, with a median of 6.8%.
3.3.2. High-risk clinical populations
Among high-risk clinical populations (table 2), our search identified 21 prevalence measures/strata, ranging from 7.8 to 68.0%, with a median of 34.5%. Among thalassaemia patients (n = 12), HCV prevalence ranged from 7.7 to 60.0%, with a median of 42.2%. Among haemodialysis patients (n = 7), HCV prevalence ranged from 16.4 to 68.0%, with a median of 28.0%. Only one study was conducted for each of haemophilia patients (prevalence of 51.4%) and multi-transfused patients (prevalence of 54.2%).
Table 2.
Studies reporting hepatitis C virus (HCV) prevalence among high-risk clinical populations in Pakistan.
| author (citation) | year(s) of data collection | province or city | study site | study design | study sampling procedure | population | sample sizea | HCV prevb (%) |
|---|---|---|---|---|---|---|---|---|
| Mujeeb [117] | — | Karachi | medical centre | CS | Conv | thalassaemia patients | 91 | 50.5 |
| Gul [118] | 1999 | Lahore | haemodialysis unit | CC | Conv | haemodialysis patients | 50 | 68.0 |
| Khokhar [119] | 2002–2003 | Islamabad | hospital | CS | Conv | haemodialysis patients | 97 | 23.7 |
| Mumtaz [120] | 2008 | Lahore | hospital | CS | Conv | haemodialysis male patients | 50 | 28.0 |
| Ullah [121] | — | Karachi | hospital | CS | Conv | thalassaemia patients | 79 | 43.0 |
| Khan [122] | 2010 | Khyber Pakhtunkhwa | hospitals | CS | SRS | haemodialysis patients | 384 | 29.2 |
| Borhany [80] | 2007–2009 | Karachi | specialized clinic | CS | Conv | haemophilia patients | 173 | 51.4 |
| Riaz [123] | 2009 | Karachi | hospital | CS | Conv | thalassaemia patients (multi-transfused) | 79 | 45.5 |
| Ansari [88] | 2010 | Karachi | specialized clinic | CS | Conv | thalassaemia patients | 160 | 13.1 |
| Sadiq [124] | 2008–2009 | Lahore | hospital | CS | Conv | transfusion dependent children | 120 | 54.2 |
| Daud [125] | 2008–2012 | Islamabad | HIV care centre | CS | Conv | HIV patients who use drugs (non-intravenously) | 81 | 6.2 |
| Din [126] | 2013 | Rawalpindi | transfusion unit | CS | Conv | thalassaemia patients | 95 | 49.5 |
| Mahmud [127] | 2012–2013 | Karachi | — | CS | Conv | haemodialysis patients | 189 | 16.4 |
| Chishti [128] | 2010–2011 | Karachi | medical centre | CS | Conv | haemodialysis patients (multi-transfused) | 200 | 29.0 |
| Khan [129] | 2013–2014 | Khyber Pakhtunkhwa | hospitals | CS | Conv | thalassaemia patients | 180 | 7.8 |
| Yasmeen [130] | 2012–2013 | — | hospital | CS | Conv | thalassaemia patients | 300 | 47.3 |
aThe table reports only studies whose sample size is greater than or equal to 50 participants. For space considerations, the table shows the overall HCV measure of each study rather than stratifications within population subgroups.
bThe decimal places of the prevalence figures are as reported in the original report, but prevalence figures with more than one decimal were rounded to one decimal place, with the exception of those below 0.1%. Prev, prevalence; CC, case-control; CS, cross-sectional; Conv, convenience; SRS, simple random sampling.
3.3.3. Intermediate risk populations
Among intermediate risk populations (electronic supplementary material, table S2), our search identified 64 prevalence measures/strata, ranging from 0.0 to 70.9%, with a median of 12.9%. Among hospitalized populations (n = 25), HCV prevalence ranged from 2.5 to 71.0%, with a median of 13.2%. Among HCWs (n = 11), HCV prevalence ranged from 0.0 to 5.6%, with a median of 3.2%. Among prisoners and/or volunteer prisoner blood donors (n = 9), HCV prevalence ranged from 8.7 to 18.2%, with a median of 13.1%. Among diabetics (n = 6), HCV prevalence ranged from 5.1 to 43.0%, with a median of 15.5%. Among household contacts of HCV index patients (n = 4), HCV prevalence ranged from 4.4 to 38.0%, with a median of 18.3%. A study conducted in Karachi among men who use roadside barbers measured HCV prevalence at 38.0% [145].
3.3.4. Special clinical populations
Among special clinical populations (electronic supplementary material, table S3), our search identified 18 prevalence measures/strata, ranging from 1.0 to 81.0%, with a median of 15.5%. Among patients with skin disorders (n = 4), HCV prevalence ranged from 3.0 to 23.4%, with a median of 7.7%. Among patients with urological conditions (n = 4), HCV prevalence ranged from 1.0 to 25.9%, with a median of 9.6%.
3.3.5. Populations with liver-related conditions
Among populations with liver-related conditions (electronic supplementary material, table S4), our search identified 73 prevalence measures/strata, ranging from 3.0 to 100.0%, with a median of 63.5%. Among chronic liver disease patients (n = 20), HCV prevalence ranged from 4.9 to 78.4%, with a median of 41.1%. Among cirrhosis patients (n = 21), HCV prevalence ranged from 28.0 to 100.0%, with a median of 68.0%. Among hepatocellular carcinoma patients (n = 18), HCV prevalence ranged from 33.3 to 92.0%, with a median of 70.1%. Among acute viral hepatitis patients (n = 6), HCV prevalence ranged from 6.4 to 57.1%, with a median of 20.9%.
3.3.6. People who inject drugs
Among PWID (table 3), our search identified 15 prevalence measures/strata, ranging from 8.0 to 94.3%, with a median of 44.7%.
3.4. Overview of HCV RNA prevalence among HCV antibody-positive individuals
Our search identified a total of 12 HCV RNA prevalence measures among HCV antibody-positive individuals (HCV viraemic rate). The details of these measures can be found in the electronic supplementary material, table S6. HCV viraemic rate ranged from 44.4 to 98.0%, with a median of 74.2%.
3.5. Pooled mean HCV prevalence estimates
Pooled mean estimates for HCV prevalence for the six risk populations are summarized in table 4. The pooled mean prevalence for the general population (populations at low risk) was estimated at 6.2% (95% CI: 5.7–6.7%). Meanwhile, the pooled mean HCV prevalence was estimated at 34.5% (95% CI: 27.0–42.3%) for high-risk clinical populations, 12.8% (95% CI: 10.8–15.1%) for intermediate risk populations, 16.9% (95% CI: 6.2–31.3%) for special clinical populations, 55.9% (95% CI: 49.2–62.5%) for populations with liver-related conditions and 53.6% (95% CI: 36.2–70.6) for PWID.
Table 4.
Pooled mean estimates for hepatitis C virus (HCV) prevalence for each of the six risk population categories in Pakistan.
| studies | samples | HCV prevalence |
pooled HCV prevalence |
heterogeneity measures |
|||||
|---|---|---|---|---|---|---|---|---|---|
| risk population | total n | total N | range (%) | median (%) | mean (%) | 95% CI | Q (p-value)a | I2 (confidence limits)b | prediction interval (%)c |
| general population (populations at low risk) | 148 | 1 352 080 | 0.4–50.6 | 5.3 | 6.2 | 5.7–6.7 | 17 552.0 (<0.0001) | 99.2% (99.1–99.2%) | 1.7–13.0 |
| high-risk clinical populations | 21 | 2377 | 7.8–68.0 | 33.3 | 34.5 | 27.0–42.3 | 294.3 (<0.0001) | 93.2% (90.9–94.9%) | 5.5–72.0 |
| populations at intermediate risk | 64 | 156 623 | 0.0–70.9 | 12.9 | 12.8 | 10.8–15.1 | 8680.5 (<0.0001) | 99.3% (99.2–99.3%) | 1.2–33.7 |
| special clinical populations | 20 | 11 940 | 1.1–80.8 | 15.5 | 16.9 | 6.2–31.3 | 5666.9 (<0.0001) | 99.7% (99.6–99.7%) | 0.0–90.2 |
| populations with liver-related conditions | 73 | 23 132 | 3.1–100.0 | 63.5 | 55.9 | 49.2–62.5 | 7028.9 (<0.0001) | 99.0% (98.9–99.1%) | 6.7–98.2 |
| PWID | 15 | 2815 | 7.8–93.8 | 44.7 | 53. 6 | 36.2–70.6 | 1181.9 (<0.0001) | 98.8% (98.6–99.0%) | 0.0–100 |
aQ: the Cochran's Q-statistic, a measure assessing the existence of heterogeneity in effect size.
bI²: a measure assessing the magnitude of between-study variation that is due to differences in effect size across studies rather than chance.
cPrediction interval: estimates the 95% interval in which the true effect size in a new HCV study will lie.
Of note, the GLMM meta-analyses produced similar pooled mean estimates for all risk populations. For example, the pooled mean HCV prevalence for special clinical populations, that showed the largest difference between the fixed effects result and the random-effects result, was 13.1% (95% CI: 6.9–31.3) using the GLMM method versus 16.9% (95% CI: 6.2–31.3%) using the Freeman–Tukey type arcsine square-root transformation method.
Statistically significant heterogeneity in effect size (that is HCV prevalence) was observed in all meta-analyses (Cochrane's Q-statistic's p-value was always less than 0.0001; table 4). Most of the variation across pooled studies was due to true difference in effect size rather than chance (I2 > 93.7%). The prediction intervals were generally very wide. The totality of these heterogeneity measures indicates high heterogeneity in HCV prevalence measures in each risk population category.
The pooled mean HCV RNA prevalence among HCV antibody-positive individuals (HCV viraemic rate) was estimated at 74.1% (95% CI: 59.5–86.5%).
The meta-analyses performed prior to and after 2005 among the general population, as part of our sensitivity analysis, estimated a pooled mean HCV prevalence of 5.0% (95% CI: 4.0–6.0%), and 6.5% (95% CI: 5.9–7.0%), respectively.
3.6. Risk factors for HCV infection
Risk factors for HCV seropositivity were assessed in 11 studies using multivariable regression analyses. Healthcare-related risk factors were most commonly reported, including history of blood transfusions [54,71,146], dental work [51,71,147], surgery [54,71,146], medical injections [42,51,147] and being a HCW [87].
Injecting drug-use-related risk factors were also commonly reported, including history of injecting drug use [54,87,95,146,148], duration of injecting drug use [131], sharing of needles or syringes [54], source of needles or syringes [135] and ‘jerking’ (drawing blood into a syringe while injecting) [131]. Sexual risk factors were also reported, including sex work (females and males), and sex for drugs [146].
3.7. Quality assessment of HCV incidence and prevalence measures
Findings of the quality assessment are summarized in the electronic supplementary material, table S5. Only one study was identified for HCV incidence [144] (not shown in the electronic supplementary material, table S5), in which there were greater than or equal to 100 participants, and was therefore classified as having high precision. As it was based on convenience sampling, it had high ROB for this domain. Meanwhile, it had low ROB in HCV ascertainment and in the response rate domains.
The majority of HCV prevalence studies (86.7%) was based on samples with greater than or equal to 100 participants, and were therefore classified as having high precision. Most studies (67.7%) reported specific details about HCV ascertainment, but nearly 70% did not report the assay generation. When information was provided, 94.2% of studies reported use of third or fourth generation assays.
A sensitivity analysis was performed to assess whether HCV prevalence in the general population differed prior to and after 2005, because the vast majority of studies after this year were likely to have been conducted using third or fourth generation assays. The confidence intervals of the estimated pooled mean HCV prevalence prior to and after 2010 overlapped, indicating HCV prevalence was not significantly different between these two time durations.
The majority of HCV prevalence studies (92.3%) used convenience, non-probability-based sampling approach. Nearly half of studies had low ROB in the response rate domain and 48.8% had missing information—only 1.6% of studies had high ROB in this domain.
To summarize, 78.6% of studies had low ROB based on at least one domain, and 41.1% had low ROB based on at least two domains. Furthermore, 1.2% of studies had high ROB based on two domains, and no study had high ROB based on three domains. The totality of the quality assessment measures indicates reasonable study quality.
4. Discussion
We presented a systematic review and synthesis of HCV incidence and prevalence in Pakistan. Our results affirm that Pakistan has one of the highest HCV infection levels in both MENA [11–17] and worldwide [149–151]. HCV prevalence in the population at large is at about 5%—one in every 20 Pakistanis has been already exposed to HCV infection. HCV prevalence was also found to be high in all risk populations, testifying to the scale of the epidemic in this country. Our results further supported a major role for HCV infection in liver disease burden in Pakistan—over half of the populations with liver-related conditions were found HCV antibody-positive.
Our results collectively indicate a major role for healthcare in HCV transmission. High HCV prevalence was observed in the populations exposed to healthcare in one form or another. In high-risk clinical populations, the pooled mean HCV prevalence was high at 34.5% (95% CI: 27.0–42.3%) (table 4), with HCV prevalence ranging across studies from 7.8 to 68.0% (table 2)—much higher than that found in the general population. In special clinical populations, the pooled mean HCV prevalence was also high at 16.9% (95% CI: 6.2–31.3%) (table 4), with HCV prevalence ranging across studies from 1.0 to 81.0% (electronic supplementary material, table S3). In all identified reports on hospitalized populations, HCV prevalence ranged from 2.5 to 71.0%, with a median of 13.2% (electronic supplementary material, table S2).
Our assessment of HCV risk factors further indicates that HCV transmission appears to be primarily driven by healthcare-related exposures, such as therapeutic injections, intravenous infusions and poor sterilization of medical equipment [42,51,54,71,87]. Injecting drug use and other community-based exposures appear also to play a role, but their relative (as opposed to absolute) role is probably small compared with healthcare procedures [152]. These findings demonstrate the urgency of addressing the HCV epidemic in Pakistan, one of the world's largest, and where 10% of the global number of chronically infected people are living [6,21].
The apparent major role for healthcare in HCV transmission distinguishes Pakistan from most other countries. Though healthcare plays a role in both developing and developed countries [11–17,153–155], healthcare practices appear to have driven HCV prevalence to atypically high levels in this country, a pattern seen only in a limited number of countries globally, such as Egypt [13,22,23] and former Soviet republics [156]. This role for healthcare is not only manifested in the high HCV prevalence in the different clinical populations (table 4) and in the reported risk factors in analytical epidemiologic studies [42,51,54,71,87], but also in the outcomes of viral hepatitis surveillance [157]. For example, the recently established viral hepatitis surveillance system in Pakistan indicated that healthcare-related exposures appear to be behind most newly reported HCV viral hepatitis cases [157]. Importantly, the surveillance demonstrated also that HCV accounted for over half of reported viral hepatitis cases [157], highlighting the special role of HCV infection in viral hepatitis disease burden in this country.
Of healthcare exposures, unnecessary therapeutic injections and reuse of syringes and needles were highlighted often as key factors [157,158]. Pakistan has one of the highest rates of therapeutic injections worldwide [159,160]—with widespread perception that injectable medications are more effective than oral medications [161–163]. Financial incentives appear also to sustain this preference for injectable medications, as healthcare providers can charge more for medications when they are administered by injections [163]. Though Pakistan has attempted to enhance provision and use of disposable injections and passed regulations for the management of disposable medical devices [164], implementation has been challenging in a country where the private sector accounts for 70% of healthcare services [157,162,165]. It bears notice that despite a possible key role for therapeutic injections, the totality of the evidence synthesized in the present study suggests that HCV healthcare exposures occur through multiple and diverse healthcare procedures.
The regional context of Pakistan and drug trafficking routes [166] support a conducive environment for injecting drug use. Our results indicated a high HCV prevalence among PWID (table 4), and evidence for injecting drug use as a mode of HCV exposure [54,87,131,157]. However, with an estimate of only 104 804 active PWID in Pakistan [167–169], the relative contribution of injecting drug use to HCV incidence is probably substantially smaller than that of healthcare, although the exact quantitative contribution remains uncertain.
Our results highlight the urgent and immediate need for expansion of HCV treatment and prevention programmes in Pakistan. High HCV prevalence was observed among all risk populations (table 4), with about one in every 20 Pakistanis being infected. Furthermore, three-quarters of all HCV antibody-positive individuals in Pakistan, per the meta-analysis of HCV viraemic rate (Section: Pooled mean HCV prevalence estimates), are chronically infected with HCV and can transmit the infection further. In spite of heavily discounted prices for DAAs in Pakistan [170], treatment scale-up has been limited, with only 311 000 chronic infections treated since 2013 [171]. To reach the WHO global target of reducing incidence by 80% by 2030, a recent modelling study indicated that the annual number of treatments must reach 490 000 and be sustained at this level for at least a decade [24]. To address the alarmingly high burden of HCV and achieve WHO global targets by 2030, Pakistan has recently developed the first National Hepatitis Strategic Framework, emphasizing the scale-up of interventions in healthcare settings and of HCV screening and treatment as well as harm reduction services [172].
Our study has identified key gaps and weaknesses in HCV epidemiological evidence in Pakistan. Despite the large epidemic, only one (now outdated) nationally representative and probability-based population-based survey was conducted in this country [10]. Repeating and enhancing this survey is critical to assess trends in prevalence and risk factors, as well as potential changes in the epidemiology. Such surveys have played an instrumental role in elucidating our knowledge of HCV transmission and in informing HCV response in other countries, such as in Egypt [173–179] and the USA [180].
Despite the major role for healthcare, a relatively small number of studies have been conducted among clinical populations, or investigated healthcare-related exposures. This is to be contrasted, for example, with Iran where a large number of studies investigated the role of healthcare—despite the relatively small role of this mode of exposure in this country [16]. Hardly any analytical cohort studies have been conducted in Pakistan despite the large epidemic, in contrast to Egypt [13,22], another MENA country with a large HCV epidemic [23]. Despite some suggestive evidence for community-based exposures [152], such as visiting roadside barbers [161], this mode of exposures remains to be clarified with concrete analytical studies. Though HCV vertical transmission appears to account for a quarter of HCV infections among children under 5 years of age in Pakistan [181], only one study appears to have investigated this mode of exposure in this country [32].
Our study is limited by the quantity and quality of reviewed studies, as well as their representativeness of the different risk populations—most studies used convenience sampling as opposed to probability-based population-based sampling. Only PubMed and Embase databases were searched, but other HCV data may exist in unpublished (grey literature) form, or are published in non-indexed journals. There was extensive heterogeneity in HCV prevalence measures in each risk population—possibly because of variability within the specific studied subpopulation, geographical location, sex and age-group representation in the sample, sampling technique and participant recruitment, year of study and study quality.
Despite these limitations, the main strength of our study is that we identified a large number of studies that covered different risk populations, and that facilitated a comprehensive synthesis of evidence and identification of gaps and weaknesses that preclude a satisfactory understanding of HCV epidemiology in Pakistan.
5. Conclusion
Pakistan is enduring an HCV epidemic of historical proportions—one in every 20 Pakistanis has been already infected with this infection playing a major role in liver disease burden in this country. HCV prevalence is high in all risk populations with most transmission apparently driven by healthcare procedures. Though our knowledge of the specific modes of exposure that drive transmission is improving, our understanding is still hampered by key gaps and weaknesses in available evidence. Conduct of repeated and comprehensive nationally representative and probability-based population-based surveys is critical to assess HCV prevalence and trends, identify risk factors and modes of exposure, examine the spatial variability in prevalence, and assess HCV knowledge and attitudes.
HCV treatment and prevention must become a national priority in Pakistan. Although Pakistan has made efforts to increase coverage of safe injection and blood screening and to improve infection control [164,182–184], commitment to prevention in all segments of the healthcare system, including the private sector, should be secured for this country to accomplish the HCV elimination target by 2030. Major expansion of infection control in healthcare facilities, and of harm reduction services for PWID, are warranted, as well as adoption of the WHO guidelines for the use of safety-engineered syringes [185,186].
Supplementary Material
Acknowledgement
The authors thank Dr Karima Chaabna for methodological expertise support.
Data accessibility
The datasets supporting this article have been uploaded as part of the manuscript and electronic supplementary material.
Competing interests
The authors have no competing interests to declare.
Authors' contributions
Z.A.K. conducted the systematic review of the literature, data retrieval, extraction, analyses and wrote the first draft of the article. S.M. conducted analyses and drafting of the article. S.P.K. contributed to the systematic review of the literature, data retrieval and extraction. L.J.A. conceived and led the design of the study, analyses and drafting of the article. All authors have read and approved the final manuscript.
Funding
This publication was made possible by NPRP grant no. 9-040-3-008 from the Qatar National Research Fund (a member of Qatar Foundation). The findings achieved herein are solely the responsibility of the authors. The authors are also grateful for infrastructure support provided by the Biostatistics, Epidemiology, and Biomathematics Research Core at Weill Cornell Medicine in Qatar.
References
- 1.Stanaway JD, et al. 2016. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 388, 1081–1088. (doi:10.1016/S0140-6736(16)30579-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen SL, Morgan TR. 2006. The natural history of hepatitis C virus (HCV) infection. Int. J. Med. Sci. 3, 47 (doi:10.7150/ijms.3.47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen HR, Martin P. 2000. Viral hepatitis in the liver transplant recipient. Infect. Dis. Clin. 14, 761–784. [DOI] [PubMed] [Google Scholar]
- 4.Lauer GM, Walker BD. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345, 41–52. (doi:10.1056/NEJM200107053450107) [DOI] [PubMed] [Google Scholar]
- 5.Bouvard V, et al. 2009. A review of human carcinogens—part B: biological agents. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 6.World Health Organization (WHO). 2017. Global hepatitis report. See http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/.
- 7.Wedemeyer H, Dore G, Ward J. 2015. Estimates on HCV disease burden worldwide–filling the gaps. J. Viral Hepat 22, 1–5. (doi:10.1111/jvh.12371) [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO). Combating hepatitis B and C to reach elimination by 2030: advocacy brief. 2016. (http://apps.who.int/iris/handle/10665/206453)
- 9.World Health Organization (WHO). Global health sector strategy on viral hepatitis 2016–2021. 2016. (http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/)
- 10.Qureshi H, Bile KM, Jooma R, Alam SE, Afridi HUR. 2010. Prevalence of hepatitis B and C viral infections in Pakistan: findings of a national survey appealing for effective prevention and control measures. East Mediterr. Health J. 16, S15–S23. [PubMed] [Google Scholar]
- 11.Mohamoud YA, Riome S, Abu-Raddad LJ. 2016. Epidemiology of hepatitis C virus in the Arabian Gulf countries: systematic review and meta-analysis of prevalence. Int. J. Infect. Dis. 46, 116–125. (doi:10.1016/j.ijid.2016.03.012) [DOI] [PubMed] [Google Scholar]
- 12.Chemaitelly H, Chaabna K, Abu-Raddad LJ. 2015. The epidemiology of hepatitis C virus in the fertile crescent: systematic review and meta-analysis. PLoS ONE 10, e0135281 (doi:10.1371/journal.pone.0135281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohamoud YA, Mumtaz GR, Riome S, Miller D, Abu-Raddad LJ. 2013. The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis. BMC Infect. Dis. 13, 288 (doi:10.1186/1471-2334-13-288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fadlalla FA, Mohamoud YA, Mumtaz GR, Abu-Raddad LJ. 2015. The epidemiology of hepatitis C virus in the Maghreb region: systematic review and meta-analyses. PLoS ONE 10, e0121873 (doi:10.1371/journal.pone.0121873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chemaitelly H, Mahmud S, Rahmani AM, Abu-Raddad LJ. 2015. The epidemiology of hepatitis C virus in Afghanistan: systematic review and meta-analysis. Int. J. Infect. Dis. 40, 54–63. (doi:10.1016/j.ijid.2015.09.011) [DOI] [PubMed] [Google Scholar]
- 16.Mahmud S, Akbarzadeh V, Abu-Raddad LJ. 2018. The epidemiology of hepatitis C virus in Iran: systematic review and meta-analyses. Sci. Rep. 8, 150 (doi:10.1038/s41598-017-18296-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaabna K, Kouyoumjian SP, Abu-Raddad LJ. 2016. Hepatitis C virus epidemiology in Djibouti, Somalia, Sudan, and Yemen: systematic review and meta-analysis. PLoS ONE 11, e0149966 (doi:10.1371/journal.pone.0149966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmud S, Al-Kanaani Z, Chemaitelly H, Chaabna K, Kouyoumjian SP, Abu-Raddad LJ. 2018. Hepatitis C virus genotypes in the Middle East and North Africa: distribution, diversity, and patterns. J. Med. Virol. 90, 131–141. (doi:10.1002/jmv.24921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harfouche M, Chemaitelly H, Mahmud S, Chaabna K, Kouyoumjian S, Al Kanaani Z, Abu-Raddad LJ. 2017. Epidemiology of hepatitis C virus among hemodialysis patients in the Middle East and North Africa: systematic syntheses, meta-analyses, and meta-regressions. Epidemiol. Infect. 145, 3243–3263. (doi:10.1017/S0950268817002242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harfouche M, Chemaitelly H, Kouyoumjian SP, Mahmud S, Chaabna K, Al-Kanaani Z, Abu-Raddad LJ. 2017. Hepatitis C virus viremic rate in the Middle East and North Africa: systematic synthesis, meta-analyses, and meta-regressions. PLoS ONE 12, e0187177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayoub H, Al Kanaani Z, Abu-Raddad LJ. 2018. Characterizing the temporal evolution of the hepatitis C virus epidemic in Pakistan. J. Viral Hepat. Epub ahead of print (doi:10.1111/jvh.12671) [DOI] [PubMed] [Google Scholar]
- 22.Kouyoumjian S, Chemaitelly H, Abu-Raddad LJ. 2018. Characterizing hepatitis C virus epidemiology in Egypt: systematic reviews, meta-analyses, and meta-regressions. Sci. Rep. 8, 1661 (doi:10.1038/s41598-017-17936-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayoub H, Abu-Raddad LJ. 2017. Impact of treatment on hepatitis C virus transmission and incidence in Egypt: a case for treatment as prevention. J. Viral Hepat. 24, 486–495. (doi:10.1111/jvh.12671) [DOI] [PubMed] [Google Scholar]
- 24.Ayoub H, Abu-Raddad LJ. Submitted. Treatment as prevention for hepatitis C virus in Pakistan: is elimination possible by 2030?
- 25.Higgins JP, Green S. 2008. Cochrane handbook for systematic reviews of interventions. Wiley Online Library.
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 (doi:10.1371/journal.pmed.1000097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244, 359–362. (doi:10.1126/science.2523562) [DOI] [PubMed] [Google Scholar]
- 28.Kuo G. 1990. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Pediatr. Infect. Dis. J. 9, 378 (doi:10.1097/00006454-199005000-00025) [DOI] [PubMed] [Google Scholar]
- 29.Agboatwalla M, Isomura S, Miyake K, Yamashita T, Morishita T, Akram DS. 1994. Hepatitis A, B and C seroprevalence in Pakistan. Indian J. Pediatr. 61, 545–549. (doi:10.1007/BF02751716) [DOI] [PubMed] [Google Scholar]
- 30.Kakepoto GN, Bhally HS, Khaliq G, Kayani N, Burney IA, Siddiqui T, Khurshid M. 1996. Epidemiology of blood-borne viruses: a study of healthy blood donors in Southern Pakistan. Southeast Asian J. Trop. Med. Public Health 27, 703–706. [PubMed] [Google Scholar]
- 31.Luby SP, Qamruddin K, Shah AA, Omair A, Pahsa O, Khan AJ, McCormick JB, Hoodbhouy F, Fisher-Hoch S. 1997. The relationship between therapeutic injections and high prevalence of hepatitis C infection in Hafizabad, Pakistan. Epidemiol. Infect. 119, 349–356. (doi:10.1017/S0950268897007899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker SP, Khan HI, Cubitt WD. 1999. Detection of antibodies to hepatitis C virus in dried blood spot samples from mothers and their offspring in Lahore, Pakistan. J. Clin. Microbiol. 37, 2061–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdul Mujeeb S, Aamir K, Mehmood K. 2000. Seroprevalence of HBV, HCV and HIV infections among college going first time voluntary blood donors. J. Pak. Med. Assoc. 50, 269–270. [PubMed] [Google Scholar]
- 34.Khan AJ, et al. 2000. Unsafe injections and the transmission of hepatitis B and C in a periurban community in Pakistan. Bull. World Health Organ. 78, 956–963. [PMC free article] [PubMed] [Google Scholar]
- 35.Mujeeb SA, Shahab S, Hyder AA. 2000. Geographical display of health information: study of hepatitis C infection in Karachi, Pakistan. Public Health 114, 413–415. (doi:10.1038/sj.ph.1900669) [PubMed] [Google Scholar]
- 36.Aslam M, Aslam J. 2001. Seroprevalence of the antibody to hepatitis C in select groups in the Punjab region of Pakistan. J. Clin. Gastroenterol. 33, 407–411. (doi:10.1097/00004836-200111000-00013) [DOI] [PubMed] [Google Scholar]
- 37.Khattak MF, Salamat N, Bhatti FA, Qureshi TZ. 2002. Seroprevalence of hepatitis B, C and HIV in blood donors in northern Pakistan. J. Pak. Med. Assoc. 52, 398–402. [PubMed] [Google Scholar]
- 38.Qureshi H, Ahsan T, Mujeeb SA, Jawad F, Mehdi I, Ahmed W, Alam SE. 2002. Diabetes mellitus is equally frequent in chronic HCV and HBV infection. J. Pak. Med. Assoc. 52, 280–283. [PubMed] [Google Scholar]
- 39.Mumtaz S. 2002. Frequency of seropositive blood donors for hepatitis B, C and HIV viruses in railway hospital Rawalpindi. Pak. J. Med. Res. 41, 51–53. [Google Scholar]
- 40.Asif N, Khokhar N, Ilahi F. 2004. Seroprevalence of HBV, HCV and HIV infection among voluntary non remunerated and replacement donors in northern Pakistan. Pak. J. Med. Sci. 20, 24–28. [Google Scholar]
- 41.Khokhar N, Raja KS, Javaid S. 2004. Seroprevalence of hepatitis C virus infection and its risk factors in pregnant women. J. Pak. Med. Assoc. 54, 135. [PubMed] [Google Scholar]
- 42.Aslam M, Aslam J, Mitchell BD, Munir KM. 2005. Association between smallpox vaccination and hepatitis C antibody positive serology in Pakistani volunteers. J. Clin. Gastroenterol. 39, 243–246. (doi:10.1097/01.mcg.0000153286.02694.14) [DOI] [PubMed] [Google Scholar]
- 43.Jaffery T, Tariq N, Ayub R, Yawar A. 2005. Frequency of hepatitis C in pregnancy and pregnancy outcome. J. Coll. Physicians Surg. Pak. 15, 716–719. [PubMed] [Google Scholar]
- 44.Muhammad N, Jan MA. 2005. Frequency of hepatitis ‘C’ in Buner, NWFP. J. Coll. Physicians Surg. Pak. 15, 11–14. [PubMed] [Google Scholar]
- 45.Jafri W, et al. 2006. Hepatitis B and C: prevalence and risk factors associated with seropositivity among children in Karachi, Pakistan. BMC Infect. Dis. 6, 101 (doi:10.1186/1471-2334-6-101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mujeeb SA, Nanan D, Sabir S, Altaf A, Kadir M. 2006. Hepatitis B and C infection in first-time blood donors in Karachi: a possible subgroup for sentinel surveillance. East Mediterr. Health J. 12, 735–741. [PubMed] [Google Scholar]
- 47.Rifat uz Z. 2006. Prevalence of hepatitis B and hepatitis C viruses in human urban population of Bahawalpur district, Pakistan. J. Med. Sci. 6, 367–373. (doi:10.3923/jms.2006.367.373) [Google Scholar]
- 48.Ahmad N, Asgher M, Shafique M, Qureshi JA. 2007. An evidence of high prevalence of hepatitis C virus in Faisalabad, Pakistan. Saudi Med. J. 28, 390–395. [PubMed] [Google Scholar]
- 49.Bhatti FA, Ullah Z, Salamat N, Ayub M, Ghani E. 2007. Anti-hepatitis B core antigen testing, viral markers, and occult hepatitis B virus infection in Pakistani blood donors: implications for transfusion practice. Transfusion 47, 74–79. (doi:10.1111/j.1537-2995.2007.01066.x) [DOI] [PubMed] [Google Scholar]
- 50.Sultan F, Mehmood T, Mahmood MT. 2007. Infectious pathogens in volunteer and replacement blood donors in Pakistan: a ten-year experience. Int. J. Infect. Dis. 11, 407–412. (doi:10.1016/j.ijid.2006.10.004) [DOI] [PubMed] [Google Scholar]
- 51.Abbas Z, Jeswani NL, Kakepoto GN, Islam M, Mehdi K, Jafri W. 2008. Prevalence and mode of spread of hepatitis B and C in rural Sindh, Pakistan. Trop. Gastroenterol. 29, 210–216. [PubMed] [Google Scholar]
- 52.Butt T, Amin MS. 2008. Seroprevalence of hepatitis B and C infections among young adult males in Pakistan. East Mediterr. Health J. 14, 791–797. [PubMed] [Google Scholar]
- 53.Hakim S, Kazmi S, Bagasra O. 2008. Seroprevalence of hepatitis B and C genotypes among young apparently healthy females of Karachi-Pakistan. Libyan J. Med. 3, 66–70. (doi:10.3402/ljm.v3i2.4760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Idrees M, Lal A, Naseem M, Khalid M. 2008. High prevalence of hepatitis C virus infection in the largest province of Pakistan. J. Dig. Dis. 9, 95–103. (doi:10.1111/j.1751-2980.2008.00329.x) [DOI] [PubMed] [Google Scholar]
- 55.Khattak MN, Akhtar S, Mahmud S, Roshan TM. 2008. Factors influencing hepatitis C virus sero-prevalence among blood donors in north west Pakistan. J. Public Health Policy 29, 207–225. (doi:10.1057/jphp.2008.7) [DOI] [PubMed] [Google Scholar]
- 56.Mujeeb SA, Pearce MS. 2008. Temporal trends in hepatitis B and C infection in family blood donors from interior Sindh, Pakistan. BMC Infect. Dis. 8, 43 (doi:10.1186/1471-2334-8-43) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abbas SZ, Ali M, Muhammad AH, Shaw S, Abbas SQ. 2009. Frequency of HCV infection and its genotypes among patients attending a liver clinic and voluntary blood donors in a rural area of Pakistan. Pak. J. Med. Sci. 25, 579–582. [Google Scholar]
- 58.Ali M, Kanwal L, Tassaduqe K, Iqbal R. 2009. Prevalence of hepatitis C virus (HCV) in relation to its promotive factors among human urban population of Multan, Pakistan. Eur. J. Gen. Med. 6, 41–45. (doi:10.29333/ejgm/82647) [Google Scholar]
- 59.Bangash MH, Bangash TH, Alam S. 2009. Prevalance of hepatitis B and hepatatis C among healthy blood donors at Kurram Agency. J. Postgrad. Med. Inst. 23, 140–145. [Google Scholar]
- 60.Bangash MH, Bangash TH, Ali J. 2009. Frequency of hepatitis B and C in healthy subjects in Parachinar. J. Postgrad. Med. Inst. 23, 347–351. [Google Scholar]
- 61.Gul N, Sarwar J, Idris M, Farid J, Rizvi F, Suleman M, Shah SH. 2009. Seroprevalence of hepatitis C in pregnant females of Hazara division. J. Ayub Med. Coll. Abbottabad 21, 83–86. [PubMed] [Google Scholar]
- 62.Jalbani A, Ansari IA, Shah AH, MalGurabakhashani K, Chutto M, Jalbani MA. 2009. Prevalence of hepatitis-C virus infection in Khairpurnathan Shah and Shahdakot a city based screening program. Med. Forum Mon. 20, 15–17. [Google Scholar]
- 63.Junejo SA, Khan NA, Lodhi AA. 2009. Prevalence of hepatitis B and C infection in patients admitted at tertiary eye care centre: a hospital based study. Pak. J. Med. Sci. 25, 597–600. [Google Scholar]
- 64.Mukhtar Hussain Sangji Z. 2009. Prevalence of blood screening markers in blood donor population of Pakistan at Husaini Haematology and Oncology Trust. Khi. Pak. Vox Sang. 97, 148. [Google Scholar]
- 65.Sami S, Korejo R, Bhutta SZ. 2009. Prevalence of hepatitis B and C: a Jinnah postgraduate medical centre experience. J. Obstet. Gynaecol. Res. 35, 533–538. (doi:10.1111/j.1447-0756.2008.00991.x) [DOI] [PubMed] [Google Scholar]
- 66.Shaikh FH, Ali Abro H, Ali Chhutto M, Abbasi PA, Shaikh AW, Ali Buriro S. 2009. Hepatitis C: frequency and risk factors associated with sero-positivity among adults in Larkana City. J. Ayub Med. Coll. Abbottabad 21, 107–109. [PubMed] [Google Scholar]
- 67.Sheikh SM. 2009. Hepatitis B and C: value of universal antenatal screening. J. Coll. Physicians Surg. Pak. 19, 179–182. [PubMed] [Google Scholar]
- 68.Abbas M, Hussain MF, Raza S, Shazi L. 2010. Frequency and awareness of hepatitis B and C in visitors of hepatitis awareness mela. J. Pak. Med. Assoc. 60, 1069–1071. [PubMed] [Google Scholar]
- 69.Ali A, Ahmad H, Ali I, Khan S, Zaidi G, Idrees M. 2010. Prevalence of active hepatitis C virus infection in District Mansehra Pakistan. Virol. J. 7, 334 (doi:10.1186/1743-422X-7-334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aziz S, Khanani R, Noorulain W, Rajper J. 2010. Frequency of hepatitis B and C in rural and periurban Sindh. J. Pak. Med. Assoc. 60, 853–857. [PubMed] [Google Scholar]
- 71.Hashmi A, Saleem K, Soomro JA. 2010. Prevalence and factors associated with hepatitis C virus seropositivity in female individuals in Islamabad, Pakistan. Int. J. Prev. Med. 1, 252–256. [PMC free article] [PubMed] [Google Scholar]
- 72.Hyder O, Ijaz M, Arshad MA, Zahira T. 2010. Age-specific frequency of screen-detected hepatitis C virus seropositivity in men from the Punjab province of Pakistan. J. Med. Screen 17, 214–216. (doi:10.1258/jms.2010.010101) [DOI] [PubMed] [Google Scholar]
- 73.Jadoon N, Shahzad A, Yaqoob R. 2010. Frequency of hepatitis C virus infection in Pakistani patients with type 2 diabetes mellitus. Int. J. Infect. Dis. 14, S68 (doi:10.1016/S1201-9712(10)60208-1) [Google Scholar]
- 74.Jadoon NA, Shahzad MA, Yaqoob R, Hussain M, Ali N. 2010. Seroprevalence of hepatitis C in type 2 diabetes: evidence for a positive association. Virol. J. 7, 304 (doi:10.1186/1743-422X-7-304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jamil MS, Ali H, Shaheen R, Basit A. 2010. Prevalence, knowledge and awareness of hepatitis C among residents of three union councils in Mansehra. J. Ayub Med. Coll. Abbottabad 22, 192–196. [PubMed] [Google Scholar]
- 76.Janjua NZ, Hamza HB, Islam M, Tirmizi SFA, Siddiqui A, Jafri W, Hamid S. 2010. Health care risk factors among women and personal behaviours among men explain the high prevalence of hepatitis C virus infection in Karachi, Pakistan. J. Viral Hepat. 17, 317–326. (doi:10.1111/j.1365-2893.2009.01230.x) [DOI] [PubMed] [Google Scholar]
- 77.Shah SM, Khattak IU, Ali A, Tariq M. 2010. Seropositivity for hepatitis B and C in voluntary blood donors. J. Ayub Med. Coll. Abbottabad 22, 149–151. [PubMed] [Google Scholar]
- 78.Taseer IU, Ishaq F, Hussain L, Safdar S, Mirbahar AM, Faiz SA. 2010. Frequency of anti-HCV, HBsAg and related risk factors in pregnant women at Nishtar Hospital, Multan. J. Ayub Med. Coll. Abbottabad 22, 13–16. [PubMed] [Google Scholar]
- 79.Aziz S, Hossain N, Karim SA, Rajper J, Soomro N, Noorulain W, Qamar R, Khanani R. 2011. Vertical transmission of hepatitis C virus in low to middle socio-economic pregnant population of Karachi. Hepatol. Int. 5, 677–680. (doi:10.1007/s12072-010-9229-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Borhany M, et al. 2011. Transfusion transmitted infections in patients with hemophilia of Karachi, Pakistan. Clin. Appl. Thromb. Hemost. 17, 651–655. (doi:10.1177/1076029611398122) [DOI] [PubMed] [Google Scholar]
- 81.Iqbal A, Akram M, Ali H, Akhtar N, Nazir SUR, Ahmad I, Awan A, Asif HM. 2011. Prevalence of hepatitis C virus (HCV) in Gadap town Karachi, Pakistan. J. Med. Plant Res. 5, 6102–6104. [Google Scholar]
- 82.Khan NU, et al. 2011. Prevalence of active HCV infection among the blood donors of Khyber Pakhtunkwa and FATA region of Pakistan and evaluation of the screening tests for anti-HCV. Virol. J. 8, 154 (doi:10.1186/1743-422X-8-154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rauf A, Nadeem MS, Ali A, Iqbal M, Mustafa M, Latif MM, Latif MZ, Ahmed N, Shakoori AR. 2011. Prevalence of hepatitis B and C in internally displaced persons of war against terrorism in Swat, Pakistan. Eur. J. Public Health 21, 638–642. (doi:10.1093/eurpub/ckq084) [DOI] [PubMed] [Google Scholar]
- 84.Safi SZ, Afzal MS, Waheed Y, Butt UJ, Fatima K, Parvez Y, Qadri I. 2011. Seroprevalence of hepatitis C and human immunodeficiency viruses in blood donors of northwestern Pakistan. Asian Biomed. 5, 389–392. [Google Scholar]
- 85.Saleem M, Ahmad W, Sarwar J, Jamshed F, Gul N, Idrees M. 2011. Frequency of hepatitis C in asymptomatic patients in district headquarters hospital Kotli, Azad Kashmir. J. Ayub Med. Coll. Abbottabad 23, 59–62. [PubMed] [Google Scholar]
- 86.Yousaf MZ, Idrees M, Saleem Z, Rehman IU, Ali M. 2011. Expression of core antigen of HCV genotype 3a and its evaluation as screening agent for HCV infection in Pakistan. Virol. J. 8, 364 (doi:10.1186/1743-422X-8-364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahmed F, Irving WL, Anwar M, Myles P, Neal KR. 2012. Prevalence and risk factors for hepatitis C virus infection in Kech District, Balochistan, Pakistan: most infections remain unexplained. A cross-sectional study. Epidemiol. Infect. 140, 716–723. (doi:10.1017/S0950268811001087) [DOI] [PubMed] [Google Scholar]
- 88.Ansari SH, Shamsi TS, Khan MT, Perveen K, Farzana T, Erum S, Ansari I. 2012. Seropositivity of hepatitis C, hepatitis B and HIV in chronically transfused beta-thalassaemia major patients. J. Coll. Physicians Surg. Pak. 22, 610–611. [PubMed] [Google Scholar]
- 89.Attaullah S, Khan S, Khan J. 2012. Trend of transfusion transmitted infections frequency in blood donors: provide a road map for its prevention and control. J. Transl. Med. 10, 20 (doi:10.1186/1479-5876-10-20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bhutta AZ, Tahir Z, Ayub S, Mushtaq S. 2012. Seroprevalence of anti-HCV in non-professional blood donors. Pak. J. Med. Health Sci. 6, 175–178. [Google Scholar]
- 91.Hafeez ud d, Siddiqui TS, Lahrasab W, Sharif MA. 2012. Prevalence of hepatitis B and C in healthy adult males of paramilitary personnel in Punjab. J. Ayub Med. Coll. Abbottabad 24, 138–140. [PubMed] [Google Scholar]
- 92.Ijaz R, Bhatti S, Ullah S. 2012. Prevalence of hepatitis B and C in healthy blood donors in a peripheral hospital: Ghurki trust hospital, Lahore. Pak. J. Med. Health Sci. 6, 568–569. [Google Scholar]
- 93.Khan MI, Muhammad M. 2012. Frequency of hepatitis B and C in patients visiting outpatient department of district head quarters hospital Lakki. J. Postgrad. Med. Inst. 26, 55–60. [Google Scholar]
- 94.Khan S. 2012. Improving the safety of blood products through stringent donor selection, pre and post donation screening of blood. Vox Sang. 103, 98–99. [Google Scholar]
- 95.Memon AR, Shafique K, Memon A, Draz AU, Rauf MUA, Afsar S. 2012. Hepatitis B and C prevalence among the high risk groups of Pakistani population. A cross sectional study. Arch. Public Health 70, 9 (doi:10.1186/0778-7367-70-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muhammad SK, Chandio MA, Soomro MA, Shaikh BA. 2012. Hepatitis C virus infection in non-Hodgkin's lymphoma: a case-control study. Hepatitis Mon. 12, 16–22. (doi:10.5812/kowsar.1735143X.4311) [Google Scholar]
- 97.Nawaz A, Chaudhry A, Nawaz S, Nawaz M, Alvi A, Riaz M, Yousaf I. 2012. Risk factors for development of chronic hepatitis in the developing world: risk factors and attitudes. Am. J. Gastroenterol. 107, S167 (doi:10.1038/ajg.2011.410) [Google Scholar]
- 98.Waheed U, Zaheer HA, Astori S. 2012. Transfusion transmitted infections among blood donors of a teaching hospital in Islamabad, Pakistan. Vox Sang. 103, 157. [Google Scholar]
- 99.Abbas F, Mengal MA, Hanif M, Ali M, Pirkarni GS. 2013. Seroprevalence of hepatitis C virus in general population of Balochistan, Pakistan. Pak. J. Med. Health Sci. 7, 180–184. [Google Scholar]
- 100.Butt KK, Shafiq F, Yousaf MA. 2013. Prevalence of HIV, ANTI-HCV, HBsAg and VDRL positive cases in blood donors of bhatti international Trust Hospital, Kasur. Pak. J. Med. Health Sci. 7, 662–663. [Google Scholar]
- 101.Irfan SM, Uddin J, Zaheer HA, Sultan S, Baig A. 2013. Trends in transfusion transmitted infections among replacement blood donors in Karachi, Pakistan. Turk. J. Hematol. 30, 163–167. (doi:10.4274/Tjh.2012.0132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khan A, Tareen AM, Ikram A, Rahman H, Wadood A, Qasim M, Khan K. 2013. Prevalence of HCV among the young male blood donors of Quetta region of Balochistan, Pakistan. Virol. J. 10, 83 (doi:10.1186/1743-422X-10-83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khan FS, et al. 2013. The burden of non-communicable disease in transition communities in an Asian megacity: baseline findings from a cohort study in Karachi, Pakistan. PLoS ONE 8, e56008 (doi:10.1371/journal.pone.0056008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qadeer MI, Hasnain S, Yasmeen H. 2013. Sero-prevalence of sexually transmitted disease (HIV, syphilis, hepatitis-B and hepatitis-C) in volunteer donors of gaol inmates and student community in Punjab province of Pakistan. Sex. Transm. Infect. Conf. STI AIDS World Congr. 89, A264.1–A264. (doi:10.1136/sextrans-2013-051184.0820) [Google Scholar]
- 105.Rauf M, Saleem MD, Anwer MO, Ahmed G, Aziz S, Memon MA. 2013. HIV, hepatitis B and hepatitis C in garbage scavengers of Karachi. J. Pak. Med. Assoc. 63, 798–802. [PubMed] [Google Scholar]
- 106.Seema B. 2013. Seroprevelance and risk factors for hepatitis C virus (HCV) infection in pregnant women attending public sector tertiary care hospital in Hyderabad Sindh. Int. J. Obstet. Gynaecol. 120, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zaffar G, Ali R, Ayyub M, Ahmed A, Ali A et al. 2013. Frequency and trends of infectious pathogen in blood donors at a tertiary care hospital in Pakistan. Hepatol. Int. 7, S346. [Google Scholar]
- 108.Ali S, et al. 2014. Genotyping of HCV RNA reveals that 3a is the most prevalent genotype in Mardan, Pakistan. Adv. Virol. 2014, 1–5. (doi:10.1155/2014/606201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ilyas M, Ahmad I. 2014. Chemiluminescent microparticle immunoassay based detection and prevalence of HCV infection in district Peshawar Pakistan. Virol. J. 11, 127 (doi:10.1186/1743-422X-11-127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moiz B, Moatter T, Shaikh U, Adil S, Ali N, Mahar F, Shamsuddin N, Khurshid M. 2014. Estimating window period blood donations for human immunodeficiency virus type 1, hepatitis C virus, and hepatitis B virus by nucleic acid amplification testing in Southern Pakistan. Transfusion 54, 1652–1659. (doi:10.1111/trf.12521) [DOI] [PubMed] [Google Scholar]
- 111.Parveen S, Latif A, Ashraf M. 2014. Seroprevalence of hepatitis C virus (HCV) in southern Punjab. Med. Forum Mon. 25, 2–4. [Google Scholar]
- 112.Kumari K, Seetlani NK, Akhter R. 2015. The emergent concern of seropositive status of hepatitis-B virus and hepatitis-C virus in the pregnant females attending a tertiary care hospital. J. Ayub Med. Coll. Abbottabad 27, 155–157. [PubMed] [Google Scholar]
- 113.Niazi SK, Bhatti FA, Salamat N, Ghani E, Tayyab M. 2015. Impact of nucleic acid amplification test on screening of blood donors in Northern Pakistan. Transfusion 55, 1803–1811. (doi:10.1111/trf.13017) [DOI] [PubMed] [Google Scholar]
- 114.Sheikh A. 2015. Seroprevalence of blood borne viruses among blood donors attended during an earthquake campaign at Gwadar Port, a south-west coastal area of Pakistan. Ann. Oncol. 26, ix159 (doi:10.1093/annonc/mdv535.14) [Google Scholar]
- 115.Donchuk D, Rossi G, Bjorklund Y, Zainal HM, Auat R et al. 2016. Hepatitis C treatment in a primary care clinic in the high HCV burden setting in Karachi, Pakistan. Hepatol. Int. 10, S34. [Google Scholar]
- 116.Karim F, Nasar A, Alam I, Alam I, Hassam S, Gul R, Ullah S, Rizwan M. 2016. Incidence of active HCV infection amongst blood donors of Mardan District, Pakistan. Asian Pac. J. Cancer Prev. 17, 235–238. (doi:10.7314/APJCP.2016.17.1.235) [DOI] [PubMed] [Google Scholar]
- 117.Mujeeb SA, Shiekh MA, Khanani R, Jamal Q. 1997. Prevalence of hepatitis C virus infection among beta-thalassaemia major patients. Trop. Doct. 27, 105 (doi:10.1177/004947559702700220) [DOI] [PubMed] [Google Scholar]
- 118.Gul A, Iqbal F. 2003. Prevalence of hepatitis C in patients on maintenance haemodialysis. J. Coll. Physicians Surg. Pak. 13, 15–18. [PubMed] [Google Scholar]
- 119.Khokhar N, Alam AY, Naz F, Mahmood SN. 2005. Risk factors for hepatitis C virus infection in patients on long-term hemodialysis. J. Coll. Physicians Surg. Pak. 15, 326–328. [PubMed] [Google Scholar]
- 120.Mumtaz A, Anees M, Barki MH, Sami W, Hussain S, Nazir M. 2009. Erectile dysfunction in haemodialysis patients. J. Ayub Med. Coll. Abbottabad 21, 4–7. [PubMed] [Google Scholar]
- 121.Ullah F, et al. 2010. To assess the sero-prevalence of viral hepatitis B, C and HIV in multi-transfused thalassemia major patients of Civil Hospital, Karachi, Pakistan. Eur. J. Med. Res. 15, 119. [Google Scholar]
- 122.Attaullah S, Ali I, Ayaz S, Naseemullah, Khan S, Siraj S, Khan J. 2011. Rising burden of hepatitis C virus in hemodialysis patients. Virol. J. 8, 438 (doi:10.1186/1743-422X-8-438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Riaz H, et al. 2011. Assessment of the seroprevalence of viral hepatitis B, viral hepatitis C and HIV in multitransfused thalassaemia major patients in Karachi, Pakistan. Trop. Doct. 41, 23–25. (doi:10.1258/td.2010.100158) [DOI] [PubMed] [Google Scholar]
- 124.Sadiq F, Ashraf T, Ahmed N. 2012. Frequency of hepatitis B and hepatitis C virus infections in transfusion dependant children. Pak. Paediatr. J. 36, 19–22. [Google Scholar]
- 125.Daud MY, Qazi RA, Bashir N. 2014. Anti-retroviral drugs compliance in intravenous and non intravenous drug abusers. J. Ayub Med. Coll. Abbottabad 26, 437–440. [PubMed] [Google Scholar]
- 126.Dasti JI, Uddin SG, Malik MS. 2014. Prevalence of HCV infection among thalassemia patients; a perspective from a multi-ethnic population of Islamabad region. FEBS J. 281, 154. [DOI] [PubMed] [Google Scholar]
- 127.Mahmud HM, Siddiqui M, Bashir B, Ali SF, Baloch AA, Masroor M. 2014. Hemodialysis patients profile at Dow University of Health Sciences, Karachi, Pakistan. Pak. J. Med. Sci. 30, 1327–1330. (doi:10.12669/pjms.306.5364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chishti SMI, Khan AM, Bashir F. 2015. Serological monitoring of HCV marker in hemodialysis patients from tertiary care hospitals of Karachi. Med. Forum Mon. 26, 6–11. [Google Scholar]
- 129.Khan MS, Ahmed M, Khan RA, Mushtaq N, Shah MWU. 2015. Consanguinity ratio in β-thalassaemia major patients in District Bannu. J. Pak. Med. Assoc. 65, 1161–1163. [PubMed] [Google Scholar]
- 130.Yasmeen H, Hasnain S. 2015. Prevalence of transfusion transmitted infections in individuals with thalassemia in Pakistani population. Haematologica 100, 774–775. [Google Scholar]
- 131.Kuo I, ul-Hasan S, Galai N, Thomas DL, Zafar T, Ahmed MA, Strathdee SA. 2006. High HCV seroprevalence and HIV drug use risk behaviors among injection drug users in Pakistan. Harm Reduct. J. 3, 26 (doi:10.1186/1477-7517-3-26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Achakzai M, Kassi M, Kasi PM. 2007. Seroprevalences and co-infections of HIV, hepatitis C virus and hepatitis B virus in injecting drug users in Quetta, Pakistan. Trop. Doct. 37, 43–45. (doi:10.1258/004947507779951989) [DOI] [PubMed] [Google Scholar]
- 133.Altaf A, Shah SA, Zaidi NA, Memon A, Nadeem ur R, Wray N. 2007. High risk behaviors of injection drug users registered with harm reduction programme in Karachi, Pakistan. Harm Reduct. J. 4, 7 (doi:10.1186/1477-7517-4-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Abbasi S, Faqir F, Khan S, Zaidi SK, Ahmed SQ, Sattar A, Satti M. 2009. A serological study of hepatitis C and human immunodeficiency virus in a cohort of intravenous drug users in Quetta, Balochistan. J. Postgrad. Med. Inst. 23, 3–6. [Google Scholar]
- 135.Platt L, Vickerman P, Collumbien M, Hasan S, Lalji N, Mayhew S, Muzaffar R, Andreasen A, Hawkes S. 2009. Prevalence of HIV, HCV and sexually transmitted infections among injecting drug users in Rawalpindi and Abbottabad, Pakistan: evidence for an emerging injection-related HIV epidemic. Sex. Transm. Infect. 85, ii17–ii22. (doi:10.1136/sti.2008.034090) [DOI] [PubMed] [Google Scholar]
- 136.Rehan N, Bokhari A, Nizamani NM, Jackson D, Naqvi HR, Qayyum K, Mansoor S, Muzaffar R. 2009. National study of reproductive tract infections among high risk groups of Lahore and Karachi. J. Coll. Physicians Surg. Pak. 19, 228–231. [PubMed] [Google Scholar]
- 137.Ur Rehman L, et al. 2011. Active hepatitis C infection and HCV genotypes prevalent among the IDUs of Khyber Pakhtunkhwa. Virol. J. 8, 327 (doi:10.1186/1743-422X-8-327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Freeman MF, Tukey JW. 1950. Transformations related to the angular and the square root. Ann. Math. Stat. 21, 607–611. (doi:10.1214/aoms/1177729756) [Google Scholar]
- 139.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. 2009. Front matter. In Introduction to meta-analysis (ed. Sharples K), p. 421 Chichester, UK: John Wiley & Sons, Ltd. [Google Scholar]
- 140.Schwarzer G, Abu-Raddad LJ, Chemaitelly H, Rucker G. Submitted. Seriously misleading result using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. [DOI] [PMC free article] [PubMed]
- 141.R Core Team 2013. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/) [Google Scholar]
- 142.Schwarzer G.2017. General Package for Meta-Analysis. Version 4.1-0. (http://cran.r-project.org/web/packages/meta/meta.pdf. )
- 143.The Cochrane collaboration. 2008. Cochrane handbook for systematic reviews of interventions. Hoboken, NJ: Wiley-Blackweill. [Google Scholar]
- 144.Zuberi BF, Zuberi FF, Hasan SR, Kumar R, Memon SA, Afsar S. 2009. Frequency of acute hepatitis C after needle stick injury and its treatment outcome. Pak. J. Med. Sci. 25, 766–769. [Google Scholar]
- 145.Makheja KD, Abro AH, Kumar S. 2010. Sero-prevalence of hepatitis C antibodies in the people visiting roadside barbers. Pak. J. Med. Sci. 26, 402–406. [Google Scholar]
- 146.Kazi AM, Shah SA, Jenkins CA, Shepherd BE, Vermund SH. 2010. Risk factors and prevalence of tuberculosis, human immunodeficiency virus, syphilis, hepatitis B virus, and hepatitis C virus among prisoners in Pakistan. Int. J. Infect. Dis. 14, e60–e66. (doi:10.1016/j.ijid.2009.11.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sarwar J, Gul N, Idris M, Anis ur R, Farid J, Adeel MY. et al. 2008. Seroprevalence of hepatitis B and hepatitis C in health care workers in Abbottabad. J. Ayub Med. Coll. Abbottabad 20, 27–29. [PubMed] [Google Scholar]
- 148.Maan MA, Fatma H, Muhammad J. 2014. Epidemiology of hepatitis C viral infection in Faisalabad, Pakistan: a retrospective study (2010–2012). Afr. Health Sci. 14, 810–815. (doi:10.4314/ahs.v14i4.6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lavanchy D. 2011. Evolving epidemiology of hepatitis C virus. Clin. Microbiol. Infect. 17, 107–115. (doi:10.1111/j.1469-0691.2010.03432.x) [DOI] [PubMed] [Google Scholar]
- 150.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. 2013. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 57, 1333–1342. (doi:10.1002/hep.26141) [DOI] [PubMed] [Google Scholar]
- 151.Cornberg M, et al. 2011. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 31, 30–60. (doi:10.1111/j.1478-3231.2011.02539.x) [DOI] [PubMed] [Google Scholar]
- 152.Trickey A, et al. 2017. Importance and contribution of community, social, and healthcare risk factors for hepatitis C infection in Pakistan. Am. J. Trop. Med. Hyg. 97, 1920–1928. (doi:10.4269/ajtmh.17-0019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alter MJ. 2007. Epidemiology of hepatitis C virus infection. World J. Gastroenterol. 13, 2436 (doi:10.3748/wjg.v13.i17.2436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shepard CW, Finelli L, Alter MJ. 2005. Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 5, 558–567. (doi:10.1016/S1473-3099(05)70216-4) [DOI] [PubMed] [Google Scholar]
- 155.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. 2006. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann. Intern. Med. 144, 705–714. (doi:10.7326/0003-4819-144-10-200605160-00004) [DOI] [PubMed] [Google Scholar]
- 156.Botheju W, Zghyer F, Mahmud S, Abu-Raddad LJ. In preparation. The epidemiology of hepatitis C virus in Central Asia: Systematic review and meta-analyses. [DOI] [PMC free article] [PubMed]
- 157.Centers for Disease Control Prevention (CDC). 2011. Establishment of a viral hepatitis surveillance system--Pakistan, 2009–2011. Morb. Mortal. Wkly. Rep. 60, 1385–1390. 1545-861X1545-861X. [PubMed] [Google Scholar]
- 158.Arshad A, Ashfaq UA. 2017. Epidemiology of hepatitis C infection in Pakistan: current estimate and major risk factors. Crit. Rev. Eukaryot. Gene Expr. 27, 63–77. (doi:10.1615/CritRevEukaryotGeneExpr.2017018953) [DOI] [PubMed] [Google Scholar]
- 159.Simonsen L, Kane A, Lloyd J, Zaffran M, Kane M. 1999. Unsafe injections in the developing world and transmission of bloodborne pathogens: a review. Bull. World Health Organ. 77, 789–800. [PMC free article] [PubMed] [Google Scholar]
- 160.Qureshi H.2015. Pakistan: prevention & control of viral hepatitis. PMRC.
- 161.Centers for Disease Control. 2011. Prevention establishment of a viral hepatitis surveillance system—Pakistan, 2009–2011. Morb. Mortal. Wkly. Rep. 60, 1385. [PubMed] [Google Scholar]
- 162.Ahmad K. 2004. Pakistan: a cirrhotic state? Lancet 364, 1843–1844. (doi:10.1016/S0140-6736(04)17458-8) [DOI] [PubMed] [Google Scholar]
- 163.Janjua NZ, Hutin YJ, Akhtar S, Ahmad K. 2006. Population beliefs about the efficacy of injections in Pakistan's Sindh province. Public Health 120, 824–833. (doi:10.1016/j.puhe.2006.05.004) [DOI] [PubMed] [Google Scholar]
- 164.Government of Pakistan, Ministry of Environment. 2005. Hospital waste management rules.
- 165.Ejaz I, Shaikh BT, Rizvi N. 2011. NGOs and government partnership for health systems strengthening: a qualitative study presenting viewpoints of government, NGOs and donors in Pakistan. BMC Health Serv. Res. 11, 122 (doi:10.1186/1472-6963-11-122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.United Nations Office on Drugs and Crime (UNODC). 2017. World Drug Report 2017. (https://www.unodc.org/wdr2017/index.html)
- 167.Mumtaz GR, Awad SF, Feizzadeh A, Weiss HA, Abu-Raddad LJ. 2017. HIV incidence among people who inject drugs in the Middle East and North Africa: mathematical modeling analysis. J. Int. AIDS Soc. 21, e25102 (doi:10.1002/jia2.25102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mumtaz GR, et al. 2014. HIV among people who inject drugs in the Middle East and North Africa: systematic review and data synthesis. PLoS Med. 11, e1001663 (doi:10.1371/journal.pmed.1001663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pakistan Global AIDS Response Progress Report (GARPR) 2015. National AIDS Control Program. Ministry of National Health Services, Regulation and Coordination. See http://www.aidsdatahub.org/pakistan-global-aids-response-progress-report-2015-national-aids-control-program-2015 .
- 170.The News International. 2016. Price of hepatitis-C drug fixed at Rs 5,868. See https://www.thenews.com.pk/print/97674-Price-of-Hepatitis-C-drug-fixed-at-Rs5868 .
- 171.Polaris Observatory. See http://cdafound.org/polaris-hepC-dashboard/ (accessed 2017).
- 172.Ministry of National Health Services, Regulations and Coordination (NHSRC). 2017. National Hepatitis Strategic Framework (NHSF) for Pakistan 2017–2021. See phrc.org.pk/Extra/NHSF.pdf.
- 173.El-Zanaty F WA. Egypt Demographic and Health Survey. 2008. Cairo: Egyptian Ministry of Health, National Population Council, El-Zanaty and Associates, and ORC Macro. https://dhsprogram.com/publications/publication-fr220-dhs-final-reports.cfm. 2009.
- 174.Cuadros DF, Branscum AJ, Miller FD, Abu-Raddad LJ. 2014. Spatial epidemiology of hepatitis C virus infection in Egypt: analyses and implications. Hepatology 60, 1150–1159. (doi:10.1002/hep.27248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Miller FD, Abu-Raddad LJ. 2010. Evidence of intense ongoing endemic transmission of hepatitis C virus in Egypt. Proc. Natl Acad. Sci. USA 107, 14 757–14 762. (doi:10.1073/pnas.1008877107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chemaitelly H, Abu-Raddad LJ, Miller FD. 2013. An apparent lack of epidemiologic association between hepatitis C virus knowledge and the prevalence of hepatitis C infection in a national survey in Egypt. PLoS ONE 8, e69803 (doi:10.1371/journal.pone.0069803) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Benova L, Awad SF, Miller FD, Abu-Raddad LJ. 2015. Estimation of hepatitis C virus infections resulting from vertical transmission in Egypt. Hepatology 61, 834–842. (doi:10.1002/hep.27596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guerra J, Garenne M, Mohamed M, Fontanet A. 2012. HCV burden of infection in Egypt: results from a nationwide survey. J. Viral Hepat. 19, 560–567. (doi:10.1111/j.1365-2893.2011.01576.x) [DOI] [PubMed] [Google Scholar]
- 179.Health Mo, Population/Egypt, El-Zanaty, Associates/Egypt, ICF International. 2015. Egypt health issues survey 2015. Cairo, Egypt: Ministry of Health and Population/Egypt and ICF International. [Google Scholar]
- 180.NHANES. National Health and Nutrition Examination Survey 1999–2016. See http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 181.Benova L, Awad SF, Abu-Raddad LJ. 2017. Estimate of vertical transmission of hepatitis C virus in Pakistan in 2007 and 2012 birth cohorts. J. Viral Hepat. 24, 1177–1183. (doi:10.1111/jvh.12748) [DOI] [PubMed] [Google Scholar]
- 182.Zaheer HA, Waheed U. 2014. Blood safety system reforms in Pakistan. Blood Transfus. 12, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zafar A, Habib F, Hadwani R, Ejaz M, Khowaja K, Khowaja R, Irfan S. 2009. Impact of infection control activities on the rate of needle stick injuries at a tertiary care hospital of Pakistan over a period of six years: an observational study. BMC Infect. Dis. 9, 78 (doi:10.1186/1471-2334-9-78) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ikram A, Shah SIH, Naseem S, Absar SF, Ullah S, Ambreen T, Sabeeh SM, Niazi SK. 2010. Status of hospital infection control measures at seven major tertiary care hospitals of northern Punjab. J. Coll. Physicians Surg. Pak. 20, 266–270. [PubMed] [Google Scholar]
- 185.World Health Organization. 2015. Making all injections safe. Ginebra, OMS.
- 186.World Health Organization. 2015. WHO guideline on the use of safety-engineered syringes for intramuscular, intradermal and subcutaneous injections in health-care settings. WHO/HIS/SDS/2015.5.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the manuscript and electronic supplementary material.