Abstract
The ubiquitin–proteasome system (UPS) controls cellular functions by maintenance of a functional proteome and degradation of key regulatory proteins. Central to the UPS is the proteasome that adjusts the abundance of numerous proteins, thereby safeguarding their activity or initiating regulatory events. Here, we demonstrate that the essential Saccharomyces cerevisiae protein Yjr141w/Ipa1 (Important for cleavage and PolyAdenylation) belongs to the HECT_2 (homologous to E6-AP carboxyl terminus_2) family. We found that five cysteine residues within the HECT_2 family signature and the C-terminus are essential for Ipa1 activity. Furthermore, Ipa1 interacts with several ubiquitin-conjugating enzymes in vivo and localizes to the cytosol and nucleus. Importantly, Ipa1 has an impact on proteasome activity, which is indicated by the activation of the Rpn4 regulon as well as by decreased turnover of destabilized proteasome substrates in an IPA1 mutant. These changes in proteasome activity might be connected to reduced maturation or modification of proteasomal core particle proteins. Our results highlight the influence of Ipa1 on the UPS. The conservation within the HECT_2 family and the connection of the human HECT_2 family member to an age-related degeneration disease might suggest that HECT_2 family members share a conserved function linked to proteasome activity.
Keywords: proteasome, protein degradation, ubiquitin–proteasome system, polyadenylation and RNA cleavage, Saccharomyces cerevisiae
PROTEIN degradation by the ubiquitin–proteasome system (UPS) is controlling nearly every cellular process in eukaryotes, placing the UPS at the very heart of regulatory events (Hershko and Ciechanover 1998; Pickart 2001). Not surprisingly, reduced activity of the main proteolytic machinery, the proteasome, is connected to aging and development of diseases in higher eukaryotes (Vilchez et al. 2014). The proteasome is a 2.5 MDa particle, composed of > 30 different subunits and divided in two subcomplexes with distinct functions: the regulatory particle for substrate recognition and unfolding, and the core particle for proteolysis (Inobe and Matouschek 2014). A chaperone-guided regimen is followed during assembly of the proteasome (Budenholzer et al. 2017). Moreover, several β-type subunits mature by cleavage after assembly of the proteasome core particle and most proteasome subunits are modified by Nα-acetylation, Nα-myristoylation, phosphorylation, succinylation, or ubiquitylation, which has impact on proteasome activity (Chen and Hochstrasser 1996; Heinemeyer et al. 1997; Kikuchi et al. 2010; Swaney et al. 2013; Weinert et al. 2013).
Proteasome activity is tightly regulated due to its fundamental role in protein homeostasis. In budding yeast, proteasome levels are feedback-regulated by the transcriptional activator Rpn4. Among the Rpn4 targets are proteasome subunits and maturation factors, as well as proteins involved in DNA repair, stress responses, and the UPS. Constant Rpn4 degradation by the proteasome closes the feedback loop (Xie and Varshavsky 2001; Dohmen et al. 2007).
Proteasomal substrates are marked for degradation through covalent attachment of a linear chain of ubiquitin moieties, which is catalyzed by an enzyme cascade of ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin-protein ligase (E3). The latter enzymes catalyze the transfer of activated ubiquitin from the E2 onto a lysine residue of the substrate (Hershko and Ciechanover 1998; Finley et al. 2012).
The main E3 families are the HECT (homologous to E6-AP carboxyl-terminus) E3s, the RING (really interesting new gene)-domain E3s, the structurally related U-box-domain E3s, and the RING-between-RING E3s. In absolute numbers, most E3 ubiquitin ligases belong to the group of RING-domain E3s (Finley et al. 2012). The unifying characteristic of the RING domains is the coordination of two Zn2+ ions by eight residues (four residues for each Zn2+) in a cross-braced arrangement. In most cases, cysteines are present as coordinating residues, but often one or two of them are replaced by histidines; in some RING domains, an acidic residue has been observed in one of the coordinating positions as well (Lipkowitz and Weissman 2011). The U-box domain has a similar structure as RING domains, but is lacking the coordination of Zn2+ ions (Metzger et al. 2014). HECT-domain E3s are characterized by a 350-amino acid catalytic domain, which contains a cysteine in its C-terminus that is involved in the ubiquitylation mechanism (Ardley and Robinson 2005).
Two distinct mechanisms of ubiquitin transfer have been identified so far. In the case of RING E3s, the activated ubiquitin is directly transferred from the E2 to the substrate. HECT domain and RING-between-RING E3s use an intermediate step: ubiquitin is covalently attached to a cysteine within the E3 before it is transferred onto the substrate by formation of an isopeptide bond with a lysine residue (Hershko and Ciechanover 1998; Ardley and Robinson 2005; Metzger et al. 2014).
Substrate ubiquitylation is connected to diverse regulatory processes: it induces proteasomal proteolysis or acts through nonproteolytic mechanisms (Finley et al. 2012). Thus, E3s influence numerous diverse cellular processes and malfunctions in E3s, or the UPS are linked to several severe diseases in humans (Vilchez et al. 2014). Recently, the human E3 UBE3D was connected to macular degeneration in aged humans in East Asia (Huang et al. 2015). Originally, this E3 was identified through its interaction with the E2 UbcH10. UBE3D was shown to ubiquitylate itself and cyclin B in vitro, and the C-terminus of it is HECT-like (Kobirumaki et al. 2005). Moreover, a threonine cysteine motif in the HECT-like C-terminus was found to be important for in vitro ubiquitylation activity. Recently, a group of proteins with a common signature and HECT-like C-terminus has been added to the Pfam database as HECT_2 domain (http://pfam.xfam.org/; Finn et al. 2016). This family includes human UBE3D, Saccharomyes cerevisiae Yjr141w/Ipa1 (Important for cleavage and PolyAdenylation), and Schizosaccharomyces pombe SPBC1734.10c, which are all essential for proliferation and have not been studied extensively (Giaever et al. 2002; Kim et al. 2010; Hayles et al. 2013; Blomen et al. 2015). High-throughput analyses of genetic interactions in yeast and functional tests have linked Ipa1 with pre-mRNA cleavage and polyadenylation (Costanzo et al. 2016). However, the molecular activity of Ipa1 has not been clarified so far.
In this study, we investigated the molecular function of Ipa1. We provide evidence that Ipa1 is localized to the cytosol and nucleus and interacts with ubiquitin-conjugating enzymes. Many residues of the HECT_2-family signature were mutated in Ipa1 without loss-of-function, yet five conserved cysteines and the HECT-like C-terminus were identified as being important for functionality. Furthermore, loss of Ipa1 has an impact on proteasome activity.
Materials and Methods
Yeast strains, growth conditions, and plasmids
The S. cerevisiae strains are derivatives of the S288C strain ESM356 and the SK1 strain YKS32 (Knop and Strasser 2000; Pereira et al. 2001). All strains are listed together with their relevant genotypes in Supplemental Material, Table S9. For growth, standard preparations of media were used (Sherman 2002). For liquid cultures, low-fluorescence medium (LFM) was used to grow yeast cells (Usherenko et al. 2014). For serial dilution experiments, cells were diluted 1:5 and spotted on solid YPD medium. The petri dishes were incubated at 30° in darkness or exposed to blue light (465 nm, 30 µmol m−2 s−1). Sporulation of yeast cells was performed as described (Jungbluth et al. 2012). Chromosomal tagging of genes was performed with PCR products as described (Janke et al. 2004); yeast strains with photo-sensitive degron (psd) module-modified genes were obtained accordingly (Lutz et al. 2016). The lithium acetate method was used for transformations (Schiestl and Gietz 1989). Yeast cells were illuminated with blue light using high-power light-emitting diode (LED) stripes (465 nm; revoART, Borsdorf, Germany) or StrawHat LED clusters (six clusters of 42 LEDs, 465 nm; revoART); both sets were equipped with a dimmer to select an appropriate light flux (30 μmol m−2 s−1). The light flux was checked before the experiment at the level of the yeast cells (distance of yeast cells to LEDs ∼ 10 cm) with an optometer (P2000, equipped with light-detector PD-9306-2; Gigahertz-Optik, Türkenfeld, Germany). For depletion of Ipa1-psd, cells were exposed to blue light for 5–6 hr. Plasmids were constructed by standard procedures (Ausubel et al. 1995) and are listed in Table S10; details and sequences of the used vectors are available on request.
Microscopy, image processing, and tetrad dissection
Live-cell imaging of yeast cells was performed as described (Jungbluth et al. 2010) with a Zeiss (Carl Zeiss, Thornwood, NY) Axiovert 200M equipped with a Hamamatsu camera, and DAPI, enhanced GFP, enhanced yellow fluorescent protein (YFP), and rhodamine filter sets using a 63× Plan Apochromat oil lens (NA 1.4). Transmitted-light (TL) images were collected in a single plane, and fluorescence images in a single plane or as z-stack (0 0.3–0.5 µm intervals) using the image-acquisition software Volocity 5.03 (Perkin Elmer [Perkin Elmer-Cetus], Norwalk, CT). The software ImageJ was used for image processing, deconvolution, and fluorescence quantification (Collins 2007). Tetrad dissection was performed as described (Sherman 2002).
Immunoblotting, cycloheximide-chase assay, quantitative measurements, and statistics
Immunoblotting experiments with samples obtained from yeast by alkaline lysis were performed as described (Jungbluth et al. 2010). At least four biological replicates measured on two different days were obtained for each experiment. Cycloheximide chases were performed as follows. Yeast cells were grown in LFM in darkness or exposed to blue light until logarithmic growth phase was reached. The first sample (t = 0 hr) was taken and the translation inhibitor cycloheximide (end concentration 100 µg/ml) was added to stop protein synthesis. Cells were kept in darkness or exposed to blue light for the rest of the experiment. Equal amounts of sample were collected at each time point and subjected to alkaline lysis and western blotting. Immunoblotting was performed as described (Renicke et al. 2013b). Antibodies detecting myc, Tub1, and Hemagglutinin (HA) were obtained by commercial suppliers.
Quantification was done with the software ImageJ (Collins 2007) using the gel analyzer tool after background subtraction (rolling ball radius 50 pixels). The software LibreOffice Calc was used to generate graphs; error bars show the SEM or SD (as indicated in figure legends). Box plots were generated with the software QtiPlot; boxes show values ranging from 25 to 75 and whiskers indicate the full range of measurements. The median of a measurement is indicated by a horizontal line. Statistical analysis (two-sided unpaired Student’s t-test) was done with the software LibreOffice Calc.
Sequence alignment
The software ClustalX with standard settings was used to perform sequence alignments (Thompson et al. 1997). The sequences were obtained from databases maintained by the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov). Sequences of all Pfam HECT_2-family members were used for the sequence similarity network (SSN). The SSN was created by feeding the sequences to the Enzyme Similarity Tool (http://efi.igb.illinois.edu/efi-est/index.php). Pairwise alignment of the sequences resulted in the SSN; a cut-off value of 50% sequence identity was used for each node. The SSN was visualized with the software cytoscape.
Microarray analysis
Transcriptional profiling was performed in triplicate using Yeast Genome 2.0 expression arrays (Affymetrix, High Wycombe, UK) following standard protocols. Strains were grown to logarithmic phase in LFM, harvested, and rapidly frozen in liquid nitrogen. For extraction of total RNA, the cell pellet was mixed with 600 µl glass beads, 600 µl 1× acetate/EDTA/SDS-buffer, and 600 µl Roti phenol/chloroform/isoamyl alcohol. After vortexing for 8 min at 4°, the cell suspension was transferred to 65° for 4 min to lyse the cells completely. All subsequent steps were conducted as described (Schmitt et al. 1990). The complementary RNA for array hybridization was synthesized following the GeneChip 3′IVT Plus Kit manual (Affymetrix) using 100 ng of total RNA. Briefly, in vitro transcription labeling was carried out for 16 hr; the fragmented samples were analyzed with an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). For each sample, 5 µg of labeled amplified RNA was hybridized to an Affymetrix GeneChip Yeast Genome 2.0 expression array (169 format) for 16 hr, followed by washing and staining using an Affymetrix Fluidics 450 station. An Affymetrix Gene-Chip Scanner 3000 7G was used to scan the arrays. Data were processed with the Affymetrix GeneChip Command Console software. Differentially expressed genes were obtained with the Bioconductor 3.2 library AffylmGUI in R (https://www.R-project.org/; Wettenhall et al. 2006; Huber et al. 2015) using standard settings (normalization method: robust multichip analysis) and a P-value of 0.05 as cut-off (Table S5 and Table S6). The lists were used for serial pattern of expression levels locator (SPELL) analyses (http://spell.yeastgenome.org/) to obtain the lists of gene ontology (GO) terms enriched in the differentially expressed genes. Array data are available from the ArrayExpress database (http://www.ebi.ac.uk) under accession number E-MTAB-5022. The list of genes containing Rpn4-binding sites in the promoter and the one comprising experimentally determined Rpn4 targets were obtained from YEASTRACT (http://www.yeastract.com; Teixeira et al. 2014). GeneVenn (http://genevenn.sourceforge.net/) was used to compare these lists with the list of genes differentially expressed in Ipa1-depleted cells.
Quantitative real-time PCR
Quantitative real-time PCR was performed by reverse transcription of 500 ng of DNase-treated total RNA into complementary DNA (cDNA), using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA), and subsequent PCR reactions were performed in 96-well plates using a CFX real-time PCR detection system (Bio-Rad). Independent PCRs were performed using the same cDNA for both the gene of interest and CDC28 as a reference with gene-specific primers (Table S11). The reaction mix (20 μl final volume) consisted of 10 μl of 2× iQ SYBR green supermix (Bio-Rad), 0.75 μl of each primer (0.4 μM final concentration), 7.5 μl H2O, and 1 μl of cDNA. A control without template was incorporated in each assay. The thermocycling program consisted of one hold at 95° for 30 sec, followed by 45 cycles of 15 sec at 95°, 30 sec at 60°, and 30 sec at 72°. After completion of these cycles, melting curve data were collected to determine PCR specificity, contamination, and the absence of primer dimers. The detection of the threshold cycle was done automatically by the cycler software. Quantification of gene expression was carried out with the 2(−ΔΔCt) method (Livak and Schmittgen 2001).
Bimolecular fluorescence complementation and quantitative yeast two-hybrid analysis
For bimolecular fluorescence complementation (BiFC) quantification, haploid yeast strains harboring E2s tagged with the C-terminal part of the YFP Venus (VC; VC173) at chromosomal level and Rpn7 tagged with red fluorescent protein (RFP), as well as a control strain without VC, were transformed with a plasmid (pCT351) containing PGAL1-IPA1-VN (N-terminal part of Venus, VN) or with an empty plasmid (pRS316). LFM containing 2% galactose and lacking uracil was used to grow the cells to logarithmic growth phase. Next, 2 × 107 cells were transferred into a black multiwell plate to measure yellow and red fluorescence with a SynergyMX multidetection reader (BioTek). The yellow fluorescence signal was normalized to the red fluorescence signal. The normalized BiFC signals were obtained by dividing the values of Ipa1-VN-containing strains by the values of the corresponding cells carrying an empty plasmid.
For the BiFC fluorescence microscopy analysis, the same strains as for the fluorimeter measurements were used and grown to logarithmic phase using the same conditions. Live-cell imaging was performed as described above; single images were recorded using TL, the YFP channel (BiFC), and the rhodamine channel (RFP).
The quantitative yeast two-hybrid was essentially done as described (Ausubel et al. 1995). Briefly, haploid cells bearing plasmids with potential interaction partners were crossed on YPD and diploid cells were obtained by growth on selective medium. Liquid selective medium containing 2% raffinose was used to grow the strains to logarithmic growth phase; expression of the fusion proteins was induced by the addition of 2% galactose. Next, 2 × 107 cells were lysed in 250 µl Z-buffer containing 25 µl chloroform and 5 µl SDS solution (0.4%); activity of β-galactosidase was measured after addition of 150 µl O-nitrophenyl β-D-galactopyranoside (2.4 mg/ml in Z-buffer) as substrate. Samples were kept at 30° for 60 min and the reaction was stopped by addition of 250 µl Na2CO3 (1 M) solution. The optical density at 420 nm was measured with a SynergyMX multidetection reader (BioTek). Activity units were calculated as described (Ausubel et al. 1995).
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplemental Material files). Additionally, microarray data are available from the ArrayExpress database (http://www.ebi.ac.uk) under accession number E-MTAB-5022. The Supplemental Material files contain additional Figures S1–S9 in File S1, and descriptions of Table S1, Table S2, Table S3, Table S4, Table S5, Table S6, Table S7, Table S8, Table S9, Table S10, and Table S11. Table S1 contains the Pfam protein names and organism identifiers for the network shown in Figure 1B. Table S2 contains microarray data (Ipa1-psd strain vs. control strain ESM356-1 in blue light). Table S3 contains microarray data (Ipa1-psd strain vs. ESM356-1 in darkness). Table S4 contains microarray data (ESM356-1 strain in blue light vs. ESM356-1 in darkness). Table S5 contains the list of upregulated genes in Ipa1-psd cells. Table S6 contains the list of downregulated genes in Ipa1-psd cells. Table S7 contains the list of enriched GO terms (upregulated genes). Table S8 contains the list of enriched GO terms (downregulated genes). Table S9 contains the list of yeast strains. Table S10 contains the list of plasmids. Table S11 contains the list of oligos used for the quantitative real-time PCR analysis. Strains and plasmids are available upon request.
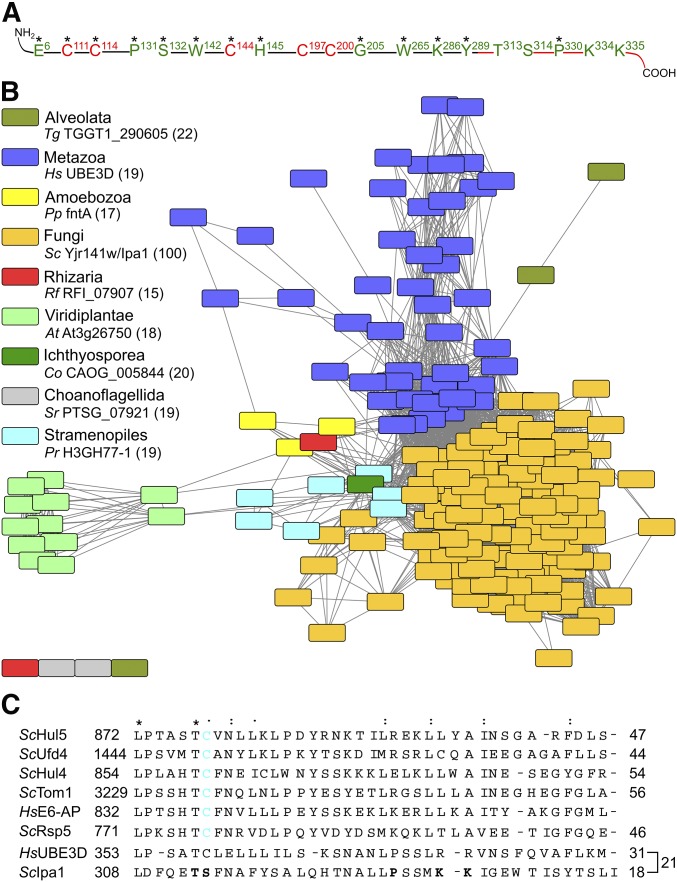
Figure 1.
The yeast protein Yjr141w/Ipa1 belongs to the eukaryotic HECT_2 family. (A) Conserved residues within Yjr141w/Ipa1 match the family signature of HECT_2-family members. Highly conserved residues are marked with an asterisk. Residues or parts of the protein that are essential for Yjr141w/Ipa1 function are marked in red, residues in green are dispensable. (B) Sequence similarity network of HECT_2-family members. Sequences with an identity > 50% were clustered together in one node. The coloring shows affiliation of the protein(s) to one of the eukaryotic subgroups (source: taxonomy database of UniProt). For each subgroup, one protein is given as example; its sequence identity to S. cerevisiae Yjr141w/Ipa1 is given in parentheses. The Pfam identifiers for each node are given in Table S1. (C) Alignment of the C-termini of S. cerevisiae HECT-family members Hul5, Ufd4, Hul4, Tom1, and Rsp5 with the prototype HECT-domain protein human E6-AP and HECT_2-family members Yjr141w/Ipa1 and human homolog UBE3D. The conservation grade of a residue is shown by asterisk, double point, and point to indicate strict conservation, and higher and lower similarity, respectively. The percentage of identical residues between E6-AP and the other sequences is given on the right side as well as the percentage of identical residues between UBE3D and Ipa1. The cysteine residue important for ubiquitylation in HECT domain E3s is highlighted in cyan, residues that have been mutated in Ipa1 to assay for functionality are marked in bold letters. At, Arabidopsis thaliana; Co, Capsaspora owczarzaki; E3, ubiquitin-protein ligase; HECT, homologous to E6-AP carboxyl terminus; Hs, Homo sapiens; Pp, Polysphondylium pallidum; Pr, Phytophthora ramorum; Rf, Reticulomyxa filosa; Sc: S. cerevisiae; Sr, Salpingoeca rosetta; Tg, Toxoplasma gondii.
Results
S. cerevisiae Yjr141w/Ipa1 is an essential member of the HECT_2-domain family
The sequence of the yeast protein Yjr141w/Ipa1 was subjected to a Position-Specific Iterated Basic Local Alignment Search Tool search. This revealed that Ipa1 contains a pattern of conserved amino acids (Figure 1A and Figure S1A in File S1). Proteins with a similar pattern are common to diverse eukaryotic organisms (Figure 1B). These proteins have been classified as members of the HECT_2-protein family that comprises, among others, the human protein UBE3D (Table S1). UBE3D was characterized earlier as a ubiquitin-protein ligase with a C-terminus showing low identity to human E6-AP (Kobirumaki et al. 2005). Most HECT_2-family members, including UBE3D and Ipa1, are single-domain proteins that contain only the HECT_2 domain (Figure S1B in File S1).
We probed the importance of the HECT_2-family signature of Ipa1 using a deletion mutant complementation assay (Figure S2A in File S1). Five cysteines were identified that are important to sustain viability, whereas mutation of other conserved residues had no impact on viability. Moreover, we mutated all cysteine residues that are present in Ipa1 and several amino acids that are conserved in close relatives of Ipa1. However, no further residues were identified that are important for Ipa1 functionality (Figure 1A and Figure S2, A and B in File S1).
Four of the five essential cysteines in Ipa1 are arranged in two pairs spaced by two other amino acids, similar as in RING-finger or other Zinc-binding domains (Metzger et al. 2014). In the Pfam database, three somehow-related families exist [zinc ribbon 6, zinc ribbon 10, and C-terminal domain of RIG-I (RIG-I_C-RD)]. Interestingly, the sequence of RIG-I_C-RD contains five cysteines that are essential for its function (Cui et al. 2008) and arranged similarly to the five functionally relevant cysteines of Ipa1. However, an alignment of Ipa1 with sequences of RING-finger-domain proteins or RIG-I_C-RD failed to indicate a relationship between them (Figure S3, A and B in File S1). Sequence identity of the aligned sequences was low and the position of important cysteine residues rarely matched, which makes it unlikely that a RING-like fold coordinating two Zn2+-ions is present in Ipa1.
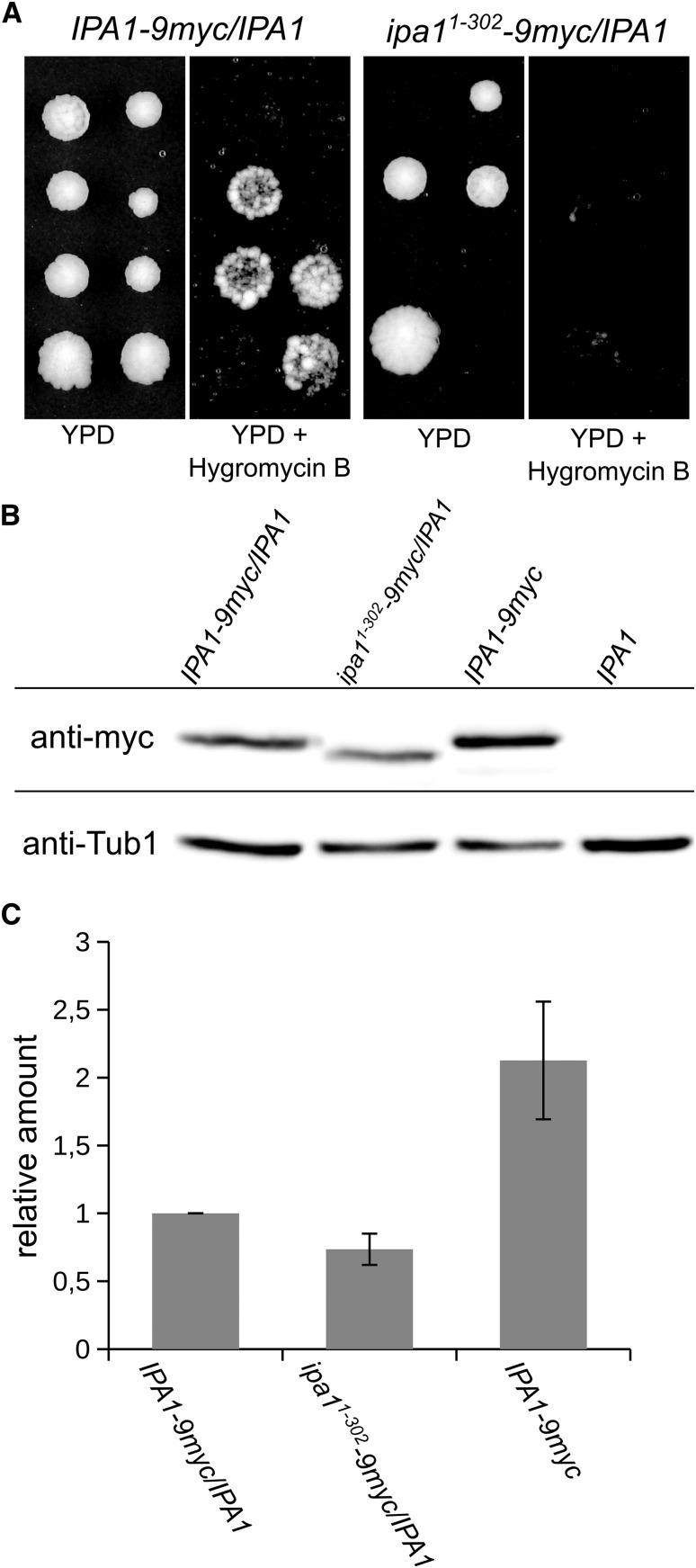
Previously, it was shown that the C-terminus of human UBE3D, which is part of the HECT_2-family signature, has low homology to human E6-AP and contains a cysteine that was found to be important for autoubiquitylation in vitro (Kobirumaki et al. 2005). An alignment of the C-termini of all yeast HECT-family members, E6-AP, UBE3D, and Ipa1 revealed sequence identities between 44 and 56% of the yeast HECT-family members with E6-AP. In contrast, UBE3D and Ipa1 showed only 31 and 18% identity, respectively (Figure 1C). The identity between Ipa1 and UBE3D is also fairly low (21%) in the C-terminus; moreover, Ipa1 contains a serine (residue Ser 314) at the position of the catalytic cysteine residue of HECT-domain proteins (Figure 1C). Functionality of Ipa1 is not affected by mutation of this serine residue, threonine 313, or proline 330, which is part of the HECT_2-consensus sequence, nor of two lysine residues (334; 335) within the C-terminus (Figure 1A and Figure S2, A and B in File S1). Therefore, we tested whether the C-terminus is essential for functionality of Ipa1. Tetrad analysis with truncated Ipa1 (residues 1–302) showed that the C-terminus is required for viability (Figure 2A). In diploid cells containing full-length Ipa1 and Ipa11–302-9myc, the shortened variant was clearly present in comparable amounts to full length Ipa1-9myc (Figure 2, B and C). In summary, Ipa1 is an essential HECT_2-family protein in S. cerevisiae, whose in vivo functionality depends on five conserved cysteine residues that belong to the HECT_2-family signature and on the C-terminus.
Figure 2.
The C-terminus of Ipa1 is important for viability of yeast cells. (A) Tetrad analysis of cells with a C-terminal deletion variant of Ipa1. The epitope 9myc was added at the very C-terminus of Ipa1 or after position R302 in diploid yeast cells to create heterozygously marked Ipa1 variants. After tetrad analysis, colonies growing on YPD were probed for growth on YPD + Hygromycin B (selection marker with the epitope inserted on the chromosome). (B) Analysis of Ipa11–302-9myc abundance compared to full-length Ipa1-9myc. Whole-cell extracts were prepared from cells growing logarithmically in liquid cultures. The blot was probed with anti-myc and anti-Tub1 (tubulin 1, loading control) antibodies. (C) Quantification of Ipa1-9myc abundance. Exemplary blot is shown in (B). Graph shows the mean of four independent measurements, Ipa1-9myc levels were normalized to Tub1 signal (error bar, SEM).
Ipa1 interacts with ubiquitin-conjugating enzymes
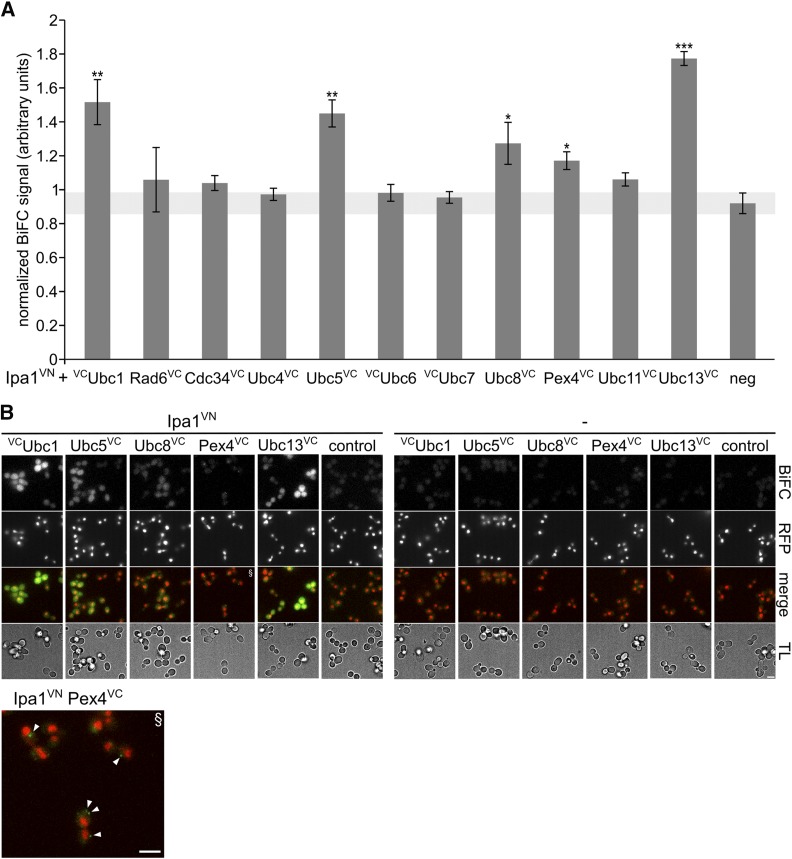
Next, we measured whether Ipa1 interacts with ubiquitin-conjugating enzymes by BiFC (Hu et al. 2002) as well as quantitative yeast two-hybrid assay (Fields and Sternglanz 1994). Using quantitative BiFC, we found that Ipa1 tagged with a VN fragment (Ipa1-VN), which is able to rescue an IPA1 deletion, gives positive results with several E2s modified with VC fragments. Specifically these are: Ubc1-VC, Ubc5-VC, Ubc8-VC, Pex4-VC, and Ubc13-VC (Figure 3A and Figure S4A in File S1). The interactions are localization-specific, as we observed dot-like BiFC signals for Ipa1-VN with Pex4-VC and signals in the cytosol and nucleus for the other positive combinations (Figure 3B). Moreover, we observed additional interactions between Ipa1 and Ubc7, as well as Ubc11, by quantitative yeast two-hybrid assay (Figure S4B in File S1). In agreement with our results, an interaction between Ipa1 and Ubc1 has been reported recently (Costanzo et al. 2016). Overall, we concluded that Ipa1 interacts with several E2s. Subsequently, we tested whether Ipa1 shows in vitro self-ubiquitylation activity like the human protein UBE3D. However, our experiments were inconclusive due to problems in obtaining purified Ipa1 (data not shown).
Figure 3.
Ipa1 interacts with E2s. (A) Quantification of BiFC signals in cells containing Ipa1-VN- and VC-tagged E2s. Plasmid-based PGAL1-IPA1-VN (nc: empty plasmid) was expressed by addition of galactose in yeast strains containing chromosomal insertions of VC at genes encoding for E2s (as indicated) as well as Rpn7-RFP. BiFC signals were quantified by fluorimeter measurements. (n = 4; error bar, SEM; statistical significance of differences was tested by a two-sided Student’s t-test; *** P < 0.001, ** P < 0.01, and * P < 0.05). (B) Fluorescence microscopy BiFC analysis of Ipa1-VN with Ubc1-VC, Ubc5-VC, Ubc8-VC, Pex4-VC, and Ubc13-VC. The same strains as in (A) were used, exemplary images are shown. The arrowheads in the enlarged Pex4 merge image point to dot-like BiFC signals. Bar size corresponds to 3 µm. BiFC, bimolecular fluorescence complementation; E2s, ubiquitin-conjugating enzymes; neg, negative control; RFP, red fluorescent protein; VC, C-terminal Venus fragment; VN, N-terminal Venus fragment.
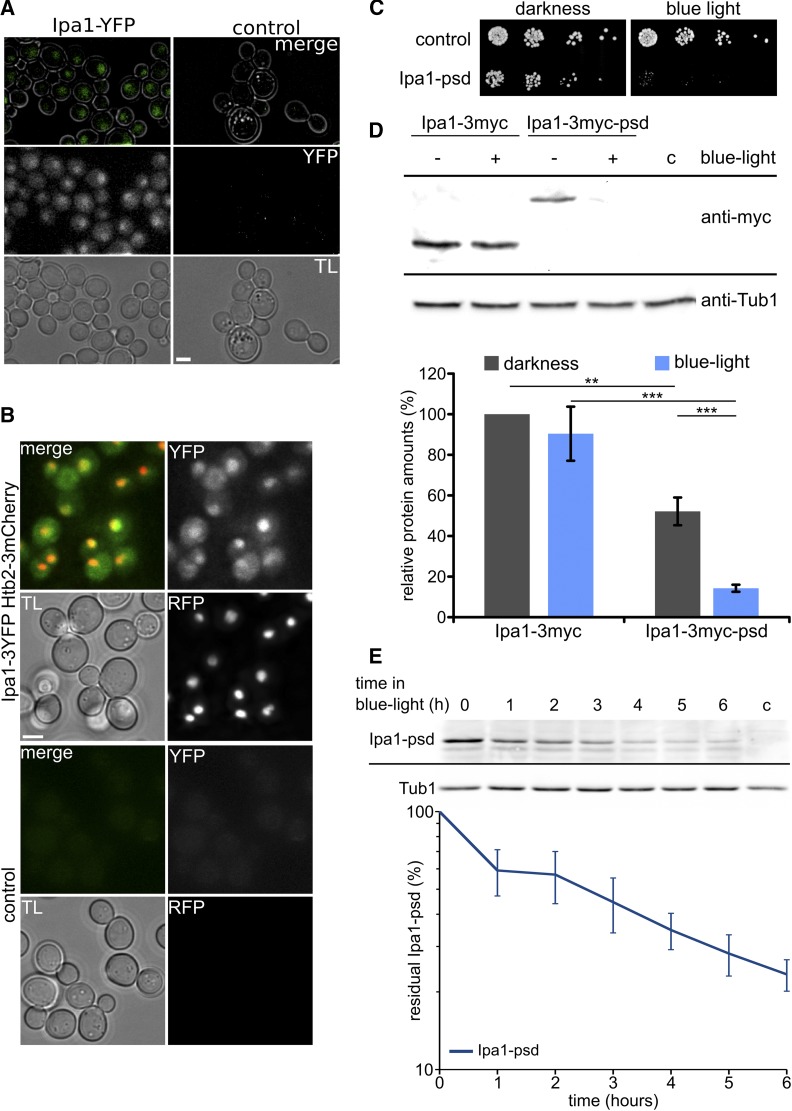
Ipa1 localizes to the cytosol and nucleus
Cellular localization of Ipa1 was investigated by fluorescence microscopy with YFP fused to its C-terminus, resulting in weak signals that could indicate localization in the cytosol and the nucleus (Figure 4A). This finding was corroborated by genomic fusion of 3YFP to Ipa1 in a yeast strain carrying a 3mCherry-tagged version of histone Htb2 as nuclear marker. There, we observed colocalization of the nuclear YFP and RFP signals, as well as weak Ipa1-3YFP signals in the cytosol (Figure 4B).
Figure 4.
Ipa1 localizes to the cytosol and nucleus and is essential for growth. (A) Ipa1-YFP localizes to cytosol and the nucleus. Cells in logarithmic growth phase were subjected to fluorescence microscopy. TL image, maximum projections of deconvolved z-stacks for fluorescence channel, and TL/YFP overlays are shown (bar size 2 µm). (B) Colocalization analysis of Ipa1-3YFP with Htb2-3mCherry. Maximum projections of deconvolved z-stacks are shown for fluorescence channels (bar size 2 µm). (C) Serial dilution drop assay with control cells compared to Ipa1-psd cells in darkness and exposed to blue light (465 nm 30 µmol m−2 s−1). Serial dilutions (1:5) were spotted on solid YPD medium. (D) Abundance of Ipa1-3myc compared to Ipa1-3myc-psd. Cell extracts were prepared from cells growing logarithmically in liquid cultures kept in darkness or exposed to blue light for 5 hr. The blot was probed with anti-myc and anti-Tub1 (loading control) antibodies. Cropped blots are shown, no relevant parts of the blot were removed. For quantification, Ipa1 levels were normalized to Tub1 signal; Ipa1-3myc in darkness was used as reference (n = 4; error bar, SEM; statistical significance of differences was tested by a two-sided Student’s t-test; ** P ≤ 0.01 and *** P ≤ 0.001). (E) Kinetics of Ipa1-3myc-psd depletion after exposure to blue light. Example blot and graph showing quantification of four independent experiments. Ipa1 levels were normalized to Tub1 signal (n = 4; error bar, SEM). psd, photo-sensitive degron; RFP, red fluorescent protein; TL, transmitted light; Tub, tubulin; YFP, yellow fluorescent protein.
To address the physiological role(s) of Ipa1, we created a conditional loss-of-function variant by tagging it with a psd module, which allows control of protein stability with blue light via ubiquitin-independent proteasomal degradation (Renicke et al. 2013a). Specifically, we fused Ipa1 to an improved psd variant (psdK91R E132A N139E N148D E155G) that confers degradation with higher efficiency at restrictive conditions (Usherenko et al. 2014). Using this system, we obtained a yeast strain that was viable in darkness but was unable to grow when exposed to blue light (Figure 4C). A comparison between Ipa1-3myc-psd and Ipa1-3myc revealed that Ipa1-3myc-psd protein levels were reduced by roughly 50% under permissive and by around 90% under restrictive conditions (Figure 4D). We measured the kinetics of Ipa1-psd depletion after transfer to blue light. This showed that Ipa1 levels decreased continuously over time. We concluded that 5–6 hr exposure to blue light should be sufficient to inactivate Ipa1-psd considerably (Figure 4E). The observed interaction of Ipa1 with Pex4 may indicate an involvement of Ipa1 in peroxisome biogenesis or peroxisomal protein import. However, we did not find differences in either the number of peroxisomes, their distribution, or the localization of the peroxisomal marker GFP-Sps19 in Ipa1-depleted cells (Figure S5 in File S1).
Ipa1 affects expression of genes involved in the UPS, stress responses, and DNA-damage repair
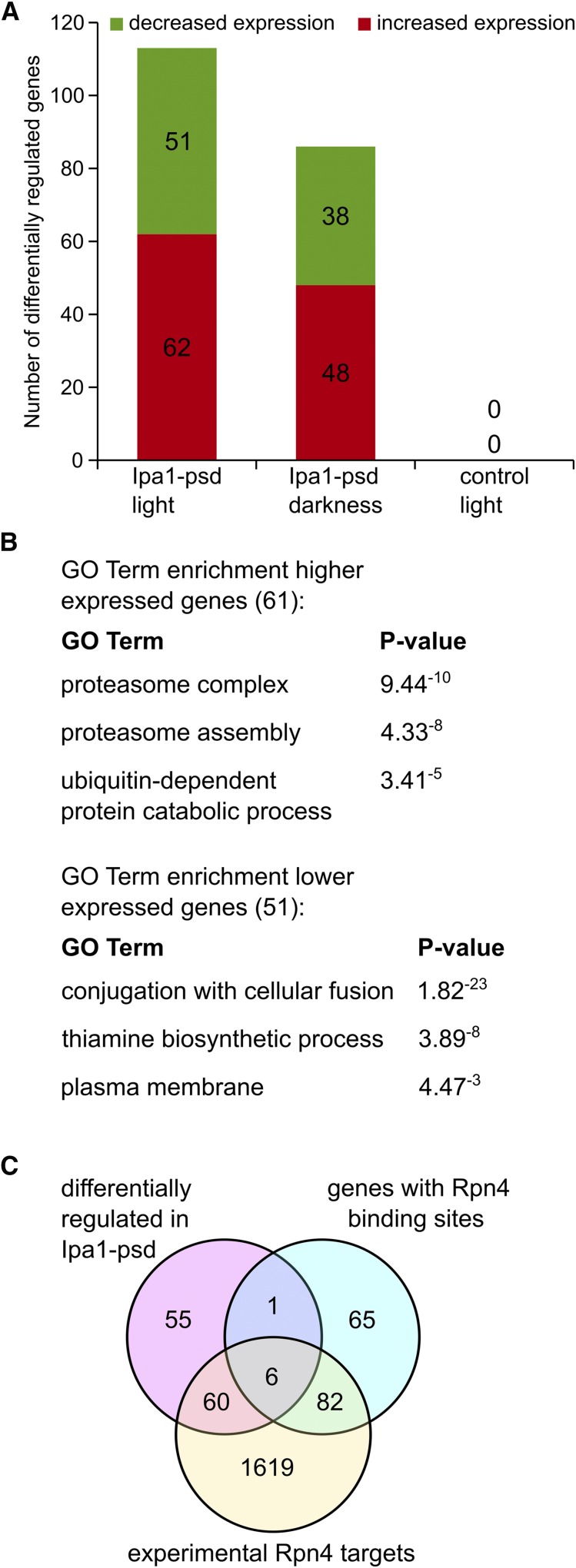
To investigate the consequences of Ipa1 depletion on a genome-wide scale, we compared the transcriptomes of the conditional loss-of-function variant Ipa1-psd grown under either permissive or restrictive conditions. We found that in comparison to a control strain, transcript levels of 62 genes were more than twofold increased in Ipa1-psd cells exposed to blue light, whereas expression of 51 genes was significantly reduced (Figure 5A, Table S2, Table S5, and Table S6). In darkness, we found higher transcript levels for 48 genes and reduced levels for 38 genes (Figure 5A, Table S3, Table S5, and Table S6). Moreover, a large fraction of the differentially expressed genes in the Ipa1-psd population was shared between darkness and blue light conditions, indicating that the reduction of Ipa1 protein levels observed in the Ipa1-psd strain (see above; Figure 4D) is responsible for this effect (Figure S6A in File S1). Exposing yeast cells to blue light did not result in significant changes of gene expression (Figure 5A and Table S4). The mRNA abundance of several differentially expressed genes was measured by real-time quantitative RT-PCR. Compared with the microarray chip analysis, highly consistent results were obtained (Figure S6B in File S1).
Figure 5.
Transcriptome analysis of Ipa1-depleted cells. (A) Number of genes with significantly altered mRNA levels (more than twofold). Ipa1-psd (photo-sensitive degron) cells exposed to light or kept in darkness were compared to control cells exposed to the same conditions; control cells exposed to light were compared to control cells kept in darkness. Cells were treated with blue light (465 nm, 30 µmol m−2 s−1) for 5 hr prior to cell lysis as indicated. (B) serial pattern of expression levels locator and gene ontology (GO)-term enrichment analysis of genes with higher and lower mRNA abundance in Ipa1-depleted cells compared to control cells. Genes significantly changed in expression by more than twofold were used for the analysis. (C) Genes differentially regulated in Ipa1-psd cells are Rpn4 targets. Venn diagram showing the number of genes belonging to the group of direct Rpn4 targets, experimentally identified Rpn4 targets, and differentially regulated in Ipa1-psd cells.
SPELL (Hibbs et al. 2007) and GO-term enrichment analysis using the set of genes with higher mRNA abundance (Table S5) revealed that expression of a significant number of genes involved in the UPS, stress responses, and DNA-damage repair were affected in Ipa1-psd cells (Figure 5B and Table S7). Furthermore, SPELL and GO-term enrichment analysis revealed an enrichment of genes connected to cell conjugation, thiamine biosynthesis, and the plasma membrane among the set of lower-expressed genes (Figure 5B and Table S8). In total, 55% of the genes affected by Ipa1 depletion (higher and lower expressed) are potential or experimentally demonstrated targets of the transcriptional activator Rpn4 (Figure 5C, Table S5 and Table S6).
As transcript levels of UFD1 were found to be increased in Ipa1-psd cells, we measured protein levels of the Cdc48-Npl4-Ufd1 complex, which is an important regulatory component of the UPS and is involved in the degradation of numerous substrates (Jentsch and Rumpf 2007; Förster et al. 2014). In agreement with the SPELL analysis, we found an increase in protein abundance of all three components of the complex (Figure S7, A–D in File S1). Whereas the abundance of Ufd1-6HA was found to be only slightly raised in Ipa1-psd cells, the levels of Cdc48-GFP and Npl4-6HA were significantly increased. This demonstrates that reduction of Ipa1 abundance not only influences expression of the Rpn4 regulon, but also leads to changes in protein levels as well.
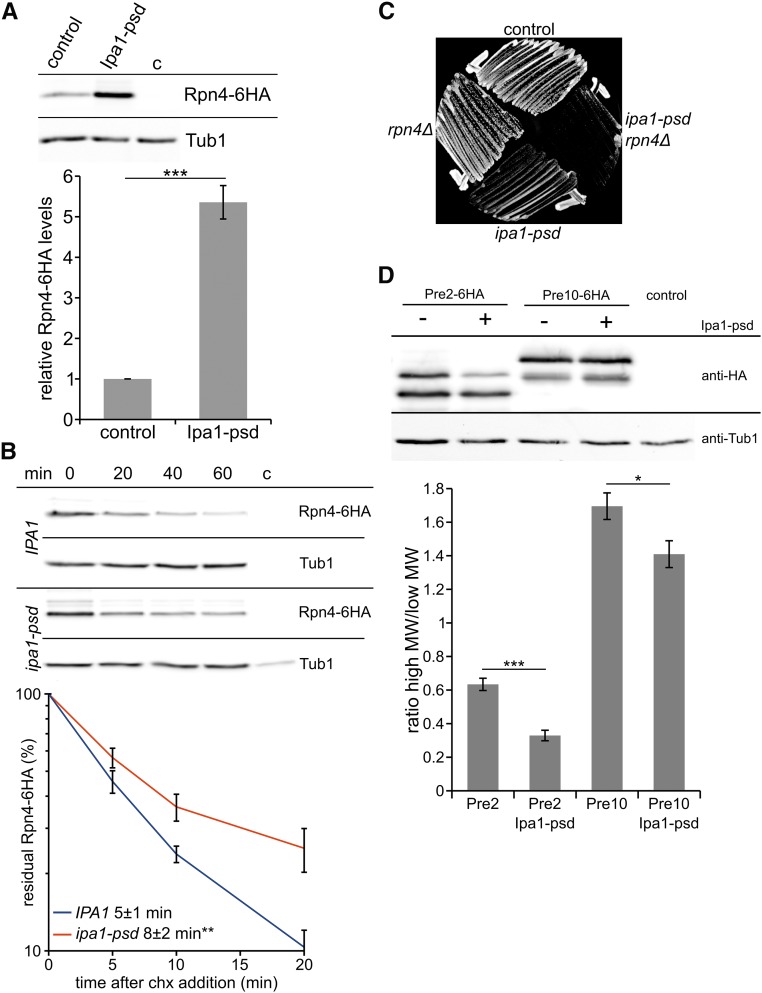
A possible explanation for the activation of the Rpn4 regulon and increased abundance of UPS components (Figure 5 and Figures S6 and S7 in File S1) might be decreased proteasome activity or proteasome overload, which both induce increased activity of Rpn4 by negative feedback regulation (Dohmen et al. 2007). Thus, we determined whether Ipa1 affects the abundance and stability of Rpn4. We found that Rpn4-6HA levels and its half-life are increased in Ipa1-depleted cells (Figure 6, A and B), which is in agreement with the results of the transcriptome analysis. To address, whether the effect of Ipa1 on proteasomal gene expression depends on Rpn4, we created an rpn4Δ ipa1-psd double mutant. Interestingly, this strain showed a severe growth defect compared to the single mutants and the control strain (Figure 6C), indicating that deletion of RPN4 is not epistatic over ipa1-psd. This suggests that Ipa1 does not affect expression of genes involved in the UPS, stress responses, and DNA-damage repair by directly targeting Rpn4, but causes this effect indirectly.
Figure 6.
The abundance and half-life of Rpn4 is increased in Ipa1-depleted cells. (A) Abundance of Rpn4-6HA in control cells compared to Ipa1-psd cells. Whole-cell extracts have been prepared from cells growing logarithmically in liquid cultures exposed to blue light. The blot was probed with anti-HA and anti-Tub1 (loading control) antibodies. Cropped blots are shown, no relevant parts of the blot were removed. Graph shows the mean of eight independent measurements, Rpn4-HA levels were normalized to Tub1 signal (error bar, SEM; statistical significance of half-life differences was tested by a two-sided Student’s t-test, *** P ≤ 0.001). (B) Analysis of Rpn4-6HA stability. The translation inhibitor chx was added to the cells after removal of the first sample (t = 0 min). Samples were analyzed by western blot, four independent measurements were performed for the graph (error bar, SEM; statistical significance of differences was tested by a two-sided Student’s t-test, ** P ≤ 0.01). (C) Growth assay of control cells, rpn4Δ, ipa1-psd, and ipa1-psd rpn4Δ cells in darkness. Cells were streaked on solid YPD medium and incubated at 30° for 2 days. (D) Appearance of Pre2-6HA and Pre10-6HA in presence and absence of Ipa1. Whole-cell extracts were prepared from cells growing logarithmically in liquid cultures exposed to blue light. The blot was probed with anti-HA and anti-Tub1 (loading control) antibodies. The graph shows the ratio of high-molecular weight signals of Pre2 and Pre10 compared to low-molecular weight signals (n = 4; error bar = SEM; statistical significance of differences was tested by a two-sided Student’s t-test; * P ≤ 0.05 and *** P ≤ 0.001). Chx, cycloheximide; HA, hemagglutinin; psd, photo-sensitive degron; Tub, tubulin.
Next, we visualized Pre2 and Pre10, two components of the proteasome core complex. Previous studies have shown that the proteolytically-active β-type subunit Pre2 matures by cleavage after assembly of the proteasome core particle, and is post-translationally modified by phosphorylation and ubiquitylation, whereas the α-type subunit Pre10 is modified by phosphorylation and succinylation (Chen and Hochstrasser 1996; Heinemeyer et al. 1997; Kikuchi et al. 2010; Swaney et al. 2013; Weinert et al. 2013). We compared maturation of Pre2-6HA and Pre10-6HA in control cells and cells depleted for Ipa1. This revealed that the ratios between high-molecular and low-molecular weight species of Pre2 and Pre10 were significantly altered in cells depleted for Ipa1 (Figure 6D). We also investigated whether depletion of Ipa1 changes the localization of Pre2 or Pre10. No change of localization was detected in logarithmically growing cells depleted for Ipa1 (Figure S8, A and B in File S1). In quiescent cells, proteasomes relocalize from the nucleus to storage granules in the cytosol (Laporte et al. 2008). After induction of starvation, localization of Pre10-YFP to storage granules was not altered in Ipa1-psd cells when compared to control cells (Figure S8C in File S1). During these fluorescence microscopy experiments, we noticed that Ipa1-psd cells showed an increase in cell size compared to control cells (Figure S8, A–C in File S1). Quantification of cell size distribution revealed a small but significant enlargement of Ipa1-psd cells (Figure S8D in File S1). Furthermore, analysis of cell cycle stages revealed that Ipa1-depleted cells are delayed in transition from metaphase to anaphase (Figure S8E in File S1). In summary, our experiments indicate that maturation and/or modification of at least some proteasome subunits is affected in Ipa1-depleted cells. As both maturation and modification of proteasome subunits has an impact on proteasomal activity (Chen and Hochstrasser 1996; Heinemeyer et al. 1997; Kikuchi et al. 2010), we measured the degradation of a ubiquitin-dependent and a ubiquitin-independent substrate.
Absence of Ipa1 affects proteasomal degradation
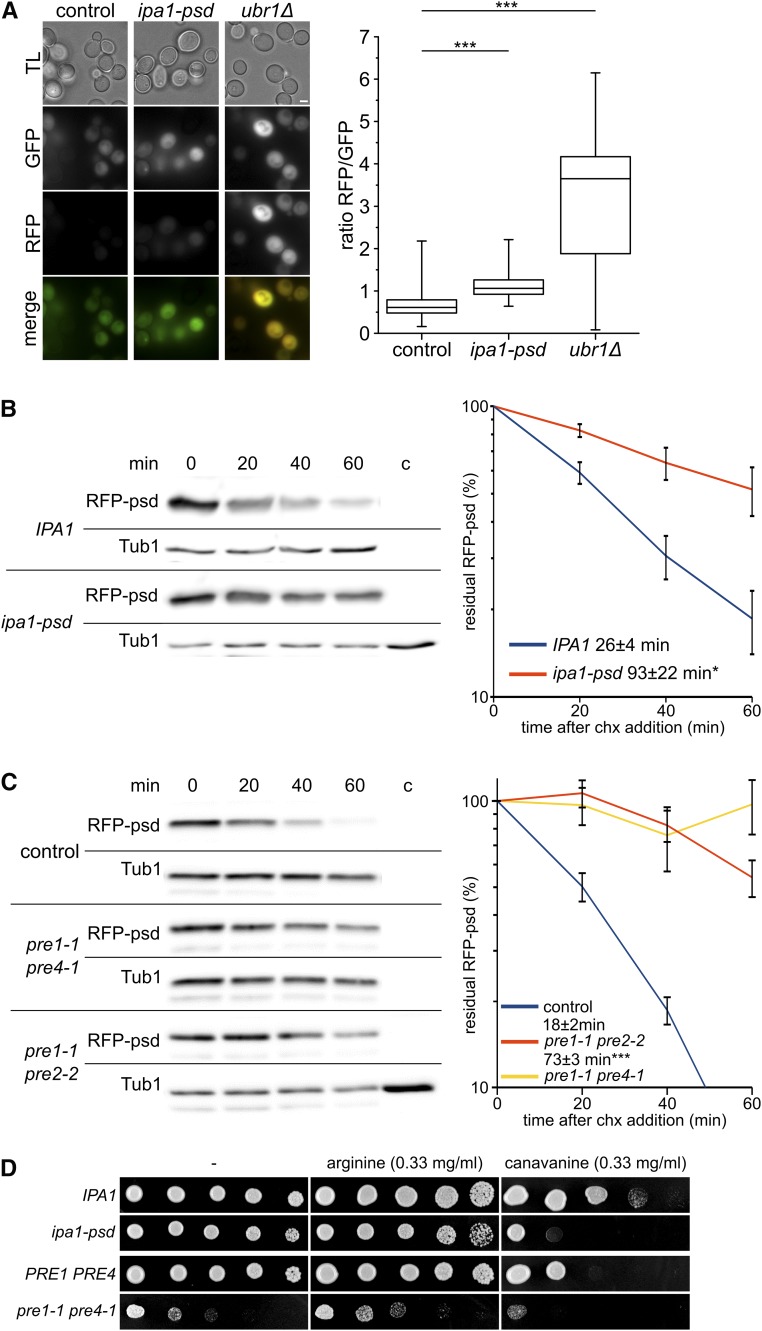
First, degradation of an N-degron based on a ubiquitin-fusion construct was followed with a tandem fluorescence protein (FP)-based construct, which allows direct quantification of UPS proteolysis by simple fluorescence measurements (Khmelinskii et al. 2012). We found a weak but consistent decrease of UPS-reporter degradation in Ipa1-depleted cells, indicated by an increase in the RFP/GFP ratio (Figure 7A). However, a much higher increase in the RFP/GFP ratio was observed in cells lacking UBR1, which is essential for degradation of the ubiquitin-R-construct. Furthermore, we measured proteasomal proteolysis of a ubiquitin-independent substrate. We observed decreased turnover of RFP-psd in Ipa1-psd cells (Figure 7B). In comparison, we tested the effect of mutations in genes encoding for subunits of the proteasome core complex. These mutations (pre1-1 pre2-2 and pre1-1 pre4-1) had a much more profound effect on RFP-psd degradation (Figure 7C). We also measured, whether Ipa1 depletion affects the growth of yeast cells in the presence of the arginine analog canavanine. Among other things, reduced proteasomal degradation enhances canavanine sensitivity due to increased substrate load that exceeds the reduced proteolytic capacity of the proteasome (Heinemeyer et al. 1991; Hilt et al. 1993; Elsasser et al. 2004). Here, we found that canavanine induced a slow-growth phenotype in an Ipa1-psd strain that is comparable to that of the proteasomal pre1-1 pre4-1 mutant strain (Figure 7D). In conclusion, Ipa1 is necessary to maintain a normal turnover rate of proteasome substrates. Our findings suggest that in Ipa1-psd cells, proteasome activity is decreased or that a general increase of substrate degradation takes place that leads to decreased turnover of the tested substrates.
Figure 7.
Proteasome activity is reduced in cells depleted for Ipa1. (A) Single-cell measurement of tandem-fluorescent protein substrate Ub-Arg-RFP-GFP abundance (using plasmid pMaM101) in control cells, ipa1-psd cells, and ubr1Δ cells exposed to blue light. Fluorescence microscopy images were recorded in TL, GFP, and RFP channels (bar size 2 µm). The graph shows quantification of GFP/RFP fluorescence intensity ratio in single cells. Whiskers comprise values of all single-cell measurements (n ≥ 350). Statistical significance of half-life differences was tested by a two-sided Student’s t-test (*** P ≤ 0.001). (B) Stability of RFP-psd in control cells compared to cells depleted for Ipa1-psd. Chx was added to the cultures after removing the first sample (t = 0 min). Cells were exposed to blue light before and during the experiment. Samples were analyzed by western blot and the abundance of RFP-psd quantified. The half-life was determined by fitting single experiments to an exponential decay with the software Qtiplot. Graphs show the mean of at least six independent experiments; error bars, SEM; statistical significance of differences was tested by a two-sided Student’s t-test (* P ≤ 0.05). (C) Stability of RFP-psd in control cells compared to cells compromised in proteasomal activity (pre1-1 pre2-2 and pre1-1 pre4-1). Experimental conditions as in (C). Graphs show the mean of four independent experiments; error bars, SEM; statistical significance of half-life differences was tested by a two-sided Student’s t-test (*** P ≤ 0.001). Please note that in the case of strain pre1-1 pre4-1, the half-life could not be obtained by fitting for some of the experiments, therefore no significance testing was performed. (D) Canavanine growth assay of ipa1-psd cells, pre1-1 pre4-1 cells, and isogenic control cells. Serial dilutions (1:5) of cells were spotted on minimal medium and grown in darkness for 4 days at 30°. Arginine or canavanine was added at a concentration of 0.33 mg/ml to the plate. Chx, cycloheximide; psd, photo-sensitive degron; RFP, red fluorescent protein; TL, transmitted light; Tub, tubulin; Ub, ubiquitin.
Discussion
In this study, we provide an in vivo characterization of a HECT_2-domain family member, the S. cerevisiae protein Ipa1/Yjr141w. Our study demonstrates that Ipa1 localizes to the cytosol and nucleus. Five cysteines of the HECT_2-family signature and the C-terminus are essential for in vivo function of Ipa1. Upregulation of the Rpn4 regulon and decreased turnover of proteasome substrates in Ipa1-psd cells indicate that Ipa1 has an impact on the UPS. One may speculate that the HECT_2 family has conserved functions: like Ipa1 in S. cerevisiae, UBE3D is essential for cell proliferation in a haploid human cell line and the HECT_2-domain protein SPBC1734.10c is essential for proliferation in Sc. pombe (Giaever et al. 2002; Kim et al. 2010; Hayles et al. 2013; Blomen et al. 2015). For the latter protein, nuclear and cytosolic localization has also been observed in a global analysis (Matsuyama et al. 2006).
The unifying signature of HECT_2-family members is their architecture as single-domain proteins containing only few conserved amino acids, among them several cysteines. The latter aspect is similar to the well-studied family of ubiquitin-protein ligases bearing a RING domain (Metzger et al. 2014). Our mutational analysis of the HECT_2-family signature of Ipa1 revealed five critical cysteines. Other amino acids of the HECT_2-family signature as well as other cysteines were not important for functionality of Ipa1. Importantly, the hallmark catalytic cysteine of HECT-domain E3s, which is present in human UBE3D, is not present in Ipa1. Assays with Ipa1 containing Escherichia coli extracts did not show self-ubiquitylation activity of Ipa1 (data not shown).
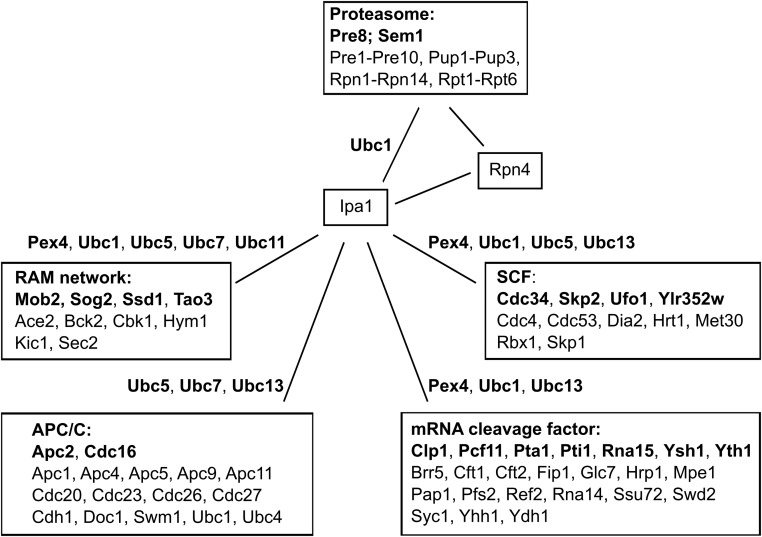
Ipa1 showed a number of interactions with different E2s in the BiFC and yeast two-hybrid assay. None of these E2s is essential for cell viability, which suggests that Ipa1 is acting together with more than one E2. We compared genetic interactions of Ipa1, E2s, Rpn4, and subunits of the proteasome, relying on the recently completed genetic interaction map and other genetic screens (Wilmes et al. 2008; Costanzo et al. 2016; Usaj et al. 2017). This revealed that several E2s share genetic interactions with Ipa1 toward subunits of the RAM network [regulation of Ace2p transcription factor and polarized morphogenesis (Saputo et al. 2012)], the APC/C [anaphase-promoting complex (Acquaviva and Pines 2006)], the mRNA cleavage factor (Mandel et al. 2008), and the SCF [Skp, Cullin, F-box-containing complex (Willems et al. 2004)]. These four complexes show negative genetic interactions with subunits of the proteasome as well. With the exception of Ubc8, all E2s that we found to interact with Ipa1 showed genetic interactions with subunits of the proteasome or the four aforementioned complexes, in most cases several E2s showed genetic interactions with subunits of a complex (Figure 8). This might be a hint that Ipa1 has context-dependent interactions with several E2s.
Figure 8.
Genetic interactions between Ipa1 and subunits of the proteasome, the RAM (regulation of Ace2p transcription factor and polarized morphogenesis) network, the APC/C (anaphase-promoting complex), the mRNA cleavage factor, and the SCF (Skp, Cullin, F-box-containing complex) are shared by E2s (ubiquitin-conjugating enzymes) interacting with Ipa1. Negative genetic interactions are indicated by a line, the individual subunits of a complex showing a genetic interaction with Ipa1 are given in bold. The E2s that interacted with Ipa1 and have been published to have a genetic interaction with subunits of the RAM network, the APC/C, the mRNA cleavage factor, and the SCF are placed next to the line. Please note that also other E2s that did not show interaction with Ipa1 have genetic interactions with these complexes. Similarly, we omitted to indicate the genetic interactions between subunits of the proteasome with subunits of the RAM network, the APC/C, the mRNA cleavage factor, and the SCF, as well as genetic interactions between Rpn4 with subunits of the RAM network, the APC/C, and the mRNA cleavage factor.
Targeted protein depletion has been used before to create conditional mutants of essential proteins using a temperature shift (Dohmen et al. 1994; Sanchez-Diaz et al. 2004). Here, Ipa1 functions were investigated using a fusion to the psd module that allows conditional downregulation using blue light. Although Ipa1-psd cells grow robustly in darkness, further analysis revealed that Ipa1-levels were already lowered at permissive conditions and specific changes of the transcriptome were observable. Thus, the psd module is a valuable tool for functional characterization of essential proteins, but the modification of the target protein might result in a partial loss-of-function at permissive conditions. Nevertheless, the usage of blue light as a switch has its advantages, as it does not influence the expression of yeast genes considerably (Figure 5A and Table S4). In contrast, a temperature shift induces the heat-shock response that alters the physiology of the cell dramatically (Verghese et al. 2012).
An unresolved question is the molecular mechanism that leads to reduced proteasome activity. Degradation of a target protein is independent of the strength of gene expression (Khmelinskii et al. 2012; Renicke et al. 2013a). The proteolytic capacity of the proteasome is high enough to ensure efficient degradation, even with very high target gene expression. Here, we measured the half-life of RFP-psd expressed from the TDH3 promoter to be 26 ± 4 min in wild-type cells (Figure 7B). A similar value (20 min) was originally measured for RFP-psd expressed by the ADH1 promoter, which is of lower strength (Renicke et al. 2013). The low abundance (< 100 molecules) of Ipa1 in cells (Kulak et al. 2014) suggests that the lowered proteasome activity in Ipa1-psd cells does not result from competition of Ipa1-psd with other proteasome substrates. If a slight increase of a few molecules of proteasome substrates would influence proteasomal processivity, one would expect a much more pronounced difference for RFP-psd degradation produced by the PTDH3 compared to the PADH1 promoter. Similarly, Ipa1-depleted cells exhibited slower transition from metaphase to anaphase indicated by increased abundance of large-budded cells (Figure S8E in File S1). The importance of proteasomal proteolysis during that cell cycle stage (King et al. 1996; Zachariae and Nasmyth 1999) makes it unlikely that Ipa1-depleted cells are trapped in a cell cycle stage with low proteasome activity. Moreover, the changes in the maturation or modification of two proteasome subunits suggest that Ipa1-psd depletion impinges directly on proteasome activity.
The influence of Ipa1 on proteasome activity might not only be restricted to yeast cells. Loss of UPS activity has been connected with aging and age-related diseases in humans and aging of yeast cells (Shang and Taylor 2012; Saez and Vilchez 2014; Vilchez et al. 2014; Ferrington et al. 2016). The human HECT_2-domain protein UBE3D, specifically a variant with a mutation in the HECT-like C-terminus (V379M), has been linked to macular degeneration in aged humans in East Asia (Huang et al. 2015). Thus, it might be worth determining whether proteasomal activity in human cells is influenced by the UBE3D V379M variant. Interestingly, most of the close homologs of UBE3D from, e.g., Mus musculus, Pan troglodytes, or Pongo abelii contain a methionine at the corresponding position (Figure S9 in File S1).
Our finding that Ipa1-psd cells show reduced proteasome activity and lowered tolerance toward canavanine indicate that Ipa1-psd cells are sensitive to conditions that induce proteotoxic stress. Previous studies have shown similar phenotypes for yeast strains with reduced proteasome activity (Heinemeyer et al. 1991; Hilt et al. 1993; Elsasser et al. 2004). However, canavanine sensitivity appears also in mutants with reduced endocytosis or increased canavanine uptake (Cabrera et al. 2013). How Ipa1 impinges on proteasome activity at the molecular level remains obscure at the moment. The HECT domain ubiquitin-protein ligase Hul5 is localized directly at the proteasome and affects proteasome activity mainly by increasing ubiquitin-chain length on proteasome-bound substrates (Leggett et al. 2002; Crosas et al. 2006). Recently, it was shown that Ipa1 physically interacts with 12 subunits of the proteasome, five of the six AAA-ATPases, four non-ATPases of the 19S regulatory particle, and three subunits of the 20S core, as well as the UPS-connected proteins Ubc1, Cue5, and Mpe1 (Costanzo et al. 2016). Yet, it is quite unlikely that Ipa1 influences proteasome activity by complex formation due to its very low abundance in the cell (Kulak et al. 2014), which means that only a few proteasome particles would actually carry Ipa1. The activation of the Rpn4 regulon that we observed in the absence of Ipa1 is rather unlikely in such a scenario. The available data points more to an indirect or enzymatic function of Ipa1.
One of the possibilities is that Ipa1 might affect the post-translational modification or maturation of proteasome subunits, or might lead to an imbalance in the synthesis of proteasome subunits. Ipa1 has been connected to RNA cleavage and polyadenylation (Costanzo et al. 2016). Missing Ipa1 activity might disturb protein synthesis and result in proteotoxic stress or unbalanced protein synthesis, and the latter could result in an overload of the UPS degradation capacity due to increased demand for proteolysis. Interestingly, it has been shown that pre-mRNA cleavage and polyadenylation requires ubiquitylation, functional proteasomes, and proteasomal degradation (Lee and Moore 2014). In regard of our finding that proteasomal activity is influenced by Ipa1, it is not clear whether the polyadenylation defects in an IPA1 mutant are directly caused by missing Ipa1 activity or indirectly evoked by changes in proteasome activity. However, the former might result in an unbalancing of protein biosynthesis in general and of proteasomal subunits in particular, which would result in inefficient maturation of proteasome complexes. Further studies are necessary to clarify the connection of pre-mRNA cleavage and polyadenylation with the proteasome.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.118.300744/-/DC1.
Acknowledgments
We thank Michael Knop and Gwenaël Rabut for constructs, Alfred Batschauer for equipment, and Daniela Störmer for her excellent technical assistance. C.T. was supported by the Deutsche Forschungsgemeinschaft (grants TA320-3-1 and TA320-7-1).
Author contributions: C.T. designed the experiments; A.P.L., S.S., C.R., and C.T. performed the experiments; A.P.L., S.S., H.-U.M., R.S., and C.T. analyzed and interpreted data; C.T. prepared the manuscript; and all authors read and approved the manuscript. The authors declare that they have no competing financial interests.
Footnotes
Communicating editor: S. Biggins
Literature Cited
- Acquaviva C., Pines J., 2006. The anaphase-promoting complex/cyclosome: APC/C. J. Cell Sci. 119: 2401–2404. 10.1242/jcs.02937 [DOI] [PubMed] [Google Scholar]
- Alabrudzinska M., Skoneczny M., Skoneczna A., 2011. Diploid-specific [corrected] genome stability genes of S. cerevisiae: genomic screen reveals haploidization as an escape from persisting DNA rearrangement stress. PLoS One 6: e21124 (erratum: PLoS One 6) 10.1371/journal.pone.0021124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardley H. C., Robinson P. A., 2005. E3 ubiquitin ligases. Essays Biochem. 41: 15–30. 10.1042/bse0410015 [DOI] [PubMed] [Google Scholar]
- Ausubel, F. M., R. E. Kingston, F. G. Seidman, K. Struhl, D. D. Moore et al. (Editors), 1995 Current Protocols in Molecular Biology. John Wiley and Sons, New York. [Google Scholar]
- Blomen V. A., Majek P., Jae L. T., Bigenzahn J. W., Nieuwenhuis J., et al. , 2015. Gene essentiality and synthetic lethality in haploid human cells. Science 350: 1092–1096. 10.1126/science.aac7557 [DOI] [PubMed] [Google Scholar]
- Budenholzer L., Cheng C. L., Li Y., Hochstrasser M., 2017. Proteasome structure and assembly. J. Mol. Biol. 429: 3500–3524. 10.1016/j.jmb.2017.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera M., Arlt H., Epp N., Lachmann J., Griffith J., et al. , 2013. Functional separation of endosomal fusion factors and the class C core vacuole/endosome tethering (CORVET) complex in endosome biogenesis. J. Biol. Chem. 288: 5166–5175. 10.1074/jbc.M112.431536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Hochstrasser M., 1996. Autocatalytic subunit processing couples active site formation in the 20S proteasome to completion of assembly. Cell 86: 961–972. 10.1016/S0092-8674(00)80171-3 [DOI] [PubMed] [Google Scholar]
- Collins T. J., 2007. ImageJ for microscopy. Biotechniques 43(1 Suppl.): 25–30. 10.2144/000112517 [DOI] [PubMed] [Google Scholar]
- Costanzo M., VanderSluis B., Koch E. N., Baryshnikova A., Pons C., et al. , 2016. A global genetic interaction network maps a wiring diagram of cellular function. Science 353: aaf1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosas B., Hanna J., Kirkpatrick D. S., Zhang D. P., Tone Y., et al. , 2006. Ubiquitin chains are remodeled at the proteasome by opposing ubiquitin ligase and deubiquitinating activities. Cell 127: 1401–1413. 10.1016/j.cell.2006.09.051 [DOI] [PubMed] [Google Scholar]
- Cui S., Eisenacher K., Kirchhofer A., Brzozka K., Lammens A., et al. , 2008. The C-terminal regulatory domain is the RNA 5′-triphosphate sensor of RIG-I. Mol. Cell 29: 169–179. 10.1016/j.molcel.2007.10.032 [DOI] [PubMed] [Google Scholar]
- Dohmen R. J., Wu P., Varshavsky A., 1994. Heat-inducible degron: a method for constructing temperature-sensitive mutants. Science 263: 1273–1276. 10.1126/science.8122109 [DOI] [PubMed] [Google Scholar]
- Dohmen R. J., Willers I., Marques A. J., 2007. Biting the hand that feeds: Rpn4-dependent feedback regulation of proteasome function. Biochim. Biophys. Acta 1773: 1599–1604. 10.1016/j.bbamcr.2007.05.015 [DOI] [PubMed] [Google Scholar]
- Elsasser S., Chandler-Militello D., Muller B., Hanna J., Finley D., 2004. Rad23 and Rpn10 serve as alternative ubiquitin receptors for the proteasome. J. Biol. Chem. 279: 26817–26822. 10.1074/jbc.M404020200 [DOI] [PubMed] [Google Scholar]
- Ferrington D. A., Sinha D., Kaarniranta K., 2016. Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration. Prog. Retin. Eye Res. 51: 69–89. 10.1016/j.preteyeres.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Sternglanz R., 1994. The two-hybrid system: an assay for protein-protein interactions. Trends Genet. 10: 286–292. 10.1016/0168-9525(90)90012-U [DOI] [PubMed] [Google Scholar]
- Finley D., Ulrich H. D., Sommer T., Kaiser P., 2012. The ubiquitin-proteasome system of Saccharomyces cerevisiae. Genetics 192: 319–360. 10.1534/genetics.112.140467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R. D., Coggill P., Eberhardt R. Y., Eddy S. R., Mistry J., et al. , 2016. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 44: D279–D285. 10.1093/nar/gkv1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster F., Schuller J. M., Unverdorben P., Aufderheide A., 2014. Emerging mechanistic insights into AAA complexes regulating proteasomal degradation. Biomolecules 4: 774–794. 10.3390/biom4030774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G., Chu A. M., Ni L., Connelly C., Riles L., et al. , 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418: 387–391. 10.1038/nature00935 [DOI] [PubMed] [Google Scholar]
- Hayles J., Wood V., Jeffery L., Hoe K. L., Kim D. U., et al. , 2013. A genome-wide resource of cell cycle and cell shape genes of fission yeast. Open Biol. 3: 130053 10.1098/rsob.130053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemeyer W., Kleinschmidt J. A., Saidowsky J., Escher C., Wolf D. H., 1991. Proteinase yscE, the yeast proteasome/multicatalytic-multifunctional proteinase: mutants unravel its function in stress induced proteolysis and uncover its necessity for cell survival. EMBO J. 10: 555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemeyer W., Fischer M., Krimmer T., Stachon U., Wolf D. H., 1997. The active sites of the eukaryotic 20 S proteasome and their involvement in subunit precursor processing. J. Biol. Chem. 272: 25200–25209. 10.1074/jbc.272.40.25200 [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A., 1998. The ubiquitin system. Annu. Rev. Biochem. 67: 425–479. 10.1146/annurev.biochem.67.1.425 [DOI] [PubMed] [Google Scholar]
- Hibbs M. A., Hess D. C., Myers C. L., Huttenhower C., Li K., et al. , 2007. Exploring the functional landscape of gene expression: directed search of large microarray compendia. Bioinformatics 23: 2692–2699. 10.1093/bioinformatics/btm403 [DOI] [PubMed] [Google Scholar]
- Hilt W., Enenkel C., Gruhler A., Singer T., Wolf D. H., 1993. The PRE4 gene codes for a subunit of the yeast proteasome necessary for peptidylglutamyl-peptide-hydrolyzing activity. Mutations link the proteasome to stress- and ubiquitin-dependent proteolysis. J. Biol. Chem. 268: 3479–3486. [PubMed] [Google Scholar]
- Hu C. D., Chinenov Y., Kerppola T. K., 2002. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell 9: 789–798. 10.1016/S1097-2765(02)00496-3 [DOI] [PubMed] [Google Scholar]
- Huang L. Z., Li Y. J., Xie X. F., Zhang J. J., Cheng C. Y., et al. , 2015. Whole-exome sequencing implicates UBE3D in age-related macular degeneration in East Asian populations. Nat. Commun. 6: 6687 10.1038/ncomms7687 [DOI] [PubMed] [Google Scholar]
- Huber W., Carey V. J., Gentleman R., Anders S., Carlson M., et al. , 2015. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 12: 115–121. 10.1038/nmeth.3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inobe T., Matouschek A., 2014. Paradigms of protein degradation by the proteasome. Curr. Opin. Struct. Biol. 24: 156–164. 10.1016/j.sbi.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C., Magiera M. M., Rathfelder N., Taxis C., Reber S., et al. , 2004. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21: 947–962. 10.1002/yea.1142 [DOI] [PubMed] [Google Scholar]
- Jentsch S., Rumpf S., 2007. Cdc48 (p97): a “molecular gearbox” in the ubiquitin pathway? Trends Biochem. Sci. 32: 6–11. 10.1016/j.tibs.2006.11.005 [DOI] [PubMed] [Google Scholar]
- Jungbluth M., Renicke C., Taxis C., 2010. Targeted protein depletion in Saccharomyces cerevisiae by activation of a bidirectional degron. BMC Syst. Biol. 4: 176 10.1186/1752-0509-4-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungbluth M., Mosch H. U., Taxis C., 2012. Acetate regulation of spore formation is under the control of the Ras/cyclic AMP/protein kinase A pathway and carbon dioxide in Saccharomyces cerevisiae. Eukaryot. Cell 11: 1021–1032. 10.1128/EC.05240-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khmelinskii A., Keller P. J., Bartosik A., Meurer M., Barry J. D., et al. , 2012. Tandem fluorescent protein timers for in vivo analysis of protein dynamics. Nat. Biotechnol. 30: 708–714. 10.1038/nbt.2281 [DOI] [PubMed] [Google Scholar]
- Kikuchi J., Iwafune Y., Akiyama T., Okayama A., Nakamura H., et al. , 2010. Co- and post-translational modifications of the 26S proteasome in yeast. Proteomics 10: 2769–2779. 10.1002/pmic.200900283 [DOI] [PubMed] [Google Scholar]
- Kim D. U., Hayles J., Kim D., Wood V., Park H. O., et al. , 2010. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 28: 617–623 (erratum: Nat. Biotechnol. 28: 1308) 10.1038/nbt.1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. W., Deshaies R. J., Peters J. M., Kirschner M. W., 1996. How proteolysis drives the cell cycle. Science 274: 1652–1659. 10.1126/science.274.5293.1652 [DOI] [PubMed] [Google Scholar]
- Knop M., Strasser K., 2000. Role of the spindle pole body of yeast in mediating assembly of the prospore membrane during meiosis. EMBO J. 19: 3657–3667. 10.1093/emboj/19.14.3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobirumaki F., Miyauchi Y., Fukami K., Tanaka H., 2005. A novel UbcH10-binding protein facilitates the ubiquitinylation of cyclin B in vitro. J. Biochem. 137: 133–139. 10.1093/jb/mvi026 [DOI] [PubMed] [Google Scholar]
- Kulak N. A., Pichler G., Paron I., Nagaraj N., Mann M., 2014. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods 11: 319–324. 10.1038/nmeth.2834 [DOI] [PubMed] [Google Scholar]
- Laporte D., Salin B., Daignan-Fornier B., Sagot I., 2008. Reversible cytoplasmic localization of the proteasome in quiescent yeast cells. J. Cell Biol. 181: 737–745. 10.1083/jcb.200711154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. D., Moore C. L., 2014. Efficient mRNA polyadenylation requires a ubiquitin-like domain, a zinc knuckle, and a RING finger domain, all contained in the Mpe1 protein. Mol. Cell. Biol. 34: 3955–3967. 10.1128/MCB.00077-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett D. S., Hanna J., Borodovsky A., Crosas B., Schmidt M., et al. , 2002. Multiple associated proteins regulate proteasome structure and function. Mol. Cell 10: 495–507. 10.1016/S1097-2765(02)00638-X [DOI] [PubMed] [Google Scholar]
- Lipkowitz S., Weissman A. M., 2011. RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat. Rev. Cancer 11: 629–643. 10.1038/nrc3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Method. Methods 25: 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lutz A. P., Renicke C., Taxis C., 2016. Controlling protein activity and degradation using blue light. Methods Mol. Biol. 1408: 67–78. 10.1007/978-1-4939-3512-3_5 [DOI] [PubMed] [Google Scholar]
- Mandel C. R., Bai Y., Tong L., 2008. Protein factors in pre-mRNA 3′-end processing. Cell. Mol. Life Sci. 65: 1099–1122. 10.1007/s00018-007-7474-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama A., Arai R., Yashiroda Y., Shirai A., Kamata A., et al. , 2006. ORFeome cloning and global analysis of protein localization in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 24: 841–847 (erratum: Nat. Biotechnol. 24: 1033) 10.1038/nbt1222 [DOI] [PubMed] [Google Scholar]
- Metzger M. B., Pruneda J. N., Klevit R. E., Weissman A. M., 2014. RING-type E3 ligases: master manipulators of E2 ubiquitin-conjugating enzymes and ubiquitination. Biochim. Biophys. Acta 1843: 47–60. 10.1016/j.bbamcr.2013.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G., Tanaka T. U., Nasmyth K., Schiebel E., 2001. Modes of spindle pole body inheritance and segregation of the Bfa1p-Bub2p checkpoint protein complex. EMBO J. 20: 6359–6370. 10.1093/emboj/20.22.6359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart C. M., 2001. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70: 503–533. 10.1146/annurev.biochem.70.1.503 [DOI] [PubMed] [Google Scholar]
- Renicke C., Schuster D., Usherenko S., Essen L. O., Taxis C., 2013a A LOV2 domain-based optogenetic tool to control protein degradation and cellular function. Chem. Biol. 20: 619–626. 10.1016/j.chembiol.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Renicke C., Spadaccini R., Taxis C., 2013b A tobacco etch virus protease with increased substrate tolerance at the P1′ position. PLoS One 8: e67915 10.1371/journal.pone.0067915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez I., Vilchez D., 2014. The mechanistic links between proteasome activity, aging and age-related diseases. Curr. Genomics 15: 38–51. 10.2174/138920291501140306113344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Diaz A., Kanemaki M., Marchesi V., Labib K., 2004. Rapid depletion of budding yeast proteins by fusion to a heat-inducible degron. Sci. STKE 2004: PL8. [DOI] [PubMed] [Google Scholar]
- Saputo S., Chabrier-Rosello Y., Luca F. C., Kumar A., Krysan D. J., 2012. The RAM network in pathogenic fungi. Eukaryot. Cell 11: 708–717. 10.1128/EC.00044-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D., 1989. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 16: 339–346. 10.1007/BF00340712 [DOI] [PubMed] [Google Scholar]
- Schmitt M. E., Brown T. A., Trumpower B. L., 1990. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 18: 3091–3092. 10.1093/nar/18.10.3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang F., Taylor A., 2012. Roles for the ubiquitin-proteasome pathway in protein quality control and signaling in the retina: implications in the pathogenesis of age-related macular degeneration. Mol. Aspects Med. 33: 446–466. 10.1016/j.mam.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F., 2002. Getting started with yeast. Methods Enzymol. 350: 3–41. 10.1016/S0076-6879(02)50954-X [DOI] [PubMed] [Google Scholar]
- Swaney D. L., Beltrao P., Starita L., Guo A., Rush J., et al. , 2013. Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat. Methods 10: 676–682. 10.1038/nmeth.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira M. C., Monteiro P. T., Guerreiro J. F., Goncalves J. P., Mira N. P., et al. , 2014. The YEASTRACT database: an upgraded information system for the analysis of gene and genomic transcription regulation in Saccharomyces cerevisiae. Nucleic Acids Res. 42: D161–D166. 10.1093/nar/gkt1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G., 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25: 4876–4882. 10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usaj M., Tan Y., Wang W., VanderSluis B., Zou A., et al. , 2017. TheCellMap.org: a web-accessible database for visualizing and mining the global yeast genetic interaction network. G3 (Bethesda) 7: 1539–1549. 10.1534/g3.117.040220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usherenko S., Stibbe H., Musco M., Essen L. O., Kostina E. A., et al. , 2014. Photo-sensitive degron variants for tuning protein stability by light. BMC Syst. Biol. 8: 128 10.1186/s12918-014-0128-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J., Abrams J., Wang Y., Morano K. A., 2012. Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol. Mol. Biol. Rev. 76: 115–158. 10.1128/MMBR.05018-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez D., Saez I., Dillin A., 2014. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat. Commun. 5: 5659 10.1038/ncomms6659 [DOI] [PubMed] [Google Scholar]
- Weinert B. T., Scholz C., Wagner S. A., Iesmantavicius V., Su D., et al. , 2013. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 4: 842–851. 10.1016/j.celrep.2013.07.024 [DOI] [PubMed] [Google Scholar]
- Wettenhall J. M., Simpson K. M., Satterley K., Smyth G. K., 2006. AffylmGUI: a graphical user interface for linear modeling of single channel microarray data. Bioinformatics 22: 897–899. 10.1093/bioinformatics/btl025 [DOI] [PubMed] [Google Scholar]
- Willems A. R., Schwab M., Tyers M., 2004. A hitchhiker’s guide to the cullin ubiquitin ligases: SCF and its kin. Biochim. Biophys. Acta 1695: 133–170. 10.1016/j.bbamcr.2004.09.027 [DOI] [PubMed] [Google Scholar]
- Wilmes G. M., Bergkessel M., Bandyopadhyay S., Shales M., Braberg H., et al. , 2008. A genetic interaction map of RNA-processing factors reveals links between Sem1/Dss1-containing complexes and mRNA export and splicing. Mol. Cell 32: 735–746. 10.1016/j.molcel.2008.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Varshavsky A., 2001. RPN4 is a ligand, substrate, and transcriptional regulator of the 26S proteasome: a negative feedback circuit. Proc. Natl. Acad. Sci. USA 98: 3056–3061. 10.1073/pnas.071022298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariae W., Nasmyth K., 1999. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 13: 2039–2058. 10.1101/gad.13.16.2039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplemental Material files). Additionally, microarray data are available from the ArrayExpress database (http://www.ebi.ac.uk) under accession number E-MTAB-5022. The Supplemental Material files contain additional Figures S1–S9 in File S1, and descriptions of Table S1, Table S2, Table S3, Table S4, Table S5, Table S6, Table S7, Table S8, Table S9, Table S10, and Table S11. Table S1 contains the Pfam protein names and organism identifiers for the network shown in Figure 1B. Table S2 contains microarray data (Ipa1-psd strain vs. control strain ESM356-1 in blue light). Table S3 contains microarray data (Ipa1-psd strain vs. ESM356-1 in darkness). Table S4 contains microarray data (ESM356-1 strain in blue light vs. ESM356-1 in darkness). Table S5 contains the list of upregulated genes in Ipa1-psd cells. Table S6 contains the list of downregulated genes in Ipa1-psd cells. Table S7 contains the list of enriched GO terms (upregulated genes). Table S8 contains the list of enriched GO terms (downregulated genes). Table S9 contains the list of yeast strains. Table S10 contains the list of plasmids. Table S11 contains the list of oligos used for the quantitative real-time PCR analysis. Strains and plasmids are available upon request.
Figure 1.
The yeast protein Yjr141w/Ipa1 belongs to the eukaryotic HECT_2 family. (A) Conserved residues within Yjr141w/Ipa1 match the family signature of HECT_2-family members. Highly conserved residues are marked with an asterisk. Residues or parts of the protein that are essential for Yjr141w/Ipa1 function are marked in red, residues in green are dispensable. (B) Sequence similarity network of HECT_2-family members. Sequences with an identity > 50% were clustered together in one node. The coloring shows affiliation of the protein(s) to one of the eukaryotic subgroups (source: taxonomy database of UniProt). For each subgroup, one protein is given as example; its sequence identity to S. cerevisiae Yjr141w/Ipa1 is given in parentheses. The Pfam identifiers for each node are given in Table S1. (C) Alignment of the C-termini of S. cerevisiae HECT-family members Hul5, Ufd4, Hul4, Tom1, and Rsp5 with the prototype HECT-domain protein human E6-AP and HECT_2-family members Yjr141w/Ipa1 and human homolog UBE3D. The conservation grade of a residue is shown by asterisk, double point, and point to indicate strict conservation, and higher and lower similarity, respectively. The percentage of identical residues between E6-AP and the other sequences is given on the right side as well as the percentage of identical residues between UBE3D and Ipa1. The cysteine residue important for ubiquitylation in HECT domain E3s is highlighted in cyan, residues that have been mutated in Ipa1 to assay for functionality are marked in bold letters. At, Arabidopsis thaliana; Co, Capsaspora owczarzaki; E3, ubiquitin-protein ligase; HECT, homologous to E6-AP carboxyl terminus; Hs, Homo sapiens; Pp, Polysphondylium pallidum; Pr, Phytophthora ramorum; Rf, Reticulomyxa filosa; Sc: S. cerevisiae; Sr, Salpingoeca rosetta; Tg, Toxoplasma gondii.