Abstract
Background
Monoclonal antibodies blocking the programmed cell death-1 (PD-1) or its ligand (PD-L1) are a group of immune checkpoints inhibitors (ICIs) with proven antitumor efficacy. However, their use is complicated by immune-related adverse events (irAEs), including endocrine adverse events (eAEs).
Purpose
We review the incidence, time to onset and resolution rate of dysthyroidism induced by PD-1/PD-L1 Ab, and the clinical, biological and radiological findings. We aim to discuss the potential mechanisms of PD-1/PD-L1 Ab-induced dysthyroidism, and to propose a management algorithm.
Methods
We performed a literature search of available clinical trials regarding PD-1/PD-L1 Ab in the PubMed database. We selected all English language clinical trials that included at least 100 patients. We also present selected case series or reports, retrospective studies and reviews related to this issue.
Findings
In patients treated with PD-1 Ab, hypothyroidism occurred in 2–10.1% and hyperthyroidism occurred in 0.9–7.8%. When thyroiditis was reported separately, it occurred in 0.34–2.6%. Higher rates were reported when PD-1 Ab were associated with other ICI or chemotherapy. The median time to onset of hyperthyroidism and hypothyroidism after PD-1 Ab initiation was 23–45 days and 2–3.5 months, respectively. Regarding PD-L1 Ab, hypothyroidism occurred in 0–10% and hyperthyroidism in 0.5–2% of treated patients. The average time to onset of dysthyroidism after PD-L1 Ab was variable and ranged from 1 day after treatment initiation to 31 months.
Conclusion
Dysthyroidism occurs in up to 10% of patients treated with PD-1/PD-L1 Ab. Hypothyroidism and reversible destructive thyroiditis are the most frequent endocrine adverse events (eAE) in PD-1/PD-L1 treated patients. Immune and non-immune mechanisms are potentially involved, independently of the presence of thyroid antibodies.
Keywords: thyroid, immunology
Introduction
A paradigm shift in the landscape of cancer treatment is in progress. Immune checkpoint inhibitors (ICIs) have emerged as new arms in the panoply of antitumor therapy. Nowadays, the interest in ICIs, such as programmed cell death-1 antibodies (PD-1 Ab) and its ligand programmed cell death ligand 1 antibodies (PD-L1 Ab), has undoubtedly been proven in the management of several advanced cancers, namely melanoma, non-small cell lung cancers (NSCLC), urothelial cancers, head and neck cancers, among others. While their antitumor action has become an undebatable issue, the emergent immune related adverse effects (irAEs) have become a real challenge in the daily practice not only of oncologists, but also of several other primary care givers and specialists. Other than endocrinologists, primary care doctors and other specialists, such as pneumologists, gastroenterologists, dermatologists, hematologists and neurologists, are concerned by these irAEs. This review addresses the endocrine- and thyroid-related adverse events induced by PD1/PD-L1 Ab. The antitumor effect of PD-1/PD-L1 Ab is not within the scope of this article.
Programmed cell death-1 (PD-1) and programmed cell death ligand 1 and 2 (PD-L1/2) pathways
The PD-1/PD-L1,2 pathway is one of the immune check points (IC), which is a key regulator in the peripheral tolerance. It is exploited by tumor cells to evade the immune response; therefore, it represents a target in the novel era of antitumor immune therapy. In 1992, a team of researchers identified the PD-1 gene as a novel member of the immunoglobulin gene superfamily and explained its role via the PD-protein in the classical type of programmed cell death (1). In 1996, the same team found that the protein (PD-1 or CD279) produced by this gene is a trans-membranous heavily glycosylated receptor of 50–55 kDa, which contains an immune receptor tyrosine-based inhibitory motif in its cytoplasmic tail. PD-1 was found to be present particularly on the surface of stimulated T cells (in the thymus, lymph nodes and spleen), B cells (spleen) and myeloid cells (2). In 1998, Nishimura et al. found that PD-1 is involved in the negative regulation of particular aspects of B cell proliferation and differentiation (3). The same team, based on the development of a characteristic lupus-like proliferative arthritis and glomerulonephritis with predominant IgG3 deposition in PD-1-deficient (PD-1−/−) mice, suggested that PD-1 is involved in the maintenance of peripheral self-tolerance acting as a negative regulator of immune responses (4). In 2000, Freeman et al. described the involvement of a B7 superfamily member (programmed cell death ligand 1/PD-L1 also called B7-H1) to achieve the negative immune regulation of PD-1. PD-L1 is expressed by antigen presenting cells (APC) including stimulated peripheral monocytes, activated dendritic cells, as well as in non-lymphoid tissues such as the heart and lungs (5). Another study showed expression of PD-L1 in the pancreas, brain, spleen and syncytiotrophoblasts in the placenta (6). The equilibrium between this inhibitory signal (PD-1/PD-L1) and a co-stimulatory signal (CD-28 with B7) was suggested to determine, along with other co-signals, the threshold level between immune tolerance and triggering an immune reaction (5). A second PD-1 ligand (PD-L2 also called B7-DC) was identified by Latchman et al. in 2001 (7). Unlike PD-L1, the expression of PD-L2 is more restricted in humans, with differences in its distribution compared to mice, while the expression of PD-L1 has more similarities between mice and humans (6, 7). The role of PD-L2 is to this day, still not fully understood and its potential role as an antitumor target has been less investigated compared to PD-L1 (8). What is known, however, is that it does not behave in the same manner as PD-L1 despite competing with the same affinity to bind PD-1. Blocking PD-1 prevents the binding of both PD-L1 and PD-L2 and enhances T-cell activity and proliferation (9). A recent report has found that overexpression of PD-L2 is associated with poorer prognosis in hepatocellular carcinoma (10) and salivary gland tumors (11). PD-L1 is highly expressed by certain tumor cells in the tumor microenvironment and infiltrative cells, and its expression confers immune resistance to tumors with a poor outcome in certain epithelial cancers. Therefore, blocking the inhibitory interaction between PD-1 and its ligand PD-L1 provides an antitumor effect (12, 13). The PD-1/PD-L1 pathway regulates this response in the effector phase peripherally within tissues and tumors (14). PD-1 antibodies interrupt PD-1 interaction with its ligands PD-L1 and PD-L2, whereas anti-PD-L1 Ab blocks the interaction of PD-L1 with both PD-1 and B7-1 (CD80), which is a member of the B7 family on the APC and has a downregulatory role on the T-cell response (15). These interesting findings led to the development and clinical evaluation of specific monoclonal antibodies against either PD-1 or PD-L1. Figure 1 illustrates immune checkpoint pathways and their inhibitors.
Figure 1.
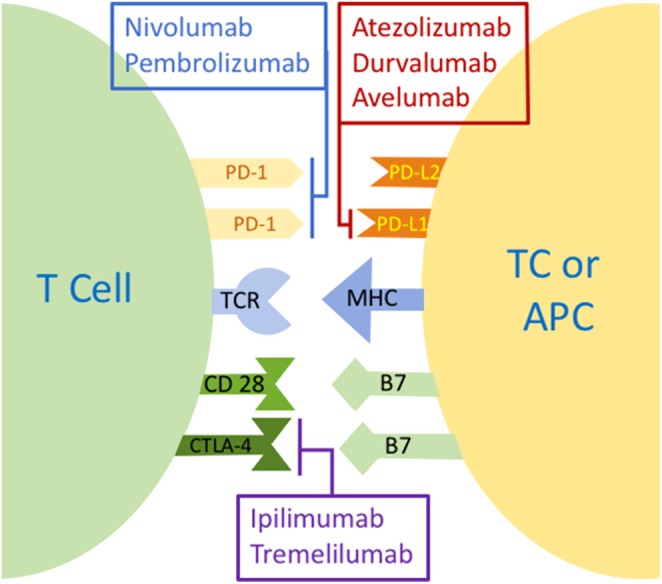
Immune checkpoint pathways and their inhibitors. APC, antigen presenting cell; CD28, cluster of differentiation 28; CTLA-4, cytotoxic T-lymphocyte antigen-4; MHC, major histocompatibility complex; PD-1, programmed cell death; PD-L1, programmed death ligand-1; PD-L2, programmed death ligand-2; TC, tumor cell; TCR, T-cell receptor.
PD-1/PD-L1 antibodies approval and indications
PD-1 antibodies
One of the first studies evaluating safety, pharmacokinetic and clinical activity of nivolumab (or MDX-1106), an anti-PD-1 antibody, showed evidence of antitumor activity by blocking the PD-1 check point in 39 patients with different solid tumors such as advanced metastatic melanoma, colorectal cancer (CRC), renal cell carcinoma (RCC), NSCLC and castration-resistant prostate cancer (13). The role of this human IgG4 monoclonal antibody mAb has been largely proved as an immune checkpoint inhibitor (16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30). In the light of these results, nivolumab was approved by the FDA in 2014 for unresectable or metastatic melanoma and disease progression after treatment with ipilimumab or a BRAF inhibitor. This approval was extended in April 2015 to metastatic squamous NSCLC with progression on or after platinum-based chemotherapy. It is now approved for other cancers such as Hodgkin’s lymphoma and head and neck cancer previously treated locally advanced or metastatic urothelial carcinoma, and in some metastatic CRC that have progressed following usual treatment.
Pembrolizumab is another PD-1 antibody for which the safety and the maximal tolerated dose were assessed in a phase I study including 30 patients with advanced solid tumors, showing a durable antitumor activity with a good tolerance (31). Following this observation, several studies have shown the antitumor beneficial effects of pembrolizumab in advanced melanoma (32, 33, 34, 35), advanced non-small cell lung cancer (36, 37, 38, 39), recurrent or metastatic squamous cell carcinoma of the head and neck (40), advanced Merkel cell carcinoma (41) and PD-L1-positive advanced gastric cancer (42), and as a second-line therapy for advanced urothelial carcinoma (43, 44) and CRC with mismatch-repair deficiency (45). By May 2017, pembrolizumab was approved by the FDA for use in almost all previously cited tumors.
PD-L1 antibodies
Atezolizumab is an engineered high-affinity human monoclonal IgG1 anti-PD-L1 antibody. Its safety and antitumor potential were demonstrated in a study of 171 patients with solid tumors including NSCLC, RCC, melanoma, CRC and gastric cancer (46) and in a population of metastatic RCC (47). Another phase I expansion study has shown the success of this anti-PD-L1 in the treatment of metastatic urothelial bladder cancer (UBC) highly expressing PD-L1. This is especially of interest as atezolizumab has a favorable renal toxicity profile and most of the patients with this type of cancer are elderly and often have renal failure (48). In March 2016, the POPLAR study, an open-label, phase 2 randomized controlled trial which randomized 144 patients with NSCLC whose disease progressed during or following platinum-containing chemotherapy, showed a better overall survival in the atezolizumab-treated group compared to the docetaxel-treated group (12.6 months and 9.7 months respectively), with a clear association between the percentage of PD-L1 expression on infiltration immune cells in the microenvironment of the tumor and the extent of positive response (49). In the same period, another single-arm, multicenter, phase 2 trial has shown in patients with inoperable locally advanced or metastatic urothelial carcinoma whose disease had progressed after previous platinum-based chemotherapy, a better objective response rate (15%) under PD-L1 antibody compared with a historical control overall response rate (10%). Once again, this response was correlated with the expression status of PD-L1 on infiltrating immune cells in the tumor microenvironment (50). In a single-arm, multicenter, phase 2 trial, atezolizumab has shown encouraging response rates, survival and tolerability as a first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma (51). Few other studies have also illustrated the beneficial antitumor effect in PD-L1 selected advanced NSCLC (52, 53, 54). There are also several ongoing studies whose aim is to demonstrate positive effects in extensive-stage SCLC (55), and following adjuvant cisplatin-based chemotherapy in patients with resected stage IB-IIIA NSCLC compared with best supportive care (56). In May 2017, the FDA approved atezolizumab for the treatment of patients with progressive advanced urothelial carcinoma after platinum-containing chemotherapy (57) and in August 17 approved it for treatment of patients with metastatic NSCLC whose disease progressed during or following platinum-containing chemotherapy (58).
The antitumor activity of avelumab, a human IgG1 lambda monoclonal antibody anti-PD-L1, was shown in 2015 (59). This anti-PD-L1 was approved by the FDA in March 2017 for patients with Merkel cell carcinoma (MCC) following the result of a multicenter, single group, open-label phase 2 trial. This study enrolled 88 patients from 35 centers with stage IV chemotherapy-refractory, histologically confirmed MCC (aged ≥18 years), and showed an objective response in 28 (31.8%) of 88 patients, including 8 complete and 20 partial responses, regardless of the PD-L1 tumor expression (60). A more recent study published in 2017 has shown a beneficial antitumor effect with an acceptable safety profile of avelumab in patients with progressive or platinum-resistant metastatic or recurrent NSCLC (61).
Durvalumab (MEDI4736) is a human IgG1 anti-PD-L1 Ab whose safety and antitumor activity have been evaluated in a phase 1b trial in association with tremelimumab, an anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) mAb. This trial included patients with advanced NSCLC, and the tolerance profile of this association was acceptable, with antitumor activity regardless of PD-L1 status (62). This finding has been also found in an early stage of the ARCTIC study in the group of patients treated with durvalumab alone (63). The ongoing ARCTIC study is a phase III randomized, open-label multicenter study comparing durvalumab with or without tremelimumab to the standard of care chemotherapy for previously treated patients with advanced NSCLC (64). On May 2017, the FDA approved durvalumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease has progressed on or after platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. This approval was based on the result of single-arm trial of 182 patients with locally advanced or metastatic urothelial carcinoma with disease progression after prior platinum-containing chemotherapy. The global objective response rate (ORR) was 17.8%, with a higher ORR (27.6%) in highly expressing compared with 5.1% in negative or low expressing PD-L1 tumors (65). Table 1 summarizes the currently available anti-PD-1 and anti-PD-L1.
Table 1.
Current anti-PD1 and anti-PD-L1 antitumor therapy.
| Antibody | Other names | Brand name | Type | Brand |
|---|---|---|---|---|
| PD-1 antibodies | ||||
| Nivolumab | BMS-936558, MDX-1106, ONO-4538 | Opdivo | Fully human IgG4 mAb against PD-1 | Bristol-Myers Squibb |
| Pembrolizumab | MK-3475 lambrolizumab | Keytruda | Humanized IgG4-kappa mAb against PD-1 | Merck |
| PD-L1 Ab | ||||
| Atezolizumab | MPDL3280ARG7446RO5541267 | Tecentriq | Fully humanized, engineered monoclonal antibody of IgG1 isotype anti PDL-L1 | Roche |
| Avelumab | MSB0010718C | Bavencio | Human IgG1 lambda monoclonal antibody against PD-L1 | EMD SeronoPfizer |
| Durvalumab | MEDI4736 | Imfinzi | Engineered human IgG1 monoclonal antibody against PD-L1 | AstraZeneca |
mAb, monoclonal antibody; PD-1, programmed cell death-1; PD-L1, programmed death ligand-1; PD-L2, programmed death ligand-2.
Incidence of dysthyroidism induced by PD-1/PD-L1 antibodies
We performed a literature search of clinical prospective trials using PD-1/PD-L1 Ab, including 100 patients or more published by August 2017 in English. We then compiled the reported thyroid-related eAE in these trials (shown in Tables 3 and 4). The standard reporting system of immune adverse effects used by these studies is the Common Terminology Criteria for Adverse Events V4.0 (CTCAE) (66). Regarding thyroid adverse effects, the CTCAE classifies hyperthyroidism or hypothyroidism in five grades, elucidated in Table 2. Some studies have reported thyroiditis separately of hyperthyroidism and classified it in 5 grades by severity of the adverse event.
Table 3.
Dysthyroidism induced by PD-1 antibodies according to pathology type.
| Study (authors and publication year) | Study phase (or name) | Patient’s number | Pathology | Treatment | Hypothyroidism (%) | Hyperthyroidism (%) | Thyroiditis (%) |
|---|---|---|---|---|---|---|---|
| Robert et al. (2014) (34) | Phase 1 trial | 173 | Advanced melanoma which progressed after at least two ipilimumab doses | i.v. pembrolizumab at 2 mg/kg every 3 weeks or 10 mg/kg every 3 weeks | 4 | 1.7 | NR |
| Robert et al. (2015) (33) | Phase 3 study (KEYNOTE-006) | 834 | Advanced melanoma | 1:1:1 pembrolizumab 10 mg/kg every 2 weeks or every 3 weeks or four doses of ipilimumab 3 mg/kg every 3 weeks | 10.1/8.7/2 | 6.5/3.2/2.3 | NR |
| Garon et al. (2015) (38) | Phase 1 study (KEYNOTE-001) | 495 | Advanced NSCLC | Pembrolizumab 2 mg or 10 mg/kg every 3 weeks or 10 mg/kg every 2 weeks | 6.9 | 1.8 | NR |
| Ribas et al. (2016) (35) | Phase 1b study | 655 | Advanced or metastatic melanoma | Pembrolizumab 10 mg/kg/2 weeks, 10 mg/kg/3 weeks, or 2 mg/kg/3 weeks | 7 | 2 | 1 |
| Langer et al. (2016) (39) | Phase 2 study (KEYNOTE-021) | 123 | Stage IIIB or IV NSCLC without targetable EGFR or ALK genetic aberrations | 4 cycles of pembrolizumab 200 mg plus carboplatin AUC 5 mg/mL/min and pemetrexed 500 mg/m2 every 3 weeks followed by pembrolizumab for 24 months (60 patients) vs the same treatment without pembrolizumab (63 patients) | 15 (pembrolizumab + chemotherapy) | 8 (pembrolizumab + chemotherapy) | NR |
| Reck et al. (2016) (37) | Phase 3 study (KEYNOTE-024) | 305 | Previously untreated advanced NSCLC with PD-L1 expression ≥50% of tumor cells and no sensitizing mutation of the EGFR gene or translocation of the ALK gene | Pembrolizumab 200 mg every 3 weeks (154 patients) or the investigator’s choice of platinum-based chemotherapy (151 patients) | 9.1 | 7.8 | 2.6 |
| Seiwert et al. (2016) (40) | Phase 1b study (KEYNOTE-012) | 104 | Recurrent or metastatic squamous cell carcinoma of the head and neck | Pembrolizumab 10 mg/kg intravenously every 2 weeks | 7 | 2 | NR |
| Bellmunt et al. (2017) (43) | Phase 3 study (KEYNOTE-045) | 542 | Advanced urothelial cancer that recurred or progressed after platinum-based chemotherapy | Pembrolizumab 200 mg every 3 weeks vs the investigator’s choice of chemotherapy with paclitaxel, docetaxel, or vinflunine | 6.4 | 3.8 | 0.8 |
| Topalian et al. (2012) (68) | Phase 1 study | 296 | Advanced melanoma, NSCLC, castration-resistant prostate cancer, or renal cell or colorectal cancer | Nivolumab 0.1–10.0 mg/kg every 2 weeks | 2 | 1 | NR |
| Topalian et al. (2014) (69) | Phase III trials | 107 | Advanced melanoma | Nivolumab i.v. 1, 3, or 10 mg/kg/2 weeks | 5.6 | 1.9 | NR |
| Borghaei et al. (2015) (25) | Phase III trials | 580 | NSCLC that had progressed during or after platinum-based doublet chemotherapy | Nivolumab at a dose of 3 mg/kg/2 weeks (292 patients) or docetaxel at a dose of 75 mg/m2 of body-surface area every 3 weeks (290 patients) | 7 | 1 | 0.34 |
| Larkin et al. (2015) (27) | Phase III trial (CheckMate 067) | 945 | Unresectable stage III or IV melanoma | 1:1:1 nivolumab alone, nivolumab plus ipilimumab, or ipilimumab alone | 8.6/15/4.2 | 4.2/9.9/1 | NR |
| Brahmer et al. (2015) (70) | Phase III trial (CheckMate 017) | 272 | Advanced NSCLC disease progression during or after first-line chemotherapy with limited treatment options | Nivolumab, at a dose of 3 mg/kg/2 weeks (135 patients), or docetaxel, at a dose of 75 mg/m2 of body-surface area every 3 weeks (137 patients) | 4/0 | NR | NR |
| Rizvi et al. (2015) (29) | Phase II trial (CheckMate 063) | 117 | Advanced, refractory, squamous non-small-cell lung cancer | Nivolumab i.v. 3 mg/kg every 2 weeks | 3 | 1 | 1 |
| Motzer et al. (2015) (26) | Phase III trial (CheckMate 025) | 821 | Advanced clear-cell RCC and previous treatment with one or two regimens of antiangiogenic therapy | 1:1 Nivolumab i.v. 3 mg/kg/2 weeks (410 patients) or a 10-mg everolimus tablet orally once daily (411 patients) | NR | NR | NR |
| Weber et al. (2015) (28) | Phase III trial (CheckMate 037) | 405 | Unresectable or metastatic melanoma, and progressed after ipilimumab, or ipilimumab and a BRAF inhibitor if BRAFV600 mutation-positive | 2:1 Nivolumab i.v. 3 mg/kg/2 weeks (272 patients) or ICC (dacarbazine 1000 mg/m2/3 weeks or paclitaxel 175 mg/m2 combined with carboplatin area under the curve 6 every 3 weeks (133 patients) | 5.9/0 | 1.9/0 | NR |
| Ferris et al. (2016) (19) | Phase III trial (CheckMate 141) | 361 | Recurrent SCC of the head and neck with disease progression within 6 months after platinum-based chemotherapy | Nivolumab 3 mg/kg/2 weeks (240 patients) or standard, single-agent systemic therapy (methotrexate, docetaxel, or cetuximab) 121 patients | 3.8/0.9 | 0.8/0 | 0.8/0 |
| Sharma et al. (2017) (16) | Phase II trial (CheckMate 275) | 270 | Metastatic or surgically unresectable locally advanced urothelial carcinoma | Nivolumab 3 mg/kg intravenously every 2 weeks | 8 | NR | NR |
ALK, anaplastic lymphoma kinase; AUC, area under curve; EGFR, epidermal growth factor receptor; ICC, investigator’s choice of chemotherapy; i.v., intravenous; NCSLC, non-small-cell lung cancer; NR, not reported; RCC, renal cell carcinoma; SCC, squamous cell carcinoma.
Table 4.
Dysthyroidism induced by PD-L1 Ab according to pathology type.
| Study (authors and publication year) | Study phase (or name) | Number of patients | Pathology | Treatment | Hypothyroidism (%) | Hyperthyroidism (%) | Thyroiditis (%) |
|---|---|---|---|---|---|---|---|
| Herbst et al. (2014) (71) | Phase 1 | 277 | Multiple types of advanced cancers (melanoma, RCC, NSCLC, CRC, GC and HNSCC, etc.) | Atezolizumab i.v. 0.1–20 mg/kg/3 weeks | NR | NR | NR |
| Fehrenbacher et al. (2016) (49) | Phase II trial (POPLAR) | 277 | Previously treated, advanced or metastatic NSCLC | Atezolizumab i.v. 1200 mg/3 weeks (142 patients) docetaxel 75 mg/m2/3 weeks (135 patients) | 6/0 | NR | NR |
| Rosenberg et al. (2016) (50) | Phase 2 trial | 310 | Inoperable locally advanced or metastatic urothelial carcinoma which progressed after previous platinum-based chemotherapy | Atezolizumab i.v. 1200 mg/3 weeks | NR | NR | NR |
| Peters et al. (2017) (52) | Phase II trial (BIRCH) | 659 | Advanced-stage NSCLC, no CNS metastases, with or without prior chemotherapy | Atezolizumab i.v. 1200 mg/3 weeks first line; n = 139; second line; n = 268; third line or higher n = 252 | 5 | NR | NR |
| Rittmeyer et al. (2017) (54) | Phase III trial (OAK) | 850 | Stage IIIB or IV NSCLC with one to two previous cytotoxic chemotherapy regimens with ≥1 platinum-based combination therapies | 1:1 (425 patients for each group) i.v. atezolizumab 1200 mg or docetaxel 75 mg/m2 every 3 weeks | NR | NR | NR |
| Balar et al. (2017) (51) | Phase 2 trial (IMvigor210) | 119 | Locally advanced or metastatic urothelial cancer which were cisplatin-ineligible | Atezolizumab i.v. 1200 mg/3 weeks | 8 | NR | NR |
| Gulley et al. (2017) (61) | Phase 1b (JAVELIN Solid Tumor) | 184 | Advanced, platinum-treated NSCLC | Avelumab i.v. monotherapy 10 mg/kg every 2 weeks | 6 | NR | NR |
| Antonia et al. (2016) (62) | Phase 1b study | 102 | Locally advanced or metastatic NSCLC | Durvalumab i.v. 3, 10, 15, or 20 mg/kg q4w or 10 mg/kg q2w were combined with tremelimumab 1, 3, or 10 mg/kg q4w for six doses then q12w for three doses | 10 | NR | NR |
| Powles et al. (2017) (65) | Phase 1/2 study | 191 | Locally advanced/metastatic UC with disease progression, or ineligibility for or refusal for prior chemotherapy | Durvalumab i.v. 10 mg/kg q2w | 5.2 | 5.2 | NR |
ALK, anaplastic lymphoma kinase; AUC, area under curve; EGFR, epidermal growth factor receptor; GC, gastric cancer; HNSCC, head and neck squamous cell carcinoma; ICC, investigator’s choice of chemotherapy; i.v., intravenous; NCSLC, non-small-cell lung cancer; NR, not reported; q2w, every 2 weeks; q4w, every 4 weeks; RCC, renal cell carcinoma; SCC, squamous cell carcinoma; UC, urothelial cancer.
Table 2.
Common terminology criteria for adverse events V4.0 (CTCAE) for thyroid adverse events.
| Adverse event | Grade | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Hypothyroidism | Asymptomatic; clinical or diagnostic observations only; intervention not indicated | Symptomatic; thyroid replacement indicated; limiting instrumental ADL | Severe symptoms; limiting self-care ADL; hospitalization indicated | Life-threatening consequences; urgent intervention indicated | Death |
| Hyperthyroidism | Asymptomatic; clinical or diagnostic observations only; intervention not indicated | Symptomatic; thyroid suppression therapy indicated; limiting instrumental ADL | Severe symptoms; limiting self-care ADL; hospitalization indicated | Life-threatening consequences; urgent intervention indicated | Death |
ADL, activities of daily living.
Based on data extracted from Table 3, hypothyroidism induced by pembrolizumab occurred in 4–10.1% of patients when used alone and 15% when pembrolizumab was associated with chemotherapy (Cx). Hyperthyroidism (hyperT) induced by pembrolizumab alone has been reported in 1.7–7.8% of patients and when pembrolizumab was associated with Cx, hyperT occurred in 8% of treated patients. When thyroiditis was reported separately of hyperT, it occurred in 1–2.6% of pembrolizumab-treated patients.
When nivolumab is used alone, it leads to eAEs in 3–13.4% of treated patients, but this percentage increases to 32.6% when it is associated with ipilimumab, a CTLA-4 mAb. Hypothyroidism was found in 2–8.6% of patients treated with nivolumab alone and again in 15% of patients in association with ipilimumab. Hyperthyroidism has been reported in 0.9–4.2% of patients treated with nivolumab alone, and in 9.9% of patients treated concomitantly with nivolumab and ipilimumab. Finally, when thyroiditis was reported separately from hyperthyroidism it occurred in 0.34–1% of patients treated with nivolumab. In one study, no dysthyroidism was reported under treatment with nivolumab in 420 patients with advanced RCC, previously treated with antiangiogenic therapy (26). In a retrospective analysis of 64 patients with advanced NSCLC who had been treated by nivolumab in one center in Japan, the incidence of hypothyroidism was 7.8% (67).
PD-L1-related adverse events are summarized in Table 4. The main type of dysthyroidism in patients treated with atezolizumab is hypothyroidism which is reported to occur between 0 and 8% of cases. There is no reported hyperthyroidism in the existing studies or thyroiditis. Surprisingly, no dysthyroidism was reported in one of the largest studies of atezolizumab in NSCLC, nor any other endocrine adverse effects (54). Avelumab treatment was complicated in 6% of patients by hypothyroidism in 188 patients with previously platinum-treated advanced NSCLC. There was no reported hyperT or thyroiditis (61). In a phase II study of avelumab in 88 patients with chemotherapy-refractory metastatic MCC (not included in Table 4 as the number of patients was less than 100), hypothyroidism occurred in 3% while hyperT in 2% of patients.
Finally, 5.2% patients with urothelial carcinoma (UTC) treated solely with durvalumab developed hyperthyroidism, with the same percentage of patients presenting with hypothyroidism (65). When combined with tremelimumab (CTLA-4 antibody) in patients with advanced NSCLC, durvalumab was complicated in 10% of patients by hypothyroidism, while no patients presented with hyperT (62). In a small safety phase 1/2 study of 61 patients with advanced UBC treated with durvalumab 10 mg/kg every 2 weeks, no dysthyroidism or any other endocrine adverse effects were reported (72).
It is worth noting that the clear majority of PD-1 and PD-L1 induced dysthyroidism was of grade I or II, whereas grades III and IV were very rare and no grade V was reported in all the trials in this review.
Time to onset and resolution of dysthyroidisms after initiation of PD-1/PD-L1 blocking therapy
Table 5 summarizes the time to onset, and when it is available, the rate and time to resolution of hyperT and hypothyroidism induced by PD-1 and PD-L1 Ab based on available data on the FDA drug information site in September 2017. These numbers are based on the major safety trials conducted by the manufacturers. As shown in Table 5, in nivolumab-treated patients, the median time to onset of hyperT and hypothyroidism after nivolumab initiation was 23–45 days and 2–3 months, respectively. In pembrolizumab-treated patients, the median time to onset of hyperT was 1.4 month and for hypothyroidism 3.5 months. Regarding anti-PD-L1 Ab, the median time to onset of hyperT in patients treated with atezolizumab was 3.2 months in UTC and 4.9 months in NSCLC patients, while the median time to onset of hypothyroidism was 5.4 months in UTC and 4.8 months in NSCLC. In patients on treatment with avelumab, median time to onset of thyroid-related iAEs was 2.8 months. Finally, the median time to onset of hypothyroidism and hyperT in patients treated with durvalumab was 42 and 43 days, respectively. In fact, the range of time to the occurrence of a thyroid-related iAE was very large and varied from 1 day after treatment initiation to 31 months, with certain iAE arising even after cessation of treatment (73).
Table 5.
Median time to onset and when available rate and time to recovery of thyroid irAEs induced by PD-1 and PD-L1 Ab.
| PD-1 antibody | Type of irAE | Median time to onset of dysthyroidism | Rate of recovery | Median time to resolution |
|---|---|---|---|---|
| Nivolumab | Hyperthyroidism | 23–45 day (range from 1 day to 14.2 month) | Almost all evolve to eu- or hypothyroidism (may require management with methimazole and corticosteroids) | |
| Nivolumab | Hypothyroidism | 2–3 month (range 1 day–16.6 month) | Frequently stay in hypothyroidism with long-term THR | |
| Pembrolizumab | Hyperthyroidism | 1.4 month (range: 1 day to ~22 month) | Three-fourths of affected patients | 2.1 months (range: 3 day to over 15 month) |
| Pembrolizumab | Hypothyroidism | 3.5 month (range: 1 day to 19 month) | One-fifth of affected patients | (range 2 day to over 27 month) |
| Atezolizumab | Hyperthyroidism | 3.2 month in UTC (range: 1.4–5.8 month)4.9 months in NSCLC (range: 21 days to 31 months) | ||
| Atezolizumab | Hypothyroidism | 5.4 months in UTC (range: 21 day to 11.3 month)4.8 months in NSCLC (range 15 day to 31 month) | ||
| Avelumab | Immune medicated thyroid disorders | 2.8 month (range: 2 week to 13 month) | 7% | Not estimable (range: 6 day to over 26 month) |
| Durvalumab | Hypothyroidism or thyroiditis leading to hypothyroidism | 42 day (range: 15–239 day) | ||
| Durvalumab | Hyperthyroidism or thyroiditis leading to hyperthyroidism | 43 day (range: 14–71 day) |
NSCLC, non-small cell lung carcinoma; THR, thyroid hormone replacement; UTC, urothelial carcinoma.
In a recent retrospective study of 93 patients with advanced cancer treated by pembrolizumab, 13 (14%) patients presented thyroid irAEs, from whom 7 patients developed thyroiditis with recovery in four patients, while the three other patients progressed to hypothyroidism. New onset hypothyroidism was diagnosed in 3 patients and levothyroxine dose doubling was necessary in 3 patients with known hypothyroidism. The median time to onset of thyroid irAEs was 6 weeks after initiation of pembrolizumab, and when recovery to a euthyroid state occurred, the median time was 6.5 weeks after presentation of irAEs (74).
The incidence and characteristics of thyroid disorders were evaluated prospectively in 99 patients with advanced melanoma who were treated with pembrolizumab. 18 thyroid irAEs were reported in 17 patients. Thyrotoxicosis occurred in 12 patients from whom 9 evolved to hypothyroidism. On the other hand, isolated hypothyroidism was reported in 6 patients. 10 of 15 patients with hypothyroidism were initiated on thyroid hormone replacement (THR) (75). The median time to onset of hypothyroidism after starting pembrolizumab was 5.7 weeks (range, 3–40), for thyrotoxicosis progressing to hypothyroidism it was 3.1 weeks (range, 3–21 week), and for isolated thyrotoxicosis it was 8.6 weeks (range, 6–11.1) (75).
Clinical manifestations
Because of the abundance of irAEs and the focus on antitumor effects, the signs and symptoms related to thyroid disorders during PD-1/PD-L1 Ab trials have not been specifically reported. In addition, there is an overlap in symptoms due to advanced cancer and its complications on the one hand, and symptoms of thyroid disorders on the other hand. One of the major adverse events of PD-1/PD-L1 Ab is fatigue which represents an important symptom of dysthyroidism. Weight change is a nonspecific symptom in the setting of advanced cancer. Tachycardia may be provoked by other causes like dehydration or fever as well as perspiration that might be a consequence of the tumor itself or an infectious complication. Hair loss or nails problems are also nonspecific in this case, and finally the psychological state of patients is largely determined by the cancer course. These clinical difficulties render the diagnosis challenging and this should drive clinicians to have a low-suspicion threshold when looking for irAEs. Thyroid irAEs are commonly asymptomatic and nonspecific (76, 77). In the case of hyperthyroidism and thyroiditis, patients might present cervical or throat tenderness or pain, tachycardia, palpitations, sweating and insomnia. Other symptoms also reported are myalgia, fever and abrupt worsening of quality of life (73, 76). In a recently published case report, a 53-year-old woman treated with nivolumab for metastatic squamous cell carcinoma of the lung was admitted to the hospital because of a myxedema crisis, but frequency of TFT control beforehand was not reported in this case report (78).
Laboratory findings
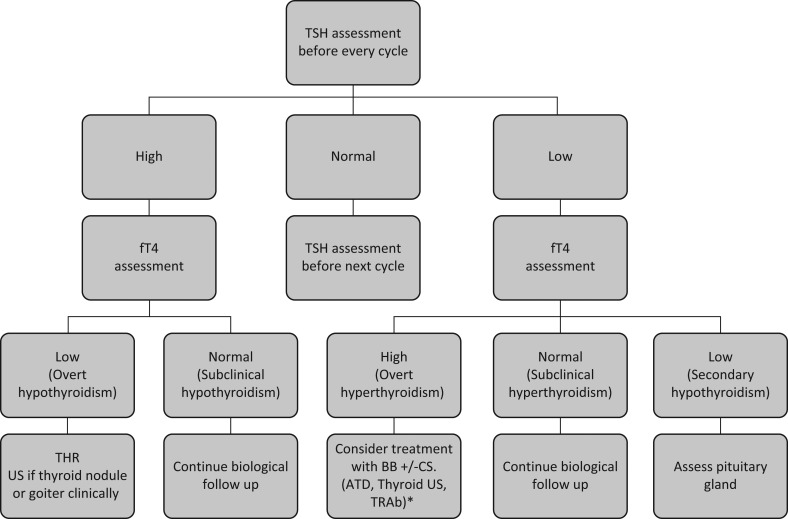
Due to the non-negligible frequency of thyroid irAEs in patients treated with PD-1 and PD-L1 Ab, and the paucity of specific symptoms, manufacturers and health authorities recommend to assess thyroid function at baseline and before every treatment (57, 58, 79, 80). TSH is first assessed and if TSH is <0.5× lower limit of normal or >2× upper limit of normal, free T4 (fT4) is then measured in the same blood sample. Overt hyperT is diagnosed when TSH is low or suppressed and fT4 is higher than the normal range, and subclinical hyperthyroidism is diagnosed in the case of significantly low TSH with a normal fT4. On the other hand, hypothyroidism is defined by a significantly high TSH with fT4 lower than the normal range, whereas fT4 in subclinical hypothyroidism remains in the normal range. In hyperT, measurement of TSH receptor antibodies (TRAb) is useful to exclude Graves’ disease (81), but the utility of thyroperoxidase (TPO) antibodies is debatable in the setting of hypothyroidism induced by ICI (67, 74, 75). Of note, drug interactions such as high-dose corticosteroid, heparin and biotin, should be taken to consideration as they may affect the measurement of TSH and fT4 (82). Finally, a low TSH with low fT4 indicates hypophysitis induced by these antibodies (16, 33, 34, 35, 37, 62). If this arises, the anterior pituitary axis should be fully assessed.
Radiological findings
In general, when hyperT is suspected based on biological results, thyroid ultrasound (US) is useful to identify nodule(s) and to assess thyroid vascularization. In addition, thyroid scintigraphy permits the quantification of radioactive iodine uptake (RAIU) (82). In the case of hypothyroidism, thyroid US is restricted to cases with a clinical suspicion of thyroid nodules, and radioactive iodine uptake is not indicated (83). Currently, there is no specific recommendation regarding performing of thyroid US or scintigraphy in the setting of dysthyroidisms induced by PD-1 and PD-L1 Ab.
F-18 FDG PET/CT shows an increased uptake in patients with Hashimoto thyroiditis (84) and to a lesser degree in Graves’ disease (85). The incidental thyroid findings when using F-18 FDG PET/CT for cancer initial assessment and follow-up have been mentioned in some studies (74, 75). In one of them, 13 out of 93 patients who were treated with pembrolizumab for advanced metastatic melanoma or NSCLC presented thyroid disorders, from which 7 with thyroiditis. While only 2 patients had FDG uptake at baseline, 7 new patients showed an increasing thyroid FDG uptake after initiation of pembrolizumab with a median time to onset of uptake of 12 weeks (74). This finding is in line with results obtained from an earlier study and indicates that PD-1/PD-L1 Ab induce thyroid inflammation (75). Nevertheless, the essential role of F-18 FDG PET/CT remains in the assessment of the oncological situation.
Mechanism and predictive factors of thyroid-related iAE induced by PD-1/PD-L1 antibodies
The mechanism of PD-1/PD-L1 Ab-induced dysthyroidism is not fully understood, and related studies are sparse. It was hypothesized that the presence of anti-thyroid antibodies such as TPO and thyroglobulin (Tg) antibodies is a positive predictive factor for developing hypothyroidism in 5 patients who developed hypothyroidism in a Japanese cohort of 64 patients with advanced NSCLC treated with nivolumab (67). Likewise, Osorio et al., in a prospective study of 51 patients with NSCLC treated with pembrolizumab, showed that thyroid dysfunction was closely associated with thyroid antibodies with 80% of thyroiditis patients (8 out of 10) having thyroid antibodies, suggesting a modulating effect of PD-1 antibodies on humoral immunity (86). Nevertheless, in a case series of 10 patients who had developed ICI-induced (either CTLA-4 Ab of PD-L1 Ab) hypothyroidism after thyroiditis, TPO Ab and thyroid-stimulating immunoglobulin were not consistently present and the authors concluded that the pathogenesis of ICI-induced dysthyroidism involves both immune and non-immune mechanisms (87). In line with this finding, thyroid antibodies were not always found in three other studies, suggesting a bystander role of these antibodies (74, 75, 88). To better understand the mechanism of thyroid destruction by PD-1 antibodies, Delivanis et al., performed a peripheral blood flow cytometry in patients who developed thyroiditis under treatment with pembrolizumab, and compared them with patients with autoimmune thyroiditis. They showed an increasing circulating number of CD56+ CD16+ natural killer (NK) cells and high HLA-DR surface expression in CD14+ CD16+ monocytes that mediate the inflammation in patients treated with anti-PD-1 therapy. This suggests the implication of pathways mediated by T cells, NK cells and/or monocytes, with two potential mechanisms of thyroiditis. First, monocyte’s HLA-DR upregulation leading to their activation, and secondly, PD-1 dependent T-cell mediated destruction of the thyroid. It is noteworthy that only 31% of patients (4 out of 13) with thyroiditis had thyroid antibodies (74). In another recent study, the presence of mRNA and protein expression of PD-L1 and PD-L2 was found in normal thyroid tissue by reverse transcription polymerase chain reaction and Western blotting, and the authors hypothesized that anti-PD-1 decreases the immune tolerance of T cells to normal thyroid tissue and leads to development of thyroiditis (77). A recent study compared the presenting patterns and the dynamic evolution of thyroid disorders in 46 patients treated with either anti-PD-1 alone or in combination with anti-CTLA-4. In this study, the most common initial thyroid irAE was thyrotoxicosis, occurring in 93% and 56% of patients on combination therapy and monotherapy, respectively. Later, 76% and 90% of the patients with thyrotoxicosis in the combination and monotherapy groups, respectively, developed hypothyroidism. The median time of shift from thyrotoxicosis to hypothyroidism was 42 days in the two groups (89). Even though T cells are the main target of immune checkpoints blockade, Das et al draw attention to a role played by B lymphocytes. They showed that patients with advanced melanoma treated by a combined checkpoint blockade (CCB) who developed high-grade irAEs compared to those who did not, had a decreased total peripheral B lymphocyte count with increased plasmablasts and a subset of B lymphocytes called CD21lo PD1+ memory cells (90, 91). These changes occur as early as the first three weeks after treatment initiation. They also showed that a 30% decrease in total B lymphocyte count with a 2-fold increase in CD21lo PD1+ B cell is associated with a higher likelihood of developing high-grade irAEs as compared to patients without B cell changes (91, 92). Another mechanism explaining the irAEs may be derived from the robust broadening of T-cell receptor (TCR) repertoire that was noted within 2 weeks after initiation of ipilimumab in patients who developed irAEs compared to those who did not, with a better antitumor response with increased TCR repertoire, but this remains to be demonstrated with PD-1/PD-L1 antibodies (93). In addition, it is suggested that some epitopes might be shared between the tumor and healthy tissues, leading to the irAE in theses tissues. The frequent incidence of vitiligo in patients with melanoma who are treated by immune checkpoint blockade may support this theory (94). This was also postulated in one of two cases of fulminant myocarditis following a CCB (95). Another mechanism that might underlie the irAEs is the ‘flexibility and plasticity’ of T cells, which briefly means that T cells can switch from an exhausted to activated state, and could also be converted from one subtype to another and thus change their target tissue (93). Finally, the role of cytokines was evoked in a study that found elevated levels of interleukin-17 (IL-17) in patients with ipilimumab-induced colitis; whether IL-17 or other cytokine elevation is involved in dysthyroidism induced by immune checkpoint blockade is not known (96). In the current state of knowledge, we postulate that dysthyroidism in the setting of PD-1/PD-L1 Ab treatment is the result of auto-reactivation of T-cells which become intolerant to thyroid tissue with subsequent thyroid inflammation and destruction with a potential modulating impact on humoral autoimmunity and a possible epitope resemblance between tumor and thyroid tissue. However, further studies should be carried out to better understand the role of thyroid antibodies, determine the predisposing factors and elucidate the reasons why certain complications occur in a group of patients, but not in others. In the light of this hypothesis, PD-1 and PD-L1 may constitute a therapeutic option in thyroid cancer.
Management
Most eAEs could be managed by the treating oncologist, and an endocrinology consultation is mainly reserved for complicated cases. While subclinical hypothyroidism can be followed up biologically and does not require treatment, hypothyroidism induced by PD-1/PD-L1 Ab, although usually low grade (grade 1–2) and does not need antitumor immunotherapy cessation (Table 2), should be managed by oral THR. Generally, the recommended daily dose of levothyroxine in overt hypothyroidism is around 1.2–1.6 microgram per kilogram of body weight (83). However, some authors suggest starting with a dose of 50 µg per day (73). In case of grade 3–4 hypothyroidism, which is rare, transient antitumor treatment withdrawal is recommended and intravenous steroids (such as methylprednisolone) could be considered, followed by oral steroids (such as prednisone) with a dose tapering upon improvement to grade 1, all along with an adequate THR (66, 97). A concurrent adrenal insufficiency should be excluded before starting THR because of the risk of adrenal crisis in the case of masked adrenal insufficiency associated with hypothyroidism.
In patients presenting with grade 1 hyperT, symptomatic treatment with beta-blockers could be considered with frequent surveillance of TFTs, but in grade 2 and more hyperT, it is worth considering a temporary discontinuation of, as well as intravenous followed by oral corticosteroids, along with symptomatic treatment. In case of thyroid hyperactivity as opposed to condition that lead to thyroid tissue destruction, anti-thyroid hormones are indicated, though it is not always easy to distinguish between these two presentations (66, 97). Corticosteroid doses are then tapered upon improvement to grade 1 hyperthyroidism. Subsequent TFTs monitoring is essential because the course of hyperT is not predictable and it could persist, resolve or convert into hypothyroidism. Figure 2 presents a proposed algorithm for the management of PD1/PD-L1 Ab-induced dysthyroidisms.
Figure 2.
Management of dysthyroidism induced by PD-1/PD-L1 antibodies. ATD, anti-thyroid drugs; BB, beta-blocker; CS, corticosteroid; fT4, free thyroxine; TRAb, TSH receptor antibodies; TRH, thyroid hormone replacement; TSH, thyroid-stimulating hormone; US, ultrasound. *If persistence of hyperthyroidism with suspicion of autoimmune underlying cause.
Conclusion
Considering the emergent immune therapy and its wide use in the management of several types of cancer, various patterns of adverse effects have appeared which differ in comparison to those of traditional chemotherapy. PD-1 and PD-L1 Ab have demonstrated a positive antitumor effect in several cancers and are amply employed in the treatment of some advanced cancers such as melanoma, NSCLC, and head and neck and urothelial cancers, among others. Along with a broad irAEs affecting several organs and systems, eAES are a frequent issue and should be known not only by oncologists, but also by primary care doctors and namely endocrinologists who will inevitably be confronted with these eAEs. Dysthyroidism is a frequent eAEs and routine TFTs assessment before such treatment has become the standard of care. While low-grade dysthyroidism can be managed without cessation of antitumor immune therapy, high-grade dysthyroidisms may require transient antitumor immune therapy interruption and a standardized management with a close collaboration between oncologists and endocrinologists. Finally, as the thyroid gland is frequently affected by PD-1/PD-L1 Ab, some iodine refractory thyroid cancers could potentially be managed with these antibodies.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO Journal 1992. 11 3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. International Immunology 1996. 8 765–772. ( 10.1093/intimm/8.5.765) [DOI] [PubMed] [Google Scholar]
- 3.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. International Immunology 1998. 10 1563–1572. ( 10.1093/intimm/10.10.1563) [DOI] [PubMed] [Google Scholar]
- 4.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999. 11 141–151. ( 10.1016/S1074-7613(00)80089-8) [DOI] [PubMed] [Google Scholar]
- 5.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. Journal of Experimental Medicine 2000. 192 1027–1034. ( 10.1084/jem.192.7.1027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. European Journal of Immunology 2003. 33 2706–2716. ( 10.1002/eji.200324228) [DOI] [PubMed] [Google Scholar]
- 7.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nature Immunology 2001. 2 261–268. ( 10.1038/85330) [DOI] [PubMed] [Google Scholar]
- 8.Rozali EN, Hato SV, Robinson BW, Lake RA, Lesterhuis WJ. Programmed death ligand 2 in cancer-induced immune suppression. Clinical and Developmental Immunology 2012. 2012 656340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghiotto M, Gauthier L, Serriari N, Pastor S, Truneh A, Nunès JA, Olive D. PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1. International Immunology 2010. 22 651–660. ( 10.1093/intimm/dxq049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung HI, Jeong D, Ji S, Ahn TS, Bae SH, Chin S, Chung JC, Kim HC, Lee MS, Baek MJ. Overexpression of PD-L1 and PD-L2 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Research and Treatment 2017. 49 246–254. ( 10.4143/crt.2016.066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang H, Kim JS, Choi YJ, Cho J-G, Woo J-S, Kim A, Kim JS, Kang EJ. Overexpression of PD-L2 is associated with shorter relapse-free survival in patients with malignant salivary gland tumors. OncoTargets and Therapy 2017. 10 2983–2992. ( 10.2147/OTT.S134589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. PNAS 2002. 99 12293–12297. ( 10.1073/pnas.192461099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. Journal of Clinical Oncology 2010. 28 3167–3175. ( 10.1200/JCO.2009.26.7609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature Medicine 2002. 8 793–800. ( 10.1038/nm730) [DOI] [PubMed] [Google Scholar]
- 15.Dolan DE, Gupta S. PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control 2014. 21 231–237. ( 10.1177/107327481402100308) [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S, et al Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncology 2017. 18 312–322. ( 10.1016/S1470-2045(17)30065-7) [DOI] [PubMed] [Google Scholar]
- 17.Sharma P, Callahan MK, Bono P, Kim J, Spiliopoulou P, Calvo E, Pillai RN, Ott PA, de Braud F, Morse M, et al Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncology 2016. 17 1590–1598. ( 10.1016/S1470-2045(16)30496-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ, Juergens RA, et al Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncology 2017. 18 31–41. ( 10.1016/S1470-2045(16)30624-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al Nivolumab for recurrent squamous-cell carcinoma of the head and neck. New England Journal of Medicine 2016. 375 1856–1867. ( 10.1056/NEJMoa1602252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncology 2016. 17 1558–1568. ( 10.1016/S1470-2045(16)30366-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, Gerber DE, Shepherd FA, Antonia S, Goldman JW, et al Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. Journal of Clinical Oncology 2016. 34 2980–2987. ( 10.1200/JCO.2016.66.9929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizvi NA, Hellmann MD, Brahmer JR, Juergens RA, Borghaei H, Gettinger S, Chow LQ, Gerber DE, Laurie SA, Goldman JW, et al Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. Journal of Clinical Oncology 2016. 34 2969–2979. ( 10.1200/JCO.2016.66.9861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F, et al Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncology 2016. 17 883–895. ( 10.1016/S1470-2045(16)30098-5) [DOI] [PubMed] [Google Scholar]
- 24.Weber JS, Gibney G, Sullivan RJ, Sosman JA, Slingluff CL, Lawrence DP, Logan TF, Schuchter LM, Nair S, Fecher L, et al Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncology 2016. 17 943–955. ( 10.1016/S1470-2045(16)30126-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. New England Journal of Medicine 2015. 373 1627–1639. ( 10.1056/NEJMoa1507643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, et al Nivolumab versus everolimus in advanced renal-cell carcinoma. New England Journal of Medicine 2015. 373 1803–1813. ( 10.1056/NEJMoa1510665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. New England Journal of Medicine 2015. 373 23–34. ( 10.1056/NEJMoa1504030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Jr, Lao CD, et al Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncology 2015. 16 375–384. ( 10.1016/S1470-2045(15)70076-8) [DOI] [PubMed] [Google Scholar]
- 29.Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, et al Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncology 2015. 16 257–265. ( 10.1016/S1470-2045(15)70054-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al Nivolumab in previously untreated melanoma without BRAF mutation. New England Journal of Medicine 2015. 372 320–330. ( 10.1056/NEJMoa1412082) [DOI] [PubMed] [Google Scholar]
- 31.Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, Drengler R, Chen C, Smith L, Espino G, et al Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clinical Cancer Research 2015. 21 4286–4293. ( 10.1158/1078-0432.CCR-14-2607) [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki N, Takenouchi T, Fujimoto M, Ihn H, Uchi H, Inozume T, Kiyohara Y, Uhara H, Nakagawa K, Furukawa H, et al Phase 1b study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced melanoma (KEYNOTE-041). Cancer Chemotherapy and Pharmacology 2017. 79 651–660. ( 10.1007/s00280-016-3237-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al Pembrolizumab versus ipilimumab in advanced melanoma. New England Journal of Medicine 2015. 372 2521–2532. ( 10.1056/NEJMoa1503093) [DOI] [PubMed] [Google Scholar]
- 34.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et al Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014. 384 1109–1117. ( 10.1016/S0140-6736(14)60958-2) [DOI] [PubMed] [Google Scholar]
- 35.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, Joshua AM, Patnaik A, Hwu WJ, Weber JS, et al Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016. 315 1600–1609. ( 10.1001/jama.2016.4059) [DOI] [PubMed] [Google Scholar]
- 36.Hui R, Garon EB, Goldman JW, Leighl NB, Hellmann MD, Patnaik A, Gandhi L, Eder JP, Ahn MJ, Horn L, et al Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non-small cell lung cancer: a phase 1 trial. Annals of Oncology 2017. 28 874–881. ( 10.1093/annonc/mdx008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. New England Journal of Medicine 2016. 375 1823–1833. ( 10.1056/NEJMoa1606774) [DOI] [PubMed] [Google Scholar]
- 38.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al Pembrolizumab for the treatment of non-small-cell lung cancer. New England Journal of Medicine 2015. 372 2018–2028. ( 10.1056/NEJMoa1501824) [DOI] [PubMed] [Google Scholar]
- 39.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, et al Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncology 2016. 17 1497–1508. ( 10.1016/S1470-2045(16)30498-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, et al Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncology 2016. 17 956–965. ( 10.1016/S1470-2045(16)30066-3) [DOI] [PubMed] [Google Scholar]
- 41.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, et al PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. New England Journal of Medicine 2016. 374 2542–2552. ( 10.1056/NEJMoa1603702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncology 2016. 17 717–726. ( 10.1016/S1470-2045(16)00175-3) [DOI] [PubMed] [Google Scholar]
- 43.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee J-L, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, et al Pembrolizumab as second-line therapy for advanced urothelial carcinoma. New England Journal of Medicine 2017. 376 1015–1026. ( 10.1056/NEJMoa1613683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plimack ER, Bellmunt J, Gupta S, Berger R, Chow LQM, Juco J, Lunceford J, Saraf S, Perini RF, O’Donnell PH. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncology 2017. 18 212–220. ( 10.1016/S1470-2045(17)30007-4) [DOI] [PubMed] [Google Scholar]
- 45.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al PD-1 blockade in tumors with mismatch-repair deficiency. New England Journal of Medicine 2015. 372 2509–2520. ( 10.1056/NEJMoa1500596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herbst RS, Gordon MS, Fine GD, Sosman JA, Soria J-C, Hamid O,, Powderly JD, Burris HA, Mokatrin A, Kowanetz M, et al A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. Journal of Clinical Oncology 2013. 31 (Supplement 15) 3000. [Google Scholar]
- 47.McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, Powderly JD, Infante JR, Fassò M, Wang YV, et al Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. Journal of Clinical Oncology 2016. 34 833–842. ( 10.1200/JCO.2015.63.7421) [DOI] [PubMed] [Google Scholar]
- 48.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et al MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014. 515 558–562. ( 10.1038/nature13904) [DOI] [PubMed] [Google Scholar]
- 49.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, et al Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016. 387 1837–1846. ( 10.1016/S0140-6736(16)00587-0) [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y, et al Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016. 387 1909–1920. ( 10.1016/S0140-6736(16)00561-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, et al Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017. 389 67–76. ( 10.1016/S0140-6736(16)32455-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peters S, Gettinger S, Johnson ML, Jänne PA, Garassino MC, Christoph D, Toh CK, Rizvi NA, Chaft JE, Carcereny Costa E, et al Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). Journal of Clinical Oncology 2017. 35 2781–2789. ( 10.1200/JCO.2016.71.9476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wakelee H, Patel JD, Heist R, Balmanoukian A, Besse B, Felip E, Carcereny Costa E, Chow LQ, Koczywas M, Garassino MC, et al ORAL01.04: phase II trial of atezolizumab for patients with PD-L1-selected advanced NSCLC (BIRCH): updated efficacy and exploratory biomarker results: topic: medical oncology. Journal of Thoracic Oncology 2016. 11 S251–S252. ( 10.1016/j.jtho.2016.09.009)27969443 [DOI] [Google Scholar]
- 54.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017. 389 255–265. ( 10.1016/S0140-6736(16)32517-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horn L, Reck M, Mok T, Johnson ML, Tang X, Lam S, Waterkamp D, Lopez-Chavez A, Sandler A, Giacconne G, et al PS01.57: IMpower133: a phase I/III study of 1L atezolizumab with carboplatin and etoposide in patients with extensive-stage SCLC: topic: medical oncology. Journal of Thoracic Oncology 2016. 11 S305–S306. ( 10.1016/j.jtho.2016.09.092)27969524 [DOI] [Google Scholar]
- 56.Vallieres E, Felip E, Altorki N, Zhou C, Zuo Y, Howland M, Xia F, Hoang T, Sandler A, Wakelee H. PS01.55: IMpower010: phase III study of atezolizumab vs BSC after adjuvant chemotherapy in patients with completely resected NSCLC: topic: medical oncology. Journal of Thoracic Oncology 2016. 11 S304 ( 10.1016/j.jtho.2016.09.090) [DOI] [Google Scholar]
- 57.Ning Y-M, Suzman D, Maher VE, Zhang L, Tang S, Ricks T, Palmby T, Fu W, Liu Q, Goldberg KB, et al FDA Approval Summary: atezolizumab for the treatment of patients with progressive advanced urothelial carcinoma after platinum-containing chemotherapy. Oncologist 2017. 22 743–749. ( 10.1634/theoncologist.2017-0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinstock C, Khozin S, Suzman D, Zhang L, Tang S, Wahby S, Goldberg KB, Kim G, Pazdur R. U.S. Food and Drug Administration Approval Summary: atezolizumab for metastatic non-small cell lung cancer. Clinical Cancer Research 2017. 23 4534–4539. ( 10.1158/1078-0432.CCR-17-0540) [DOI] [PubMed] [Google Scholar]
- 59.Boyerinas B, Jochems C, Fantini M, Heery CR, Gulley JL, Tsang KY, Schlom J. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunology Research 2015. 3 1148–1157. ( 10.1158/2326-6066.CIR-15-0059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, Shih KC, Lebbé C, Linette GP, Milella M, et al Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncology 2016. 17 1374–1385. ( 10.1016/S1470-2045(16)30364-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gulley JL, Rajan A, Spigel DR, Iannotti N, Chandler J, Wong DJL, Leach J, Edenfield WJ, Wang D, Grote HJ, et al Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncology 2017. 18 599–610. ( 10.1016/S1470-2045(17)30240-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, Narwal R, Steele K, Gu Y, Karakunnel JJ, et al Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncology 2016. 17 299–308. ( 10.1016/S1470-2045(15)00544-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rizvi NA Brahmer JR Ou S-HI Segal NH Khleif S Hwu W-J, Gutierrez M Schoffski P Hamid O Weiss J, et al Safety and clinical activity of MEDI4736, an anti-programmed cell death-ligand 1 (PD-L1) antibody, in patients with non-small cell lung cancer (NSCLC). Journal of Clinical Oncology 2015. 33 (Supplement 15) 8032. [Google Scholar]
- 64.Planchard D, Yokoi T, McCleod MJ, Fischer JR, Kim Y-C, Ballas M, Shi K, Soria JC. A phase III study of durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced NSCLC: rationale and protocol design of the ARCTIC study. Clinical Lung Cancer 2016. 17 232.e1–236.e1. ( 10.1016/j.cllc.2016.03.003) [DOI] [PubMed] [Google Scholar]
- 65.Powles T, O’Donnell PH, Massard C, Arkenau H-T, Friedlander TW, Hoimes CJ, Lee JL, Ong M, Sridhar SS, Vogelzang NJ, et al Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncology 2017. 3 e172411 ( 10.1001/jamaoncol.2017.2411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cancer Therapy Evaluation Program. CTCAE v4.0 Terms with Lay Terms. Bethesda, MD, USA: NIH, 2014. (available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_4_with_lay_terms.pdf) [Google Scholar]
- 67.Maekura T, Naito M, Tahara M, Ikegami N, Kimura Y, Sonobe S, Kobayashi T, Tsuji T, Minomo S, Tamiya A, et al Predictive factors of nivolumab-induced hypothyroidism in patients with non-small cell lung cancer. In Vivo 2017. 31 1035–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New England Journal of Medicine 2012. 366 2443–2454. ( 10.1056/NEJMoa1200690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. Journal of Clinical Oncology 2014. 32 1020–1030. ( 10.1200/JCO.2013.53.0105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. New England Journal of Medicine 2015. 373 123–135. ( 10.1056/NEJMoa1504627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014. 515 563–567. ( 10.1038/nature14011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, Curiel TJ, Colon-Otero G, Hamid O, Sanborn RE, et al Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. Journal of Clinical Oncology 2016. 34 3119–3125. ( 10.1200/JCO.2016.67.9761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sznol M, Postow MA, Davies MJ, Pavlick AC, Plimack ER, Shaheen M, Veloski C, Robert C. Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treatment Reviews 2017. 58 70–76. ( 10.1016/j.ctrv.2017.06.002) [DOI] [PubMed] [Google Scholar]
- 74.Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S, Dietz AB, Ryder M. Pembrolizumab-induced thyroiditis: comprehensive clinical review and insights into underlying involved mechanisms. Journal of Clinical Endocrinology and Metabolism 2017. 102 2770–2780. ( 10.1210/jc.2017-00448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, Bravenboer B. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. Journal of Clinical Endocrinology and Metabolism 2016. 101 4431–4439. ( 10.1210/jc.2016-2300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanaka R, Fujisawa Y, Maruyama H, Nakamura Y, Yoshino K, Ohtsuka M, Fujimoto M. Nivolumab-induced thyroid dysfunction. Journal of Clinical Oncology 2016. 46 575–579. ( 10.1093/jjco/hyw036) [DOI] [PubMed] [Google Scholar]
- 77.Yamauchi I, Sakane Y, Fukuda Y, Fujii T, Taura D, Hirata M, Hirota K, Ueda Y, Kanai Y, Yamashita Y, et al Clinical features of nivolumab-induced thyroiditis: a case series study. Thyroid 2017. 27 894–901. ( 10.1089/thy.2016.0562) [DOI] [PubMed] [Google Scholar]
- 78.Khan U, Rizvi H, Sano D, Chiu J, Hadid T. Nivolumab induced myxedema crisis. Journal for Immunotherapy of Cancer 2017. 5 13 ( 10.1186/s40425-017-0213-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merck Sharp, Dohme Corp. Keytruda Prescribing Information. Whitehouse Station, NJ, USA: Merck & Co. Inc, 2017. (available at: https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf) [Google Scholar]
- 80.Bristol-Myers Squibb. Opdivo Prescribing Information. New York, NY, USA: Bristol-Myers Squibb, 2018. (available at: https://packageinserts.bms.com/pi/pi_opdivo.pdf) [Google Scholar]
- 81.De Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet 2016. 388 906–918. ( 10.1016/S0140-6736(16)00278-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, et al 2016 American Thyroid Association Guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016. 26 1343–1421. ( 10.1089/thy.2016.0229) [DOI] [PubMed] [Google Scholar]
- 83.Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA. & American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocrine Practice 2012. 18 988–1028. ( 10.4158/EP12280.GL) [DOI] [PubMed] [Google Scholar]
- 84.Karantanis D, Bogsrud TV, Wiseman GA, Mullan BP, Subramaniam RM, Nathan MA, Peller PJ, Bahn RS, Lowe VJ. Clinical significance of diffusely increased 18F-FDG uptake in the thyroid gland. Journal of Nuclear Medicine 2007. 48 896–901. ( 10.2967/jnumed.106.039024) [DOI] [PubMed] [Google Scholar]
- 85.Chen Y-K, Chen Y-L, Cheng R-H, Yeh C-L, Lee C-C, Hsu C-H. The significance of FDG uptake in bilateral thyroid glands. Nuclear Medicine Communications 2007. 28 117–122. ( 10.1097/MNM.0b013e328013eaf7) [DOI] [PubMed] [Google Scholar]
- 86.Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok JD, et al Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Annals of Oncology 2017. 28 583–589. ( 10.1093/annonc/mdw640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alhusseini M, Samantray J. Hypothyroidism in cancer patients on immune checkpoint inhibitors with anti-PD1 agents: insights on underlying mechanisms. Experimental and Clinical Endocrinology and Diabetes 2017. 125 267–269. ( 10.1055/s-0042-119528) [DOI] [PubMed] [Google Scholar]
- 88.Orlov S, Salari F, Kashat L, Walfish PG. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. Journal of Clinical Endocrinology and Metabolism 2015. 100 1738–1741. ( 10.1210/jc.2014-4560) [DOI] [PubMed] [Google Scholar]
- 89.Lee H, Hodi FS, Giobbie-Hurder A, Ott PA, Buchbinder EI, Haq R, Tolaney S, Barroso-Sousa R, Zhang K, Donahue H, et al Characterization of thyroid disorders in patients receiving immune checkpoint inhibition therapy. Cancer Immunology Research 2017. 5 1133–1140. ( 10.1158/2326-6066.CIR-17-0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liudahl SM, Coussens LM. B cells as biomarkers: predicting immune checkpoint therapy adverse events. Journal of Clinical Investigation 2018. 128 577–579. ( 10.1172/JCI99036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R, Dhodapkar MV, et al Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. Journal of Immunology 2015. 194 950–959. ( 10.4049/jimmunol.1401686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Das R, Bar N, Ferreira M, Newman AM, Zhang L, Bailur JK, Bacchiocchi A, Kluger H, Wei W, Halaban R, et al Early B cell changes predict autoimmunity following combination immune checkpoint blockade. Journal of Clinical Investigation 2018. 128 715–720. ( 10.1172/JCI96798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nature Medicine 2017. 23 540–547. ( 10.1038/nm.4321) [DOI] [PubMed] [Google Scholar]
- 94.Byrne EH, Fisher DE. Immune and molecular correlates in melanoma treated with immune checkpoint blockade. Cancer 2017. 123 2143–2153. ( 10.1002/cncr.30444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL,et al Fulminant myocarditis with combination immune checkpoint blockade. New England Journal of Medicine 2016. 375 1749–1755. ( 10.1056/NEJMoa1609214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Callahan MK, Yang A, Tandon S, Xu Y, Subudhi SK, Roman RA, Heine AI, Pogoriler E, Kuk D, Panageas K, et al Evaluation of serum IL-17 levels during ipilimumab therapy: correlation with colitis. Journal of Clinical Oncology 2011. 29 (Supplement 15) 2505 ( 10.1200/jco.2011.29.15_suppl.2505) [DOI] [Google Scholar]
- 97.Linardou H, Gogas H. Toxicity management of immunotherapy for patients with metastatic melanoma. Annals of Translational Medicine 2016. 4272 ( 10.21037/atm.2016.07.10) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a