Abstract
Studies have suggested PTSD to be associated with an inflammatory state, although few studies have examined the balances between stimulatory and inhibitory immune mediators in PTSD. An exploratory approach was taken to assess the immune imbalances between Th1 stimulatory, inflammatory and inhibitory mediators associated with PTSD. This approach focused on a tightly-controlled and relatively homogeneous population of Veterans, all with similar levels of combat exposure in the Afghanistan and Iraq wars, but some testing negative and others testing positive for PTSD. Although the sample size was small (6 controls and 7 with PTSD) and a limitation of this study, the results showed significant imbalances in immune cytokines favoring a Th1 and inflammatory state, with reduced levels of inhibitory cytokines in Veterans with PTSD. This was particularly prominent in the saliva of PTSD subjects compared to in their plasma.
Keywords: Cytokines, Immune regulation, Inflammation, Plasma, Post-traumatic stress disorder, PTSD, Saliva
1. Introduction
Over 2.5 million U.S. service members have been deployed to Operation Iraq Freedom/Operation Enduring Freedom (OEF/OIF) arenas since 2001. An unprecedented proportion of these service members develop post-traumatic stress disorder (PTSD) following combat exposure in Afghanistan and Iraq [1,2]. According to reports from the Department of Veterans Affairs, 15.7% of these OEF/OIF Veterans have PTSD [1]. PTSD is not limited to combat but is also seen following natural disasters, sexual trauma or loss of family members [3,4]. Therefore, PTSD has a tremendous economic and social impact on the community at large. In the past decade, extensive research has been devoted to identify the biological causes of PTSD. However, the precise mechanisms of pathogenesis of PTSD remain unclear.
Studies involving human subjects with PTSD have shown correlations between inflammation and PTSD following trauma exposure, though it is unlikely that inflammation is the primary cause of PTSD. These measurements of the various immune mediators in PTSD subjects have been somewhat haphazard, but collectively the results of these studies have suggested immune alterations toward increased levels of inflammatory cytokines [4–8]. Studies have also shown increased levels of inflammatory mediators in saliva following stress exposure, although such studies of salivary cytokine levels are relatively few [3,9]. A study of hospitalized patients with traumatic orthopedic injuries showed that those who developed PTSD had increased blood levels of inflammatory mediators and reduced inhibitory mediators, and suggested that this could be used as a biomarker for PTSD [7]. A prospective study of deployed Marines showed that pre-deployment levels of C-reactive protein, an indicator of inflammation, predicted post-deployment PTSD and suggested inflammation to be a contributor to PTSD [10].
Studies using animal models for PTSD have been more comprehensive and definitive than studies with PTSD in human subjects. Animal models of PTSD have shown peripheral inflammation triggering neuroinflammation in the CNS [11–13], dependence on immune function for anxiety behavior following stress sensitization [14,15], and the tempering of maladaptation to acute psychological stress by neutralizing the inflammatory cytokine IL1 [16] or by treatment with immune inhibitory Treg cells [17]. Induction of peripheral inflammation in animal models causes an increase in activation of brain microglia and in levels of inflammatory mediators in the hippocampus [13]. Studies with stress-sensitized mice have shown that trafficking of monocytes from the periphery to the brain re-established anxiety [14]. Analyses of aggressor-exposed mice that were subjected to trauma reminders showed increased immune inflammatory signaling pathways in both the periphery and neuroinflammation in the CNS of this PTSD model [18,19].
Most studies assessing the immune linkage with PTSD have been hampered by the variability in the type and severity of trauma exposure that led to PTSD. We conducted an exploratory study in which levels of stimulatory as well as inhibitory cytokines were measured in a limited, but a tightly-controlled and relatively homogeneous population of OEF/OIF Veterans, all with similar levels of combat exposure, but some testing negative and others testing positive for PTSD. The results of this study showed an imbalance in immune cytokines favoring a Th1 and inflammatory state, with reduced inhibitory cytokines. This was particularly prominent in the saliva of PTSD subjects compared to in their plasma.
2. Methods
2.1. Research participants
All the subjects participating in this study were OEF/OIF Veterans that were recruited through the local VA Medical Center, either through the OEF/OIF program or upon referral by the OEF/OIF Program to the PTSD clinic. The research protocol of this study was approved by the Institutional Review Boards (IRB) of the Medical University of South Carolina, which is the IRB of record for the Ralph H. Johnson VA Medical Center. The brief description and voluntary nature of this study were explained to participants by a trained research assistant, and those who were interested in participating in this research were screened to determine eligibility for study involvement with inclusion and exclusion criteria described below. Written informed consent was obtained from all of the participants before the formal interview.
2.2. Procedure
After collecting the demographic and deployment information, participants were assessed by a trained research assistant for the presence of PTSD or other psychiatric disorders with Mini-International Neuropsychiatric Interview (MINI) [20]. MINI is valid and reliable structured diagnostic instruments that assess the presence of DSM-IV diagnoses. A board-certified psychiatrist (ZW) interviewed participants for combat exposure history and PTSD status, as well as other major psychiatric illnesses that would be exclusionary (see inclusion/exclusion section below). Therefore, full diagnostic level data and demographic data are available for all 13 participants. All participants provided a peripheral blood and saliva sample via standard methods.
All research participants completed the Combat Exposure Scale (CES) [21], a 7-item self-report measure, used to obtain information regarding exposure to wartime stressor events. The measure has total scores ranging from 1 to 41, with a higher number reflecting a higher severity of combat exposure. These participants also were interviewed using the Clinician Administered PTSD Scale (CAPS) [22], a diagnostic interview for current and lifetime PTSD. The CAPS has been used in over 200 studies and has excellent psychometric properties. The CAPS demonstrates high inter-rater reliability (i.e., above 0.86) and internal consistency on each of the three PTSD symptom clusters (range 0.63 to 0.89), and correlates strongly (i.e., above 0.61) with other measures of PTSD [23,24]. Developed by researchers at the National Center of PTSD, this structured interview assesses all 17 symptoms of PTSD for frequency (scored on a 0 [never] to 4 [daily or almost every day]) and intensity (0 [none] to 4 [extreme, incapacitating distress]). This measure also assesses subjective distress, functional impairment, and onset and duration of symptoms, and includes response validity items. In addition, the Hamilton Depression (Ham-D) Scale [25] was used to for assess the symptom severity of depression.
Inclusion criteria for enrollment into the research study were: combat Veterans of the OEF/OIF arenas, male or female, age 25–55 with good physical health, evidence of combat as defined by a history of deployment to a combat zone (a DD214) and trauma exposure sufficient to meet Category A of PTSD criteria [26]. The study allowed participation of subjects with depressive disorders, but only if the depressive episodes were secondary to PTSD; subjects with prior episodes of depression were excluded from this study. Exclusion criteria were age younger than 25 or greater than 55, an active or lifetime major mental health diagnosis as determined by DSM-IV Axis I Disorders, major traumatic brain injury, current substance dependence and abuse within 6 months, females who are pregnant or likely to become pregnant, active suicidal thoughts and behavior, conditions known to prominently impact on immune reactivity such as recent chemotherapy or HIV. Using the method of extreme discordant phenotype (EDP) [27], only distinct phenotypes were enrolled into either Group I (PTSD) or Group II (controls), based on diagnosis via the CAPS-IV scores. Therefore, OEF/OIF combat-exposed Veterans that were eligible to participate in the study (13 of the over 20 screened OEF/OIF Veterans) were divided into two groups: control combat-exposed Veterans without PTSD (as defined by CAPS-IV ≤ 35), and combat-exposed Veterans with PTSD (as defined by positive DSM-IV Diagnosis, and CAPS-IV ⩾ 60). Once enrolled into the study, blood and saliva samples we collected from control Veterans and those with PTSD prior to receiving any pharmacotherapy using uniform procedures for both groups of subjects. Blood was collected into heparinized tubes. For collection of unstimulated saliva, subjects first performed a mouth rinse with water and, after a 10 min wait, saliva was collected by the passive drool approach. The blood and saliva were collected concurrently. The time of specimen collection was in the mid-afternoon, although the precise timing was not standardized. These blood and saliva specimens were cryopreserved until used for cytokine measurements as described below.
2.3. Cytokine measurements
The blood and saliva specimens collected from OEF/OIF Veterans with PTSD and OEF/OIF Veteran controls were used for measurements of select Th1 stimulatory cytokines (IL-2 and IFN-γ), inflammatory cytokines (IL-6, IL-17 and TNF-α) and immune inhibitory cytokines (IL-4 and IL-10). Blood specimens were centrifuged for collection of plasma, while saliva was centrifuged to remove particulate debris. Both plasma and saliva were cryopreserved until analyzed for cytokine levels by cytokine bead array.
To measure levels of immune mediators, the frozen plasma and saliva samples were first thawed and allowed to return to room temperature. Levels of cytokines in plasma and saliva were then measured using human cytometric bead array flex sets for Th1/Th2/Th17 cytokines as directed by the manufacturer’s instructions (BD Biosciences, San Jose, CA, USA). A FACS Canto (BD Biosciences) flow cytometer was used to quantify cytokine profiles and relative amounts of each cytokine were analyzed using FCAP Array Software (manufactured by Soft Flow Hungary Ltd. for BD Biosciences, San Jose, CA, USA). Any values that were below the level of detection by the bead array flex sets were considered as 0 values.
2.4. Statistical analyses
Data were presented as boxplots depicting the minimum, 25th, 50th (median), 75th percentiles, and maximum for cytokine levels in the plasma and saliva. As values were not assumed to be normally distributed, a Mann-Whitney U test was used to determine significance of differences in values between control versus PTSD subjects (GraphPad Prism version 6.03 for Windows, GraphPad Software, La Jolla, CA, USA). The Cohen’s d was used as the effect size measure for the Student’s t test. The Spearman’s rank correlation was calculated to assess the strength of the correlation between cytokine levels in the plasma versus in the serum in subjects. The Fisher’s exact test was used to determine significance of differences in race between the groups of subjects. Significance for all analyses was reported at the 95% confidence interval.
3. Results
3.1. Study participants
This exploratory study involving a tightly controlled and relatively homogeneous group of OEF/OIF combat-exposed Veterans compared levels of select Th1, inflammatory and inhibitory immune mediators among control Veterans and those with PTSD. Shown in Table 1 is a summary of the combat-exposed Veterans whose cytokine levels were measured. A total of 13 OEF/OIF combat-exposed Veterans were enrolled into this exploratory study. Seven of these 13 Veterans met the full criteria for PTSD, but had not yet received pharmacotherapy for PTSD. The remaining 6 Veterans tested negative for PTSD. As the subjects were all in combat during their military service, the majority were males, although the study was open to both males and females. There were no significant differences in age or racial distribution among Veterans without PTSD and those with PTSD. There was a significant difference in PTSD severity between the two groups, as determine by the CAPS total score. Based on a cut-off HAM-D score of 20 to define subjects with versus without depression, 3 of the PTSD subjects were classified as not being depressed and 4 as having depression. However, those with active or lifetime major mental health diagnosis or with severe traumatic brain injury were excluded from this study. A review of the medical records of the research subjects identified two PTSD participants and one control participant had reported a prior history of allergic rhinitis. However, there were no other outstanding health differences, including no other ongoing causes of inflammation and no problems associated with oral health.
Table 1.
Descriptive and clinical information on research subjects.
| Controls (n = 6) |
PTSD (n = 7) |
p | |
|---|---|---|---|
| Age | 39.0 ± 7.5 | 44.4 ± 9.6 | NS |
| Males | 5 (83.3%) | 7 (100%) | NS |
| Race | NS | ||
| Caucasian | 5 (83.3%) | 4 (57.1%) | |
| African American | 0 (0.0%) | 3 (42.9%) | |
| Hispanic | 1 (16.7%) | 0 (0.0%) | |
| Combat exposure severity | 9.67 ± 9.09 | 13.43 ± 5.71 | NS |
| PTSD severity | 11.7 ± 22.8 | 75.9 ± 13.3 | <0.0005 |
| HAM-D | 4.0 ± 6.2 | 22.7 ± 10.6 | <0.002 |
Combat exposure severity = CES total score; PTSD severity = CAPS total score. The p-values represent results of t-tests between groups except for the p-value for race, which is the result of Fisher’s exact test.
3.2. Increased plasma levels of Th1 and inflammatory cytokines, and diminished levels of immune inhibitory cytokines in combat-exposed Veterans with PTSD
Three categories of cytokines were measured in combat-exposed Veterans with and without PTSD: Th1, inflammatory and inhibitory cytokines. While limited by the low number of subjects, results show a dysregulated immune state of OEF/OIF combat Veterans with PTSD (Fig. 1). Subjects with PTSD had increased plasma levels of the Th1 cytokine IL-2. Levels of the Th1 cytokine IFN-γ were elevated in subjects with PTSD, but this increase was not statistically significant. Of the inflammatory mediators that were measured, plasma levels of both IL-6 and IL-17 were increased in subjects with PTSD over levels in controls. Plasma levels of TNF-α were low in both groups of subjects, although the levels tended to be higher in the PTSD group of subjects. In contrast to the increases in Th1 and inflammatory cytokine levels, plasma levels of the inhibitory mediator IL-4 were reduced in PTSD subjects. IL-10, which is also an inhibitory mediator, was in lower levels in the plasma of PTSD subjects, but the decrease was not significant. For each of the cytokines measured, the effect size (Cohen’s d) was large, ranging from 1.186 to 4.453, with the weakest being for IFN-γ and IL-10, and the strongest for IL-2 and IL-4 (Fig. 1 legend). These results indicate immune imbalance in subjects with PTSD toward an increase in Th1 and inflammatory cytokines and a reduction in the immune inhibitory control mediators.
Fig. 1.
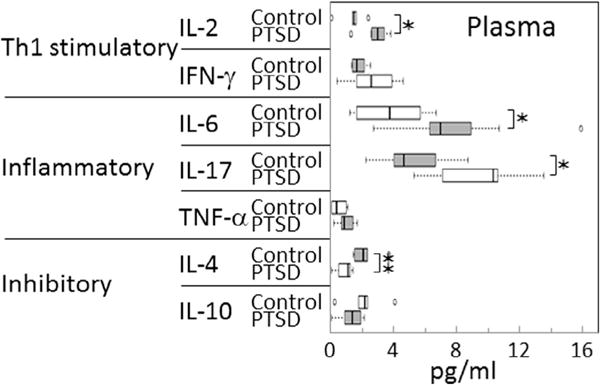
Increased levels of stimulatory and inflammatory cytokines, and reduced levels of immune inhibitory cytokines in blood plasma of Veterans with PTSD. Plasma levels of cytokines were measured and compared between Veterans with PTSD or control Veterans without PTSD, both groups having similar levels of combat exposure. Significance of the difference between plasma cytokine levels in controls versus Veterans with PTSD were measured by the Mann Whitney U test: ✶ = p < 0.05, ✶✶ = p < 0.01. The effect sizes (Cohen’s d) are as follows: IL-2 = 4.453, IFN-γ = 1.690, IL-6 = 2.605, IL-17 = 2.781, TNF-α = 2.067, IL-4 = 3.400, IL-10 = 1.186.
3.3. Prominent increases in levels of Th1 and inflammatory cytokines in saliva, with diminished salivary levels of inhibitory cytokines in Veterans with PTSD
While most prior studies examining cytokine levels in subjects with PTSD have measured levels in plasma, studies looking at stressful events related to and unrelated to PTSD have suggested that alterations in inflammatory cytokine levels may occur in saliva [3,4,9,28]. In addition to measuring the more commonly measured inflammatory cytokines, the present study also measured levels of Th1 and inhibitory cytokines in saliva of the same subjects whose blood plasma levels of cytokines were described above. These measurements showed several similarities to what was seen in plasma cytokine levels, but also revealed differences (Fig. 2). Consistent with the tendency seen for cytokines in plasma, levels of Th1 and inflammatory cytokines were all significantly increased in subjects with PTSD compared to levels in control subjects. This included both of the Th1 cytokines, IL-2 and IFN-γ. It also included the inflammatory cytokines, IL-6 and IL-17. While levels of TNF-α were increased in saliva of PTSD subjects, the difference was not significant. Contrasting with these increases, levels of both of the inhibitory cytokines, IL-4 and IL-10, were significantly lower in saliva of Veterans with PTSD compared to levels in control Veterans. The effect sizes (Cohen’s d) for differences in cytokine levels in the saliva of control versus PTSD subjects were large for all measured cytokines, ranging from 0.941 to 5.927, with the weakest being for IL-4, and the strongest for IL-6 and IL-10 (Fig. 2 legend).
Fig. 2.
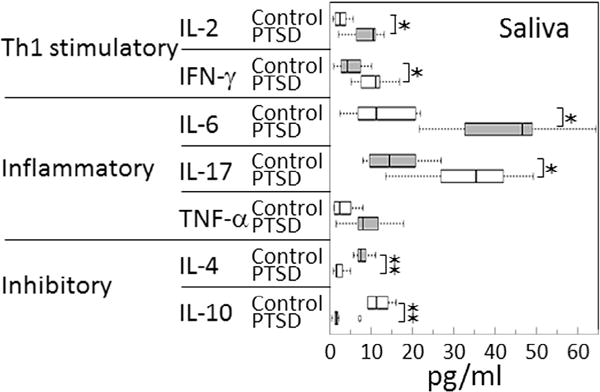
Increased levels of stimulatory and inflammatory cytokines, and reduced levels of immune inhibitory cytokines in saliva of Veterans with PTSD. Levels of cytokines in saliva were measured and compared between Veterans with PTSD or control Veterans without PTSD, both groups having similar levels of combat exposure. Significance of the difference between plasma cytokine levels in controls versus Veterans with PTSD were measured by the Mann-Whitney U test: ✶ = p < 0.05, ✶✶ = p < 0.01. The effect sizes (Cohen’s d) are as follows: IL-2 = 2.752, IFN-γ = 2.440, IL-6 = 5.927, IL-17 = 3.538, TNF-α = 2.312, IL-4 = 0.941, IL-10 = 4.570.
Assessment of correlation between levels of cytokines in plasma versus saliva of each control subject showed no significant correlations. In contrast, there was a strong correlation in levels of IL-6 that were present in plasma and saliva of Veterans with PTSD (R = 0.7143). Also, there was a strong correlation in levels of the immune inhibitory mediator IL-10 between plasma and saliva of subjects with PTSD (R = 0.8571).
One very prominent difference between cytokine levels in plasma and in saliva is the higher absolute level of each cytokine in saliva of both controls as well as PTSD subjects. This is depicted in Fig. 3 showing the percent increases in mean salivary levels over mean plasma levels for control Veterans without PTSD and Veterans with PTSD. Also shown in this figure are the cytokines whose levels are statistically significantly different between levels in plasma versus saliva. Fig. 3 indicates a more prominent increase in the Th1 and inflammatory mediators in saliva versus plasma of subjects with PTSD compared to differences in control subjects. It also shows the lower level of increase in inhibitory cytokines in saliva versus plasma of Veterans with PTSD. This is more clearly shown in Fig. 4 depicting the percent difference in the mean cytokine levels in PTSD subjects over mean levels for control subjects. This figure provides an alternate means to indicate that cytokine levels in saliva of PTSD subjects are more prominently skewed toward a Th1 and inflammatory phenotype. It also indicates more prominent reduction in mean levels of immune inhibitory mediators in saliva versus in plasma of PTSD subjects compared to levels in controls.
Fig. 3.
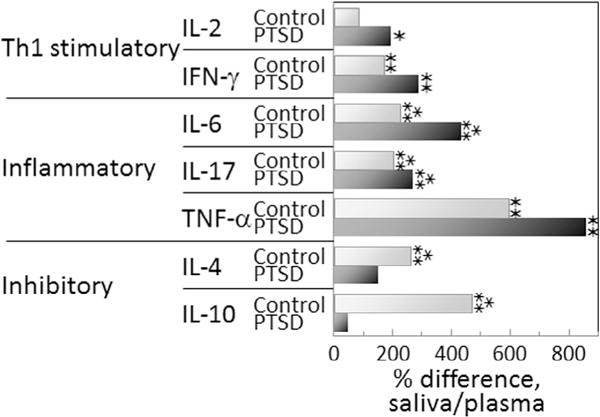
Greater cytokine levels in saliva compared to levels in plasma in both control and PTSD subject groups, but the increases in Th1 and inflammatory cytokines in saliva are more prominent for PTSD subjects. The percent increase in cytokine levels in saliva over levels in plasma were calculated from the means of the cytokine levels for each group of subjects by the following formula:
The significance of the difference in levels of each cytokine in plasma versus saliva was determined by the Mann-Whitney U test: ✶ = p < 0.05, ✶✶ = p < 0.01;
 = p < 0.005.
= p < 0.005.
Fig. 4.
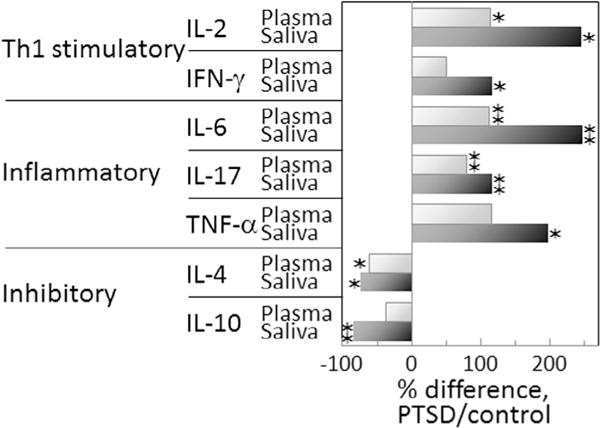
Th1 and inflammatory cytokine levels are greater and inhibitory cytokine levels reduced in PTSD subjects compared to levels in controls, with the differences being more prominent in saliva versus in plasma. The percent difference in cytokine levels in subjects with PTSD over levels in controls were calculated from the means of the cytokine levels for each group of subjects using the following formula: The significance of the difference in levels of each cytokine in subjects with or without PTSD was determined by the Student’s t-test: ✶ = p < 0.05, ✶✶ = p < 0.01.
3.4. Comparison of cytokine levels among PTSD subjects with or without depression
As depression has previously been associated with an increase in inflammatory mediators [29,30], the contribution of depression to the immune skewing in PTSD subjects was determined by comparing cytokine levels in plasma and saliva between PTSD subjects with versus without a diagnosis of depression (HAM-D ⩾ 20). When comparing plasma cytokine levels, there were no differences in levels of any of the cytokines between PTSD subjects with versus without depression (data not shown). The levels of IL-17 in saliva of PTSD subjects with depression was slightly greater than that for PTSD subjects without depression, but this difference was not statistically significant (p = 0.189). Levels of the other cytokines in saliva were similar among the two groups of PTSD subjects. While this comparison was between subgroups of a small number of subjects, they do not support depression as a major contributor to the immune skewing demonstrated above in PTSD subjects.
4. Discussion
Prior studies have indicated heightened inflammatory activity in subjects with PTSD. Such immune alterations in PTSD subjects include immune skewing toward increases in levels of inflammatory cytokines such as IL-6 and TNF-α [4–8,31]. Very few studies have also examined the balance between stimulatory and inhibitory mediators in subjects with PTSD. A significant challenge to studying the immune association with PTSD is the variability in the extent and type of trauma exposure. The present study was exploratory to test a novel approach to limit such variables so as to more accurately visualize the immune influence of PTSD. With that goal, this study used a tightly defined population of Veterans, all of whom had similar levels of OEF/OIF combat exposure and all being in good health, except some of the subjects were diagnosed with PTSD and others were tested negative for PTSD. Also, none of the subjects had received any pharmacotherapy for PTSD. Our analysis of a spectrum of immune mediators showed skewing toward Th1 and inflammatory mediators and a reduction in inhibitory mediators in subjects with PTSD compared to levels in combat-exposed Veterans without PTSD. This was seen in both plasma and saliva, with skewing being significantly more pronounced in saliva than in plasma. In the saliva, levels of each cytokines in the Th1 and inflammatory groupings were increased and levels of each of the inhibitory mediators were reduced in Veterans with PTSD compared to levels in control Veterans.
Previously conducted studies that have suggested PTSD to be associated with an inflammatory state have traditionally measured levels of inflammatory cytokines in the blood plasma. However, the results of the present study suggest that the altered balance among Th1/inflammatory and inhibitory immune mediators in saliva more prominently depicts the immune dysregulated immune state in subjects with PTSD. It has previously been suggested that levels of immune mediators in the saliva can be influenced by sympathetic nervous system innervation, rather than being secondary to blood levels of the mediators. This suggestion comes from demonstrations of earlier appearance of inflammatory cytokines in saliva following an acute stress than the increases that were seen systemically [9,32]. Thus, salivary cytokine levels may be more reflective of the mental health status than would plasma cytokine levels.
Cytokine levels in plasma and saliva can be influenced by a multitude of factors. While there were no noted differences in oral health or in other health conditions between the two groups of subjects, it is important to note that the salivary cytokine composition can be biased by the subject’s health status, including oral health status. For example, levels of inflammatory cytokines are increased in subjects with periodontitis [33], premalignant oral lesions [34] or oral cancer [35]. In addition, depression has been suggested to be associated with an inflammatory state [29,30]. This study’s analysis of cytokine levels in PTSD subjects with or without depression showed no significant differences in levels in either the plasma or saliva suggesting that depression was not a significant contributor to immune skewing associated with PTSD. While this comparison is weakened by the low number of subjects in the subgroups, it is consistent with a prior study showing that the pro-inflammatory phenotype of subjects with PTSD is independent of depression [36].
Studies using animal models for PTSD have shown that the dysregulation of cytokines that is seen peripherally is also seen in select brain regions where levels of the inflammatory mediator IL-1 are increased, while levels of the inhibitory cytokines IL-4 and IL-10 are reduced [37]. Other animal models have shown that induction of peripheral inflammation causes an increase in the number of activated microglia and in levels of inflammatory mediators IL-1, IL-6 and TNF-α in the hippocampus [13]. Immune-brain communication is further intertwined such as by the trafficking of monocytes from the periphery to the brain, resulting in the reestablishment of anxiety in stress-sensitized mice [14]. That immune mediators such as IL-1, IL-6, and TNF-α are able to cross the blood-brain barrier further emphasizes how peripheral immune reactivity can have CNS impacts [11].
Studies showing peripheral immune activation results in inflammation in the brain further underscore their intercommunication and its impact on behavior. For example, a population study showed low-grade inflammation to be associated with depressive disorder and that this was independent of other diseases such as diabetes, cardiovascular disease, or kidney disease [29]. Levels of the inflammatory markers IL-1Ra and TNF-R1 were increased in patients with schizophrenia [38]. Inflammation associated with viral or bacterial infectious burden was associated with Alzheimer’s disease [39]. While none of these studies prove inflammation to be responsible for mental health disorders, the association provides support for more definitive investigations into the pathogenic role of inflammation. Such studies are more feasible in animal models than with human subjects. In one such study, endotoxin-induced neuroinflammation resulted in depressive-like behaviors [40]. In a separate study, treatment of rodents with endotoxin induced despair-like behavior, which was shown to be inflammation-mediated as it was abolished by treatment with antibody against neutrophils [41].
Although this study demonstrated immune imbalances in Veterans with PTSD, this study has weaknesses and has left several unanswered questions. A major weakness of this study is the limited number of subjects that were studied. As this was an exploratory study for future analysis of cytokines or balances in cytokine levels that might be useful as biomarkers for PTSD, the number of subjects enrolled in this pilot study was limited. However, despite this limitation, the study yielded statistically significant results and clearly showed the immune dysregulation in PTSD subjects. This may have been achieved as a result of the study design having an emphasis on limiting variability among study participants, which is often a challenge due to variability in the extent and type of trauma exposure. What information is available about health behaviors that vary as a function of mental health (e.g., smoking status, body mass index) and that are also reliably related to inflammation? This pertains to general status (e.g., smoker/non-smoker) and also health behaviors prior to saliva collection (e.g., smoking, use of caffeine, etc.). If this information is not available, it should be addressed in the discussion as a limitation. In future studies, the immunological dysfunction will need to be analyzed in the context of the subjects’ PTSD clinical status so as to determine if the immune imbalances are more pronounced in subjects with more severe PTSD or if select CAPS cluster scores more closely correlate with the inflammatory phenotype than other cluster scores. Also needed in future analyses is whether the immune balances are restored with pharmacotherapy for PTSD in those patients that are responsive or remain unresponsive to treatment. Not yet explored are mechanisms underlying the loss of immune regulation in PTSD subjects. Also, the alternations in signaling pathways that allow Th1 and inflammatory mediators to be increased and which suppress levels of inhibitory regulatory cytokines have yet to be studied. Despite these unanswered questions, the present study shows immune imbalances toward an increased Th1/inflammatory state with diminished negative controls, and a loss of coordination of levels of cytokines with stimulatory or inhibitory activity.
Acknowledgments
This work was supported by the Clinical Sciences Research & Development Program of the Department of Veterans Affairs (Merit Review Grants 1 I01 CX000851 to MRIY and 1 I01 CX000487 to ZW).
Footnotes
There are no conflicts of interest to disclose.
References
- 1.Dursa EK, Reinhard MJ, Barth SK, Schneiderman AI. Prevalence of a positive screen for PTSD among OEF/OIF and OEF/OIF-era veterans in a large population-based cohort. J Trauma Stress. 2014;27:542–549. doi: 10.1002/jts.21956. [DOI] [PubMed] [Google Scholar]
- 2.Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44:4–19. doi: 10.3109/00048670903393597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An K, Salyer J, Kao HF. Psychological strains, salivary biomarkers, and risks for coronary heart disease among hurricane survivors. Biol Res Nurs. 2015;17:311–320. doi: 10.1177/1099800414551164. [DOI] [PubMed] [Google Scholar]
- 4.Newton TL, Fernandez-Botran R, Miller JJ, Burns VE. Interleukin-6 and soluble interleukin-6 receptor levels in posttraumatic stress disorder: associations with lifetime diagnostic status and psychological context. Biol Psychol. 2014;99:150–159. doi: 10.1016/j.biopsycho.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sommershof A, Aichinger H, Engler H, Adenauer H, Catani C, Boneberg EM, Elbert T, Groettrup M, Kolassa IT. Substantial reduction of naive and regulatory T cells following traumatic stress. Brain Behav Immun. 2009;23:1117–1124. doi: 10.1016/j.bbi.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 6.von Kanel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Cohen M, Meir T, Klein E, Volpin G, Assaf M, Pollack S. Cytokine levels as potential biomarkers for predicting the development of posttraumatic stress symptoms in casualties of accidents. Int J Psychiatry Med. 2011;42:117–131. doi: 10.2190/PM.42.2.b. [DOI] [PubMed] [Google Scholar]
- 8.Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, Groettrup M, Elbert T, Kolassa IT. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13:40. doi: 10.1186/1471-244X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slavish DC, Graham-Engeland JE, Smyth JM, Engeland CG. Salivary markers of inflammation in response to acute stress. Brain Behav Immun. 2015;44:253–269. doi: 10.1016/j.bbi.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O’Connor DT, Baker DG. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71:423–431. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood–brain barrier. NeuroImmunoModulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 12.Vargas-Caraveo A, Perez-Ishiwara DG, Martinez-Martinez A. Chronic psychological distress as an inducer of microglial activation and leukocyte recruitment into the area postrema. NeuroImmunoModulation. 2015 doi: 10.1159/000369350. [DOI] [PubMed] [Google Scholar]
- 13.Ho YH, Lin YT, Wu CW, Chao YM, Chang AY, Chan JY. Peripheral inflammation increases seizure susceptibility via the induction of neuroinflammation and oxidative stress in the hippocampus. J Biomed Sci. 2015;22:46. doi: 10.1186/s12929-015-0157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, Godbout JP. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry. 2014;75:970–981. doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKim DB, Patterson JM, Wohleb ES, Jarrett BL, Reader BF, Godbout JP, Sheridan JF. Sympathetic release of splenic monocytes promotes recurring anxiety following repeated social defeat. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones ME, Lebonville CL, Barrus D, Lysle DT. The role of brain interleukin-1 in stress-enhanced fear learning. Neuropsychopharmacology. 2015;40:1289–1296. doi: 10.1038/npp.2014.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, Schwartz M, Kipnis J. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J Neurobiol. 2006;66:552–563. doi: 10.1002/neu.20249. [DOI] [PubMed] [Google Scholar]
- 18.Muhie S, Gautam A, Meyerhoff J, Chakraborty N, Hammamieh R, Jett M. Brain transcriptome profiles in mouse model simulating features of post-traumatic stress disorder. Mol Brain. 2015;8:14. doi: 10.1186/s13041-015-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gautam A, D’Arpa P, Donohue DE, Muhie S, Chakraborty N, Luke BT, Grapov D, Carroll EE, Meyerhoff JL, Hammamieh R, Jett M. Acute and chronic plasma metabolomic and liver transcriptomic stress effects in a mouse model with features of post-traumatic stress disorder. PLoS One. 2015;10:e0117092. doi: 10.1371/journal.pone.0117092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl. 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- 21.Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, Mora C. Clinical evaluation of a measure to assess combat exposure. Psychol Assess. 1989;1:53–55. [Google Scholar]
- 22.Weathers FW, Blake DD, Schnurr PP, Marx BP, Keane TM. Clinician-Administered PTSD Scale for DSM-5 (CAPS-5), Department of Veterans Affairs. National Center for PTSD. 2015 online. [Google Scholar]
- 23.Hyer L, Summers MN, Boyd S, Litaker M, Boudewyns P. Assessment of older combat veterans with the clinician-administered PTSD scale. J Trauma Stress. 1996;9:587–593. doi: 10.1007/BF02103667. [DOI] [PubMed] [Google Scholar]
- 24.Radnitz CL, Hsu L, Willard J, Perez-Strumolo L, Festa J, Lillian LB, Walczak S, Tirch DD, Schlein IS, Binks M, Broderick CP. Posttraumatic stress disorder in veterans with spinal cord injury: trauma-related risk factors. J Trauma Stress. 1998;11:505–520. doi: 10.1023/A:1024404729251. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton M. The Hamilton rating scale for depression. J Oper Psychiatr. 1979;10:149–165. [Google Scholar]
- 26.Breslau N, Kessler RC. The stressor criterion in DSM-IV posttraumatic stress disorder: an empirical investigation. Biol Psychiatry. 2001;50:699–704. doi: 10.1016/s0006-3223(01)01167-2. [DOI] [PubMed] [Google Scholar]
- 27.Nebert DW. Extreme discordant phenotype methodology: an intuitive approach to clinical pharmacogenetics. Eur J Pharmacol. 2000;410:107–120. doi: 10.1016/s0014-2999(00)00809-8. [DOI] [PubMed] [Google Scholar]
- 28.Pesce M, Fratta IL, Ialenti V, Patruno A, Ferrone A, Franceschelli S, Rizzuto A, Tatangelo R, Campagna G, Speranza L, Felaco M, Grilli A. Emotions, immunity and sport: winner and loser athlete’s profile of fighting sport. Brain Behav Immun. 2015;46:261–269. doi: 10.1016/j.bbi.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 29.van Dooren FE, Schram MT, Schalkwijk CG, Stehouwer CD, Henry RM, Dagnelie PC, Schaper NC, van der Kallen CJ, Koster A, Sep SJ, Denollet J, Verhey FR, Pouwer F. Associations of low grade inflammation and endothelial dysfunction with depression – the Maastricht study. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, Allen NB, Stuart AL, Hayley AC, Byrne ML, Maes M. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J, Nagarkatti P, Zhong Y, Ginsberg JP, Singh NP, Zhang J, Nagarkatti M. Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS One. 2014;9:e94075. doi: 10.1371/journal.pone.0094075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minetto MA, Gazzoni M, Lanfranco F, Baldi M, Saba L, Pedrola R, Komi PV, Rainoldi A. Influence of the sample collection method on salivary interleukin-6 levels in resting and post-exercise conditions. Eur J Appl Physiol. 2007;101:249–256. doi: 10.1007/s00421-007-0484-x. [DOI] [PubMed] [Google Scholar]
- 33.Ebersole JL, Nagarajan R, Akers D, Miller CS. Targeted salivary biomarkers for discrimination of periodontal health and disease(s) Front Cell Infect Microbiol. 2015;5:62. doi: 10.3389/fcimb.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma M, Bairy I, Pai K, Satyamoorthy K, Prasad S, Berkovitz B, Radhakrishnan R. Salivary IL-6 levels in oral leukoplakia with dysplasia and its clinical relevance to tobacco habits and periodontitis. Clin Oral Investig. 2011;15:705–714. doi: 10.1007/s00784-010-0435-5. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan R, Thayalan DK, Padmanaban R, Ramadas R, Annasamy RK, Anandan N. Association of serum and salivary tumor necrosis factor-alpha with histological grading in oral cancer and its role in differentiating premalignant and malignant oral disease. Asian Pac J Cancer Prev. 2014;15:7141–7148. doi: 10.7314/apjcp.2014.15.17.7141. [DOI] [PubMed] [Google Scholar]
- 36.Lindqvist D, Wolkowitz OM, Mellon S, Yehuda R, Flory JD, Henn-Haase C, Bierer LM, Abu-Amara D, Coy M, Neylan TC, Makotkine I, Reus VI, Yan X, Taylor NM, Marmar CR, Dhabhar FS. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun. 2014;42:81–88. doi: 10.1016/j.bbi.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Wilson CB, McLaughlin LD, Ebenezer PJ, Nair AR, Dange R, Harre JG, Shaak TL, Diamond DM, Francis J. Differential effects of sertraline in a predator exposure animal model of post-traumatic stress disorder. Front Behav Neurosci. 2014;8:256. doi: 10.3389/fnbeh.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morch RH, Dieset I, Faerden A, Hope S, Aas M, Nerhus M, Gardsjord ES, Joa I, Morken G, Agartz I, Aukrust P, Djurovic S, Melle I, Ueland T, Andreassen OA. Inflammatory evidence for the psychosis continuum model. Psychoneuroendocrinology. 2016;67:189–197. doi: 10.1016/j.psyneuen.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Bu XL, Yao XQ, Jiao SS, Zeng F, Liu YH, Xiang Y, Liang CR, Wang QH, Wang X, Cao HY, Yi X, Deng B, Liu CH, Xu J, Zhang LL, Gao CY, Xu ZQ, Zhang M, Wang L, Tan XL, Xu X, Zhou HD, Wang YJ. A study on the association between infectious burden and Alzheimer’s disease. Eur J Neurol. 2015;22:1519–1525. doi: 10.1111/ene.12477. [DOI] [PubMed] [Google Scholar]
- 40.Tang MM, Lin WJ, Pan YQ, Guan XT, Li YC. Hippocampal neurogenesis dysfunction linked to depressive-like behaviors in a neuroinflammation induced model of depression. Physiol Behav. 2016 doi: 10.1016/j.physbeh.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 41.Aguilar-Valles A, Kim J, Jung S, Woodside B, Luheshi GN. Role of brain transmigrating neutrophils in depression-like behavior during systemic infection. Mol Psychiatry. 2014;19:599–606. doi: 10.1038/mp.2013.137. [DOI] [PubMed] [Google Scholar]


