Abstract
Lethal and sub lethal effects of fresh and old residues of azadirachtin, spinosad, Bacillus thuringiensis var. kurstaki (Bt var. k), and deltamethrin, were evaluated at their recommended field doses against adult and immature stages of Trichogramma brassicae under in vitro conditions. The experiments were carried out at the Entomology section of Division of Crop Protection, ICAR Research Complex for NEH region, Umiam, Meghalaya, in 2012–2013. The effects of different pesticides were determined by bioassays using the residual film method, the diet contamination method, the pupal dip method and the topical application technique. The four pesticides were found harmful to adult T. brassicae after ingestion, however surface contact bioassays revealed that Bt var. k was the least toxic pesticide. Except Bt var. k, other three pesticides were found harmful also to the immature stages of T. brassicae and significantly affected parasitism potential, adult emergence, longevity of adults, and sex ratio of the progeny. Deltamethrin and azadirachtin were the most harmful, even after 15 days of application. Spinosad was found to be relatively safe to T. brassicae after 15 days of application. As Bt appeared to be the least toxic pesticide for T. brassicae, it could be used for the management of severe infestations of lepidopteran pests in cruciferous ecosystems.
If essential, spinosad may be used 15 days after parasitoid release, thus minimizing the chances of parasitoid exposure.
Keywords: Azadirachtin, Bacillus thuringiensis, Deltamethrin, Spinosad
1. Introduction
Cole crops are a major component in human diets. Many lepidopteran pests attack cole crops in various growth stages; the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) and the cabbage butterfly, Pieris brassicae (Lepidoptera: Pieridae) are the most destructive among all (Firake et al., 2012a, Lytan and Firake, 2012). The caterpillars of both pest species feed voraciously on the leaves, defoliate the plants and make pesticidal applications obligatory in the cultivation of cole crops (Kulye et al., 2007, Firake et al., 2012b). In addition to cultural practices, eco-friendly management of cruciferous pests has traditionally relied on exploiting potential parasitoids and entomopathogens through conservation and augmentation. The wasp Trichogramma brassicae (Bezdenko) (Hymenoptera: Trichogrammatidae) is used worldwide against several lepidopteran pests of agricultural and horticultural crops (Polaszek, 2010, Ghoneim, 2014). Augmentative releases of T. brassicae in cruciferous crops are usually done at the rate of 6,00,000 parasitoids per hectare concurring with the egg laying period of the target pests (Anonymous, 2009, Krishnamoorthy, 2012). Due to the very high fecundity of the cabbage butterfly and the diamondback moth and a low market tolerance of cole crops to pest damage, intervention of pesticides becomes crucial for better yields. Several pesticides, including synthetic inorganic, botanicals and of microbial origin are being used in cruciferous ecosystems to manage major lepidopteran pests. Azadirachtin, spinosad, Bacillus thuringiensis var. kurstaki and deltamethrin are the commonly used pesticides against lepidopteran pests in cruciferous ecosystem.
The application of pesticides on a field with natural enemies of crop pests is often critical. Under field conditions, T. brassicae wasps are frequently affected by direct contact with the treated plant surfaces during foraging, finding mates and feeding on contaminated water or honeydew available on plant surfaces in the sprayed field. They are also indirectly affected since during their immature stages, they live and feed inside the host eggs. In several cases, these parasitoids die along with their hosts. Being a weak flier, T. brassicae habitually stays for a longer time on plant surfaces presenting comparatively high risks than any other parasitoids.
Besides direct mortality (lethal effect), pesticides also affect several important biological traits of parasitoids, including parasitism potential, longevity, sex ratio, and adult emergence (Firake and Khan, 2010, Croft, 1990, Desneux et al., 2007). It may also affect foraging behaviour, intra-specific communication, and courtship. Consequently, there is an urgent need to understand the risks (lethal and sub-lethal effects) of commonly used pesticides on T. brassicae and also to identify the pesticides with appropriate timing of application that controls pests without adversely affecting their natural enemies (Hafez et al., 1999). Although effects of pesticides on Trichogramma wasp have been extensively studied, information on the effects of four commonly used pesticides viz., azadirachtin, spinosad, Bt var. kurstaki and deltamethrin to T. brassicae is still not available. Considering all possible direct and indirect risks, these pesticides were evaluated at their recommended field doses against T. brassicae under laboratory conditions. In addition, residual toxicity tests were conducted to know the persistence of these pesticides after 15 days, so that a decision can be made to release T. brassicae in the field in an integrated pest management programme.
2. Materials and methods
All experiments were carried out in Biological Control Laboratory at Division of Crop Protection (Entomology), ICAR Research Complex for NEH region, Umiam, Meghalaya in 2012–2013. On the basis of literature, four commonly used pesticides at their recommended field doses against cabbage butterfly were selected for this study viz., spinosad 45 SC (0.05%), azadirachtin 0.15 EC (0.2%), B. thuringiensis var. kurstaki 8 SP (0.2%) and deltamethrin 2.5 EC (0.1%).
2.1. Source and mass rearing of egg parasitoid T. brassicae
Fresh culture of T. brassicae was procured from the State Biological Control Laboratory, Upper Shillong, Meghalaya and further reared on the eggs of rice moth, Corcyra cephalonica Stainton (Lepidoptera: Pyralidae) in the laboratory (25 ± 1 °C temperature, 75 ± 5% R.H. and 16:8, light: dark period). The C. cephalonica was reared on a standard diet containing maize, Zea mays L. (Poales: Poaceae) following appropriate protocol (Firake and Khan, 2010, Firake and Khan, 2013) with little modification.
Paper strips containing about 5000 UV-light-treated eggs of C. cephalonica were exposed to mated females of T. brassicae @ (1:6, parasitoid: host ratio). Fine streak of 50% honey solution was provided as a food to the adult parasitoids. After 24 h of exposure, the parasitized egg strips were removed and reared separately until the emergence of T. brassicae. About 7–9 days were required for T. brassicae to complete its immature stages. Adults of T. brassicae were used for experiments on diet contamination bioassay and residual film method.
Besides, a large number of P. brassicae adults were collected from the field and released inside the specially developed cages (45 × 45 × 45 cm) for egg laying on potted cabbage plants. Egg masses were used for studying the effect of pesticides on adult emergence, longevity, parasitism potential and sex ratio of the progeny.
2.2. Evaluation of lethal and sub-lethal effects of fresh and 15 day old residues of pesticides on egg parasitoid, T. brassicae
Pesticide solutions with required concentrations were made in the clean and sterilized glass jar with the help of micropipette (Tarsons®). Distilled water along with 0.1% Triton X-100 was used for the preparation of pesticide solutions. Only 0.1% Triton X-100 solution was used as a control in each experiment. Triton X-100 is a commonly used surfactant during pesticide spraying in the field, which allows the pesticides to properly spread on the target surface. New sterilized plastic containers (Tarsons®, size 8 cm height and 6 cm diameter) with sufficient ventilation were used for the bioassay experiment. The bioassay experiment was conducted at ambient conditions (average temperature 22 ± 1 °C and 75 ± 5% relative humidity, 16:8 light: dark conditions).
Surface contact toxicity of the different pesticides to T. brassicae was studied by the residual film method. Initially plastic containers (Tarsons®, size 8 cm height and 6 cm diameter) were uniformly surface coated (except lid) with pesticides. In control, container surfaces were treated with 0.1% Triton X-100 only. Further, after drying of container surface, i.e. after 1 h, thirty parasitoids (less than 12 h of emergence) were released in each container and covered with a lid.
Lethal effect of ingestion of different pesticides to T. brassicae was studied by diet contamination bioassay. Pesticide solutions were prepared as mentioned above. Thirty newly emerged (within 12 h of emergence) parasitoids T. brassicae were released in new sterilized plastic containers (Tarsons®, size 8 cm height and 6 cm diameter) and covered with a lid. Cotton soaked with pesticide solution +50% honey solution (1:1 ratio) was provided as a food source to the adult parasitoids. In the control, cotton soaked with 0.1% Triton X-100 solution +50% honey solution (1:1 ratio) was provided as food for the adults. Food source was replaced every morning in each container.
In the residual toxicity study (15 day old residue), plastic containers were surface coated as mentioned above in the residual film method. Further, these containers were kept open in the laboratory and were used after 15 days. The remaining procedure was the same as for residual film method.
All the above three experiments were conducted in a Completely Randomized Design (CRD) and each treatment was replicated three times. Mortality of parasitoids was recorded after 6, 12, 24, 48 and 72 h of treatment. The data recorded on mortality were converted to a percentage.
The effect of pesticides on adult emergence and longevity of T. brassicae was studied by the pupal dip method. The experiment was conducted in a Completely Randomized Design (CRD) and each treatment was replicated three times. Initially, egg masses of P. brassicae were exposed to UV rays of 15 watt UV tube for 45 min to prevent hatching. The UV treated egg masses of P. brassicae were further exposed to mated females of T. brassicae (1:6, host: parasitoid ratio) for 48 h. Parasitized eggs of P. brassicae were turned to brownish black after 4–6 days of parasitization. From blackening of the host eggs until adult emergence, the parasitoid was considered to be in the prepupal and pupal stages (Dahlan and Gordh, 1996). One parasitized egg mass (about 40–50 brown eggs, after 6 days of parasitization) of P. brassicae was dipped in the required concentration of pesticide solutions for 10 s and further kept in plastic containers after drying. In the control, parasitized egg mass was dipped in only 0.1% Triton X-100 solution for 10 s. The number of adults that emerged out of the total number of parasitized eggs were recorded regularly and converted to a percentage. Emerged parasitoids were separated into new containers according to treatments and provided with fine streaks of 50% honey solution as an adult food. Observations on their longevity were recorded regularly twice a day.
In studying the effects of pesticides on parasitism potential and sex ratio, fresh and UV light treated egg masses (40–50 egg/mass) of P. brassicae were sprayed (topically) with required concentrations of pesticides. In the control, only 0.1% Triton X-100 solution was sprayed on eggs. After drying, egg masses were exposed to five newly emerged mated females of T. brassicae for 24 h. The observations on the longevity of females were recorded regularly every 6 h. After 24 h of exposure, live T. brassicae females were removed and reared separately until their death. After 4–6 days of parasitization, the parasitized eggs (brown eggs) were counted and percent parasitism was calculated. After emergence, the adults were isolated and percent females of the progeny were recorded. An adult female of T. brassicae has a black head, black thorax, a yellow striped abdomen and is about 0.6 mm. They have bent antennae with a button on the end. Males have longer antennae with long curved hairs.
3. Statistical analysis
Data on mortality, adult emergence, parasitism potential and female progeny and longevity were analysed using a one way analysis of variance (ANOVA) at a significance level of 0.05 and before conducting ‘F’ test, the homogeneity of variances between different treatments were tested by application of Levene’s (1960) test. Afterwards, Tukey’s Honestly Significant Difference (Tukey HSD) test was used to find out the significant differences in mean values. All the statistical analysis was completed by IBM SPSS 21 software.
4. Results
4.1. Surface contact toxicity of different pesticides to T. brassicae (by residual film method)
Significant differences were observed in mortality of T. brassicae exposed to surfaces treated with different pesticides within six hours of treatment and more than 50% mortality was observed in spinosad (57.67 ± 7.22%) and deltamethrin (72.33 ± 2.96%) treated surface (Table 2). Azadirachtin was found to be relatively safe at 6 h of treatment (7.33 ± 3.71%); however the toxicity was found to be increasing over the time of exposure and 100% mortality was observed after 72 h of treatment. Deltamethrin was found to be the most harmful, causing 100% mortality of T. brassicae within 12 h of treatment followed by spinosad (Table 2). Bt var. k was found to be the least toxic to T. brassicae showing the lowest percentage of mortality (only 3.33 ± 3.33%) even after 72 h of treatment.
Table 2.
Surface contact toxicity of pesticides to T. brassicae.
| Treatments | Dose (%) | Percent adult mortality (mean ± SEM) |
||||
|---|---|---|---|---|---|---|
| 6 h | 12 h | 24 h | 48 h | 72 h | ||
| Azadirachtin 0.15 EC | 0.2 | 7.33 ± 3.71a | 24.00 ± 7.02b | 44.00 ± 2.65b | 67.33 ± 2.33b | 100.00 ± 0.0b |
| Spinosad 45 SC | 0.05 | 57.67 ± 7.2b | 93.33 ± 3.53c | 100.00 ± 0.00c | 100.00 ± 0.00c | 100.00 ± 0.0b |
| Deltamethrin 2.5 EC | 0.1 | 72.33 ± 2.9b | 100.00 ± 0.00c | 100.00 ± 0.00c | 100.00 ± 0.00c | 100.00 ± 0.0b |
| Bt var. k 8 SP | 0.2 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 3.33 ± 3.33a | 3.33 ± 3.33a |
| Control | – | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 3.33 ± 3.33a |
| Critical difference (CD) at 5% | – | 9.90 | 9.01 | 3.03 | 4.66 | 5.40 |
Note: Different small letters after mean values indicate significant differences among treatments values.
4.2. Lethal effect of ingestion of different pesticides to T. brassicae (by diet contamination bioassay)
Mortality of T. brassicae was not observed within 6 h of exposure, when the diets were mixed with pesticides (Table 1). However, significantly higher mortality of T. brassicae was found in deltamethrin at 12 h of treatment (85.33 ± 2.6%) and spinosad (79 ± 3.79%) and 100% mortality was observed within 24 h in both the treatments. In case of azadirachtin, 14.33 ± 2.96%, 29.33 ± 5.21% and 80.33 ± 6.23% mortality were found at 12, 24 and 48 h of treatment, respectively with 100% mortality recorded at 72 h of treatment (Table 1). No mortality was recorded up to 24 h of treatment of Bt var. k; whereas 28.33 ± 3.33% and 40.67 ± 2.19% mortality was noticed after 48 and 72 h of treatment, respectively.
Table 1.
Toxicity of pesticides to T. brassicae by diet contamination bioassay.
| Treatments | Dose (%) | Percent adult mortality (mean ± SEM) |
||||
|---|---|---|---|---|---|---|
| 6 h | 12 h | 24 h | 48 h | 72 h | ||
| Azadirachtin 0.15 EC | 0.2 | 0.00 ± 0.00 | 14.33 ± 2.9b | 29.33 ± 5.21b | 80.33 ± 6.23c | 100.00 ± 0.00c |
| Spinosad 45 SC | 0.05 | 0.00 ± 0.00 | 79.00 ± 3.79c | 100.00 ± 0.00c | 100.00 ± 0.0d | 100.00 ± 0.00c |
| Deltamethrin 2.5 EC | 0.1 | 0.00 ± 0.00 | 85.33 ± 2.60c | 100.00 ± 0.00c | 100.00 ± 0.0d | 100.00 ± 0.00c |
| Bt var. k 8 SP | 0.2 | 0.00 ± 0.00 | 0.00 ± 0.00a | 0.00 ± 0.00a | 28.33 ± 3.33b | 40.67 ± 2.19b |
| Control | – | 0.00 ± 0.00 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Critical difference (CD) at 5% | – | – | 6.27 | 5.97 | 8.09 | 2.51 |
Note: Different small letters after mean values indicate significant differences among treatments.
4.3. Residual toxicity (surface contact) of pesticides (15 day old residue) to the egg parasitoid, T. brassicae
The adult mortality was found to be significantly higher in deltamethrin at 12 h of exposure (39 ± 5.03%) to the 15 day old residue (Fig. 1). Overall, deltamethrin was found to have a higher residual effect with 100% mortality within 72 h of exposure followed by azadirachtin (48.67 ± 5.36). However, the toxicity of spinosad was relatively less even after 72 h of continuous exposure (26.00 ± 3.05%). The Bt var. k was found to be completely safe to the parasitoids exposed to 15 day old residues.
Figure 1.
Residual toxicity of pesticides (15 day old residue) to T. brassicae (mean ± SE).
4.4. Effect of different pesticides exposed to T. brassicae pupae on their adult emergence and longevity
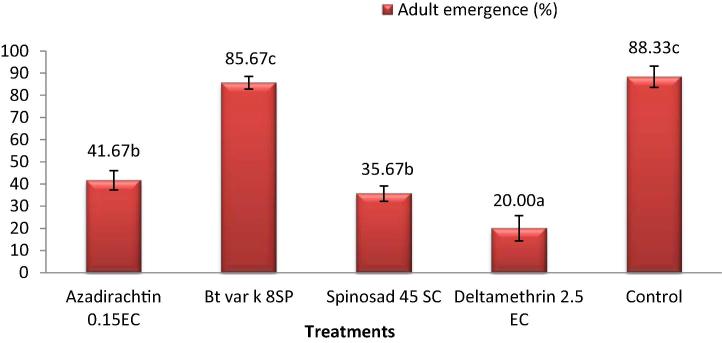
Significant differences were found in adult emergence and longevity of adult T. brassicae, when they were exposed to pesticides during the pupal stage (Fig. 2). Deltamethrin was found to be extremely harmful having the least adult emergence (20 ± 5.77%) followed by spinosad (35.67 ± 3.48%) and azadirachtin (41.67 ± 4.41%); whereas Bt var. k was found to be relatively less toxic pesticide having the highest adult emergence (85.67 ± 2.91%) (Fig. 2). Longevity of emerging adults of T. brassicae was also affected by Spinosad (2.33 ± 0.33), azadirachtin and deltamethrin (2.67 ± 0.67 each) treatment; while the longevity of Bt var. k treated adults was not affected and they survived (7.67 ± 0.33) quite as long as control (8.33 ± 0.33) (Fig. 3).
Figure 2.
Effect of pesticides on adult emergence of T. brassicae (mean ± SE). Note: Different small letters after mean values indicate significant differences among treatments.
Figure 3.
Effect of pesticides on adult longevity of T. brassicae (mean ± SE). Note: Different small letters after mean values indicate significant differences among treatments.
4.5. Effect of different pesticides sprayed on host eggs on parasitism potential and the number of females in the progeny of T. brassicae
The parasitism potential of T. brassicae was found to be significantly lower when their host eggs were treated with deltamethrin (2.67 ± 1.76%) followed by spinosad (4.00 ± 2.08%) and azadirachtin (22.00 ± 6.08%). In the majority cases, adult T. brassicae died immediately on the surface of host eggs treated with deltamethrin during foraging. The parasitism potential was not affected by Bt var. k treatment (Fig. 4). The percent female progeny was observed to be significantly higher in Bt var. k (81.89 ± 2.00%) followed by spinosad (79.67 ± 2.19), azadirachtin (68.00 ± 2.65) and deltamethrin (50.78 ± 1.47%) (Fig. 5).
Figure 4.
Effect of pesticides on parasitism potential of T. brassicae (mean ± SE). Note: Different small letters after mean values indicate significant differences among treatments.
Figure 5.
Effect of pesticides on sex ratio of T. brassicae (Mean ± SE). Note: Different small letters after mean values indicate significant differences among treatments.
5. Discussion
The diamondback moth, P. xyllostella and the cabbage butterflies, Pieris spp. are the most destructive lepidopteran pests of cruciferous crops throughout the World (Debbarma and Firake, 2013, Azad Thakur et al., 2013, Sontakke et al., 2014, Firake et al., 2014). The parasitoid wasp T. brassicae is a dominant egg parasitoid of several lepidopteran pests and widely used for the management of cruciferous lepidopteran pests. All four common pesticides used in this study were found harmful to T. brassicae, when they consumed contaminated food. Deltamethrin was found extremely harmful followed by spinosad and azadirachtin; whereas Bt var. k was found relatively less hazardous. A toxic effect of deltamethrin after oral feeding has been known in several parasitoids (Sterk et al., 1999, Hassan, 1994, Bayram et al., 2010). Some studies have also shown immediate mortality after ingestion of spinosad by Trichogramma spp. (Jay et al., 2001, Adeney et al., 2008, Charles et al., 2000, Consoli et al., 2008) and several other parasitoids (Tillman and Mulrooney, 2000).
Neem products are considered to be safe to the parasitoids and predators; however, several reports indicated their toxicity to the natural enemies (Raguraman and Singh, 1999, Lyons et al., 2003, Saber et al., 2004). The Bt var. k 8 SP is a commercial formulation of entomopathogenic bacteria, B. thuringiensis var. kurstaki and it is basically a stomach poison. It mainly acts in the presence of alkaline gut conditions and binds to the specific receptors in insects (especially in lepidopteran insects). These conditions may not be available in parasitoids gut, but based on a limited sample size used in the experiments, we observed some extent of mortality of T. brassicae after oral feeding with Bt solution. The exact mode of action of Bt in the case of parasitoids is still uncertain and parasitoid mortality after ingestion of Bt could be due to spore–crystal complex as suggested by Thomas and Watson (1986). Brunner et al., 2001, Ksentini et al., 2010 also observed mortality of Trichogramma spp. after Bt ingestion.
In the residual film method, except Bt var. kurstaki, all three pesticides were found harmful to T. brassicae. Being a stomach poison, Bt was found to be safe to the natural enemies with the residual film method. In this method, there was likely no ingestion of the Bt spores or only some ingestion through body grooming activities that adult parasitoids display. The three pesticides have different modes of action with a certain extent of contact toxicity and therefore mortality of parasitoids was found higher. In several studies, surface contact bioassay of deltamethrin showed 100% mortality of the parasitoid adults, even during short-term exposure (Longley, 1999, Sterk et al., 1999, Hassan, 1994, Bayram et al., 2010). Spinosad is also reported to be a toxic compound against many insect parasitoids, including our test parasitoids (Jay et al., 2001, Cañete, 2005, Charles et al., 2000). Consoli et al. (2008) reported spinosad as a harmful pesticide for the adults of Trichogramma galloi in a laboratory bioassay. Schmutterer (1992) found severe toxicity of neem oil formulation to the braconid parasitoid under field conditions at recommended doses.
In this study, residual toxicity of deltamethrin and azadirachtin was found to be severe even when exposed to 15 day old residues. This might be due to high persistence of deltamethrin. Furthermore, our formulation of neem i.e. azadirachtin 0.15 EC is an oil based formulation and it is a well known fact that oil formulations have relatively more residual effects than others. Interestingly, residual toxicity of spinosad was much less, comparatively, to fresh residue and it could be due to the rapid degradation property of spinosad. Schneider et al. (2003) also found little effect of spinosad to the parasitoid wasps after exposed to 10 day old residues. The effect of Bt var. k was found almost negligible. As discussed above, chances of ingestion of Bt in residual film method were very less and therefore Bt was found safer in residual toxicity test also.
The adult emergence of T. brassicae was drastically affected when their pupae were exposed to deltamethrin, spinosad and azadirachtin; while Bt var. k had no effect on adult emergence and longevity of parasitoids. Except Bt var. k, the remaining three pesticides can also act by contact and therefore substantial mortality during the pupal stage could be possible; while Bt only acts after ingestion and consequently adult emergence was not affected. In this study, the longevity of emerged adults from pesticide treated pupae was also affected and the lowest longevity was observed in the spinosad and deltamethrin treatment followed by azadirachtin; whereas longevity of parasitoids was not affected in Bt var. k treated pupae. This could be possible because, many times parasitoid adults are able to emerge from treated pupae due to sub-lethal action of some pesticides. Nevertheless, immediately after emergence, adult parasitoids frequently come in contact with treated areas and that leads to mortality. Moreover, egg stage of many insects does not have a well-developed nervous system and hence neurotoxic insecticides (deltamethrin and spinosad) do not have a significant effect on the eggs. In contrast, the larvae or pupae of T. brassicae which were in the parasitized eggs are expected to have a well-developed nervous system. Thus, the effect of deltamethrin and spinosad on the nervous system of T. brassicae could have affected adult emergence and subsequent longevity or fitness. The growth inhibitory effect of neem to T. chilonis is a well known fact (Raguraman and Singh, 1999, Firake and Khan, 2010). A toxic effect of spinosad to the immature stages of Trichogramma spp. has been reported by several authors (Jay et al., 2001, Adeney et al., 2008, Cañete, 2005, Charles et al., 2000, Consoli et al., 2008, Schneider et al., 2003). In many cases, some parasitoids could emerge from spinosad treated cocoons, but immediately died within few hours of emergence (Schneider et al., 2003, Schneider et al., 2004, Xu et al., 2004). Shoeb (2010) also reported that the emergence rate of T. evanescens an egg parasitoid of Sitotroga cerealella was very low (3–5%) and the emerged adults died within 6–12 h after treatment with spinosad. T. chilonis emergence was significantly reduced by dipping parasitized host eggs into spinosad solution (Hussain et al., 2010). Deltamethrin also severely affects the emergence rate and longevity of Trichogramma spp. (Garcia et al., 2009, Bayram et al., 2010, Meilin et al., 2012). Some studies have also shown safety of Bt to pupae of hymenopteran parasitoids (Muck et al., 1981, Temerak, 1982).
We observed a drastic effect of deltamethrin on the parasitism potential of T. brassicae, followed by spinosad and azadirachtin; while the effect of Bt var. k was almost negligible. Furthermore, the percent female progeny were highest in Bt var. k exposed females of T. brassicae, followed by spinosad, azadirachtin and deltamethrin. Williams et al. (2003) reported that about 55% spinosad is accumulated in the ovaries of females, and this may explain the reported sub-lethal effects, including a reduction in fecundity in many parasitoids after exposure to this compound. This could also be possible in case of deltamethrin, therefore parasitism and sex ratio of parasitoid in our study were affected. Bastos et al. (2006) reported that, deltamethrin was very capable of lowering the parasitoid’s ability to parasitize the eggs of its host. Garcia et al. (2006) observed drastic effect of deltamethrin on vitellogenesis process and it ultimately reduces fecundity and alters sex ratio in Trichogramma spp. Raguraman and Singh (1999) also found a significant reduction in fecundity of Trichogramma spp. fed with neem products.
6. Conclusion
The results of the present study indicate that the four commonly used pesticides against crucifer pests are harmful to egg parasitoid (T. brassicae), and therefore, the use of these pesticides should not be encouraged along with the mass release of T. brassicae against lepidopteran pests under field conditions. However, Bt var. k is a comparatively safe pesticide for T. brassicae, hence it could be used during severe infestation of cabbage butterflies. Egg parasitoids are more useful if there are sufficient host eggs in the field, consequently, their releases can be planned based on pest monitoring data and pesticide with less residual effect like spinosad may be used 15 days after parasitoid release, thus minimizing the chances of parasitoid exposure. Given the harmful effect caused by pesticides on T. brassicae, the pesticide-tolerant strain of T. brassicae could be developed for the sustainable management of major lepidopteran pests of cruciferous crops.
Acknowledgments
The authors are thankful to the Director, ICAR Research Complex for NEH Region, Umiam, Meghalaya (India) for providing necessary lab facilities during the course of study. This study is the part of M.Sc. Thesis of the first author. The authors are grateful to the student advisory committee members, Dr. N.S. Azad Thakur, ICAR RC NEH Region, Umiam and Dr. D. Majumder and Dr. T. Rajesh, CPGS (CAU), Umiam for their critical advice during the study. Our sincere thanks also go to the Kong Bashisha, Research Officer, State Biological Control Laboratory, Upper Shillong, Meghalaya for providing an initial culture of T. brassicae and C. cephalonica. Authors are also thankful to four anonymous reviewers for their critical comments and suggestions for improvement of this manuscript. The reference to some pesticide names in this manuscript is in no way an endorsement of or discrimination against these products by the authors.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
D.P. Thubru, Email: d.thubru@gmail.com.
D.M. Firake, Email: dfirake@gmail.com.
G.T. Behere, Email: ganeshbehere@gmail.com.
References
- Adeney F.B., Regiane C.O.F.B., Para J.R.P., Simone S.V. Effects of pesticides used in soybean crops to the egg parasitoid Trichogramma pretiosum. Cienc. Rural. 2008;38:1495–1503. [Google Scholar]
- Anonymous . 2009. AICRP on Biological Control of crop pests and weeds; p. 279. (Annual Progress report 2008-09 of PDBC). (Bangalore, India) [Google Scholar]
- Azad Thakur N.S., Firake D.M., Behere G.T., Firake P.D., Saikia K. Biodiversity of agriculturally important insects in north eastern Himalaya: an overview. Indian J. Hill Farm. 2013;25:37–40. [Google Scholar]
- Bastos C.S., Almeida R.P., Suinaga F.A. Selectivity of pesticides used on cotton (Gossypium hirsutum) to Trichogramma pretiosum reared on two laboratory-reared hosts. Pest. Manage. Sci. 2006;62:91–98. doi: 10.1002/ps.1140. [DOI] [PubMed] [Google Scholar]
- Bayram A., Salerno G., Onofri A., Conti E. Sub-lethal effects of two pyrethroids on biological parameters and behavioural responses to host cues in the egg parasitoid Telenomus busseolae. Biol. Control. 2010;53:153–160. [Google Scholar]
- Brunner J.F., Dunley J.E., Doerr M.D., Beers E.H. Effect of pesticides on Colpoclypeus florus (Hymenoptera: Eulophidae) and Trichogramma platneri (Hymenoptera: Trichogrammatidae), parasitoids of leafrollers in Washington. J. Econ. Entomol. 2001;94:1075–1084. doi: 10.1603/0022-0493-94.5.1075. [DOI] [PubMed] [Google Scholar]
- Cañete C.L. Universidade Federal do Paraná; Curitiba, PR: 2005. Seletividade de inseticidas a espécies de Trichogramma (Hymenoptera: Trichogrammatidae) (Dissertação (Mestrado em Zoologia)) 106f. [Google Scholar]
- Charles P.C., Orrd B., Van D.J.W. Effect of insecticides on Trichogramma exiguum (Trichogrammatidae: Hymenoptera) preimaginal development and adult survival. J. Econ. Entomol. 2000;93(3):558–577. doi: 10.1603/0022-0493-93.3.577. [DOI] [PubMed] [Google Scholar]
- Consoli F.L., Botelho P.S.M., Parra J.R.P. Selectivity of insecticides to the egg parasitoid Trichogramma galloi Zucchi 1988 (Hym., Trichogrammatidae) J. Appl. Entomol. 2008;125:37–43. [Google Scholar]
- Croft B.A. Wiley; New York: 1990. Arthropod Biological Control Agents and Pesticides; p. 723. [Google Scholar]
- Dahlan A.N., Gordh G. Development of Trichogramma australicum Girault (Hymenoptera: Trichogrammatidae) on Helicoverpa armigera (Hubner) eggs (Lepidoptera: Noctuidae) Aust. J. Entomol. 1996;35:337–344. [Google Scholar]
- Debbarma M., Firake D.M. Host generated cues alter the foraging behaviour of cabbage butterfly, Pieris brassicae and its larval parasitoids, Cotesia glomerata and Hyposoter ebeninus. J. Entomol. Acarol. Res. 2013;45(15):90–92. [Google Scholar]
- Desneux N., Decourtye A., Delpuech J.M. The sublethal effects of pesticides on beneficial arthropods. Ann. Rev. Entomol. 2007;52:81–106. doi: 10.1146/annurev.ento.52.110405.091440. [DOI] [PubMed] [Google Scholar]
- Firake D.M., Khan M.A. Influence of some plant oils on the parasitization potential and adult emergence of Trichogramma chilonis Ishii and Trichogramma poliae Nagaraja. J. Insect Sci. 2010;23(4):430–433. [Google Scholar]
- Firake D.M., Khan M.A. Alternating temperatures affect the performance of Trichogramma species. J. Insect Sci. 2013;14:1–14. doi: 10.1093/jis/14.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firake D.M., Lytan D., Behere G.T., Thakur N.S.A. Host plants alter the reproductive behavior of cabbage butterfly, Pieris brassicae (Lepidoptera: Pieridae) and its endo-larval parasitoid, Hyposoter ebeninus (Hymenoptera: Ichneumonidae) in cruciferous ecosystems. Florida Entomol. 2012;95(4):905–913. [Google Scholar]
- Firake D.M., Lytan D., Behere G.T. Bio-diversity and seasonal activity of arthropod fauna in Brassicaceous crop ecosystems of Meghalaya, north east India. Mol. Entomol. 2012;3:18–22. [Google Scholar]
- Firake D.M., Behere G.T., Azad Firake P.D., Thakur N.S., Ngachan S.V., Shockly M. Rapid technique of sex differentiation in immature stage of parasitoid wasp, Hyposoter ebeninus Gravenhorst (Ichneumonidae: Hymenoptera) Entomol. News. 2014;124:193–204. [Google Scholar]
- Garcia P., Cabral S., Oliveira L., Rodrigues A. Effects of deltamethrin on the reproduction of Trichogramma cordubensis (Hymenoptera: Trichogrammatidae) Biocontrol Sci. Technol. 2006;16:699–708. [Google Scholar]
- Garcia P., Pereira N., Oliveira L. Side-effects of organic and synthetic pesticides on cold-stored diapausing prepupae of Trichogramma cordubensis. Biocontrol. 2009;54:451–458. [Google Scholar]
- Ghoneim K. Parasitic insects and mites as potential biocontrol agents for a devastative pest of tomato, Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) in the world: a review. Int. J. Adv. Res. 2014;2(8):81–115. [Google Scholar]
- Hafez M.B., Schmitt A., Hassan S.A. The side-effects of plant extracts and metabolites of Reynoutria sachalinensis (F. Schmidt) Nakai and conventional fungicides on the beneficial organism Trichogramma cacoeciae Marchal (Hym., Trichogrammatidae) J. Appl. Entomol. 1999;123:363–368. [Google Scholar]
- Hassan S.A. Activities of the IOBC working group pesticides and beneficials. IOBC Bull. 1994;17:1–5. [Google Scholar]
- Hussain D., Akram M., Iqbal Z., Ali A., Saleem M. Effect of Some Insecticides on Trichogramma Chilonis Ishii. (Trichogrammatidae: Hymenoptera) immature and adult survival. J. Agric. Res. 2010;48:531–537. [Google Scholar]
- Jay F.B., John E.D., Michael D.D., El-Izabeth H.B. Effect of Pesticides on Colpoclypeus florus (Hymenoptera: Eulophidae) and Trichogramma platneri (Hymenoptera: Trichogrammatidae), parasitoids of leafrollers in Washington. J. Econ. Entomol. 2001;94:1075–1084. doi: 10.1603/0022-0493-94.5.1075. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy A. Exploitation of egg parasitoids for control of potential pests in vegetable ecosystems in India. Commun. Sci. 2012;3(1):1–15. [Google Scholar]
- Ksentini I., Jardak T., Zeghal N. Bacillus thuringiensis, deltamethrin and spinosad side-effects on three Trichogramma species. Bull. Insectol. 2010;63:31–37. [Google Scholar]
- Kulye M.S., Gondhalekar A.D., Chaudhari C.S., Chandele A.G. Comparative toxicity of some benzoylphenyl urea molt-inhibiting insecticides against diamondback moth (Plutella xylostella) Pestology. 2007;31:45–46. [Google Scholar]
- Levene H. Robust testes for equality of variances. In: Olkin I., editor. Contributions to Probability and Statistics. Stanford Univ. Press; Palo Alto, CA: 1960. pp. 278–292. [Google Scholar]
- Longley M. A review of pesticide effects upon immature aphid parasitoids within mummified hosts. Int. J. Pest Manage. 1999;45(2):139–145. [Google Scholar]
- Lyons D.B., Helson B.V., Bourchier R.S., Jones G.C., McFarlane J.W. Effects of azadirachtin-based insecticides on the egg parasitoid Trichogramma minutum (Hymenoptera: Trichogrammatidae) Can. Entomol. 2003;135:685–695. [Google Scholar]
- Lytan D., Firake D.M. Effects of different host plants and rearing atmosphere on life cycle of large white cabbage butterfly, Pieris brassicae (L.) Arch. Phytopathol. Plant Prot. 2012;45:1819–1825. [Google Scholar]
- Meilin A., Trisyono Y.A., Martono E., Buchori D. The effects of deltamethrin applied at sublethal concentrations on the adults of Anagrus nilaparvatae (Hymenoptera: Mymaridae) ARPN J. Agric. Biol. Sci. 2012;7(12):1990–6145. [Google Scholar]
- Muck O., Hassan S., Huger A.M., Krieg A. Zur Wirkung von Bacillus thuringiensis Berliner auf die parasitischen Hymenoptera Apanteles glomeratus L. (Braconidae) und Pimpla turionellae (L.) (Ichneumonidae) Z. Angew. Entomol. 1981;92:303–314. [Google Scholar]
- Polaszek A. Biodiversity and host associations of Trichogramma in Eurasia. In: Consoli FL, Parra JRP, Zucchi RA, editors. Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma. Progress in Biological Control. vol. 9. Springer; New York: 2010. pp. 1–479. (Chapter 9, pp. 237–266) [Google Scholar]
- Raguraman S., Singh R.P. Biological effects of neem (Azadirachta indica) seed oil on an egg parasitoid, Trichogramma chilonis. J. Econ. Entomol. 1999;92:1274–1280. [Google Scholar]
- Saber M., Hejazi M.J., Hassan S.A. Effects of azadirachtin/neemazal on different stages and adult life table parameters of Trichogramma cacoeciae. J. Econ. Entomol. 2004;97:905–910. doi: 10.1093/jee/97.3.905. [DOI] [PubMed] [Google Scholar]
- Schmutterer H. Einfl uB von azadirachtin. einer azadirachtinfreien fraktion eines alkoholischen niemsamenextraktes und von formulierten extrakten auf verpuppung, schlupfund imaginesder kohlweiglingsbrackwespe Apanteles glomeratus (L.) (Hym., Braconidae) J. Appl. Entomol. 1992;113:79–87. [Google Scholar]
- Schneider M.I., Smagghe G., Gobbi A., Viñuela E. Toxicity and pharmacokinetics of insect growth regulators and other novel insecticides on pupae of Hyposoter didymator (Thunberg 1822) (Hym., Ichneumonidae), a parasitoid of early larval instars of Noctuid pests. J. Econ. Entomol. 2003;96:1054–1065. doi: 10.1603/0022-0493-96.4.1054. [DOI] [PubMed] [Google Scholar]
- Schneider M.I., Smagghe G., Pineda G.S., Vinuel E. Action of insect growth regulator insecticides and spinosad on life history parameters and absorption in third-instar larvae of the endoparasitoid Hyposoter didymator. Biol. Control. 2004;31:189–198. [Google Scholar]
- Shoeb M.A. Effect of some insecticides on the immature stages of the egg parasitoid Trichogramma evanescens West. (Hym., Trichogrammatidae) J. Biol. Sci. 2010;3:31–38. [Google Scholar]
- Sontakke P.P., Behere G.T., Firake D.M., Thubru D.P. Evaluation of toxicity and cotoxicity of biopesticides against diamondback moth, Plutella xylostella (L.) J. Biopest. 2014;7:90–97. [Google Scholar]
- Sterk G., Hassan S.A., Baillod M., Bigkler F., Blumel S., Bogenschutz H., Boller E., Bromand B., Brun J. Results of the seventh joint pesticide testing programme carried out by the IOBC/WPRSWorking group “Pesticides and beneficial organisms”. Biocontrol. 1999;44:99–117. [Google Scholar]
- Temerak S.A. Wirkungen zwischen Bacillus thuringiensis Ber l. u nd larven der Schlupfwespe Bracon brevicornis Wesm. in bzw. an d en Rau pen von Sesamia cretica Led. b ei ver schiedenen Temperaturen. Anz. Schadlingskd. Pfl. 1982;55:137–140. [Google Scholar]
- Thomas E.M., Watson T.F. Effect of Dipel (Bacillus thuringiensis) on the survival of immature and adult Hyposoter exiguae (Hymenoptera: Ichneumonidae) J. Invertebrate Pathol. 1986;47:178–183. [Google Scholar]
- Tillman P.G., Mulrooney J.E. Effects of selected insecticides on the natural enemies Coleomegilla maculata and Hippodamia convergens (Coleoptera:Coc-cinellidae), Geocoris punctipes (Hemiptera: Lygaeidae), and Braconmellitor, Cardiochilesnigriceps, and Cotesia marginiventris (Hymenoptera:Braconidae) in cotton. J. Econ. Entomol. 2000;93:1638–1643. doi: 10.1603/0022-0493-93.6.1638. [DOI] [PubMed] [Google Scholar]
- Williams V.I.J., Valle T., Uela E. Is the naturally derived insecticide Spinosad compatible with insect natural enemies? Biocontrol Sci. Technol. 2003;13:459–475. [Google Scholar]
- Xu Yong-yu, Liu Tong-xian, Leibee Gary L., Jones Walker A. Effects of selected insecticides on Diadegma insulare (Hymenoptera: Ichneumonidae) a parasitoid of Plutella xylostella (Lepidoptera: Plutellidae) Biocontrol Sci. Technol. 2004;14:713–723. [Google Scholar]