Abstract
Renin Angiotensin System (RAS) is a hormonal system that regulates blood pressure and fluid balance through a coordinated action of renal, cardiovascular, and central nervous systems. In addition to its hemodynamic regulatory role, RAS involves in many brain activities, including memory acquisition and consolidation. This review has summarized the involvement of RAS in the pathology of Alzheimer’s disease (AD), and the outcomes of treatment with RAS inhibitors. We have discussed the effect of brain RAS in the amyloid plaque (Aβ) deposition, oxidative stress, neuroinflammation, and vascular pathology which are directly and indirectly associated with AD. Angiotensin II (AngII) via AT1 receptor is reported to increase brain Aβ level via different mechanisms including increasing amyloid precursor protein (APP) mRNA, β-secretase activity, and presenilin expression. Similarly, it was associated with tau phosphorylation, and reactive oxygen species generation. However, these effects are counterbalanced by Ang II mediated AT2 signaling. The protective effect observed with angiotensin receptor blockers (ARBs) and angiotensin converting enzyme inhibitors (ACEIs) could be as the result of inhibition of Ang II signaling. ARBs also offer additional benefit by shifting the effect of Ang II toward AT2 receptor. To conclude, targeting RAS in the brain may benefit patients with AD though it still requires further in depth understanding.
Keywords: RAS, ARB, ACEI, amyloid β, oxidative stress, vascular disease, inflammation, AD
Introduction
Renin Angiotensin System (RAS) is a hormonal system that regulates body fluid, electrolyte homostasis, and vascular tone (Yim and Yoo, 2008; Sparks et al., 2014). These classical functions of RAS are mediated by angiotensin effector peptides including Ang II, III and 1–7 (Atlas, 2007). Ang II, the primary effector peptide, is produced in the blood and exerts a number of effects on kidney, adrenal glands, sympathetic nervous system and baroreceptor reflexes (Reid, 1992; Dasgupta and Zhang, 2011). Studies have also shown the presence of local RAS in many different tissues including brain (Ganten et al., 1983; Wang et al., 1996; Vila-Porcile and Corvol, 1998; Grobe et al., 2010; Ferrão et al., 2014). In the central nervous system, angiotensinogen is synthesized by astrocytes and subsequently cleaved by renin, angiotensin converting enzyme (ACE) and aminopeptidases or ACE2 and Neprilysin (Bodiga and Bodiga, 2013). Despite some speculations, it is not clearly known where these RAS enzymes are locally synthesized in the brain (McKinley et al., 2003).
The angiotensin ligands interact with their receptors including angiotensin (AT) 1A, 1B, 2, 4 and Mas and controls various brain function (Guimond and Gallo-Payet, 2012; Premer et al., 2013). The receptors are differentially expressed in several parts of the brain (Kakar et al., 1992; Braga, 2011). AT1A is expressed in areas mainly involved in regulation of blood pressure and electrolyte balance including subfornical organ, paraventricular nucleus of the hypothalamus, lateral septum, cerebral cortex, and hippocampus (Johren et al., 1995; MacGregor et al., 1995; Lenkei et al., 1997), brainstem baroreflex arc, olivocerebellary system, and preoptic region (Lenkei et al., 1997). While AT1B is expressed in structures which involve in higher brain function and memory including cerebral cortex and hippocampus (Johren et al., 1995).
Activation of AT1 receptors is associated with increase in oxidative stress (Prusty et al., 2017), anxiety and stress (Saavedra et al., 2005; Wincewicz and Braszko, 2014), ischemic brain damage (Panahpour et al., 2014), and cognitive impairment (Nakagawa et al., 2017).
AT2 receptor, on the other hand, is observed in parts of the brain which regulate learning and memory including hippocampus, cingulate cortex, superior colliculus, lateral septum, in thalamic nuclei, in the subthalamic nucleus, in the locus coeruleus, and in the inferior olive (Millan et al., 1991; Lenkei et al., 1996). AT2 receptor is also expressed in brain structures including red nucleus, pedunculopontine tegmental nucleus, bed nucleus of the supraoptic decussation, paragenual nucleus, motor hypoglossal nucleus, cerebellar nuclei (Song et al., 1991, 1992; Tsutsumi and Saavedra, 1991; Lenkei et al., 1996), substantia nigra (Garrido-Gil et al., 2013; Valenzuela et al., 2016), and ventral tegmental area (Garrido-Gil et al., 2013). However, the extent of the receptor expression is limited after the fetal period (AbdAlla et al., 2009). AT2 receptor signaling is suggested to play beneficial role in neurogenesis (Umschweif et al., 2014), cerebral blood flow (Iwai et al., 2004; Fuchtemeier et al., 2015), neuronal plasticity (Namsolleck et al., 2013), and learning and memory (Jing et al., 2012). Activation of the receptor is also reported to attenuate inflammation (Rompe et al., 2010), oxidative stress (Lu et al., 2015) and abnormal neuronal firing (Grammatopoulos et al., 2004; Matsuura et al., 2005) observed as the result of AT1 receptor stimulation (Guimond and Gallo-Payet, 2012).
In addition to AT1 and AT2 receptors, recent evidences show the presence of other receptors in CNS including AT4 and Mas (Singh and Karnik, 2016). AT4 receptor interacts with a different angiotensin ligand called angiotensin IV, and it is reported to regulate learning and memory in brain areas including the hippocampus, neocortex and motor nuclei (Wright et al., 1999; Chai et al., 2000). The receptor is also localized in claustrum, choroid plexus, pontine nucleus, thalamic nuclei, substantia nigra pars compacta and hypothalamus (Zhuo et al., 1998; Chai et al., 2000). It is also suggested for its neuroprotective effect against cerebral ischemia (Faure et al., 2006). Mas receptor also contributes for the diverse actions of RAS in the brain (Jackson et al., 2018). The receptor is mainly localized in the hippocampus, amygdala, anterodorsal thalamic nucleus, cortex, and hypoglossal nucleus (Bunnemann et al., 1990; Becker et al., 2007; Freund et al., 2012; Lazaroni et al., 2012). Activation of the receptor by angiotensin 1–7 was found to strengthen synapses in areas involved in memory (Bunnemann et al., 1990; Hellner et al., 2005; Uekawa et al., 2016).
Brain RAS generally involves in regulating central activities including learning, memory, anxiety, depression, cognition, and emotional stress (Gard, 2004; Paul et al., 2006; de Gasparo et al., 2013), but it also complements functions of the peripheral RAS (McKinley et al., 2003). Importantly, there are growing evidence indicating the contribution of brain RAS in development of neurodegenerative disorders including AD (Zhu et al., 2011; Tian et al., 2012; AbdAlla et al., 2013; Ana Flavia et al., 2017; Takane et al., 2017). However, it is not exactly known how RAS system influences the development and progression of AD. It is not also well understood how medications acting on RAS system affect AD though some studies have shown a link between RAS and accumulation of toxic Aβ peptides (Murphy and LeVine, 2010; Gouras et al., 2015), tau phosphorylation (Tian et al., 2012), oxidative stress (Chrissobolis et al., 2012), mitochondrial dysfunction (Nozoe et al., 2008), neuroinflammation (Vargas et al., 2012) and cholinergic dysfunction (Barnes et al., 1990).
Amyloid and Alzheimer’s Disease
Aβ42 and Aβ40 are the two-predominant Aβ-proteins that are highly susceptible for aggregation to form oligomers, protofibrils, and fibrils (Shin et al., 1997; Ahmed et al., 2010). Under normal physiological conditions, brain eliminates toxic peptides via enzymatic degradation, perivascular drainage and receptor-mediated efflux transport (Higuchi et al., 2005; Wang et al., 2011; Iliff et al., 2012; Provias and Jeynes, 2014; Baranello et al., 2015). Impairment of either of these clearance mechanisms may result in accumulation of Aβ peptide. The accumulation can cause neuronal membrane damage, an increase in oxidative stress, receptor-mediated alteration of signal transduction, alteration of membrane pore, increase in intracellular level of calcium ion and mitochondrial damage (Yankner, 1996; Carrillo-Mora et al., 2014). These changes also trigger persistent loss of cholinergic projections to the neocortex (Supnet and Bezprozvanny, 2010).
Aβ deposition facilitates the formation of pathological phosphorylated tau proteins (Busciglio et al., 1995; Zheng et al., 2002; Bloom, 2014). Accumulation of toxic tau protein could also occur independent of amyloid β (Katsuno et al., 2005). The abnormal aggregation and deposition of tau protein can result in formation of neurofibrillary tangles leading to a progressive loss of neurons (Buee et al., 2000; Hanger et al., 2009; Wolfe, 2012). Tau mediated neurodegeneration could be due to sequestration of tau protein and disturbance of microtubule function (Alonso et al., 2008; Iqbal et al., 2009). This results impairment of normal axon flow and subsequent loss of neurons and their connectivity (Iqbal et al., 2009; Baird and Bennett, 2013).
Renin Angiotensin System and Aβ Peptides: In Vitro Studies
In vitro studies have shown the role of ACE in the degradation of Aβ peptides halting the halts development of amyloid plaque (Hu et al., 2001; Oba et al., 2005). The enzymatic action of ACE in the breakdown Aβ peptides have demonstrated by several studies (Hemming and Selkoe, 2005; Sun et al., 2008; Zou et al., 2009). Whilst ACE inhibitors were reported to promote Aβ aggregation (Hu et al., 2001). ACE2, a homolog of ACE, was also reported to have a catalytic role in the cleavage of Aβ43 to Aβ40 and this was inhibited by specific ACE2 inhibitor called DX600 (Liu et al., 2014). N domain part of the enzyme was found responsible for hydrolysis Aβ peptides at N-terminal position. ACE hydrolyses the most neurotoxic peptides Aβ43 and Aβ42 (Welander et al., 2009; Brouillette et al., 2012), in to amyloid peptides that are less susceptibility to aggregate and form senile plaques. ACE also metabolizes the most abundant amyloid peptide, Aβ40 with the potential to reduce the Aβ42 oligomerization and deposition (Kim et al., 2007; Murray et al., 2009). ACE reduces amyloid β peptides the main risk factor for the development and progression of AD (Karran et al., 2011) (Table 1). These studies altogether indicate the metabolic action of RAS enzymes in reducing amyloid plaque deposition via degradation of the most toxic form amyloid peptides composed of 40-43 amino acid sequences.
Table 1.
The effect of ACE-Is on Amyloid-β level: In vitro study.
| Cloned culture | Effects of ACE expression | Effects of ACE inhibition | Reference |
|---|---|---|---|
| Seminal plasma | Decrease Aβ40 level | Lisinopril promote Aβ40 production | Hu e/al., 2001 |
| Neuroblastoma | Decrease Aβ40 and Aβ42 level | Captopril promote Aβ40 and Aβ42 level | Hemming and Selkoe, 2005 |
| HEK293 | Increase break down of Aβ43 to Aβ42 | DX600 inhibit breakdown Aβ43 to Aβ42 | Liu et al., 2014 |
| COS7 cells | Increase breakdown of Aβ40 | – | Oba et al., 2005 |
| CHO cells | Increase breakdown of m and h Aβ | – | Sun et al., 2008 |
| COS7 cells | Increase breakdown of Aβ42 to Aβ40 | ACEIs inhibit conversion of Aβ42 to-Aβ40 | Zou et al., 2009 |
ACEIs, angiotensin converting enzyme inhibitors; CHO, Chinese hamster ovary; HEK, human embryonic kidney cells 293; Aβ, Amyloid-β; m Aβ, murine Amyloid- β; h Aβ, human amyloid- β.
Renin Angiotensin System and Alzheimer’s Disease: Animal Studies
In vitro studies have shown the role of ACE in degradation of Aβ peptides thereby reducing deposition and accumulation of amyloid plaque while inhibition of the enzyme is detrimental (Hemming and Selkoe, 2005; Sun et al., 2008; Zou et al., 2009; Liu et al., 2014). Ramipril (ACE inhibitor) also increased Aβ peptides in ACE10/10 mice with AD (Bernstein et al., 2014). Recent studies, however, does not support the idea that ACEIs increases accumulation of Aβ peptides in AD animal models (Eckman et al., 2006; Hemming et al., 2007; Ferrington et al., 2011, 2012). These studies challenge the notion that ACEIs inhibit degradation of Aβ peptides and favoring amyloid plaque formation. Some ACEIs even reduced Aβ peptide level in animal models of AD (AbdAlla et al., 2013). Moreover, ACEIs showed beneficial effect in reducing AD signs and symptoms (Dong et al., 2011; Tota et al., 2012; AbdAlla et al., 2015). Administration of perindopril (ACEI) has shown an instrumental effect in increasing density of normal neurons and improving learning and memory (Hou et al., 2008). A study on Tg2576 AD model demonstrated the positive role of captopril in preventing signs of neurodegeneration (AbdAlla et al., 2013). These studies support the potential benefit of ACEIs in alleviating sign and symptom of AD; however, with contrasting reports. A study on Tg2576 mice showed increase in deposition of Aβ42 after treatment with captopril (Zou et al., 2007). In line with this study, treatment with ramipril elevated brain level of Aβ42 peptide in AD+ACE (10/10) mice. Most in vivo studies have shown a positive correlation between increased expression of ACE and signs of AD but ACE inhibitors have protective effect against AD (Table 2). The protective effect of ACE inhibitors could be explained partly via suppressing brain derived neurotrophic factor decline and TNF-α release. They were also found to ameliorate oxidonitrosative stress and nitrotyrosine production (Ali et al., 2016) with that in turn reduces amyloidogenesis and subsequent Aβ deposition (Goel et al., 2016). However, further investigations are required to see if the contradicting reports were intrinsic to the specific inherent nature of the drug or methodological issue.
Table 2.
The effect of ACEIs on Brain A level: Animal studies.
| Animal model | Tested drug | Results | Reference |
|---|---|---|---|
| Aβ42 induced SDR | Perindopril | Decrease Aβ42 | Hou et al., 2008 |
| Tg2576 mice | Captopril/Enalapril | Reduced Aβ plaque and ROS accumulation | AbdAlla et al., 2013 |
| C57BL/6 × DBA2 and 3xTg AD | Captopril | No effect on Aβ levels | Hemming et al., 2007 |
| LPS induced Mice | Perindopril | Decrease Aβ level | Ali et al., 2016 |
| LPS induced WRs | Perindopril | Decrease Aβ levels and improved CBF | Goel et al., 2016 |
| A E10/10 mice | Ramipril | Increase Aβ levels | Bernstein et al., 2014 |
ACE, angiotensin converting enzyme; LPS, lipopolysaccharide; ROS, reactive oxygen species.
A review by Kehoe indicated Ang II (as with ACE) increased accumulation and deposition Aβ peptides in AD animal models (Kehoe, 2009). Ang II increases Aβ level, promotes cerebrovascular dysfunction, and micro-vascular amyloid deposition which those in turn worsens AD outcome (Faraco et al., 2016). ARBs, e.g., telmisartan, have shown to prevent cognitive decline associated with Aβ40 injection (Mogi et al., 2008). Olmesartan was also associated with improved cognitive function and hippocampal synaptic plasticity (Takeda et al., 2009). Losartan was reported to prevent neuropathological and cognitive deficits observed in AD (Ongali et al., 2014). These studies showed the beneficial roles of ARBs in animal models of AD. The protective effect could be explained in part via suppressing AT1 receptor mediated APP mRNA up regulation, Aβ peptide production and phosphorylated tau induced neurotoxicity (Zhu et al., 2011). The protective effect of these drugs could also be attributed as a result of unopposed action of Ang II on AT2 receptor (Horiuchi et al., 2010; Gallo-Payet et al., 2011) and stimulation of AngIV/AT4R signaling as observed in losartan (Royea et al., 2017). AT2 receptor mediated signaling pathways are known to prevent degeneration of neurons (Li et al., 2005; McCarthy et al., 2009). In line with these reports, valsartan have shown to attenuate oligomerization of Aβ peptides into high molecular weight oligomeric peptides and reduces cognitive deterioration (Wang et al., 2007). However, other studies with the same model have shown that Aβ induces the formation of oligomers of AT2 receptor in the hippocampus that disrupts Ang II mediated signaling. The Aβ- induced AT2 receptor oligomerization was associated with enhanced neurodegeneration. Conversely, inhibition of cross-linked AT2 receptor delayed tau phosphorylation (AbdAlla et al., 2009).
In other studies, however, valsartan or eprosartan (ARBs), did not alter accumulation of Aβ oligomers and phosphorylated tau in triple transgenic mice (Ferrington et al., 2011). The contradiction could be reconciled by the difference in AD animal models used. Variability in the dose of drug, the age and strain of animal used in the experiment could also explain the discrepancy (Ferrington et al., 2012). Despite varying result of RAS on amyloidosis, the overall effects of this system seem to favor amyloidosis. More specifically, the Ang II favors production of Aβ peptides via the most widely expressed angiotensin receptor, AT1 (Hohle et al., 1995). In addition to reduction of Aβ deposition and its consequences, RAS inhibitors have also other beneficial roles including suppression of inflammation (Saavedra, 2012), oxidative stress (Prusty et al., 2017), vascular damage/ischemia (Takeda and Morishita, 2017), and increase in acetylcholine release (Barnes et al., 1990) and glutamate uptake (Ruginsk et al., 2015) (Figure 1).
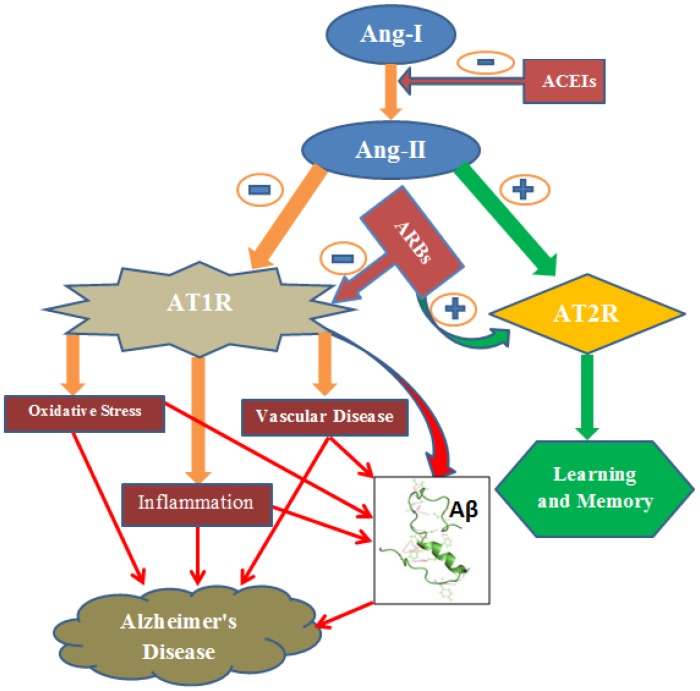
FIGURE 1.
Ang-II induces oxidative stress, inflammation and vascular disease via AT1R. Consequently, it causes accumulation of amyloid-β resulting Alzheimer’s disease. However, AT2 R signaling produces beneficial effect including learning and memory. ARBs inhibit AT1R signaling and this shifts the action of Ang-II toward the beneficial pathway (AT2R signaling). ACEIs, Angiotensin converting enzyme inhibitors; ARBs, Angiotensin Receptor blockers; AT1R, Angiotensin 1 Receptor; AT2R, Angiotensin 2 Receptor; Aβ, Amyloid-β; -, negative outcome or blockage; +, positive outcome.
Ang II enhances AT1 receptor mediated brain inflammation. Contrarily, ARBs attenuates the release of proinflammatory mediators (Lanz et al., 2010). Central infusion of Ang II increased hippocampal CD68- positive cells, indicating its hippocampal proinflammatory action (Takane et al., 2017). In contrarily, candesartan (ARB) decreased lipopolysaccharide (LPS) induced and AT1 receptor mediated release of proinflammatory mediators including TNFα, IL-1β, IκBα, iNOS, ICAM-1, and VCAM-1 in cerebral cortex (Benicky et al., 2009). In addition, candesartan attenuated brain level of NF-α, GFAP, COX-2, and NF-κB in the same animal model. They have also demonstrated the advantage of unopposed action of Ang II on AT2 receptor in addition to AT1 receptor blockage mediated amelioration of proinflammatory mediators releasesuggesting the beneficial role of AT2 receptors in reducing neuroinflammation (Goel et al., 2018). Moreover, ARBs prevents impairment and preserves the integrity of blood brain barrier which in turn reduces infiltration of inflammatory mediators observed in many neurodegenerative disease including AD (de Vries et al., 1997; Panahpour et al., 2014; So et al., 2015).
AngII via AT1 receptor is also suggested as effector of oxidative stress (Nickenig and Harrison, 2002; Marchesi et al., 2008; Chan and Chan, 2013; Seifi et al., 2014; Prusty et al., 2017). Ang II increased a reactive oxygen species called superoxide (Takane et al., 2017). On the other hand, telmisartan (ARB) was found to normalize diminished thioredoxin (Trx) system in addition to attenuating thioredoxin-interacting protein (TXNIP) expression. This reduces generation of endogenous reactive oxygen species (Erdi et al., 2016). Similarly, telmisartan reduced advanced glycation end products and 4-hydroxynonenal, which are the markers of oxidative stress and associated with Neurodegeneration (Safciuc et al., 2007; Barone et al., 2017). Candesartan also reduced brain level of free radicals by diminishing Malondialdehyde and increasing glutathione level (Tota et al., 2009). Thus partly alleviates the development and progression of AD (Gustaw-Rothenberg et al., 2010; Saharan and Mandal, 2014). Captopril (Bild et al., 2013) and losartan (Seifi et al., 2015) were also found to ameliorate oxidative stress.
Ang II is also implicated in neurovascular damage and cognitive impairment (Mogi et al., 2012; Bodiga and Bodiga, 2013; Bloch et al., 2015). Candesartan increased cerebral blood flow, reduced infarct size and improved cerebral ischemia (Ito et al., 2002; Engelhorn et al., 2004). Similarly, losartan prevented blood brain barrier disruption and restored blood flow after induction of hemorrhagic stroke. Moreover, telmisartan (Iwanami et al., 2010), valsartan (Takada et al., 2006), and olmesartan (Matsumoto et al., 2009) have shown a beneficial role in prevention of vascular damage via blockage of AT1 receptor. Suggested mechanism of ARBs on cerebral blood flow is in part explained via unblocked AT2 receptor activation (Iwai et al., 2004; Li et al., 2005; Jing et al., 2012). These studies generally show the benefit of ARBs in improving neurovascular network and cerebral blood flow after certain initial insult which in turn prevents onset and progressive neurodegeneration observed in AD (Bell and Zlokovic, 2009; Zlokovic, 2011). In addition to the above mechanisms described, Ang II is also speculated to inhibit acetylcholine release in which the deficiency is responsible for AD (Barnes et al., 1992; Tota et al., 2013). Conversely, pre-treatment with candesartan prevented Ang II induced reduction of acetylcholine level (Tota et al., 2009, 2013). This reduces cognitive impairment observed in AD (Burns, 2003; Herholz, 2008).
Renin Angiotensin System and Alzheimer’s Disease: Human Studies
Human studies have shown the involvement of RAS in the pathogenesis and progression of AD (Amouyel et al., 2000; Kolsch et al., 2005). Nevertheless, only few studies have shown a link between RAS and AD (Ellul et al., 2007; Davies et al., 2011). ACEIs and ARBs have shown a beneficial effect in slowing and reducing the cognitive impairment associated with AD (Li et al., 2012; Hsu et al., 2013; Saavedra, 2016). In a cross sectional study, patients taking ARBs and ACEIs had lower risk of cognitive deterioration (Jackson et al., 2018).
Central acting RAS inhibitors have shown a superior efficacy which imply brain RAS involvement in development and progression of AD (Hebert et al., 2013; Soto et al., 2013; Wharton et al., 2015; Zhuang et al., 2016). A prospective multicentre cohort study showed slower rate of cognitive decline on older adults taking ACE-Is (Soto et al., 2013). ARBs and ACEIs were generally found to reduce the risk and progression of AD (Hajjar et al., 2008; Li et al., 2010; Davies et al., 2011). The central acting agent including perindopril was significantly associated with a slower rate of functional decline (Davies et al., 2011). Telmisartan reduced cognitive impairment in hypertensive patients with AD (Li et al., 2012). The drug reduced amyloid β, oxidative stress and neuroinflammation. The RAS also activates peroxisome proliferator activated receptor (PPAR) gamma which has a role in prevention of neurodegeneration (Inestrosa et al., 2005; Kume et al., 2012; Li et al., 2012). Other ARBs losartan (Moriwaki et al., 2004; Hong et al., 2010), and olmesartan (Matsumoto et al., 2010) have shown beneficial effect in AD patients. In contrast, in a 4-month of pilot clinical trial ramipril was not associated with reduction of CSF Aβ1-42 level and cognitive impairment (Wharton et al., 2012). This limited effect of ramipril could be attributed to its limited blood brain barrier penetration (Sink et al., 2009). Most of these studies support the beneficial effect of RAS inhibitors in prevention and mitigation of cognitive impairments associated with AD (Table 3).
Table 3.
The effect of ACEIs and ARBs on cognitive function: Human study.
| Study design | Tested drug | Result | Reference |
|---|---|---|---|
| Cross sectional | ACE-Is and ARBs | Reduce cognitive decline | Ellul et al., 2007 |
| Observational | ACEIs | Slow decline of memory and daily functions | Hajjar et al., 2008 |
| Case Control | ACE-Is and ARBs | Decease incidence of AD | Davies et al., 2011 |
| Cohort | ARBs | Reduction in the incidence and progression of AD | Li et al., 2010 |
| Cohort | ACE-Is | Slow cognitive decline | Soto et al., 2013 |
| Observational | RAS-Ms | Slows cognitive decline | Wharton et al., 2015 |
| Observational | CACE-Is | Reduce functional decline | O’Caoimh et al., 2014 |
| Cohort | ACEIs | Not effect on cognitive decline | Zhuang et al., 2016 |
ACEIs, angiotensin converting enzyme inhibitors; ARBs, angiotensin II Receptor Blockers; RAS-Ms, renin angiotensin system medications; CACE-I, centrally acting Angiotensin converting enzyme inhibitors.
Genetic Studies
Genetic studies have also reported for the associate of ACE with AD (Elkins et al., 2004). ACE protein is coded by several genes containing various variants, specifically the insertion/deletion variant (rs1799752) have been associated with AD. Some other variants, including single nucleotide polymorphisms rs4291A > T located 240 base pair from the initiation codon, and rs4343G > A encoding a silent mutation in exon 16 were also thought to be involved in AD (Helbecque et al., 2009; Gaiteri et al., 2016). AD patients with the haplotype of rs1800764 (CC): rs4291 (TT) responded better for ACEIs that can cross the blood brain barrier (captopril or perindopril). However, the response was not significant among independent carriers of rs1800764 or rs429 (de Oliveira et al., 2014). Further stratification showed the benefit of ACEIs among ACE haplotypes (rs1800764 – T and rs4291 – A) and Apolipoprotein (APOE4) – carriers (rs1800764 – T or rs4291 – T). Nevertheless, APOE4+ carriers were non-responsive for ACEIs indicating the role of genetic variation and ACEIs response rate among AD patients (de Oliveira et al., 2018).
Conclusion
Understanding AD in terms of various pathophysiological pathways is worthwhile to unravel the complex nature of the disease process and identifying potential therapeutic targets. The brain RAS is reported to be involved in the development and progression of AD through AT1 receptor via increasing the production of amyloid-β, oxidative stress, inflammatory processes, and decreasing release of acetylcholine. However, RAS also is reported to have protective effect against AD. Through AT2 receptor activation that counterbalances the deleterious effects of AT1 receptor mediated RAS effects. With concept, beneficial effect of ARBs against AD is via the unopposed action of Ang II on AT2 receptors it as AT1 receptor is blocked these drugs increased Ang II concentration to act on AT2 receptor. ACE is reported to be involved in breakdown of amyloid β peptides, but most of the studies have contradicting result. This requires further understanding especially involvement of ACE in cleavage of amyloid β peptides in vivo. In summary, RAS through AT1 receptor is linked with AD pathology through its action on neurovascular change, oxidative stress, and inflammation as evidenced by the protective role of ARBs and ACEIs both in patients and animal models. However, the role of RAS in AD pathology is still not well established and need further in-depth understanding.
Author Contributions
AG conducted the review and prepared the first draft while all authors contributed to substantial enhancement of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- AbdAlla S., El Hakim A., Abdelbaset A., Elfaramawy Y., Quitterer U. (2015). Inhibition of ACE retards tau hyperphosphorylation and signs of neuronal degeneration in aged rats subjected to chronic mild stress. Biomed Res. Int. 2015:917156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AbdAlla S., Langer A., Fu X., Quitterer U. (2013). ACE inhibition with captopril retards the development of signs of neurodegeneration in an animal model of Alzheimer’s disease. Int. J. Mol. Sci. 14 16917–16942. 10.3390/ijms140816917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AbdAlla S., Lother H., El Missiry A., Langer A., Sergeev P., El Faramawy Y., et al. (2009). Angiotensin II AT2 receptor oligomers mediate G-protein dysfunction in an animal model of Alzheimer disease. J. Biol. Chem. 284 6554–6565. 10.1074/jbc.M807746200 [DOI] [PubMed] [Google Scholar]
- Ahmed M., Davis J., Aucoin D., Sato T., Ahuja S., Aimoto S., et al. (2010). Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils. Nat. Struct. Mol. Biol. 17 561–567. 10.1038/nsmb.1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M. R., Abo-Youssef A. M., Messiha B. A., Khattab M. M. (2016). Tempol and perindopril protect against lipopolysaccharide-induced cognition impairment and amyloidogenesis by modulating brain-derived neurotropic factor, neuroinflammation and oxido-nitrosative stress. Naunyn Schmiedebergs Arch. Pharmacol. 389 637–656. 10.1007/s00210-016-1234-6 [DOI] [PubMed] [Google Scholar]
- Alonso A. C., Li B., Grundke-Iqbal I., Iqbal K. (2008). Mechanism of tau-induced neurodegeneration in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 5 375–384. 10.2174/156720508785132307 [DOI] [PubMed] [Google Scholar]
- Amouyel P., Richard F., Berr C., David-Fromentin I., Helbecque N. (2000). The renin angiotensin system and Alzheimer’s disease. Ann. N. Y. Acad. Sci. 903 437–441. 10.1111/j.1749-6632.2000.tb06395.x [DOI] [PubMed] [Google Scholar]
- Ana Flavia A.-S., Lucas M. K., Maria Jose C.-S. (2017). The renin-angiotensin system and the neurodegenerative diseases: a brief review. Protein Pept. Lett. 24 841–853. [DOI] [PubMed] [Google Scholar]
- Atlas S. A. (2007). The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J. Manag. Care Pharm. 13(8 Suppl B), 9–20. 10.18553/jmcp.2007.13.s8-b.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird F. J., Bennett C. L. (2013). Microtubule defects & neurodegeneration. J. Genet. Syndr. Gene Ther. 4:203. 10.4172/2157-7412.1000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranello R. J., Bharani K. L., Padmaraju V., Chopra N., Lahiri D. K., Greig N. H., et al. (2015). Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer’s disease. Curr. Alzheimer Res. 12 32–46. 10.2174/1567205012666141218140953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J. M., Barnes N. M., Costall B., Coughlan J., Kelly M. E., Naylor R. J., et al. (1992). Angiotensin-converting enzyme inhibition, angiotensin, and cognition. J. Cardiovasc. Pharmacol. 19(Suppl. 6), S63–S71. 10.1097/00005344-199219006-00011 [DOI] [PubMed] [Google Scholar]
- Barnes J. M., Barnes N. M., Costall B., Horovitz Z. P., Ironside J. W., Naylor R. J., et al. (1990). Angiotensin II inhibits acetylcholine release from human temporal cortex: implications for cognition. Brain Res. 507 341–343. 10.1016/0006-8993(90)90294-L [DOI] [PubMed] [Google Scholar]
- Barone E., Head E., Butterfield D. A., Perluigi M. (2017). HNE-modified proteins in down syndrome: Involvement in development of Alzheimer disease neuropathology. Free Radic. Biol. Med. 111 262–269. 10.1016/j.freeradbiomed.2016.10.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker L. K., Etelvino G. M., Walther T., Santos R. A., Campagnole-Santos M. J. (2007). Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am. J. Physiol. Heart Circ. Physiol. 293 H1416–H1424. 10.1152/ajpheart.00141.2007 [DOI] [PubMed] [Google Scholar]
- Bell R. D., Zlokovic B. V. (2009). Neurovascular mechanisms and blood–brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 118 103–113. 10.1007/s00401-009-0522-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benicky J., Sánchez-Lemus E., Pavel J., Saavedra J. M. (2009). Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell. Mol. Neurobiol 29 781–792. 10.1007/s10571-009-9368-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein K. E., Koronyo Y., Salumbides B. C., Sheyn J., Pelissier L., Lopes D. H., et al. (2014). Angiotensin-converting enzyme overexpression in myelomonocytes prevents Alzheimer’s-like cognitive decline. J. Clin. Invest. 124 1000–1012. 10.1172/JCI66541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bild W., Hritcu L., Stefanescu C., Ciobica A. (2013). Inhibition of central angiotensin II enhances memory function and reduces oxidative stress status in rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 43 79–88. 10.1016/j.pnpbp.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Bloch S., Obari D., Girouard H. (2015). Angiotensin and neurovascular coupling: beyond hypertension. Microcirculation 22 159–167. 10.1111/micc.12193 [DOI] [PubMed] [Google Scholar]
- Bloom G. S. (2014). Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 71 505–508. 10.1001/jamaneurol.2013.5847 [DOI] [PubMed] [Google Scholar]
- Bodiga V. L., Bodiga S. (2013). Renin angiotensin system in cognitive function and dementia. Asian J. Neurosci. 2013:102602 10.1155/2013/102602 [DOI] [Google Scholar]
- Braga V. A. (2011). Differential brain angiotensin-II type I receptor expression in hypertensive rats. J. Vet. Sci. 12 291–293. 10.4142/jvs.2011.12.3.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillette J., Caillierez R., Zommer N., Alves-Pires C., Benilova I., Blum D., et al. (2012). Neurotoxicity and memory deficits induced by soluble low-molecular-weight amyloid-beta1-42 oligomers are revealed in vivo by using a novel animal model. J. Neurosci. 32 7852–7861. 10.1523/JNEUROSCI.5901-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buee L., Bussiere T., Buee-Scherrer V., Delacourte A., Hof P. R. (2000). Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 33 95–130. 10.1016/S0165-0173(00)00019-9 [DOI] [PubMed] [Google Scholar]
- Bunnemann B., Fuxe K., Metzger R., Mullins J., Jackson T. R., Hanley M. R., et al. (1990). Autoradiographic localization of mas proto-oncogene mRNA in adult rat brain using in situ hybridization. Neurosci. Lett. 114 147–153. 10.1016/0304-3940(90)90063-F [DOI] [PubMed] [Google Scholar]
- Burns A. (2003). Treatment of cognitive impairment in Alzheimer’s disease. Dialogues Clin. Neurosci. 5 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busciglio J., Lorenzo A., Yeh J., Yankner B. A. (1995). beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron 14 879–888. 10.1016/0896-6273(95)90232-5 [DOI] [PubMed] [Google Scholar]
- Carrillo-Mora P., Luna R., Colín-Barenque L. (2014). Amyloid beta: multiple mechanisms of toxicity and only some protective effects? Oxid. Med. Cell. Longev. 2014:795375. 10.1155/2014/795375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai S. Y., Bastias M. A., Clune E. F., Matsacos D. J., Mustafa T., Lee J. H., et al. (2000). Distribution of angiotensin IV binding sites (AT4 receptor) in the human forebrain, midbrain and pons as visualised by in vitro receptor autoradiography. J. Chem. Neuroanat. 20 339–348. 10.1016/S0891-0618(00)00112-5 [DOI] [PubMed] [Google Scholar]
- Chan S. H., Chan J. Y. (2013). Angiotensin-generated reactive oxygen species in brain and pathogenesis of cardiovascular diseases. Antioxid. Redox Signal. 19 1074–1084. 10.1089/ars.2012.4585 [DOI] [PubMed] [Google Scholar]
- Chrissobolis S., Banfi B., Sobey C. G., Faraci F. M. (2012). Role of Nox isoforms in angiotensin II-induced oxidative stress and endothelial dysfunction in brain. J. Appl. Physiol. 113 184–191. 10.1152/japplphysiol.00455.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta C., Zhang L. (2011). Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discov. Today 16 22–34. 10.1016/j.drudis.2010.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N. M., Kehoe P. G., Ben-Shlomo Y., Martin R. M. (2011). Associations of anti-hypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. J. Alzheimers Dis. 26 699–708. [DOI] [PubMed] [Google Scholar]
- de Gasparo M., Speth R. C., Baltatu O. C., Vanderheyden P. (2013). Brain RAS: hypertension and beyond. Int. J. Hypertens. 2013:157180. 10.1155/2013/157180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira F. F., Bertolucci P. H., Chen E. S., Smith M. C. (2014). Brain-penetrating angiotensin-converting enzyme inhibitors and cognitive change in patients with dementia due to Alzheimer’s disease. J. Alzheimers Dis. 42(Suppl. 3), S321–S324. [DOI] [PubMed] [Google Scholar]
- de Oliveira F. F., Chen E. S., Smith M. C., Bertolucci P. H. F. (2018). Pharmacogenetics of angiotensin-converting enzyme inhibitors in patients with Alzheimer’s disease dementia. Curr. Alzheimer Res. 15 386–398. 10.2174/1567205014666171016101816 [DOI] [PubMed] [Google Scholar]
- de Vries H. E., Kuiper J., De Boer A. G., Van Berkel T. J., Breimer D. D. (1997). The blood-brain barrier in neuroinflammatory diseases. Pharmacol. Rev. 49 143–156. [PubMed] [Google Scholar]
- Dong Y. F., Kataoka K., Tokutomi Y., Nako H., Nakamura T., Toyama K., et al. (2011). Perindopril, a centrally active angiotensin-converting enzyme inhibitor, prevents cognitive impairment in mouse models of Alzheimer’s disease. FASEB J. 25 2911–2920. 10.1096/fj.11-182873 [DOI] [PubMed] [Google Scholar]
- Eckman E. A., Adams S. K., Troendle F. J., Stodola B. A., Kahn M. A., Fauq A. H., et al. (2006). Regulation of steady-state beta-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. J. Biol. Chem. 281 30471–30478. 10.1074/jbc.M605827200 [DOI] [PubMed] [Google Scholar]
- Elkins J. S., Douglas V. C., Johnston S. C. (2004). Alzheimer disease risk and genetic variation in ACE: a meta-analysis. Neurology 62 363–368. 10.1212/01.WNL.0000106823.72493.FF [DOI] [PubMed] [Google Scholar]
- Ellul J., Archer N., Foy C. M., Poppe M., Boothby H., Nicholas H., et al. (2007). The effects of commonly prescribed drugs in patients with Alzheimer’s disease on the rate of deterioration. J. Neurol. Neurosurg. Psychiatry 78 233–239. 10.1136/jnnp.2006.104034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhorn T., Goerike S., Doerfler A., Okorn C., Forsting M., Heusch G., et al. (2004). The angiotensin II type 1-receptor blocker candesartan increases cerebral blood flow, reduces infarct size, and improves neurologic outcome after transient cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 24 467–474. 10.1097/00004647-200404000-00012 [DOI] [PubMed] [Google Scholar]
- Erdi F., Keskin F., Esen H., Kaya B., Feyzioglu B., Kilinc I., et al. (2016). Telmisartan ameliorates oxidative stress and subarachnoid haemorrhage-induced cerebral vasospasm. Neurol. Res. 38 224–231. 10.1080/01616412.2015.1105626 [DOI] [PubMed] [Google Scholar]
- Faraco G., Park L., Zhou P., Luo W., Paul S. M., Anrather J., et al. (2016). Hypertension enhances Abeta-induced neurovascular dysfunction, promotes beta-secretase activity, and leads to amyloidogenic processing of APP. J. Cereb. Blood Flow Metab. 36 241–252. 10.1038/jcbfm.2015.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure S., Chapot R., Tallet D., Javellaud J., Achard J. M., Oudart N. (2006). Cerebroprotective effect of angiotensin IV in experimental ischemic stroke in the rat mediated by AT(4) receptors. J. Physiol. Pharmacol. 57 329–342. [PubMed] [Google Scholar]
- Ferrão F. M., Lara L. S., Lowe J. (2014). Renin-angiotensin system in the kidney: What is new? World J. Nephrol. 3 64–76. 10.5527/wjn.v3.i3.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington L., Miners J. S., Palmer L. E., Bond S. M., Povey J. E., Kelly P. A., et al. (2011). Angiotensin II-inhibiting drugs have no effect on intraneuronal Abeta or oligomeric Abeta levels in a triple transgenic mouse model of Alzheimer’s disease. Am. J. Transl. Res. 3 197–208. [PMC free article] [PubMed] [Google Scholar]
- Ferrington L., Palmer L. E., Love S., Horsburgh K. J., Kelly P. A., Kehoe P. G. (2012). Angiotensin II-inhibition: effect on Alzheimer’s pathology in the aged triple transgenic mouse. Am. J. Transl. Res. 4 151–164. [PMC free article] [PubMed] [Google Scholar]
- Freund M., Walther T., Von Bohlen Und Halbach O. (2012). Immunohistochemical localization of the angiotensin-(1-7) receptor Mas in the murine forebrain. Cell Tissue Res. 348 29–35. 10.1007/s00441-012-1354-3 [DOI] [PubMed] [Google Scholar]
- Fuchtemeier M., Brinckmann M. P., Foddis M., Kunz A., Po C., Curato C., et al. (2015). Vascular change and opposing effects of the angiotensin type 2 receptor in a mouse model of vascular cognitive impairment. J. Cereb. Blood Flow Metab. 35 476–484. 10.1038/jcbfm.2014.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiteri C., Mostafavi S., Honey C. J., De Jager P. L., Bennett D. A. (2016). Genetic variants in Alzheimer disease - molecular and brain network approaches. Nat. Rev. Neurol. 12 413–427. 10.1038/nrneurol.2016.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo-Payet N., Guimond M. O., Bilodeau L., Wallinder C., Alterman M., Hallberg A. (2011). Angiotensin II, a neuropeptide at the frontier between endocrinology and neuroscience: is there a link between the angiotensin ii type 2 receptor and Alzheimer’s disease? Front. Endocrinol. 2:17 10.3389/fendo.2011.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganten D., Hermann K., Unger T., Lang R. E. (1983). The tissue renin-angiotensin systems: focus on brain angiotensin, adrenal gland and arterial wall. Clin. Exp. Hypertens. A 5 1099–1118. 10.3109/10641968309048844 [DOI] [PubMed] [Google Scholar]
- Gard P. R. (2004). Angiotensin as a target for the treatment of Alzheimer’s disease, anxiety and depression. Expert Opin. Ther. Targets 8 7–14. 10.1517/14728222.8.1.7 [DOI] [PubMed] [Google Scholar]
- Garrido-Gil P., Valenzuela R., Villar-Cheda B., Lanciego J. L., Labandeira-Garcia J. L. (2013). Expression of angiotensinogen and receptors for angiotensin and prorenin in the monkey and human substantia nigra: an intracellular renin–angiotensin system in the nigra. Brain Struct. Funct. 218 373–388. 10.1007/s00429-012-0402-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel R., Bhat S. A., Hanif K., Nath C., Shukla R. (2016). Perindopril attenuates lipopolysaccharide-induced amyloidogenesis and memory impairment by suppression of oxidative stress and RAGE activation. ACS Chem. Neurosci. 7 206–217. 10.1021/acschemneuro.5b00274 [DOI] [PubMed] [Google Scholar]
- Goel R., Bhat S. A., Hanif K., Nath C., Shukla R. (2018). Angiotensin II receptor blockers attenuate lipopolysaccharide-induced memory impairment by modulation of NF-kappaB-Mediated BDNF/CREB expression and apoptosis in spontaneously hypertensive rats. Mol. Neurobiol. 55 1725–1739. 10.1007/s12035-017-0450-5 [DOI] [PubMed] [Google Scholar]
- Gouras G. K., Olsson T. T., Hansson O. (2015). beta-Amyloid peptides and amyloid plaques in Alzheimer’s disease. Neurotherapeutics 12 3–11. 10.1007/s13311-014-0313-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatopoulos T. N., Johnson V., Moore S. A., Andres R., Weyhenmeyer J. A. (2004). Angiotensin type 2 receptor neuroprotection against chemical hypoxia is dependent on the delayed rectifier K+ channel, Na+/Ca2+ exchanger and Na+/K+ ATPase in primary cortical cultures. Neurosci. Res. 50 299–306. 10.1016/j.neures.2004.07.010 [DOI] [PubMed] [Google Scholar]
- Grobe J. L., Grobe C. L., Beltz T. G., Westphal S. G., Morgan D. A., Xu D., et al. (2010). The brain renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab. 12 431–442. 10.1016/j.cmet.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimond M.-O., Gallo-Payet N. (2012). The angiotensin II type 2 receptor in brain functions: an update. Int. J. Hypertens. 2012:351758 10.1155/2012/351758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustaw-Rothenberg K., Kowalczuk K., Stryjecka-Zimmer M. (2010). Lipids’ peroxidation markers in Alzheimer’s disease and vascular dementia. Geriatr. Gerontol. Int. 10 161–166. [DOI] [PubMed] [Google Scholar]
- Hajjar I. M., Keown M., Lewis P., Almor A. (2008). Angiotensin converting enzyme inhibitors and cognitive and functional decline in patients with Alzheimer’s disease: an observational study. Am. J. Alzheimers Dis. Other Demen. 23 77–83. 10.1177/1533317507309803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanger D. P., Anderton B. H., Noble W. (2009). Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 15 112–119. 10.1016/j.molmed.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Hebert P. L., Mcbean A. M., O’Connor H., Frank B., Good C., Maciejewski M. L. (2013). Time until incident dementia among Medicare beneficiaries using centrally acting or non-centrally acting ACE inhibitors. Pharmacoepidemiol. Drug Saf. 22 641–648. 10.1002/pds.3449 [DOI] [PubMed] [Google Scholar]
- Helbecque N., Codron V., Cottel D., Amouyel P. (2009). An age effect on the association of common variants of ACE with Alzheimer’s disease. Neurosci. Lett. 461 181–184. 10.1016/j.neulet.2009.06.006 [DOI] [PubMed] [Google Scholar]
- Hellner K., Walther T., Schubert M., Albrecht D. (2005). Angiotensin-(1-7) enhances LTP in the hippocampus through the G-protein-coupled receptor Mas. Mol. Cell. Neurosci. 29 427–435. 10.1016/j.mcn.2005.03.012 [DOI] [PubMed] [Google Scholar]
- Hemming M. L., Selkoe D. J. (2005). Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J. Biol. Chem. 280 37644–37650. 10.1074/jbc.M508460200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming M. L., Selkoe D. J., Farris W. (2007). Effects of prolonged angiotensin-converting enzyme inhibitor treatment on amyloid beta-protein metabolism in mouse models of Alzheimer disease. Neurobiol. Dis. 26 273–281. 10.1016/j.nbd.2007.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herholz K. (2008). Acetylcholine esterase activity in mild cognitive impairment and Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 35(Suppl. 1), S25–S29. 10.1007/s00259-007-0699-4 [DOI] [PubMed] [Google Scholar]
- Higuchi M., Iwata N., Saido T. C. (2005). Understanding molecular mechanisms of proteolysis in Alzheimer’s disease: progress toward therapeutic interventions. Biochim. Biophys. Acta 1751 60–67. 10.1016/j.bbapap.2005.02.013 [DOI] [PubMed] [Google Scholar]
- Hohle S., Blume A., Lebrun C., Culman J., Unger T. (1995). Angiotensin receptors in the brain. Pharmacol. Toxicol. 77 306–315. 10.1111/j.1600-0773.1995.tb01032.x [DOI] [PubMed] [Google Scholar]
- Hong K. S., Kang D. W., Bae H. J., Kim Y. K., Han M. K., Park J. M., et al. (2010). Effect of cilnidipine vs losartan on cerebral blood flow in hypertensive patients with a history of ischemic stroke: a randomized controlled trial. Acta Neurol. Scand. 121 51–57. 10.1111/j.1600-0404.2009.01299.x [DOI] [PubMed] [Google Scholar]
- Horiuchi M., Mogi M., Iwai M. (2010). The angiotensin II type 2 receptor in the brain. J. Renin Angiotensin Aldosterone Syst. 11 1–6. 10.1177/1470320309347793 [DOI] [PubMed] [Google Scholar]
- Hou D. R., Wang Y., Zhou L., Chen K., Tian Y., Song Z., et al. (2008). Altered angiotensin-converting enzyme and its effects on the brain in a rat model of Alzheimer disease. Chin. Med. J. 121 2320–2323. [PubMed] [Google Scholar]
- Hsu C. Y., Huang C. C., Chan W. L., Huang P. H., Chiang C. H., Chen T. J., et al. (2013). Angiotensin-receptor blockers and risk of Alzheimer’s disease in hypertension population–a nationwide cohort study. Circ. J. 77 405–410. 10.1253/circj.CJ-12-0658 [DOI] [PubMed] [Google Scholar]
- Hu J., Igarashi A., Kamata M., Nakagawa H. (2001). Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (A beta); retards A beta aggregation, deposition, fibril formation; and inhibits cytotoxicity. J. Biol. Chem. 276 47863–47868. 10.1074/jbc.M104068200 [DOI] [PubMed] [Google Scholar]
- Iliff J. J., Wang M., Liao Y., Plogg B. A., Peng W., Gundersen G. A., et al. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4:147ra111. 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa N. C., Godoy J. A., Quintanilla R. A., Koenig C. S., Bronfman M. (2005). Peroxisome proliferator-activated receptor gamma is expressed in hippocampal neurons and its activation prevents beta-amyloid neurodegeneration: role of Wnt signaling. Exp. Cell Res. 304 91–104. 10.1016/j.yexcr.2004.09.032 [DOI] [PubMed] [Google Scholar]
- Iqbal K., Liu F., Gong C. X., Alonso A. C., Grundke-Iqbal I. (2009). Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 118 53–69. 10.1007/s00401-009-0486-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Yamakawa H., Bregonzio C., Terron J. A., Falcon-Neri A., Saavedra J. M. (2002). Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an angiotensin II AT1 antagonist. Stroke 33 2297–2303. 10.1161/01.STR.0000027274.03779.F3 [DOI] [PubMed] [Google Scholar]
- Iwai M., Liu H. W., Chen R., Ide A., Okamoto S., Hata R., et al. (2004). Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation 110 843–848. 10.1161/01.CIR.0000138848.58269.80 [DOI] [PubMed] [Google Scholar]
- Iwanami J., Mogi M., Tsukuda K., Min L. J., Sakata A., Jing F., et al. (2010). Low dose of telmisartan prevents ischemic brain damage with peroxisome proliferator-activated receptor-gamma activation in diabetic mice. J. Hypertens. 28 1730–1737. 10.1097/HJH.0b013e32833a551a [DOI] [PubMed] [Google Scholar]
- Jackson L., Eldahshan W., Fagan S. C., Ergul A. (2018). Within the brain: the renin angiotensin system. Int. J. Mol. Sci. 19:876. 10.3390/ijms19030876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing F., Mogi M., Sakata A., Iwanami J., Tsukuda K., Ohshima K., et al. (2012). Direct stimulation of angiotensin II type 2 receptor enhances spatial memory. J. Cereb. Blood Flow Metab. 32 248–255. 10.1038/jcbfm.2011.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johren O., Inagami T., Saavedra J. M. (1995). AT1A, AT1B, and AT2 angiotensin II receptor subtype gene expression in rat brain. Neuroreport 6 2549–2552. 10.1097/00001756-199512150-00024 [DOI] [PubMed] [Google Scholar]
- Kakar S. S., Riel K. K., Neill J. D. (1992). Differential expression of angiotensin II receptor subtype mRNAs (AT-1A and AT-1B) in the brain. Biochem. Biophys. Res. Commun. 185 688–692. 10.1016/0006-291X(92)91680-O [DOI] [PubMed] [Google Scholar]
- Karran E., Mercken M., De Strooper B. (2011). The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 10 698–712. 10.1038/nrd3505 [DOI] [PubMed] [Google Scholar]
- Katsuno T., Morishima-Kawashima M., Saito Y., Yamanouchi H., Ishiura S., Murayama S., et al. (2005). Independent accumulations of tau and amyloid β-protein in the human entorhinal cortex. Neurology 64 687–692. 10.1212/01.WNL.0000151958.79884.86 [DOI] [PubMed] [Google Scholar]
- Kehoe P. G. (2009). Angiotensins and Alzheimer’s disease: a bench to bedside overview. Alzheimers Res. Ther. 1:3. 10.1186/alzrt3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Onstead L., Randle S., Price R., Smithson L., Zwizinski C., et al. (2007). Abeta40 inhibits amyloid deposition in vivo. J. Neurosci. 27 627–633. 10.1523/JNEUROSCI.4849-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolsch H., Jessen F., Freymann N., Kreis M., Hentschel F., Maier W., et al. (2005). ACE I/D polymorphism is a risk factor of Alzheimer’s disease but not of vascular dementia. Neurosci. Lett. 377 37–39. 10.1016/j.neulet.2004.11.062 [DOI] [PubMed] [Google Scholar]
- Kume K., Hanyu H., Sakurai H., Takada Y., Onuma T., Iwamoto T. (2012). Effects of telmisartan on cognition and regional cerebral blood flow in hypertensive patients with Alzheimer’s disease. Geriatr. Gerontol. Int. 12 207–214. 10.1111/j.1447-0594.2011.00746.x [DOI] [PubMed] [Google Scholar]
- Lanz T. V., Ding Z., Ho P. P., Luo J., Agrawal A. N., Srinagesh H., et al. (2010). Angiotensin II sustains brain inflammation in mice via TGF-beta. J. Clin. Invest. 120 2782–2794. 10.1172/JCI41709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaroni T. L., Raslan A. C., Fontes W. R., De Oliveira M. L., Bader M., Alenina N., et al. (2012). Angiotensin-(1-7)/Mas axis integrity is required for the expression of object recognition memory. Neurobiol. Learn. Mem. 97 113–123. 10.1016/j.nlm.2011.10.003 [DOI] [PubMed] [Google Scholar]
- Lenkei Z., Palkovits M., Corvol P., Llorens-Cortes C. (1996). Distribution of angiotensin II type-2 receptor (AT2) mRNA expression in the adult rat brain. J. Comp. Neurol. 373 322–339. [DOI] [PubMed] [Google Scholar]
- Lenkei Z., Palkovits M., Corvol P., Llorens-Cortes C. (1997). Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front. Neuroendocrinol. 18 383–439. 10.1006/frne.1997.0155 [DOI] [PubMed] [Google Scholar]
- Li J., Culman J., Hortnagl H., Zhao Y., Gerova N., Timm M., et al. (2005). Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. FASEB J. 19 617–619. 10.1096/fj.04-2960fje [DOI] [PubMed] [Google Scholar]
- Li N.C., Lee A., Whitmer R.A., Kivipelto M., Lawler E., Kazis L.E., et al. (2010). Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ 340:b5465. 10.1136/bmj.b5465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zhang J. W., Lu F., Ma M. M., Wang J. Q., Suo A. Q., et al. (2012). [Effects of telmisartan on the level of Abeta1-42, interleukin-1beta, tumor necrosis factor alpha and cognition in hypertensive patients with Alzheimer’s disease]. Zhonghua Yi Xue Za Zhi 92 2743–2746. [PubMed] [Google Scholar]
- Liu S., Liu J., Miura Y., Tanabe C., Maeda T., Terayama Y., et al. (2014). Conversion of Abeta43 to Abeta40 by the successive action of angiotensin-converting enzyme 2 and angiotensin-converting enzyme. J. Neurosci. Res. 92 1178–1186. 10.1002/jnr.23404 [DOI] [PubMed] [Google Scholar]
- Lu J., Wu L., Jiang T., Wang Y., Zhao H., Gao Q., et al. (2015). Angiotensin AT2 receptor stimulation inhibits activation of NADPH oxidase and ameliorates oxidative stress in rotenone model of Parkinson’s disease in CATH.a cells. Neurotoxicol. Teratol. 47 16–24. 10.1016/j.ntt.2014.11.004 [DOI] [PubMed] [Google Scholar]
- MacGregor D. P., Murone C., Song K., Allen A. M., Paxinos G., Mendelsohn F. A. (1995). Angiotensin II receptor subtypes in the human central nervous system. Brain Res. 675 231–240. 10.1016/0006-8993(95)00076-3 [DOI] [PubMed] [Google Scholar]
- Marchesi C., Paradis P., Schiffrin E. L. (2008). Role of the renin–angiotensin system in vascular inflammation. Trends Pharmacol. Sci. 29 367–374. 10.1016/j.tips.2008.05.003 [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Shimodozono M., Miyata R., Kawahira K. (2009). Benefits of the angiotensin II receptor antagonist olmesartan in controlling hypertension and cerebral hemodynamics after stroke. Hypertens. Res. 32 1015–1021. 10.1038/hr.2009.143 [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Shimodozono M., Miyata R., Kawahira K. (2010). The angiotensin II type 1 receptor antagonist olmesartan preserves cerebral blood flow and cerebrovascular reserve capacity, and accelerates rehabilitative outcomes in hypertensive patients with a history of stroke. Int. J. Neurosci. 120 372–380. 10.3109/00207450903389362 [DOI] [PubMed] [Google Scholar]
- Matsuura T., Kumagai H., Onimaru H., Kawai A., Iigaya K., Onami T., et al. (2005). Electrophysiological properties of rostral ventrolateral medulla neurons in angiotensin II 1a receptor knockout mice. Hypertension 46 349–354. 10.1161/01.HYP.0000173421.97463.ac [DOI] [PubMed] [Google Scholar]
- McCarthy C. A., Vinh A., Callaway J. K., Widdop R. E. (2009). Angiotensin AT2 receptor stimulation causes neuroprotection in a conscious rat model of stroke. Stroke 40 1482–1489. 10.1161/STROKEAHA.108.531509 [DOI] [PubMed] [Google Scholar]
- McKinley M. J., Albiston A. L., Allen A. M., Mathai M. L., May C. N., Mcallen R. M., et al. (2003). The brain renin-angiotensin system: location and physiological roles. Int. J. Biochem. Cell Biol. 35 901–918. 10.1016/S1357-2725(02)00306-0 [DOI] [PubMed] [Google Scholar]
- Millan M. A., Jacobowitz D. M., Aguilera G., Catt K. J. (1991). Differential distribution of AT1 and AT2 angiotensin II receptor subtypes in the rat brain during development. Proc. Natl. Acad. Sci. U.S.A. 88 11440–11444. 10.1073/pnas.88.24.11440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M., Iwanami J., Horiuchi M. (2012). Roles of brain angiotensin II in cognitive function and dementia. Int. J. Hypertens. 2012:169649. 10.1155/2012/169649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M., Li J. M., Tsukuda K., Iwanami J., Min L. J., Sakata A., et al. (2008). Telmisartan prevented cognitive decline partly due to PPAR-gamma activation. Biochem. Biophys. Res. Commun. 375 446–449. 10.1016/j.bbrc.2008.08.032 [DOI] [PubMed] [Google Scholar]
- Moriwaki H., Uno H., Nagakane Y., Hayashida K., Miyashita K., Naritomi H. (2004). Losartan, an angiotensin II (AT1) receptor antagonist, preserves cerebral blood flow in hypertensive patients with a history of stroke. J. Hum. Hypertens. 18 693–699. 10.1038/sj.jhh.1001735 [DOI] [PubMed] [Google Scholar]
- Murphy M. P., LeVine H. (2010). Alzheimer’s disease and the β-Amyloid peptide. J. Alzheimers Dis. 19 311–323. 10.3233/JAD-2010-1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. M., Bernstein S. L., Nyugen V., Condron M. M., Teplow D. B., Bowers M. T. (2009). Amyloid β-protein: Aβ40 Inhibits Aβ42 Oligomerization. J. Am. Chem. Soc. 131 6316–6317. 10.1021/ja8092604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Hasegawa Y., Uekawa K., Senju S., Nakagata N., Matsui K., et al. (2017). Transient mild cerebral ischemia significantly deteriorated cognitive impairment in a mouse model of Alzheimer’s disease via angiotensin AT1 receptor. Am. J. Hypertens. 30 141–150. 10.1093/ajh/hpw099 [DOI] [PubMed] [Google Scholar]
- Namsolleck P., Boato F., Schwengel K., Paulis L., Matho K. S., Geurts N., et al. (2013). AT2-receptor stimulation enhances axonal plasticity after spinal cord injury by upregulating BDNF expression. Neurobiol. Dis. 51 177–191. 10.1016/j.nbd.2012.11.008 [DOI] [PubMed] [Google Scholar]
- Nickenig G., Harrison D. G. (2002). The AT1-type angiotensin receptor in oxidative stress and atherogenesis: part I: oxidative stress and atherogenesis. Circulation 105 393–396. 10.1161/hc0302.102618 [DOI] [PubMed] [Google Scholar]
- Nozoe M., Hirooka Y., Koga Y., Araki S., Konno S., Kishi T., et al. (2008). Mitochondria-derived reactive oxygen species mediate sympathoexcitation induced by angiotensin II in the rostral ventrolateral medulla. J. Hypertens. 26 2176–2184. 10.1097/HJH.0b013e32830dd5d3 [DOI] [PubMed] [Google Scholar]
- Oba R., Igarashi A., Kamata M., Nagata K., Takano S., Nakagawa H. (2005). The N-terminal active centre of human angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide. Eur. J. Neurosci. 21 733–740. 10.1111/j.1460-9568.2005.03912.x [DOI] [PubMed] [Google Scholar]
- O’Caoimh R., Healy L., Gao Y., Svendrovski A., Kerins D. M., Eustace J., et al. (2014). Effects of centrally acting angiotensin converting enzyme inhibitors on functional decline in patients with Alzheimer’s disease. J. Alzheimers Dis. 40 595–603. [DOI] [PubMed] [Google Scholar]
- Ongali B., Nicolakakis N., Tong X. K., Aboulkassim T., Papadopoulos P., Rosa-Neto P., et al. (2014). Angiotensin II type 1 receptor blocker losartan prevents and rescues cerebrovascular, neuropathological and cognitive deficits in an Alzheimer’s disease model. Neurobiol. Dis. 68 126–136. 10.1016/j.nbd.2014.04.018 [DOI] [PubMed] [Google Scholar]
- Panahpour H., Nekooeian A. A., Dehghani G. A. (2014). Candesartan attenuates ischemic brain edema and protects the blood-brain barrier integrity from ischemia/reperfusion injury in rats. Iran. Biomed. J. 18 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M., Poyan Mehr A., Kreutz R. (2006). Physiology of local renin-angiotensin systems. Physiol. Rev. 86 747–803. 10.1152/physrev.00036.2005 [DOI] [PubMed] [Google Scholar]
- Premer C., Lamondin C., Mitzey A., Speth R. C., Brownfield M. S. (2013). Immunohistochemical localization of, and angiotensin II receptor subtypes in the rat adrenal, pituitary, and brain with a perspective commentary. Int. J. Hypertens. 2013:175428. 10.1155/2013/175428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provias J., Jeynes B. (2014). The role of the blood-brain barrier in the pathogenesis of senile plaques in Alzheimer’s disease. Int. J. Alzheimers Dis. 2014:191863. 10.1155/2014/191863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusty S. K., Sahu P. K., Subudhi B. B. (2017). Angiotensin mediated oxidative stress and neuroprotective potential of antioxidants and AT1 receptor blockers. Mini Rev. Med. Chem. 17 518–528. 10.2174/1389557516666161025094539 [DOI] [PubMed] [Google Scholar]
- Reid I. A. (1992). Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am. J. Physiol. 262 E763–E778. 10.1152/ajpendo.1992.262.6.E763 [DOI] [PubMed] [Google Scholar]
- Rompe F., Artuc M., Hallberg A., Alterman M., Stroder K., Thone-Reineke C., et al. (2010). Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension 55 924–931. 10.1161/HYPERTENSIONAHA.109.147843 [DOI] [PubMed] [Google Scholar]
- Royea J., Zhang L., Tong X. K., Hamel E. (2017). Angiotensin IV receptors mediate the cognitive and cerebrovascular benefits of losartan in a mouse model of Alzheimer’s disease. J. Neurosci. 37 5562–5573. 10.1523/JNEUROSCI.0329-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruginsk S., Vechiato F., Elias L., Antunes-Rodrigues J., Cruz J. (2015). Angiotensin II reduces mRNA expression for glutamate transporters and glutamine synthetase in cultured hypothalamic astrocytes. FASEB J. 29 968.919. [Google Scholar]
- Saavedra J. M. (2012). Angiotensin II AT(1) receptor blockers as treatments for inflammatory brain disorders. Clin. Sci. 123 567–590. 10.1042/CS20120078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J. M. (2016). Evidence to consider angiotensin II receptor blockers for the treatment of early Alzheimer’s disease. Cell. Mol. Neurobiol. 36 259–279. 10.1007/s10571-015-0327-y [DOI] [PubMed] [Google Scholar]
- Saavedra J. M., Ando H., Armando I., Baiardi G., Bregonzio C., Juorio A., et al. (2005). Anti-stress and anti-anxiety effects of centrally acting angiotensin II AT1 receptor antagonists. Regul. Pept. 128 227–238. 10.1016/j.regpep.2004.12.015 [DOI] [PubMed] [Google Scholar]
- Safciuc F., Constantin A., Manea A., Nicolae M., Popov D., Raicu M., et al. (2007). Advanced glycation end products, oxidative stress and metalloproteinases are altered in the cerebral microvasculature during aging. Curr. Neurovasc. Res. 4 228–234. 10.2174/156720207782446351 [DOI] [PubMed] [Google Scholar]
- Saharan S., Mandal P. K. (2014). The emerging role of glutathione in Alzheimer’s disease. J. Alzheimers Dis. 40 519–529. [DOI] [PubMed] [Google Scholar]
- Seifi B., Kadkhodaee M., Bakhshi E., Ranjbaran M., Ahghari P., Rastegar T. (2014). Enhancement of renal oxidative stress by injection of angiotensin II into the paraventricular nucleus in renal ischemia-reperfusion injury. Can. J. Physiol. Pharmacol. 92 752–757. 10.1139/cjpp-2014-0108 [DOI] [PubMed] [Google Scholar]
- Seifi B., Kadkhodaee M., Bakhshi E., Ranjbaran M., Zahmatkesh M., Sedaghat Z., et al. (2015). Angiotensin II in paraventricular nucleus contributes to sympathoexcitation in renal ischemia-reperfusion injury by AT1 receptor and oxidative stress. J. Surg. Res. 193 361–367. 10.1016/j.jss.2014.06.042 [DOI] [PubMed] [Google Scholar]
- Shin R. W., Ogino K., Kondo A., Saido T. C., Trojanowski J. Q., Kitamoto T., et al. (1997). Amyloid beta-protein (Abeta) 1-40 but not Abeta1-42 contributes to the experimental formation of Alzheimer disease amyloid fibrils in rat brain. J. Neurosci. 17 8187–8193. 10.1523/JNEUROSCI.17-21-08187.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K. D., Karnik S. S. (2016). Angiotensin receptors: structure, function, signaling and clinical applications. J. Cell Signal. 1:111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink K. M., Leng X., Williamson J., Kritchevsky S. B., Yaffe K., Kuller L., et al. (2009). Angiotensin converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the cardiovascular health study. Arch. Intern. Med. 169 1195–1202. 10.1001/archinternmed.2009.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- So G., Nakagawa S., Morofuji Y., Hiu T., Hayashi K., Tanaka K., et al. (2015). Candesartan improves ischemia-induced impairment of the blood-brain barrier in vitro. Cell. Mol. Neurobiol. 35 563–572. 10.1007/s10571-014-0152-8 [DOI] [PubMed] [Google Scholar]
- Song K., Allen A. M., Paxinos G., Mendelsohn F. A. (1991). Angiotensin II receptor subtypes in rat brain. Clin. Exp. Pharmacol. Physiol. 18 93–96. 10.1111/j.1440-1681.1991.tb01414.x [DOI] [PubMed] [Google Scholar]
- Song K., Allen A. M., Paxinos G., Mendelsohn F. A. (1992). Mapping of angiotensin II receptor subtype heterogeneity in rat brain. J. Comp. Neurol. 316 467–484. 10.1002/cne.903160407 [DOI] [PubMed] [Google Scholar]
- Soto M. E., Van Kan G. A., Nourhashemi F., Gillette-Guyonnet S., Cesari M., Cantet C., et al. (2013). Angiotensin-converting enzyme inhibitors and Alzheimer’s disease progression in older adults: results from the Reseau sur la Maladie d’Alzheimer Francais cohort. J. Am. Geriatr. Soc. 61 1482–1488. 10.1111/jgs.12415 [DOI] [PubMed] [Google Scholar]
- Sparks M. A., Crowley S. D., Gurley S. B., Mirotsou M., Coffman T. M. (2014). Classical renin-angiotensin system in kidney physiology. Compr. Physiol. 4 1201–1228. 10.1002/cphy.c130040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Becker M., Pankow K., Krause E., Ringling M., Beyermann M., et al. (2008). Catabolic attacks of membrane-bound angiotensin-converting enzyme on the N-terminal part of species-specific amyloid-beta peptides. Eur. J. Pharmacol. 588 18–25. 10.1016/j.ejphar.2008.03.058 [DOI] [PubMed] [Google Scholar]
- Supnet C., Bezprozvanny I. (2010). The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium 47 183–189. 10.1016/j.ceca.2009.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada J., Ibayashi S., Ooboshi H., Ago T., Ishikawa E., Kamouchi M., et al. (2006). Valsartan improves the lower limit of cerebral autoregulation in rats. Hypertens. Res. 29 621–626. 10.1291/hypres.29.621 [DOI] [PubMed] [Google Scholar]
- Takane K., Hasegawa Y., Lin B., Koibuchi N., Cao C., Yokoo T., et al. (2017). Detrimental effects of centrally administered angiotensin II are enhanced in a mouse model of Alzheimer disease independently of blood pressure. J. Am. Heart Assoc. 6:e004897. 10.1161/JAHA.116.004897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Morishita R. (2017). Angiotensin receptor blocker protects Alzheimer’s disease brain from ischemic insult. Am. J. Hypertens. 30 110–111. 10.1093/ajh/hpw158 [DOI] [PubMed] [Google Scholar]
- Takeda S., Sato N., Takeuchi D., Kurinami H., Shinohara M., Niisato K., et al. (2009). Angiotensin receptor blocker prevented beta-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension 54 1345–1352. 10.1161/HYPERTENSIONAHA.109.138586 [DOI] [PubMed] [Google Scholar]
- Tian M., Zhu D., Xie W., Shi J. (2012). Central angiotensin II-induced Alzheimer-like tau phosphorylation in normal rat brains. FEBS Lett. 586 3737–3745. 10.1016/j.febslet.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Tota S., Goel R., Pachauri S. D., Rajasekar N., Najmi A. K., Hanif K., et al. (2013). Effect of angiotensin II on spatial memory, cerebral blood flow, cholinergic neurotransmission, and brain derived neurotrophic factor in rats. Psychopharmacology 226 357–369. 10.1007/s00213-012-2913-8 [DOI] [PubMed] [Google Scholar]
- Tota S., Kamat P. K., Awasthi H., Singh N., Raghubir R., Nath C., et al. (2009). Candesartan improves memory decline in mice: involvement of AT1 receptors in memory deficit induced by intracerebral streptozotocin. Behav. Brain Res. 199 235–240. 10.1016/j.bbr.2008.11.044 [DOI] [PubMed] [Google Scholar]
- Tota S., Kamat P. K., Saxena G., Hanif K., Najmi A. K., Nath C. (2012). Central angiotensin converting enzyme facilitates memory impairment in intracerebroventricular streptozotocin treated rats. Behav. Brain Res. 226 317–330. 10.1016/j.bbr.2011.07.047 [DOI] [PubMed] [Google Scholar]
- Tsutsumi K., Saavedra J. M. (1991). Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am. J. Physiol. 261 R209–R216. 10.1152/ajpregu.1991.261.1.R209 [DOI] [PubMed] [Google Scholar]
- Uekawa K., Hasegawa Y., Senju S., Nakagata N., Ma M., Nakagawa T., et al. (2016). Intracerebroventricular infusion of angiotensin-(1-7) ameliorates cognitive impairment and memory dysfunction in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 53 127–133. 10.3233/JAD-150642 [DOI] [PubMed] [Google Scholar]
- Umschweif G., Liraz-Zaltsman S., Shabashov D., Alexandrovich A., Trembovler V., Horowitz M., et al. (2014). Angiotensin receptor type 2 activation induces neuroprotection and neurogenesis after traumatic brain injury. Neurotherapeutics 11 665–678. 10.1007/s13311-014-0286-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela R., Costa-Besada M. A., Iglesias-Gonzalez J., Perez-Costas E., Villar-Cheda B., Garrido-Gil P., et al. (2016). Mitochondrial angiotensin receptors in dopaminergic neurons. Role in cell protection and aging-related vulnerability to neurodegeneration. Cell Death Dis. 7:e2427. 10.1038/cddis.2016.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas R., Rincon J., Pedreanez A., Viera N., Hernandez-Fonseca J. P., Pena C., et al. (2012). Role of angiotensin II in the brain inflammatory events during experimental diabetes in rats. Brain Res. 1453 64–76. 10.1016/j.brainres.2012.03.021 [DOI] [PubMed] [Google Scholar]
- Vila-Porcile E., Corvol P. (1998). Angiotensinogen, prorenin, and renin are Co-localized in the secretory granules of all glandular cells of the rat anterior pituitary: an immunoultrastructural study. J. Histochem. Cytochem. 46 301–311. 10.1177/002215549804600303 [DOI] [PubMed] [Google Scholar]
- Wang B. R., Shi J. Q., Zhang Y. D., Zhu D. L., Shi J. P. (2011). Angiotensin II does not directly affect Abeta secretion or beta-/gamma-secretase activity via activation of angiotensin II type 1 receptor. Neurosci. Lett. 500 103–107. 10.1016/j.neulet.2011.06.014 [DOI] [PubMed] [Google Scholar]
- Wang J., Ho L., Chen L., Zhao Z., Zhao W., Qian X., et al. (2007). Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J. Clin. Invest. 117 3393–3402. 10.1172/JCI31547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. M., Slembrouck D., Potter W. D. (1996). Expression of angiotensinogen mRNA and localization of angiotensin II and renin in peripheral adrenergic neurons in primary culture. Biochem. Biophys. Res. Commun. 229 876–881. 10.1006/bbrc.1996.1895 [DOI] [PubMed] [Google Scholar]
- Welander H., Franberg J., Graff C., Sundstrom E., Winblad B., Tjernberg L. O. (2009). Abeta43 is more frequent than Abeta40 in amyloid plaque cores from Alzheimer disease brains. J. Neurochem. 110 697–706. 10.1111/j.1471-4159.2009.06170.x [DOI] [PubMed] [Google Scholar]
- Wharton W., Goldstein F. C., Zhao L., Steenland K., Levey A. I., Hajjar I. (2015). Renin-angiotensin-system modulation may slow the conversion from mild cognitive impairment to Alzheimer’s disease. J. Am. Geriatr. Soc. 63 1749–1756. 10.1111/jgs.13627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton W., Stein J. H., Korcarz C., Sachs J., Olson S. R., Zetterberg H., et al. (2012). The effects of ramipril in individuals at risk for Alzheimer’s disease: results of a pilot clinical trial. J. Alzheimers Dis. 32 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wincewicz D., Braszko J. J. (2014). Telmisartan attenuates cognitive impairment caused by chronic stress in rats. Pharmacol. Rep. 66 436–441. 10.1016/j.pharep.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Wolfe M. S. (2012). The role of tau in neurodegenerative diseases and its potential as a therapeutic target. Scientifica 2012:796024. 10.6064/2012/796024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. W., Stubley L., Pederson E. S., Kramar E. A., Hanesworth J. M., Harding J. W. (1999). Contributions of the brain angiotensin IV-AT4 receptor subtype system to spatial learning. J. Neurosci. 19 3952–3961. 10.1523/JNEUROSCI.19-10-03952.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner B. A. (1996). Mechanisms of neuronal degeneration in Alzheimer’s disease. Neuron 16 921–932. 10.1016/S0896-6273(00)80115-4 [DOI] [PubMed] [Google Scholar]
- Yim H. E., Yoo K. H. (2008). Renin-angiotensin system - Considerations for hypertension and kidney. Electrolyte Blood Press. 6 42–50. 10.5049/EBP.2008.6.1.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W. H., Bastianetto S., Mennicken F., Ma W., Kar S. (2002). Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience 115 201–211. 10.1016/S0306-4522(02)00404-9 [DOI] [PubMed] [Google Scholar]
- Zhu D., Shi J., Zhang Y., Wang B., Liu W., Chen Z., et al. (2011). Central angiotensin II stimulation promotes beta amyloid production in sprague dawley rats. PLoS One 6:e16037. 10.1371/journal.pone.0016037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S., Wang H. F., Wang X., Li J., Xing C. M. (2016). The association of renin-angiotensin system blockade use with the risks of cognitive impairment of aging and Alzheimer’s disease: a meta-analysis. J. Clin. Neurosci. 33 32–38. 10.1016/j.jocn.2016.02.036 [DOI] [PubMed] [Google Scholar]
- Zhuo J., Moeller I., Jenkins T., Chai S. Y., Allen A. M., Ohishi M., et al. (1998). Mapping tissue angiotensin-converting enzyme and angiotensin AT1, AT2 and AT4 receptors. J. Hypertens. 16 2027–2037. 10.1097/00004872-199816121-00026 [DOI] [PubMed] [Google Scholar]
- Zlokovic B. V. (2011). Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12 723–738. 10.1038/nrn3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K., Maeda T., Watanabe A., Liu J., Liu S., Oba R., et al. (2009). Aβ42-to-Aβ40- and angiotensin-converting activities in different domains of angiotensin-converting enzyme. J. Biol. Chem. 284 31914–31920. 10.1074/jbc.M109.011437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K., Yamaguchi H., Akatsu H., Sakamoto T., Ko M., Mizoguchi K., et al. (2007). Angiotensin-converting enzyme converts amyloid beta-protein 1-42 (Abeta(1-42)) to Abeta(1-40), and its inhibition enhances brain Abeta deposition. J. Neurosci. 27 8628–8635. 10.1523/JNEUROSCI.1549-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]