Abstract
Present investigation evaluates the effect of daidzin in dry eye rat model through the suppression of inflammation and oxidative stress in the cornea. Briefly, electron spine resonance was used for the estimation of radical scavenging activity of daidzin and COX Fluorescent Activity Assay Kit was used for the estimation of PGS activity. Dry eye rat model was developed by removing the lacrimal gland and effect of daidzin was evaluated in dry eye rat model by estimating the fluorescein score, tear volume and expressions of heme oxigenase (HO-1), TNF α, Interlukin 6 (IL-6), matrix metallopeptidase 9 (MMP-9) and PGS-2. Result of the present study suggested that daidzin possess tyrosyl radical scavenging activity and thereby decreases the oxidative stress. Activity of PGS significantly increases in dry eye which was inhibited by daidzin treatment due to competitive inhibition of PGS. It also recovers the tear volume in dry eye rat model in which lacrimal gland was removed. Thus corneal erosion was improved by daidzin in dry eye rat model. Thus present study concludes that treatment with daidzin protects the cornea in dry eye rat model by suppression inflammation and oxidative stress.
Keywords: Daidzin, Dry eye, Radical scavenging activity, Inflammation
1. Introduction
Keratoconjunctivitis sicca is a pathological condition commonly called as Dry eyes characterized by alteration in tears secretion quantity and quality wise (Nakamura et al., 2005). It is clinically manifested by infection, irritation, ulceration and blurred vision (Schaumberg et al., 2002). In dry eyes integrity of ocular surface was changes due to several factors such as age, airflow, UV radiation, temperature, chemical compound, humidity and hormonal changes (Cekiç et al., 2002, Chen et al., 2008, Craig et al., 2000, Zuclich and Connolly, 1979). All these changes generate the free radical that induces inflammation and oxidative stress (Higuchi et al., 2011). Reported literature confirms that tear contains several antioxidants such as lactoferrin, tyrosine and glutathione that protect epithelial cells of the cornea (Fujihara et al., 2000). Lack of tear and dehydration of ocular surface causes dry eyes syndrome (Tsubota and Higuchi, 2000). Thus increased inflammation and oxidative stress are involved in the dry eyes induced corneal ulcer (Antonisamy et al., 2015). So, the drugs that inhibit these factors could be a treatment option for the management of dry eyes.
Estrogen and its derivatives were reported to possess strong antioxidant and anti-inflammatory property (Rifici and Khachadurian, 1992). Moreover study performed by Higuchi et al., proves that 2-hydroxyestradiol significantly decreases inflammation and oxidative stress in dry eye syndrome rat model (Higuchi et al., 2016). Isoflavones are known to produce strong antioxidant and anti-inflammatory activity, moreover isoflavones like daidzin and genistein reported to have similar effect as that of estrogen (Xie et al., 1994). Daidzin is a isoflavones isolated from Pueraria lobata (Fabaceae) (Osman and Fett, 1983). In the recent years many medicinal plants and their metabolites were used as alternative for the treatment of many antibiotics and therapeutic agents (Balamurugan, 2015, Rathi et al., 2015, Nandhini and Stella Bai, 2015, Puthur, 2016, Sreeshma et al., 2016, Serasanambati and Chilakapati, 2016). Literature suggested that daidzin was used for the management of alcohol dependence and neurodegenerative disorders on the basis of its antioxidant and anti-inflammatory property (Rezvani et al., 2003, Zhao et al., 2002). Daidzin inhibits the proinflammatory mediators by inhibits the activation of NF-κB and STAT-1 (Hämäläinen et al., 2007). Thus present investigation evaluates the effect of daidzin in the management of dry eyes.
2. Material and methods
2.1. Animals
Healthy male wistar rats (250–300 g) at about 8 weeks of age were used for the pharmacological screening in the present study. The animals were housed at 25 ± 2 °C temperature, 12 h light/dark cycle and 60 ± 5% of relative humidity. Rats were feed with standard diet and water ad libitum. Protocols of the present investigation for all the animal studies were approved by the Institutional Animal Ethical Committee Jiangxi provincial people’s hospital, Nanchang 330006, China (19/2015).
2.2. Assessment of radical scavenging activity of daidzin
Daidzin was procured from Sigma (USA). Estimation of tyrosine radicals was performed as per the previously reported study using electron spin resonance (Miura, 2012a). The mixture was used in this study contained 5,5-dimethyl-1-pyrroline-N-oxide (DMPO; 100 mM), H2O2 and myoglobin (400 μM) in PBS with (pH 7.4) ethanol. All the steroids were poured into the above given reaction mixture and thereafter H2O2 was added to start the reaction. The ESR spectra were taken in flat cell at room temperature.
2.3. Effect of daidzin on activity of PGS
The concentration of daidzin was estimated for the further experiments by evaluating the effect of daidzin on cellular viability by using human corneal epithelial cell line CEPI-17-CL4 (Sharif et al., 1998). Epilife media which was supplemented with HCGS used to culture the CEPI cells in 96 well plates. CEPI cells were incubated in the medium with daidzi for the duration of one day. Later 10% Alamar Blue containing fresh medium was used to replace the medium of plates. This reagent was used for the estimation of cellular viability. These plates were incubated for the period of 1 h and ARVO SX multilabel reader was used for the determination of fluorescence Alamar Blue.
Evaluation of PGS inhibition activity confirms the anti inflammatory activity of drugs like catechols. CEPI cells cellular extract was used to evaluate the PGS inhibition activity of daidzin. Cellular extract was prepared by homogenizing the CEPI cells with Tris-buffer (5 mM and pH 7.4) that contains DTT and EDTA for the duration of 5 min at 10000 RPM. COX Fluorescent Activity Assay Kit was used for the estimation of activity of PGS. Activity of COX1 and COX2 enzyme was estimated by fluorescence-based method using the given assay kit in crude and purified preparations of enzyme. COX component, peroxidase was utilized in the assay used in this study. Prostaglandin G2 and resorufin was estimated at 530–40 nm wavelength for excitation and 585–95 nm for emission. ARVO SX multilabel reader was used for the estimation of resorufin.
2.4. Effect of daidzin on dry eye
Effect of daidzin was evaluated on by eye using dry eye rat model. All the rats were anesthetized and surgically lacrimal glands were removed to develop dry eye rat model. Experiment was started a day after the removal of lacrimal glands and daidzin was used with 0.1, 1 or 10 μM concentration. 10 mM concentration of daidzin was prepared by dissolving it into ethanol and in PBS at 0.1, 1 or 10 μM. In each rat 5 μl of daidzin was administered for the duration of 2 week four times a day and in vehicle control group vehicle was administered to the lacrimal glands removed rats. After the treatment period tear volume and fluorescein score was evaluated as given in previous study. Moreover, ocular surface was stained with fluorescein to evaluate degree of dry eye. Cornea photographs were separated into nine different areas and depending upon the degree of staining every area was scored between 0 and 3. Score of each area was added together to get total score called as fluorescein score. There are various factors like air stream, temperature, humidity viz. alters the fluorescein score and thus normal rats score considered as a reference (Higuchi et al., 2010).
Total RNA was isolated from the corneas of the entire groups for real time PCR. TRIzol was used to extract total RNA from the cornea and SuperScript III was used to perform reverse transcription. RT-PCR was performed for the estimation of gene expressions of heme oxigenase, TNF α, Interlukin 6 (IL-6), matrix metallopeptidase 9 (MMP-9) and PGS-2. ΔΔCt method was used to analyzed all the data. COX Fluorescent Activity Assay Kit was used to estimate the PGS activity in the extract of the corneas of normal and vehicle groups.
2.5. Statistical analysis
All the values of these experiments were articulated as mean ± SEM and the data was statistically analyzed by one-way ANOVA with Dunnett post hoc test and student t test. p < 0.05 was considered statistically significant.
3. Result
3.1. Estimation of radical scavenging activity of daidzin
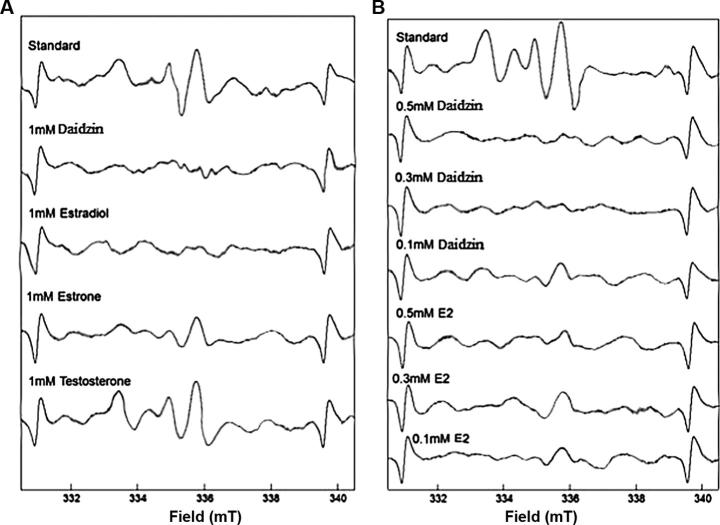
ESR study suggested that ESR signal of tyrosyl radical were reduced by 1 mM esterdiol and 1 mM of daidzin as shown in Fig.1A. Moreover 0.3–0.5 mM concentration of daidzin also significantly reduces the signals and esterdiol reduces ESR signals weekly at the same concentrations as shown in Fig.1B.
Figure 1.
Effect of steroids on ESR signals of hydroxyl radicals. (A) Effect of 1 mM steroid (B) effect of 0.1, 0.3, 0.5 mM of daidzin and E2.
3.2. Effect of daidzin on PGS activity
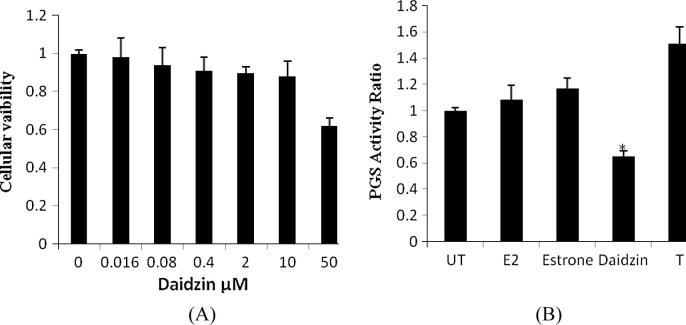
The effect of daidzin on viability of cell was estimated as shown in Fig.2A. It was observed that viability of cell up to 10 μM found to be 90% and thereafter on further increase in concentration it declines significantly. Thus for the further study concentration of daidzin was used below and up to 10 μM. Moreover daidzin at a concentration of 10 μM was significantly inhibited the PGS activity as shown in Fig.2B. Whereas, other steroid used in this study were not alters the PGS activity. Thus, on the basis of its free radical scavenging activity and inhibition of PGS activity daidzin was used for the treatment of dry eye.
Figure 2.
Effect of daidzin on inhibition of PGS activity. (A) Effect of daidzin on Viability of cells. (B) PGS activity of cellular extract in presence or absence of different steroids. UT: Untreated, E2: estradiol and T: testosterone.
Value are expressed as Mean ± SD, ∗p < 0.05 compared to untreated.
3.3. Effect of daidzin on dry eye
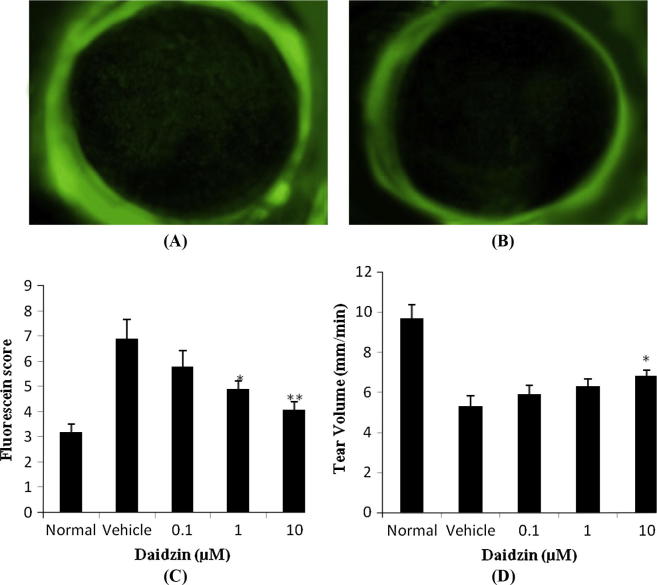
Effect of daidzin on dry eye was shown in Fig. 3. Photo of cornea stained by fluorescein reveals that in PBS treated slide fluorescein-stained areas was bigger compared to the slide treated with daidzin (Fig.3A and B). Fluorescein score was also observed in this study. There were significant increases in the fluorescein score of vehicle treated group than normal group of rats. Treatment with daidzin significantly (∗p < 0.05, ∗∗p < 0.01) decreases the score compared to vehicle treated group. Moreover, result of tear secretion suggested that there were approximately 45% decrease in the tear secretion in vehicle treated group after removal of lacrimal gland and this decrease in the tear secretion was recovered after the treatment of daidzin as shown in Fig.3D.
Figure 3.
Effect of daidzin on dry eye in lacrimal gland removed rats. (A) Treatment with PBS (B) treatment with daizin (C) fluorescein score and (D) tear volume.
Value are expressed as Mean ± SD (n = 10), ∗p < 0.05, ∗∗p < 0.01 compared to Vehicle treated group.
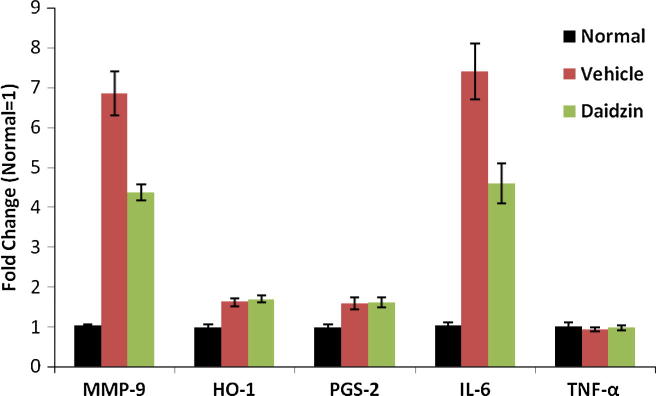
Effect of daidzin on the fold change of mRNA in dry eye was shown in Fig 4. and from ΔCt value was calculated fold change (Table 1). Treatment with daidzin suppresses the expression of MMP9 & IL6 which was found to be increased in vehicle treated group. Whereas, there were no alteration in the expressions of TNF-α in daidzin and vehicle treated group than normal rat. Although there was weakly increase in the expressions of HO-1 and PGS-2 in daidzin and vehicle treated group than normal rat.
Figure 4.
Effect of daidzin on the fold change of mRNA in the cornea of dry eye. Value are expressed as Mean ± SD, (n = 10).
Table 1.
Effect of daidzin on the expressions of mRNA in cornea of dry eye.
| Sr. No. | MMP-9 | HO-1 | PGS-2 | IL-6 | TNF-α |
|---|---|---|---|---|---|
| 1. | 11.4 ± 1.7 | 10.2 ± 1.1 | 9.3 ± 0.5 | 18.3 ± 2.9 | 9.1 ± 0.6 |
| 2. | 8.2 ± 1.3 | 9.3 ± 0.6 | 9 ± 0.7 | 15.4 ± 2.3 | 9.5 ± 0.7 |
| 3. | 9.1 ± 0.3 | 9.3 ± 0.6 | 8.8 ± 0.4 | 16.3 ± 2.4 | 9.2 ± 0.7 |
Value are expressed as Mean ± SD (n = 10).
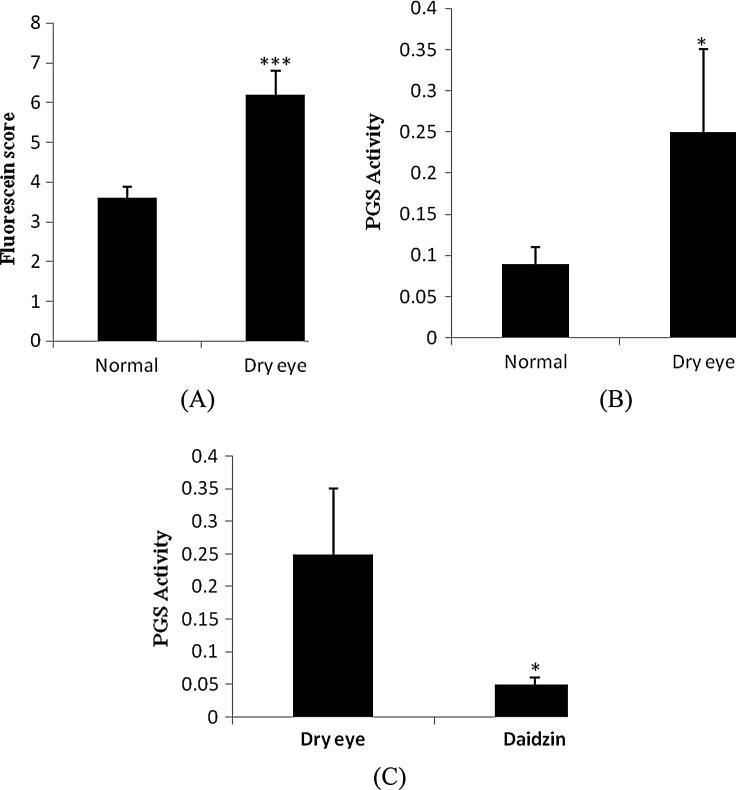
3.4. Assessment of cornea for PGS activity
Tear secretion was decreased and cornea was erosed in the rat without lacrimal gland. There were significant increase in the fluorescein score in dry eye in compared to normal side. It was observed that PGS activity found to be higher in dry eye than normal side. Moreover treatment with daidzin on significantly inhibited PGS activity compared to dry eye side (Fig 5 A–C).
Figure 5.
Assessment PGS activity on the cornea of dry eye. (A) Fluorescein score, (B) PGS activity and (C) effect of daidzin on PGS activity. Value are expressed as Mean ± SD (n = 10), ∗p < 0.05, ∗∗∗p < 0.01 compared to normal or dry eye.
4. Discussion
Present investigation evaluates the protective effect of daidzin on cornea in dry eye rat model and postulates the possible mechanism of daidzin for its action. Dry eye rat model was developed by removing the lacrimal gland. Various report suggested that this model used for the production of dry eye is simple and no chemical is given for the induction of dry eye (Higuchi et al., 2010, Kalaiselvi et al., 2016, Neelamkavil and Thoppil, 2016). Literature suggested that in dry eye there is increase in the expressions of IL-6, MMP9 & PGS2 in the cornea cells (Li et al., 2010, Valsan and Raphael, 2016, Noorudheen and Chandrasekharan, 2016, Santhosh et al., 2016, Sreeshma et al., 2016). Treatment with daidzin suppresses the expressions of IL6 and MMP9, which was increased in the dye eye. These result suggested that daidzin improves the dry eye syndrome.
Effect of daidzin was not suppresses the expression of PGS2 in CEPI cells. This suggested that in cellular extract daidzin was dissociated from PGS. Thus the study concludes that daidzin inhibits the activity of PGS through competitive inhibition. Its effect on tyrosine radicals were also assessed in the investigation. It was observed that daidzin posses tyrosine radical scavenging activity and tyrosine radical was essential for the activity of PGS (Miura, 2012b). Hence, there was a relation between tyrosine radical scavenger and PGS inhibitor confirmed. It means that daidzin inhibits the activity of PGS on the basis of its tyrosine radical scavenging activity. Moreover there were no alteration in the expressions of TNF-α found with vehicle and daidzin treatment than normal. Treatment with daidzin was partially recovers the tear secretion in dry eye rat model. These effects were observed probably due to enhanced secretion of intraorbital lacrimal glands or in ocular surface retention of water get recovered.
5. Conclusion
Thus present study concludes that treatment with daidzin protects the cornea in dry eye rat model by suppression inflammation and oxidative stress.
Acknowledgement
All the authors of this manuscript are thankful to Jiangxi provincial people’s hospital, Nanchang 330006, China for providing necessary facility for the work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Antonisamy P., Duraipandiyan V., Ignacimuthu S., Kim J.-H. Anti-diarrhoeal activity of friedelin isolated from Azima tetracantha lam. in wistar rats. South Indian J. Biol. Sci. 2015;1:34–37. [Google Scholar]
- Balamurugan R. Smilax chinensis Linn. (Liliaceae) root attenuates insulin resistance and ameliorate obesity in high diet induced obese rat. South Indian J. Biol. Sci. 2015;1:47–51. [Google Scholar]
- Cekiç O., Ohji M., Hayashi A., Fang X.Y., Kusaka S., Tano Y. Effects of humidified and dry air on corneal endothelial cells during vitreal fluid-air exchange. Am. J. Ophthalmol. 2002;134:75–80. doi: 10.1016/s0002-9394(02)01472-1. [DOI] [PubMed] [Google Scholar]
- Chen W., Zhang X., Zhang J. A murine model of dry eye induced by an intelligently controlled environmental system. Invest. Ophthalmol. Vis. Sci. 2008;49:1386–1391. doi: 10.1167/iovs.07-0744. [DOI] [PubMed] [Google Scholar]
- Craig J.P., Singh I., Tomlinson A., Morgan P.B., Efron N. The role of tear physiology in ocular surface temperature. Eye (Lond) 2000;14:635–641. doi: 10.1038/eye.2000.156. [DOI] [PubMed] [Google Scholar]
- Fujihara T., Nagano T., Endo K., Nakamura M., Nakata K. Lactoferrin protects against UV-B irradiation-induced corneal epithelial damage in rats. Cornea. 2000;19:207–211. doi: 10.1097/00003226-200003000-00015. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M., Nieminen R., Vuorela P., Heinonen M., Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007;2007:45673. doi: 10.1155/2007/45673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi A., Takahashi K., Hirashima M., Kawakita T., Tsubota K. Selenoprotein P controls oxidative stress in cornea. PLoS ONE. 2010;5:e9911. doi: 10.1371/journal.pone.0009911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi A., Kawakita T., Tsubota K. IL-6 induction in desiccated corneal epithelium in vitro and in vivo. Mol. Vision. 2011;17:2400–2406. [PMC free article] [PubMed] [Google Scholar]
- Higuchi A., Oonishi E., Kawakita T., Tsubota K. Evaluation of treatment for dry eye with 2-hydroxyestradiol using a dry eye rat model. Mol. Vision. 2016;22:446–453. [PMC free article] [PubMed] [Google Scholar]
- Kalaiselvi V., Binu T.V., Radha S.R. Preliminary phytochemical analysis of the various leaf extracts of Mimusops elengi L. South Indian J. Biol. Sci. 2016;2:24–29. [Google Scholar]
- Li N., He J., Schwartz C.E., Gjorstrup P., Bazan H.E. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J. Ocul. Pharmacol. Ther. 2010;26:431–439. doi: 10.1089/jop.2010.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T. Reactivity of nonsteroidal anti-inflammatory drugs with peroxidase: a classification of nonsteroidal anti-inflammatory drugs. J. Pharm. Pharmacol. 2012;64:1461–1471. doi: 10.1111/j.2042-7158.2012.01524.x. [DOI] [PubMed] [Google Scholar]
- Miura T. A mechanistic study of the formation of hydroxyl radicals induced by horseradish peroxidase with NADH. J. Biochem. 2012;152:199–206. doi: 10.1093/jb/mvs068. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Shibuya M., Nakashima H., Imagawa T., Uehara M., Tsubota K. D-β-hydroxybutyrate protects against corneal epithelial disorders in a rat dry eye model with jogging board. Invest. Ophthalmol. Vis. Sci. 2005;46 doi: 10.1167/iovs.04-1344. 2679-2387. [DOI] [PubMed] [Google Scholar]
- Nandhini V.S., Stella Bai G.V. In-vitro Phytopharmacological effect and cardio protective activity of Rauvolfia tetraphylla L. South Indian J. Biol. Sci. 2015;1:97–102. [Google Scholar]
- Neelamkavil S.V., Thoppil J.E. Evaluation of the anticancer potential of the traditional medicinal herb Isodon coetsa. South Indian J. Biol. Sci. 2016;2:41–45. [Google Scholar]
- Noorudheen N., Chandrasekharan D.K. Effect of ethanolic extract of Phyllanthus emblica on captan induced oxidative stress in vivo. South Indian J. Biol. Sci. 2016;2:95–102. [Google Scholar]
- Osman S., Fett W. Isoflavone glucoside stress metabolites of soybean leaves. Phytochemistry. 1983;22(9):1921. [Google Scholar]
- Puthur J.T. Antioxidants and cellular antioxidation mechanism in plants. South Indian J. Biol. Sci. 2016;2:14–17. [Google Scholar]
- Rathi M.A., Meenakshi P., Gopalakrishnan V.K. Hepatoprotective activity of ethanolic extract of alysicarpus vaginalis against nitrobenzene-induced hepatic damage in rats. South Indian J. Biol. Sci. 2015;1:60–65. [Google Scholar]
- Rezvani A., David H., Perfumi M., Massi M. Plant derivatives in the treatment of alcohol dependency. Pharmacol. Biochem. Behav. 2003;75(3):593. doi: 10.1016/s0091-3057(03)00124-2. [DOI] [PubMed] [Google Scholar]
- Rifici V.A., Khachadurian A.K. The inhibition of low-density lipoprotein oxidation by 17-beta estradiol. Metabolism. 1992;41:1110–1114. doi: 10.1016/0026-0495(92)90295-l. [DOI] [PubMed] [Google Scholar]
- Santhosh S.K., Venugopal A., Radhakrishnan M.C. Study on the phytochemical, antibacterial and antioxidant activities of Simarouba glauca. South Indian J. Biol. Sci. 2016;2:119–124. [Google Scholar]
- Schaumberg D.A., Sullivan D.A., Dana M.R. Epidemiology of dry eye syndrome. Adv. Exp. Med. Biol. 2002;506:989–998. doi: 10.1007/978-1-4615-0717-8_140. [DOI] [PubMed] [Google Scholar]
- Serasanambati M., Chilakapati S.R. Function of Nuclear Factor Kappa B (NF-kB) in human diseases-a review. South Indian J. Biol. Sci. 2016;2:368–387. [Google Scholar]
- Sharif N.A., Wiernas T.K., Howe W.E., Griffin B.W., Offord E.A., Pfeifer A.M. Human corneal epithelial cell functional responses to inflammatory agents and their antagonists. Invest. Ophthalmol. Vis. Sci. 1998;39:2562–2571. [PubMed] [Google Scholar]
- Sreeshma P.S., Raphael K.R., Baby A.A. Pharmacognostic studies of leaves of Naravelia zeylanica (Linn) DC. South Indian J. Biol. Sci. 2016;2:179–182. [Google Scholar]
- Tsubota K., Higuchi A. Serum application for the treatment of ocular surface disorders. Int. Ophthalmol. Clin. 2000;40:113–122. doi: 10.1097/00004397-200010000-00009. [DOI] [PubMed] [Google Scholar]
- Valsan A.L., Raphael K.R. Pharmacognostic profile of Averrhoa bilimbi Linn. leaves. South Indian J. Biol. Sci. 2016;2:75–80. [Google Scholar]
- Xie C.I., Lin R.C., Antony V., Lumeng L., Li T.K., Mai K., Liu C., Wang Q.D., Zhao Z.H., Wang G.F. Daidzin, an antioxidant isoflavonoid, decreases blood alcohol levels and shortens sleep time induced by ethanol intoxication. Alcohol. Clin. Exp. Res. 1994;18(6):1443–1447. doi: 10.1111/j.1530-0277.1994.tb01448.x. [DOI] [PubMed] [Google Scholar]
- Zhao L., Chen Q., Diaz Brinton R. Neuroprotective and neurotrophic efficacy of phytoestrogens in cultured hippocampal neurons. Exp. Biol. Med. (Maywood) 2002;227(7):509–519. doi: 10.1177/153537020222700716. [DOI] [PubMed] [Google Scholar]
- Zuclich J.A., Connolly J.S. Ocular damage induced by nearultraviolet laser radiation. Invest. Ophthalmol. Vis. Sci. 1979;15:760–764. [PubMed] [Google Scholar]