Abstract
Our understanding of long-term organic matter preservation comes mostly from studies in aquatic systems. In contrast, taphonomic processes in extremely dry environments are relatively understudied and are poorly understood. We investigated the accumulation and preservation of lipid biomarkers in hyperarid soils in the Yungay region of the Atacama Desert. Lipids from seven soil horizons in a 2.5 m vertical profile were extracted and analyzed using GC-MS and LC-MS. Diagnostic functionalized lipids and geolipids were detected and increased in abundance and diversity with depth. Deeper clay units contain fossil organic matter (radiocarbon dead) that has been protected from rainwater since the onset of hyperaridity. We show that these clay units contain lipids in an excellent state of structural preservation with functional groups and unsaturated bonds in carbon chains. This indicates that minimal degradation of lipids has occurred in these soils since the time of their deposition between >40,000 and 2 million years ago. The exceptional structural preservation of biomarkers is likely due to the long-term hyperaridity that has minimized microbial and enzymatic activity, a taphonomic process we term xeropreservation (i.e. preservation by drying). The degree of biomarker preservation allowed us to reconstruct major changes in ecology in the Yungay region that reflect a shift in hydrological regime from wet to dry since the early Quaternary. Our results suggest that hyperarid environments, which comprise 7.5% of the continental landmass, could represent a rich and relatively unexplored source of paleobiological information on Earth.
Keywords: preservation, lipid, biomarker, desert, Atacama, Mars, hyperarid, FAME
1. Introduction
Understanding taphonomic processes and the conditions conducive to long term preservation of organic matter has been critical for reconstructing the evolutionary history of life on Earth (e.g., Peters et al., 2005) and in developing strategies to search for evidence of life elsewhere (Eigenbrode, 2008; Summons et al., 2011). Microbial processes mediate the majority of organic matter decomposition (Skopintsev, 1981; Petsch et al., 2001; Kuzyakov, 2010), with only approximately 0.1% of the global net primary production preserved in the sediment record (Holser et al., 1988; Des Marais, 2001). This limits the quantity and quality of molecular fossils (a.k.a. biomarkers) that become preserved after organisms die.
Long-term preservation of biomarkers is enhanced when fast sedimentation or mineral encapsulation impede or mitigate microbial attack (Van Veen and Kuikman, 1990; Farmer and Des Marais, 1999), or under environmental conditions that limit microbial activity and retard organic degradation, such as low temperature and humidity (Rethemeyer et al., 2010). In this paper, we focus on the preservation of biomolecules in an environment that has been extremely dry (i.e. hyperarid, aridity index <0.05) over geologic timescales. While hyperarid deserts represent 7.5% of the Earth’s continental landmass (UNEP definition) and fossils are known to be preserved in them (Pyenson et al., 2014), biomarker degradation and taphonomic processes over geological timescales in a hyperarid environments has not been previously investigated. We hypothesized that typical pathways of organic matter degradation would be inhibited in long-lived hyperarid regions where low water activity suspends microbial activity and greatly reduces enzyme action (de Gomez-Puyou and Gomez-Puyou, 1998).
We investigated the accumulation and degree of preservation of biomolecules in million-year-old hyperarid soils in the Atacama Desert, the oldest, continuously dry desert on Earth (Clarke, 2006). We focused our investigation on lipids because they have functional groups that are susceptible to microbial attack, but also contain recalcitrant hydrocarbon cores that can be preserved over geologic time scales, recording the presence and activity of organisms living millions to billions of years ago as well as the history of diagenetic conditions since the time of deposition (Peters et al., 2005; Eigenbrode, 2008).
1.1 Study Site
The Yungay region of the Atacama Desert experiences <2 mm of precipitation annually (McKay et al., 2003), with rain events often interspaced by a decade or more. The Aridity Index value, which is defined as the ratio of mean annual precipitation to potential evapotranspiration, is approximately 0.004 in this region. This value is 30 times more arid than the Mojave or Gobi Deserts (Davila and Schulze-Makuch, 2016). Surface soils in the Yungay region primarily experience water activities that range from 0.01–0.52 over the course of a diurnal cycle (Connon et al., 2007), well below 0.6, the threshold for microbial growth (Grant et al., 2004). The extreme aridity causes an absolute lack of habitation by plants or animals and can only support a sparse microbial population in surface soils that are primarily derived from atmospheric inputs (Warren-Rhodes et al., 2006; Connon et al., 2007; Lester et al., 2007). This region has been arid to semiarid since the late Jurassic (150 million years), and has experienced continuous hyperaridity for the last ~2 million years (Hartley and Chong, 2002; Hartley et al., 2005; Jordan et al., 2014). Fluvial incision and deposition ceased at Yungay near the Plio-Pleistocene boundary coinciding with transition to hyperarid climatic conditions within the region. Subsequent to drying, landforms have retained and accumulated the aeolian deposits of atmospheric salts and dust in the upper meter of the soil column (Ewing et al., 2006).
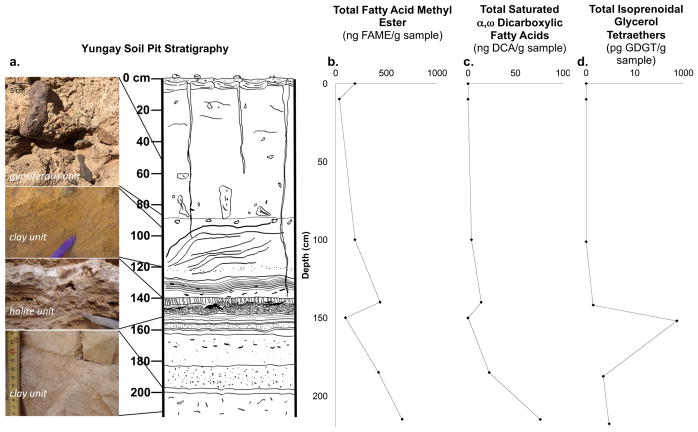
We sampled a 2.5 m stratigraphic sequence in a soil pit that was previously dug by (Sutter et al., 2007), and can be generally categorized into three major units (Fig. 1A): (i) gypsiferous soils in the top 90 cm; (ii) clay-rich units below 90 cm depth; and (iii) a 10 cm thick well-cemented, massive halite unit that interrupts the clay units at 140–150 cm depth. Within the top 2 meters of soil, 87% of the NaCl is located in this massive halite unit (Ewing et al., 2006). Sparse plant material is found in the clay units below the massive halite unit (Ewing et al., 2008; Supplementary Fig. 4).
Figure 1.
Yungay Soil Pit Stratigraphy & Key Lipid Abundances: a) The soil pit contains three major units: gypsiferous soils (0–90 cm depth), clay-rich units (> 90 cm depth), and a 10 cm-thick halite unit that interrupts clay units at 150 cm depth. Gypsiferous soils are matrix-supported and contain angular lithics. A clay unit at 100 cm depth contains centimeter-sized laminations composed of course sand. A clay unit at 140 cm depth contains fine sub-cm sized laminations (Supplementary Fig. 3). The massive, well-cemented halite unit (~140–150 cm) has two major morphologies: vertical, crystalline structures and mottled halite. Beneath are alternating bands of well-sorted clay units and contain fibrous plant fragments (cm-size) that become more concentrated with depth. A more detailed description of the stratigraphic profile is provided by (Ewing et al., 2006; 2008). b, c, d) Total abundances of FAME, DCA, and Isoprenoidal GDGT were found to increase with depth with the exception of the halite unit (150 cm). Isoprenoidal GDGT and DCA were absent from upper gypsiferous soil. Isoprenoidal GDGTs are plotted on a logarithmic scale due to the presence of a high relative abundance of Archaeol in the halite unit.
Landform ages in this region are ~2 million years based on cosmogenic radionuclide concentrations in surface boulders and Ar isotopes in interbedded volcanic ash deposits (Ewing et al., 2008). During this time, atmospheric salts have been vertically redistributed in the top ~1.5 meters of unaltered fluvial deposit and dust derived silicate matrix due to episodic wetting events Ewing et al. (2006; 2008). Small rain events (1 mm or less) do not saturate the surface soil in Yungay, nor are able to percolate past the gypsic crusts at 10–20 cm depth (Davis et al., 2010). These gypsic crusts act as a strong barrier to the diffusion of moisture into the soil (Davis et al., 2010). For larger rain events that are able to percolate down past the gypsic crust, according to (Ewing et al., 2006; 2008), the massive, well-cemented halite unit at 140–150 cm signals the maximum depth of rainwater percolation and salt distillation over the last 2 million years. This is further supported by the low concentrations of soluble ions, including chloride, in layers below the halite (Supplementary Fig. 1). Hence, the massive, well-cemented halite unit has acted as an aquiclude, preventing clay layers beneath from being exposed to rainwater and other modern surficial processes (Ewing et al., 2006). Consequently, organic matter in the deeper clay layers is of fossil origin, possibly older than 2 million years but at least >40,000 years because it is “radiocarbon dead” (Ewing et al., 2008), and represents a rare opportunity to investigate the degradation of functionalized lipid biomarkers over geological timescales in the absence of water.
2. Methods
2.1 Sample Collection
We collected seven samples from the soil pit in Yungay. Samples were collected in September 2014, before the decennial rain event that occurred in March 2015. Due to the extremely low inventory of biomass in Atacama soils (Ewing et al., 2006), samples were collected by scientists wearing full-body, sterile, clean-room suits (<1 colony forming unit per 10,000 garments), masks, glasses, and gloves to minimize the introduction of emitted anthropogenic biological contaminants (Meadow et al., 2015) during sampling. To remove surface contamination and expose fresh faces of the different soil horizons, approximately 20 cm of the pit wall was removed using a solvent-cleaned chisel (details of cleaning protocols are available in the SI). Samples were then collected in the soil pit from the bottom up using a solvent-cleaned drill bit to loosen the material. A solvent-cleaned spoon was used to scoop samples into glass jars that had been heated to 500°C for greater than 8 hours. Each sample was collected using a unique drill bit and spoon to minimize cross contamination. Samples were kept frozen until being returned to NASA Goddard Space Flight Center for storage at −20°C.
2.2 Abbreviated Lipid Analyses
For each unique sample, approximately 100 g of soil was homogeneously powdered with a solvent washed and ashed ceramic mortar and pestle. Soil samples were extracted using a modified Bligh and Dyer protocol (Bligh and Dyer, 1959; Jahnke et al., 1992). The protocol was modified primarily to minimize transfer steps and thus minimize loss from these organically lean soils. The monophasic extraction mixture of HPLC-grade water, methanol, and methylene chloride was split by further addition of methylene chloride and water to a final volume ratio of 1:1:0.9. This mixture was gravimetrically separated and the resultant total lipid extract (TLE) was collected, a portion set aside, and evaporated under a stream of N2 until a final volume of 40 μL was achieved. The TLE was subjected to a medium acid methanolysis (Kates, 1989) to cleave ester-linked membrane fatty acids and to methylate free fatty acids (FFA). This produced a total fatty acid methyl ester (FAME). The extract was subsequently derivatized using bis-(trimethylsilyl) trifluoroacetamide (BSTFA) for silylation of hydroxyl groups and remaining FFA. An aliquot of the derivatized TLE (20% of total) was analyzed on an Agilent 5975C gas chromatograph-mass spectrometer (GC-MS) (chromatographic conditions available in the SI). The lipids were quantified relative to an internal standard (5α-cholestanol, 12.5 ng on column). A portion of each TLE (10% of total) was also analyzed for glycerol di- and tetraethers on a 1260 Infinity series liquid chromatograph-mass spectrometer (LC-MS) (chromatographic conditions available in the SI).
3. Results
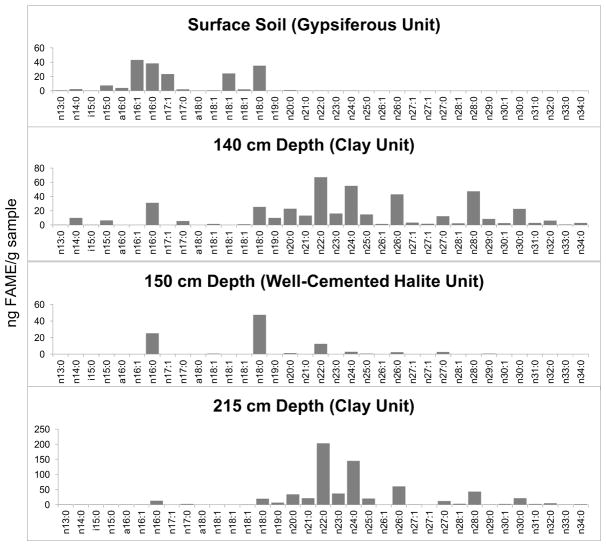
The abundance and diversity of functionalized lipid groups increased with depth throughout the profile (Fig. 1b and 2). The most abundant class of lipids detected were FAMEs, which are formed through the transesterification of esterified fatty acids (e.g., complex membrane-bound polar lipids, acylglycerols, steryl esters) as well as methylation of FFAs (Ichihara et al., 1996). Between 20 and 62 unique FAMEs were detected in each sample with alkyl chain lengths between 13 and 34 carbons, including methyl-branched FAMEs (terminal branched and mid-chain). The FAME profile composition was markedly different between horizons (Fig. 2), with diversity and abundance increasing with depth (Fig, 1b & 2). FAMEs were particularly abundant in the clay layers, with a maximum concentration in the deepest layer analyzed (215 cm) of 742 ng/g soil, comprised of 62 unique FAMEs. The abundance of FAMEs in the gypsiferous soil was lower by a factor of 4 or more. Deeper clay layers contained FAMEs with unsaturated bonds in their alkyl chains (Fig. 2). Small amounts of silylated fatty acids were also detected in soils 100 cm and below, reflecting free fatty acids not methylated during our acid methanolysis procedure (Supplementary Table 4). Additionally, α- and β-monohydroxy monocarboxylic fatty acids were detected that contain a hydroxyl (OH) group exposed to the external environment and available to be chemically altered. Based on the diagnostic fragment ions at m/z 175 and 159, α-hydroxy fatty acids were the more abundant member.
Figure 2.
Fatty Acid Methyl Ester (FAME) Profiles for Representative Yungay Pit Samples: Upper gypsiferous soils were dominated by n-16:0 and n-18:0 FAMEs. On the other hand, clay units were dominated by n-22:0 and greater chain length FAMES and contain a greater diversity in FAME content. The deepest clay unit had the greatest total FAME abundance. The well-cemented halite unit was dominated by n-16:0 and n-18:0 FAMEs. The vertical scale changes between plots.
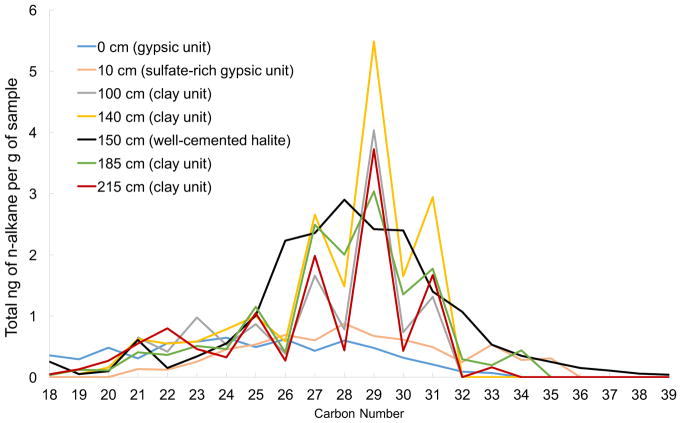
The upper gypsiferous soils were dominated by n-C16:0 and n-C18:0 FAMEs, and contained both iso and antiso FAMEs (Fig. 2). N-alkanes were also present in these units and had an unusual, slight even-over-odd chain length preference in the C25–C33 range (Fig. 3). Contamination from petroleum can be excluded by the absence of phenanthrene and other polycyclic aromatic hydrocarbons in the samples (Volkman, 2006). Hydroxy fatty acids were not detected in the gypsiferous soils, with the sole exception of a single chain length class (n-C16:0) found in the surface soil (Supplementary Table 2). Additionally, no archaeal lipids were detected within gypsiferous units.
Figure 3.
n-Alkane Content in Yungay Pit Soils: The straight chain n-alkane content of Yungay soils reveals two distinct patterns. Clay rich units exhibit an odd-over-even chain length preference. Conversely, gypsiferous soils and the halite unit exhibit a slight even-over-odd chain length preference.
Conversely, the clay-rich units below the aquiclude contained a higher abundance of lipids and included lipid classes that were not detected in the upper gypsiferous soils. The FAME profile in these units was dominated by n-C22:0 and C24:0 FAMEs (Fig. 2). Dimethyl esters of saturated α,ω-dicarboxylic fatty acids (DCA) were detected in all samples below 100 cm depth, with abundance and chain length ranges increasing with depth. The deepest clay unit below the aquiclude had the largest abundance, and also the largest range in chain length (C9–C31) (Fig. 1c; Supplementary Table 1), while only trace amounts of C22 DCA was observed in the halite unit. α- and β-monohydroxy monocarboxylic acids were also present in clay units with a relatively high abundance of n-24:0. A clay-rich sample at 185 cm depth had the highest total abundance of this lipid class as well as the greatest diversity, with chain lengths that ranged from C15 to C33. N-alkanes in these clay units contained an odd-over-even chain length preference (Fig. 3).
Isoprenoidal glycerol dialkyl glycerol tetraethers (GDGTs) (Fig. 1d) and non-isoprenoidal, branched GDGTs were detected in clay-rich layers below 100 cm depth (Supplementary Table 3), but not in the upper gypsiferous soils. An isoprenoidal GDGT (GDGT-0) was extracted from a clay-rich unit at 140 cm depth, and three additional isoprenoidal GDGTs (GDGT-1, GDGT-2, and crenarchaeol) along with five non-isoprenoidal GDGTs were identified in the clay-rich units at 185 cm and 215 cm depth respectively (Supplementary Fig. 2 and Supplementary Table 3). Branched GDGTs were found in greater abundance than isoprenoidal GDGTs.
Significantly, the well-cemented halite unit at 140–150 cm depth contained a GDGT profile distinctive from both the gypsiferous and clay-rich units. It was characterized by a relatively high abundance of isoprenoidal glycerol diether, archaeol (481 pg/g of soil), a lesser abundance of isoprenoidal GDGT-0, and the halophilic archaeal biomarker, C20–C25 extended archaeol (Supplementary Table 3).
4. Discussion
4.1 Biomarker preservation under prolonged hyperarid conditions
Fatty acids are rapidly destroyed by biological degradation such as aerobic and anaerobic respiration, fermentation, and photoheterotrophy (Brocks and Summons, 2004; Kuzyakov, 2010), and incubation experiments with phytoplankton have demonstrated complete degradation over the course of a few weeks (Sun et al., 1997). Hence, in most environments, the presence of labile lipids such as FFAs and FAMEs is indicative of extant communities or of recent biogenesis (Simoneit et al., 1998). Surprisingly, the concentration of FAMEs (Fig. 1b and 2) and FFAs (Supplementary Table 4) in Yungay soils was not only found to increase with depth in the soil sequence, but FFAs and FAMEs retained labile features such as unsaturated bonds in the alkyl chains within the deeper, sealed-off clay units. In addition, α- and β-monohydroxy monocarboxylic acids were primarily found in clay-rich units below 1 m depth (Supplementary Table 2), despite this class of fatty acid’s susceptibility to rapid diagenesis (Volkman, 2006) and hydroxyl group loss (e.g., Bordenave, 1993).
Given the short residence time of labile fatty acids in most environments, their detection throughout the soil profile suggests that they either represent extant biomass, or exceptionally well-preserved functionalized fossil lipids. We considered evidence for both scenarios. The Yungay region is one of the driest areas in the Atacama Desert. The water activity in the surface soils is well below the threshold for metabolic growth and enzymatic activity (Connon et al., 2007), and should remain very low and constant below a depth of 1 m (Azua-Bustos et al., 2015). The extremely dry conditions are reflected in the low biomass (103–105 cells cm−3) in surface soils (e.g., Navarro-González et al., 2003; Connon et al., 2007; and Crits-Christoph et al., 2013), and low organic carbon content (<102 ppm) (Connon et al., 2007 and Ewing et al., 2006), which is “radiocarbon dead” (older than 40,000 years) below a depth of 100 cm (Ewing et al., 2008). In addition, the D/L ratio of aspartic acid in the top cm of soil indicates significant racemization of biologically produced amino acids, and yields ages of 103 to 105 years (Skelley et al., 2007). Together, these data argue against substantial biological activity or recent biogenesis of lipids, even in the topmost gypsiferous soils. Instead, functionalized lipids in the top 1 m of soil likely represent relatively modern atmospheric inputs, a conclusion supported by the fact that the majority of cultivable isolates found in these soils are root-associated microbes in an environment where there has not been plant growth in millions of years (Lester et al., 2007).
On the other hand, the deeper clay layers that contain the highest concentration and diversity of lipids including plant lipids, have been isolated from rainwater as well as modern surface inputs for a period of hundreds of thousands to a few-million years by the massive halite unit at 140–150 cm depth. This halite unite is a marker of maximum water percolation (Ewing et al., 2006) and has acted as an aquiclude. Soluble ion distribution in the soil profile (Supplementary Fig. 1) reaffirms that there has been little or no percolation of rainwater through this halite unit (see below). Therefore, we argue that the functionalized lipids in the deeper clay units, and especially the archaeal and plant lipids found to be absent from the upper gypsiferous and surface soils, are fossils of organisms in an excellent state of chemical and structural preservation since their time of deposition.
The remarkable degree of preservation of functionalized fatty acids and other lipids over tens of thousands to a few million years in hyperarid soils can be explained by the extremely low water activity, a taphonomic process that we term xeropreservation (preservation by drying). Low water activity in soils arrests biological activity (e.g., both the synthesis and degradation of lipids) and suspends or greatly reduces chemical degradation, inhibiting modification of a cell’s own lipid membrane as well as the action of heterotrophs. In particular, low water activity can inactivate or slow the reaction rate of the cell’s own lytic enzymes, or those of heterotrophs, that degrade organic moieties upon cell death (e.g., de Gomez-Puyou and Gomez-Puyou, 1998). By halting aerobic or anaerobic metabolic activity, labile lipids such as fatty acids can be preserved. Such excellent lipid biomarker preservation is comparable to other geological samples that have been subjected to rapid dehydration, such as in polymerized resins like amber (Bada et al., 1999; Schweitzer, 2004), and opens the possibility for extreme longevity of other labile biomarkers such as ancient DNA. Additionally, the structural and chemical integrity of labile lipids throughout the soil profile suggests that chemical oxidation of these biomolecules has been limited despite the photochemical formation and accumulation of reactive oxidant species in these hyperarid soils (Georgiou, 2015). Again, this is likely due to the extremely low water availability in these soils, a factor exacerbated with depth, which limits soil organic oxidant chemistry.
Additionally, there are a number of abiotic conditions known to increase preservation of organic matter. Clay minerals present in the soil, which include smectite and kaolinite, may play a role enhancing the preservation of organics (e.g., polar lipids) through interaction with charged mineral surfaces (Farmer and Des Marais, 1999; Eigenbrode, 2008; Summons et al., 2011). Entombment by chemical precipitates is another mechanism of microbial fossilization (Farmer and Des Marais, 1999; Melendez et al., 2013; Williams et al., 2015). The many salts observed at Yungay could have served a similar function by encasing microorganisms or organic matter, thereby leading to increased chance of long-term preservation. It is also possible that the environmental conditions microorganisms were exposed to prior to drying had some effect on the degree of preservation of intracellular biomolecules. Stress (e.g., osmotic stress, decreases in pH, and starvation) has been shown to induce a number of survival strategies including modifications in cell membrane fatty acid composition, which afford protections to microorganisms that increase recovery after drying (Morgan et al., 2006 and references therein). The possibility that cellular modifications from environmental stressors before drying offer increased long-term preservation should be investigated further.
4.2 Microbial diversity and paleoenvironmental reconstruction based on lipid biomarkers
Certain lipids have been used as taxonomic markers for particular groups of microorganisms (e.g., Summons and Lincoln, 2012). Some recent work has called into question the utility of this approach, specifically as it relates to methylated hopanoids (e.g., Rashby et al., 2007; Welander et al., 2010). However, ester-linked membrane fatty acids are commonly used to assess microbial diversity (e.g., White et al., 1993), since the fatty acid chain length, number and position of double bonds, cyclopropane rings, and position of methyl branches allow for distinction between aerobes, anaerobes, sulfate-reducing bacteria, cyanobacteria, actinomycetes, fungi, protozoa, plants, and green algae (Vestal and White, 1989 and references therein). The excellent preservation of the labile and refractory lipids allowed us to characterize taxonomic groups present in each stratigraphic unit, perform paleoenvironmental reconstructions based on known habitats of these groups, and assess environmental change over time. We broadly used FAMEs, isoprenoidal glycerol ethers, DCAs, and alkanes to distinguish between bacteria, archaea, and higher organisms such as plants throughout the soil profile.
FAME profiles in the upper gypsiferous soils were indicative of a predominant bacterial source (Volkman, 1998; Kaneda, 1991). The slight even-over-odd carbon chain length preference in alkanes was unusual, and could indicate a distinctive microbial origin or alteration of algal detritus (Elias et al., 1997). The lack of archaeal lipids from the gypsiferous soils was consistent with previous work that noted an absence of archaeal groups based on 16S RNA analyses (Crits-Christoph et al., 2013). These data support the idea that the Yungay region soils contain a population primarily consisting of bacteria.
On the other hand, clay-rich units below 100 cm depth contained lipid biomarkers not detected in the soils above, including lipids that are typically diagnostic of plants. The long-chain DCAs detected are likely derived from plant biopolymers such suberin and cutin (Wakeham, 1999). The odd-over-even chain length preference seen in C24–C32 n-alkanes is a common biosignature of terrestrial plants (e.g., Wakeham, 1999; Volkman, 2006). The finding of plant-derived lipids in clay-rich units is consistent with the finding of cm-long fragments of fibrous plant material in samples at 185 cm and 215 cm depth (Ewing et al., 2006, Supplementary Fig. 4). Plant-derived DCAs identified in units beneath the aquiclude especially supports the argument for preservation of labile lipids under hyperarid conditions as the plant material is certainly no longer living, and hasn’t been since burial (>40,000 years ago).
The presence of α- and β-monohydroxy monocarboxylic acids almost exclusively in the clay units also points to past inputs of biological lipids. While monohydroxy monocarboxylic acids can form from the decomposition of FAMEs, the profile of α- and β-monohydroxy monocarboxylic acids in the clays differs from that of the FAME profiles and is suggestive of a unique source instead of a diagenetic product. This class of lipid is common in soil, marine, and lacustrine sediments (Volkman et al., 1998; Volkman, 2006 and references therein), and occurs in a wide range of taxonomic groups, or can be produced as intermediates in the alpha- and beta- oxidation of monocarboxylic acids, so their exact source is difficult to determine, although the most abundant monohydroxy monocarboxylic acid found (n-24:0) is known to occur in certain seagrass species (Wakeham, 1999).
Isoprenoidal GDGTs, a class of membrane lipids diagnostic for archaea (Pearson and Ingalls, 2013; Schouten et al., 2013) were also found in the clay units, along with branched GDGTs that are sourced from bacteria (Weihers et al., 2006). Non-isoprenoidal, branched GDGTs are proposed bacterial lipids (Weihers et al., 2006). Their greater abundance versus isoprenoidal GDGTs in the lower clay units is consistent with a dominant bacterial signal. Furthermore, this signal is inconsistent with offshore marine sources (e.g., Schouten et al., 2013) which argues against atmospheric sources. This, along with the absence of GDGTs in the upper gypsiferous soils suggests that both bacterial and archaeal GDGTs are syngenetic to the host clay unit. Together with the evidence for plant biomarkers, the presence of bacterial and archaeal GDGTs towards the bottom of the soil profile indicates a past wetter environment that was once capable of supporting a more diverse ecosystem than that what exists under the current hyperarid environmental conditions.
Finally, the well-cemented halite unit at approximately 140–150 cm depth contained a GDGT profile distinct from both the gypsiferous and clay-rich units. The relatively high abundance of archaeol; a lesser abundance of isoprenoidal GDGT-0; and the presence of the biomarker C20–C25 extended archaeol (Supplementary Table 3) are all diagnostic of halophilic archaea (Kates, 1978; Koga, 2005; Jahnke et al., 2008; Birgel et al., 2014). The occurrence of the halite unit between two layered clay units (Figs. 1 and Supplementary Fig. 3) and the halophilic archaeal lipid biomarkers found within the halite unit are consistent with formation in a small-scale evaporitic environment under a wetter climate regime. In this scenario, the buried halite layer could represent a paleo-surface horizon formed during a period of intense evaporation. This is in contrast to the proposed “wash-down” origin of the halite layer, whereby the salt-rich horizon formed from the episodic vertical transport of atmospheric salt during infrequent rain events and subsequent reprecipitation (Ewing et al., 2006; 2008). The presence of diagnostic archaeal lipids found only in the halite unit, and the presence of undisrupted fine laminations in the clay unit immediately above (Supplementary Fig. 3), both suggest that rainwater percolation might not be the sole mechanism responsible for the formation of the halite unit. However, soluble ion concentrations (Supplementary Fig. 1) do indicate that atmospherically-derived salts have been solubilized by rainwater and reprecipitated for the most part above the halite unit (at 100 and 137 cm depth, see supplementary information appendix for expanded results and discussion). Thus, the halite unit still marks the maximum depth for percolation of rainwater as suggested by (Ewing et al., 2006; 2008).
The wetter climate regime in the Yungay region inferred from the lipid biomarkers in the clay and halite units could be related to permanent El Niño-like conditions inferred for the central Atacama 5.6–4.7 million years (Wang et al., 2015), or with short, punctuated wet climate intervals during the last ~5 million years (Jordan et al., 2014), a timing that is consistent with the estimated age of the soil profile (> 2 million years).
In summary, extreme and prolonged dryness has been responsible for the preservation of labile biomarkers in hyperarid Atacama soils. This is likely due to the low water activity in the soils, which prevents any significant microbial activity or chemical degradation of organic compounds. The exceptional preservation of functionalized and more fragile or labile lipids, especially at the bottom of the soil profile that has been sealed-off from rainwater over a tens-of-thousands to a few million-years time scale, is comparable to the preservation of other labile biomarkers in deep-frozen permafrost or in polymerized resins like amber, and allows us to reconstruct significant taxonomic changes that may point to a shift in the hydrologic regime > 2 million years ago.
5. Conclusions
This study demonstrates that the range of stability for labile, functionalized lipid biomarkers in clays is on the order of tens-of-thousands to a few million years under extreme hyperarid conditions. Xeropreservation of biomolecules could represent an important, unexplored source of paleobiological information in the Atacama region in northern Chile, including Miocene sediments with marine vertebrates and invertebrates (Pyenson et al., 2014) and Jurassic and Cretaceous sediments with dinosaur remains (Bell and Padian, 1995; Salgado et al., 2008) and in other hyperarid desert environments. Xeropreservation could also have implications for the search for lipid biomarkers preserved under hyperarid conditions on Mars.
Supplementary Material
Acknowledgments
This work was supported primarily by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1148903 to M.B.W. Additional support was provided by a NASA Astrobiology Institute Early Career Collaboration Award to M.B.W. A.F.D. acknowledges funding from the NASA Astrobiology Institute (NAI Grant NNX15BB01A to the SETI Institute). M.N.P. was supported by NASA Exobiology grant NNX15AM17G. R.E.S. acknowledges support from the NASA Astrobiology Institute (NNA13AA90A) Foundations of Complex Life, Evolution, Preservation, and Detection on Earth and Beyond. X.-L.L. and RES were further supported by the Simons Foundation Collaboration on the Origins of Life (SCOL). S.O.R. was supported by the EU Marie Curie Actions Program and the Irish Research Council (ELEVATE Career-Development Fellowship). We thank Carolyn Colonero and Kate French of MIT for technical assistance and Terry Jordan, Lujendra Ojha, Max Bernstein, and Raechel Harnoto for helpful discussions. Finally, we thank Dr. Phil Meyers and one anonymous reviewer for helpful and constructive comments.
References
- Azua-Bustos A, Caro-Lara L, Vicuña R. Discovery and microbial content of the driest site of the hyperarid Atacama Desert, Chile. Environmental microbiology reports. 2015;7:388–394. doi: 10.1111/1758-2229.12261. [DOI] [PubMed] [Google Scholar]
- Bada JL, Wang XS, Hamilton H. Preservation of key biomolecules in the fossil record: current knowledge and future challenges. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1999;354:77–87. doi: 10.1098/rstb.1999.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CM, Padian K. Pterosaur fossils from the Cretaceous of Chile: evidence for a pterosaur colony on an inland desert plain. Geological Magazine. 1995;132:31–38. [Google Scholar]
- Birgel D, Guido A, Liu X, Hinrichs KU, Gier S, Peckmann J. Hypersaline conditions during deposition of the Calcare di Base revealed from archaeal di-and tetraether inventories. Organic Geochemistry. 2014;77:11–21. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bordenave ML. The sedimentation of organic matter. In: Bourdenave ML, editor. Applied Petroleum Geochemistry. Editions Technip; Paris: 1993. pp. 15–76. [Google Scholar]
- Brocks JJ, Summons RE. Sedimentary hydrocarbons, biomarkers for early life. In: Schlesinger WH, editor. Biogeochemistry: Treatise on Geochemistry. Vol. 8. Elsevier Pergamon; Oxford: 2004. pp. 63–115. [Google Scholar]
- Clarke JDA. Antiquity of aridity in the Chilean Atacama Desert. Geomorphology. 2006;73:101–114. [Google Scholar]
- Connon SA, Lester ED, Shafaat HS, Obenhuber DC, Ponce A. Bacterial diversity in hyperarid Atacama Desert soils. Journal of Geophysical Research: Biogeosciences. 2007:112. http://dx.doi.org/10.1029/2006JG000311.
- Crits-Christoph A, Robinson CK, Barnum T, Fricke WF, Davila AF, Jedynak B, McKay CP, DiRuggiero J. Colonization patterns of soil microbial communities in the Atacama Desert. Microbiome. 2013:1. doi: 10.1186/2049-2618-1-28. http://dx.doi.org/10.1186/2049-2618-1-28. [DOI] [PMC free article] [PubMed]
- Davila AF, Schulze-Makuch D. The Last Possible Outposts for Life on Mars. Astrobiology. 2016;16:159–168. doi: 10.1089/ast.2015.1380. [DOI] [PubMed] [Google Scholar]
- de Gomez-Puyou MT, Gomez-Puyou A. Enzymes in low water systems. Critical reviews in biochemistry and molecular biology. 1998;33:53–89. doi: 10.1080/10409239891204170. [DOI] [PubMed] [Google Scholar]
- Davis WL, de Pater I, McKay CP. Rain infiltration and crust formation in the extreme arid zone of the Atacama Desert, Chile. Planetary and Space Science. 2010;58:616–622. zzZ. [Google Scholar]
- Des Marais DJ. Isotopic evolution of the biogeochemical carbon cycle during the Precambrian. Reviews in Mineralogy and Geochemistry. 2001;43:555–578. [Google Scholar]
- Eigenbrode JL. Fossil lipids for life-detection: a case study from the early Earth record. In: Botta O, Bada JL, Gomez-Elvira J, Javaux E, Selsis F, Summons RE, editors. Strategies of Life Detection. Springer US; New York: 2008. pp. 161–185. [Google Scholar]
- Elias VO, Simoneit BR, Cardoso JN. Even n-alkane predominances on the Amazon shelf and a Northeast Pacific hydrothermal system. Naturwissenschaften. 1997;84:415–420. [Google Scholar]
- Ewing SA, Sutter B, Owen J, Nishiizumi K, Sharp W, Cliff SS, Perry K, Dietrich W, McKay CP, Amundson R. A threshold in soil formation at Earth’s arid–hyperarid transition. Geochimica et Cosmochimica Acta. 2006;70:5293–5322. [Google Scholar]
- Ewing SA, Macalady JL, Warren-Rhodes K, McKay CP, Amundson R. Changes in the soil C cycle at the arid-hyperarid transition in the Atacama Desert. Journal of Geophysical Research: Biogeosciences. 2008:113. http://dx.doi.org/10.1029/2007JG000495.
- Farmer JD, Des Marais DJ. Exploring for a record of ancient Martian life. Journal of Geophysical Research: Planets. 1999;104:26977–26995. doi: 10.1029/1998je000540. [DOI] [PubMed] [Google Scholar]
- Georgiou CD. Evidence for photochemical production of reactive oxygen species in desert soils. Nature Communications. 2015:6. doi: 10.1038/ncomms8100. http://dx.doi.org/10.1038/ncomms8100. [DOI] [PubMed]
- Grant WD. Life at low water activity. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2004;359:1249–1267. doi: 10.1098/rstb.2004.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley AJ, Chong G. Late Pliocene age for the Atacama Desert: implications for the desertification of western South America. Geology. 2002;30:43–46. [Google Scholar]
- Hartley AJ, Chong G, Houston J, Mather AE. 150 million years of climatic stability: evidence from the Atacama Desert, northern Chile. Journal of the Geological Society. 2005;162:421–424. [Google Scholar]
- Holser WT, Schidlowski M, Mackenzie FT, Maynard JB. Geochemical cycles of carbon and sulfur. In: Gregor CB, Garrels RM, Mackenzie FT, Maynard JB, editors. Chemical Cycles in the Evolution of the Earth. John Wiley & Sons; New York: 1988. pp. 105–173. [Google Scholar]
- Ichihara KI, Shibahara A, Yamamoto K, Nakayama T. An improved method for rapid analysis of the fatty acids of glycerolipids. Lipids. 1996;31:535–539. doi: 10.1007/BF02522648. [DOI] [PubMed] [Google Scholar]
- Jahnke LL, Orphan VJ, Embaye T, Turk KA, Kubo MD, Summons RE, Des Marais DJ. Lipid biomarker and phylogenetic analyses to reveal archaeal biodiversity and distribution in hypersaline microbial mat and underlying sediment. Geobiology. 2008;6:394–410. doi: 10.1111/j.1472-4669.2008.00165.x. [DOI] [PubMed] [Google Scholar]
- Jordan TE, Kirk-Lawlor NE, Blanco NP, Rech JA, Cosentino NJ. Landscape modification in response to repeated onset of hyperarid paleoclimate states since 14 Ma, Atacama Desert, Chile. Geological Society of America Bulletin. 2014;126:1016–1046. [Google Scholar]
- Kaneda T. Iso-and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiological reviews. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates M. The phytanyl ether-linked polar lipids and isoprenoid neutral lipids of extremely halophilic bacteria. Progress in Chemistry: Fats and Other Lipids. 1978;15:301–342. doi: 10.1016/0079-6832(77)90011-8. [DOI] [PubMed] [Google Scholar]
- Kates M, Work E. Laboratory techniques in biochemistry and molecular biology. Vol. 3. Elsevier; Amsterdam: 1986. [Google Scholar]
- Koga Y, Morii H. Recent advances in structural research on ether lipids from Archaea including comparative and physiological aspects. Bioscience, Biotechnology, and Biochemistry. 2005;69:2019–2034. doi: 10.1271/bbb.69.2019. [DOI] [PubMed] [Google Scholar]
- Kuzyakov Y. Priming effects: interactions between living and dead organic matter. Soil Biology and Biochemistry. 2010;42:1363–1371. [Google Scholar]
- Lester ED, Satomi M, Ponce A. Microflora of extreme arid Atacama Desert soils. Soil Biology and Biochemistry. 2007;39:704–708. [Google Scholar]
- McKay CP, Friedmann EI, Gómez-Silva B, Cáceres-Villanueva L, Andersen DT, Landheim R. Temperature and moisture conditions for life in the extreme arid region of the Atacama Desert: four years of observations including the El Niño of 1997–1998. Astrobiology. 2003;3:393–406. doi: 10.1089/153110703769016460. [DOI] [PubMed] [Google Scholar]
- Meadow JF, Altrichter AE, Bateman AC, Stenson J, Brown GZ, Green JL, Bohannan BJ. Humans differ in their personal microbial cloud. PeerJ. 2015:3. doi: 10.7717/peerj.1258. https://doi.org/10.7717/peerj.1258. [DOI] [PMC free article] [PubMed]
- Melendez I, Grice K, Schwark L. Exceptional preservation of Palaeozoic steroids in a diagenetic continuum. Scientific reports. 2013:3. doi: 10.1038/srep02768. http://dx.doi.org/10.1038/srep02768. [DOI] [PMC free article] [PubMed]
- Morgan CA, Herman N, White PA, Vesey G. Preservation of micro-organisms by drying; a review. Journal of microbiological methods. 2006;66:183–193. doi: 10.1016/j.mimet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Navarro-González R, Rainey FA, Molina P, Bagaley DR, Hollen BJ, de la Rosa J, Small AM, Quinn RC, Grunthaner FJ, Cáceres L, Gomez-Silva B. Mars-like soils in the Atacama Desert, Chile, and the dry limit of microbial life. Science. 2003;302:1018–1021. doi: 10.1126/science.1089143. [DOI] [PubMed] [Google Scholar]
- Pearson A, Ingalls AE. Assessing the use of archaeal lipids as marine environmental proxies. Annual Review of Earth and Planetary Sciences. 2013;41:359–384. [Google Scholar]
- Peters KE, Walters CC, Moldowan JM. The biomarker guide. Vol. 1. Cambridge University Press; Cambridge: 2005. [Google Scholar]
- Petsch ST, Eglinton TI, Edwards KJ. 14C-dead living biomass: evidence for microbial assimilation of ancient organic carbon during shale weathering. Science. 2001;292:1127–1131. doi: 10.1126/science.1058332. [DOI] [PubMed] [Google Scholar]
- Pyenson ND, Gutstein CS, Parham JF, Le Roux JP, Chavarría CC, Little H, Metallo A, Rossi V, Valenzuela-Toro AM, Velez-Juarbe J, Santelli CM. Repeated mass strandings of Miocene marine mammals from Atacama Region of Chile point to sudden death at sea. Proceedings of the Royal Society of London B: Biological Sciences. 2014:281. doi: 10.1098/rspb.2013.3316. http://dx.doi.org/10.1098/rspb.2013.3316. [DOI] [PMC free article] [PubMed]
- Rashby SE, Sessions AL, Summons RE, Newman DK. Biosynthesis of 2-methylbacteriohopanepolyols by an anoxygenic phototroph. Proceedings of the National Academy of Sciences. 2007;104:15099–15104. doi: 10.1073/pnas.0704912104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethemeyer J, Schubotz F, Talbot HM, Cooke MP, Hinrichs KU, Mollenhauer G. Distribution of polar membrane lipids in permafrost soils and sediments of a small high Arctic catchment. Organic Geochemistry. 2010;41:1130–1145. [Google Scholar]
- Salgado L, Cruz RD, Suárez M, Fernández M, Gasparini Z, Palma-Heldt S, Fanning M. First Late Jurassic dinosaur bones from Chile. Journal of Vertebrate Paleontology. 2008;28:529–534. [Google Scholar]
- Schouten S, Hopmans EC, Damsté JSS. The organic geochemistry of glycerol dialkyl glycerol tetraether lipids: a review. Organic geochemistry. 2013;54:19–61. [Google Scholar]
- Schweitzer MH. Molecular paleontology: some current advances and problems. Annales de paléontologie. 2004;90:81–102. [Google Scholar]
- Simoneit BR, Summons RE, Jahnke LL. Biomarkers as tracers for life on early Earth and Mars. Origins of Life and Evolution of the Biosphere. 1998;28:475–483. doi: 10.1023/a:1006508012904. [DOI] [PubMed] [Google Scholar]
- Skelley AM, Aubrey AD, Willis PA, Amashukeli X, Ehrenfreund P, Bada JL, Grunthaner FJ, Mathies RA. Organic amine biomarker detection in the Yungay region of the Atacama Desert with the Urey instrument. Journal of Geophysical Research: Biogeosciences. 2007:112. http://dx.doi.org/10.1029/2006JG000329.
- Skopintsev BA. Decomposition of Organic Matter of Plankton, Humification and Hydrolysis. Elsevier Oceanography Series. 1981;31:125–177. [Google Scholar]
- Summons RE, Lincoln SA. Biomarkers: informative molecules for studies in geobiology. In: Knoll AH, Canfield DE, Konhauser KO, editors. Fundamentals of Geobiology. Blackwell Publishing Ltd; Oxford: 2012. pp. 269–296. [Google Scholar]
- Summons RE, Amend JP, Bish D, Buick R, Cody GD, Des Marais DJ, Dromart G, Eigenbrode JL, Knoll AH, Sumner DY. Preservation of martian organic and environmental records: final report of the Mars Biosignature Working Group. Astrobiology. 2011;11:157–181. doi: 10.1089/ast.2010.0506. [DOI] [PubMed] [Google Scholar]
- Sun MY, Wakeham SG, Lee C. Rates and mechanisms of fatty acid degradation in oxic and anoxic coastal marine sediments of Long Island Sound, New York, USA. Geochimica et Cosmochimica Acta. 1997;61:341–355. [Google Scholar]
- Sutter B, Dalton JB, Ewing SA, Amundson R, McKay CP. Terrestrial analogs for interpretation of infrared spectra from the Martian surface and subsurface: Sulfate, nitrate, carbonate, and phyllosilicate-bearing Atacama Desert soils. Journal of Geophysical Research: Biogeosciences. 2007:112. http://dx.doi.org/10.1029/2006JG000313.
- Van Veen JA, Kuikman PJ. Soil structural aspects of decomposition of organic matter by micro-organisms. Biogeochemistry. 1990;11:213–233. [Google Scholar]
- Vestal JR, White DC. Lipid analysis in microbial ecology quantitative approaches to the study of microbial communities. Bioscience. 1989;39:535–541. [PubMed] [Google Scholar]
- Volkman JK. Lipid markers for marine organic matter. In: Volkman JK, editor. Marine Organic Matter: Biomarkers, Isotopes and DNA. Springer Berlin Heidelberg; Heidelberg: 2006. pp. 27–70. [Google Scholar]
- Volkman JK, Barrett SM, Blackburn SI, Mansour MP, Sikes EL, Gelin F. Microalgal biomarkers: a review of recent research developments. Organic Geochemistry. 1998;29:1163–1179. [Google Scholar]
- Wakeham SG. Monocarboxylic, dicarboxylic and hydroxy acids released by sequential treatments of suspended particles and sediments of the Black Sea. Organic Geochemistry. 1999;30:1059–1074. [Google Scholar]
- Wang F, Michalski G, Seo JH, Granger DE, Lifton N, Caffee M. Beryllium-10 concentrations in the hyper-arid soils in the Atacama Desert, Chile: Implications for arid soil formation rates and El Niño driven changes in Pliocene precipitation. Geochimica et Cosmochimica Acta. 2015;160:227–242. [Google Scholar]
- Warren-Rhodes KA, Rhodes KL, Pointing SB, Ewing SA, Lacap DC, Gomez-Silva B, Amundson R, Friedmann EI, McKay CP. Hypolithic cyanobacteria, dry limit of photosynthesis, and microbial ecology in the hyperarid Atacama Desert. Microbial Ecology. 2006;52:389–398. doi: 10.1007/s00248-006-9055-7. [DOI] [PubMed] [Google Scholar]
- Weijers JW, Schouten S, Hopmans EC, Geenevasen JA, David OR, Coleman JM, Pancost RD, Sinninghe Damsté JS. Membrane lipids of mesophilic anaerobic bacteria thriving in peats have typical archaeal traits. Environmental Microbiology. 2006;8:648–657. doi: 10.1111/j.1462-2920.2005.00941.x. [DOI] [PubMed] [Google Scholar]
- Welander PV, Coleman ML, Sessions AL, Summons RE, Newman DK. Identification of a methylase required for 2-methylhopanoid production and implications for the interpretation of sedimentary hopanes. Proceedings of the National Academy of Sciences. 2010;107:8537–8542. doi: 10.1073/pnas.0912949107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DC, Meadows P, Eglinton G, Coleman ML. In situ Measurement of Microbial Biomass, Community Structure and Nutritional Status [and Discussion] Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences. 1993;344:59–67. [Google Scholar]
- Williams AJ, Sumner DY, Alpers CN, Karunatillake S, Hofmann BA. Preserved filamentous microbial biosignatures in the Brick Flat gossan, Iron Mountain, California. Astrobiology. 2015;15:637–668. doi: 10.1089/ast.2014.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.