Abstract
Protein import into chloroplasts is an energy-requiring process mediated by a proteinaceous import apparatus. Although previous work has shown that low levels of ATP or GTP can support precursor binding, the role of GTP during the import process remains unclear. Specifically, it is unknown whether GTP plays a separate role from ATP during the early stages of protein import and whether GTP has any role in the later stages of transport. We investigated the role of GTP during the various stages of protein import into chloroplasts by using purified GTP analogs and an in vitro import assay. GTP, GDP, the nonhydrolyzable analog GMP-PNP, and the slowly hydrolyzable analogs guanosine 5′-O-(2-thiodiphosphate) and guanosine 5′-O-(3-thiotriphosphate) were used in this study. Chromatographically purified 5′-guanylyl-imido-diphosphate and guanosine 5′-O-(3-thiotriphosphate) were found to inhibit the formation of early-import intermediates, even in the presence of ATP. We also observed that GTP does not play a role during the translocation of precursors from the intermediate state. We conclude that GTP hydrolysis influences events leading to the formation of early-import intermediates, but not subsequent steps such as precursor translocation.
Most chloroplastic proteins are nuclear encoded, synthesized on cytosolic ribosomes, and imported posttranslationally across the two membranes of the chloroplastic envelope. An N-terminal extension called the transit peptide targets these precursor proteins to chloroplasts. Protein import into chloroplasts is mediated by a proteinaceous translocation apparatus that spans both the outer and inner envelope membranes. The exact functions of the proteins that comprise this apparatus have yet to be determined conclusively (for review, see Schnell, 1998; Keegstra and Cline, 1999).
The import of precursors into chloroplasts can be divided into three distinct stages. In the first, precursor proteins interact with chloroplasts in an energy-independent and readily reversible manner. It is thought that precursors initially interact with outer membrane lipids (van't Hof and de Kruijff, 1995; Bruce, 1998) and subsequently bind to import receptors (Perry and Keegstra, 1994; Ma et al., 1996). Cross-linking experiments have revealed that the initial interaction of the transit peptide with the import receptor does not require energy (Perry and Keegstra, 1994; Ma et al., 1996).
In the second stage, precursor proteins interact with the translocation apparatus in an irreversible manner. Olsen et al. (1989) showed that a low level of ATP (less than 100 μm) is required for this step; GTP can support some binding but it cannot substitute for ATP (Olsen et al., 1989; Olsen and Keegstra, 1992). The hydrolysis of ATP during this stage promotes the insertion of precursor protein into the protein-conducting complex of both the outer and inner membranes, which has recently been defined as the formation of an “early-import intermediate” (Ma et al., 1996; Nielsen et al., 1997). Although the precursor has partially inserted into the import apparatus, it remains susceptible to degradation by exogenously added proteases.
The third stage of import requires higher levels of stromal ATP (1–3 mm) for complete translocation of precursors across the envelope membranes (Pain and Blobel, 1987; Theg et al., 1989). During or after translocation, the transit peptide is removed by the stromal processing peptidase (Chua and Schmidt, 1978; Highfield and Ellis, 1978; Oblong and Lamppa, 1992; VanderVere et al., 1995; Richter and Lamppa, 1998). Newly imported proteins are then folded or further directed to the thylakoid membrane. A membrane potential across the envelope membrane is not required for protein transport into chloroplasts (Theg et al., 1989), distinguishing this process from mitochondrial protein import, which does require a membrane potential (Pfanner and Neupert, 1986).
The association of precursors with the chloroplastic translocation apparatus is postulated to involve a trimeric complex composed of Toc75, Toc86, and Toc34. The exact function of each component of the import apparatus has yet to be conclusively determined. Toc75 has been proposed to function as a component of the protein-conducting channel for the outer envelope membrane (Schnell et al., 1994; Tranel et al., 1995; Hinnah et al., 1997). Interest in the role of GTP in the import process has increased since the discovery that two components of the import apparatus, Toc34 and Toc86, are GTP-binding proteins (Hirsch et al., 1994; Kessler et al., 1994). Toc34 and Toc86 have sequence similarity to each other and are both integral membrane proteins with their GTP-binding domains exposed to the cytosol (Hirsch et al., 1994; Kessler et al., 1994; Seedorf et al., 1995). Based on cross-linking experiments, Toc86 has been proposed to function as a receptor for precursor binding (Perry and Keegstra, 1994; Ma et al., 1996); the function for Toc34 is less clear. Recently, however, Kouranov and Schnell (1997) proposed that Toc34 might regulate the transition from energy-independent binding of precursor protein to a later stage in import through a cycle of GTP binding and hydrolysis. These results and others raise many interesting questions as to the role of GTP during the formation of early-import intermediates and the translocation stage of import.
We investigated the role of GTP during the second and third stages of protein import into chloroplasts using GTP analogs and an in vitro import assay. This investigation concentrated on two main questions: First, does GTP have a separate role from ATP during the import process? Second, at what stage of import does GTP have a role? Specifically, we wanted to determine whether GTP acts before the formation of early-import intermediates or afterward. We show that GTP has a separate role that is distinct from the ATP requirement during the formation of early-import intermediates. We postulate that the GTP requirement for the generation of early-import intermediates is mediated by the GTP-binding proteins Toc34 and Toc86. We also observed that GTP does not play a role during the translocation of precursors from the intermediate state. We conclude from these results that once the import apparatus has formed early-import intermediates, ATP hydrolysis rather than GTP hydrolysis mediates the translocation step of import. Therefore, GTP hydrolysis influences the formation of early-import intermediates and not the translocation stage of import.
MATERIALS AND METHODS
Materials
Percoll, GTP, GDP, and guanosine 5′-O-(2-thiodiphosphate) (GDP-βS) were obtained from Sigma. Guanosine 5′-O-(3-thiotriphosphate) (GTP-γS) and 5′-guanylyl-imidodiphosphate (GMP-PNP) were obtained from Calbiochem. [35S]Met was purchased from DuPont/NEN. Pea (Pisum sativum var Little Marvel) seeds were supplied by the Olds Seed Company (Madison, WI).
Isolation of Chloroplasts
Intact chloroplasts were isolated from 8- to 12-d-old pea seedlings and purified over a Percoll gradient as previously described (Bruce et al., 1994). Intact chloroplasts were re-isolated and suspended in import buffer (330 mm sorbitol, 50 mm HEPES/KOH, pH 8.0) at a concentration of 1 mg chlorophyll/mL, and stored in the dark on ice prior to their use in binding and translocation experiments.
In Vitro Translation of Precursor Protein
The plasmid containing prSS (Olsen and Keegstra, 1992) was linearized with PstI, transcribed with SP6 RNA polymerase, and translated using a wheat germ system and [35S]Met, as previously described (Bruce et al., 1994). After translation, residual nucleotides were removed by gel filtration as previously described (Olsen et al., 1989).
HPLC Analysis of Nucleotides
Nucleotides were prepared as 100 mm stocks and stored at −80°C in small aliquots to minimize freeze/thaw cycles. HPLC was performed under conditions adapted from a procedure developed for the purification of ATP and ATP analogs (Horst et al., 1996). Nucleotides were purified on a phenyl ether anion-exchange column (POROS-PE, PerSeptive Biosystems, Framingham, MA) equilibrated with 0.1 mm NH4HCO3, and were eluted by a linear gradient of NH4HCO3 from 0.1 to 0.5 mm. For recovery of purified samples, appropriate fractions were pooled and precipitated by adding 0.25 volume of 7.0 m NH4Oac, followed by 11 volumes of cold ethanol. These samples were incubated at −20°C overnight and the precipitated material was recovered by centrifugation at 10,000g for 1 h at 4°C in a rotor (model HB-6, Sorvall). The pellets were rinsed once with cold ethanol and dried under vacuum for 5 min before being resuspended in import buffer. Recovery was quantitated as the A253. The purification of nucleotides removed most of the contaminant visible by chromatography, yielding products with an apparent purity of >95% (data not shown).
Formation of Early-Import Intermediates and Translocation Reactions
To reduce the endogenous levels of nucleotides present in our assay, the following steps were taken. First, to remove ATP and GTP from our wheat germ translation system, precursor proteins were subjected to gel filtration (Olsen et al., 1989). Second, chloroplasts were depleted of endogenous levels of ATP by incubation with the ionophore nigericin (described below). Third, before their addition to assays for early-import intermediate formation and translocation, all GTP analogs were purified by anion-exchange chromatography (Horst et al., 1996; data not shown). With these precautions, the effect of GTP on the second and third stages of import could then be studied with minimal interference from the presence of contaminating endogenous nucleotides.
Early-import intermediate formation and translocation assays were performed as follows: Prior to assays for early-intermediate formation or translocation, the chloroplasts were incubated with 6 μm nigericin for 10 min in the dark to deplete internal ATP levels. Each intermediate formation or import reaction (adapted from Bruce et al., 1994) received 500,000 dpm of [35S]prSS and intact chloroplasts corresponding to 25 μg of chlorophyll in a final volume of 150 μL. All nucleotides were added as either magnesium salts or equimolar magnesium acetate. ATP-depleted chloroplasts were incubated for 5 min with a 1.0 mm GTP analog prior to the addition of either 0.1 mm ATP for binding or 1 mm ATP for translocation. Early-import intermediate formation and translocation reactions were incubated in the dark for an additional 30 min at room temperature. Intact chloroplasts were then recovered by sedimentation through a 40% (v/v) Percoll cushion. The pellets were solubilized in 2× SDS-PAGE sample buffer. All fractions were analyzed by SDS-PAGE (Laemmli, 1970) and fluorography.
Translocation of Precursors Already Present as Intermediates
For translocation assays, chloroplasts were incubated with 6 μm nigericin for 10 min in the dark to deplete internal ATP levels. Early-import intermediates were generated as follows: Large-scale reactions containing 3.5 × 106 dpm of [35S]prSS, intact chloroplasts corresponding to 175 μg of chlorophyll and 0.1 mm MgATP (final concentration) in a final volume of 1050 μL were incubated in the dark for 10 min at room temperature. Intact chloroplasts containing early-import intermediates were recovered by sedimentation through a 40% (v/v) Percoll cushion. The pellet was resuspended in import buffer and centrifuged again for 5 min. This pellet was finally resuspended in import buffer and used for translocation reactions. After a 5-min dark incubation with a GTP analog and equimolar magnesium acetate, sufficient ATP (1.0 mm final concentration) was added to initiate translocation. At the times indicated, 150-μL aliquots were removed and import was quenched using HgCl2 (Reed et al., 1990). Variations in this basic protocol are described in the figure legends. Samples were analyzed by SDS-PAGE and fluorography. The extent of translocation was quantitated using a phosphor imager (model 400B, Molecular Dynamics).
RESULTS
GTP Has a Separate Role That Is Distinct from the ATP Requirement during the Formation of Import Intermediates
The formation of early-import intermediates requires low levels of ATP (less than 100 μm). Although GTP can support this process to a limited extent, it cannot substitute for ATP (Olsen et al., 1989; Olsen and Keegstra, 1992). To further investigate the GTP requirements for both early-import intermediate formation and translocation, the current investigation focused on the following questions: (a) at what stage of import is GTP required?, and (b) does GTP play a separate role from ATP during the import process?
To examine the influence of GTP on import, special attention was paid to the order of addition of the GTP analog relative to ATP. This is an important feature of our import protocol. By providing a GTP incubation time prior to ATP addition, GTP was given an opportunity to interact with the import apparatus before ATP. Therefore, the effect of GTP at various stages of import relative to ATP could be observed. This experimental design is significantly different from previous studies examining the nucleotide requirement for protein import into chloroplasts (Olsen et al., 1989; Theg et al., 1989; Olsen and Keegstra, 1992; Kessler et al., 1994). With this consideration in mind, GTP analog studies were designed as follows: Nigericin-treated chloroplasts were incubated with 1 mm GTP analog for 5 min before the addition of sufficient ATP to support formation of early-import intermediates (100 μm) or to support the entire import sequence (1 mm) (see Figs. 1 and 2).
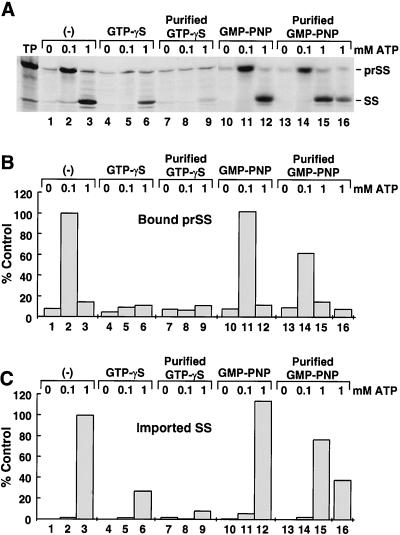
Figure 1.
GTP has a separate role that is distinct from the ATP requirement during the generation of early-import intermediates. Chloroplasts were depleted of endogenous levels of ATP by incubation with nigericin for 10 min in the dark. They were then incubated with a 1 mm concentration of GTP analog with equimolar Mg2+ for 5 min (except for the experiment shown in lane 16, in which a 5 mm analog concentration was used). Radiolabeled prSS and 0, 0.1, or 1.0 mm MgATP was added and incubated for 30 min. Samples were analyzed by SDS-PAGE and fluorography (A) and were quantitated with a phosphor imager (B and C). The control for quantitation of intermediate formation was lane 2 of A (prSS bound in the presence of 0.1 mm ATP), whereas the control for translocation was lane 3 of A (prSS translocated in the presence of 1 mm ATP). TP, Translated product (10%) added to a single reaction. Results shown are from one of three separate experiments.
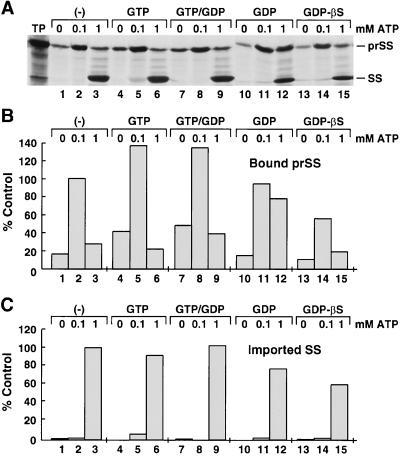
Figure 2.
Early-import intermediate formation is reduced when GTP/GDP exchange is inhibited. Chloroplasts were prepared as described in Figure 1. Chloroplasts were then incubated with a 1 mm analog concentration with equimolar Mg2+ for 5 min. Radiolabeled prSS and 0, 0.1, or 1.0 mm MgATP was added and incubated for 30 min. For the GTP/GDP competition studies (lanes 7–9), 500 μm GTP and GDP were added to chloroplasts for 5 min before the addition of MgATP. Samples were analyzed by SDS-PAGE and fluorography (A) and were quantitated with a phosphor imager (B and C). The control for quantitation of intermediate formation was lane 2 of A (prSS bound in the presence of 0.1 mm ATP), whereas the control for translocation was lane 3 of A (prSS translocated in the presence of 1 mm ATP). TP, Translated product (10%) added to a single reaction. Results shown are from one of three separate experiments.
The discovery that two components of the import machinery, Toc34 and Toc86, are specific GTP-binding proteins (Hirsch et al., 1994; Kessler et al., 1994; Seedorf et al., 1995) suggests that GTP performs a different role than ATP during import. To examine this role, GTP analogs were utilized, because they can be used to block the cycling of GTP-binding proteins. Initially, the GTP analogs GTP-γS and GMP-PNP were used to investigate the role of GTP on the formation of early-import intermediates. The results presented in Figure 1 demonstrate that the nonhydrolyzable analog GMP-PNP and the slowly hydrolyzable analog GTP-γS inhibited the formation of early-import intermediates. In agreement with earlier results, generation of early-import intermediates requires low levels of ATP (Fig. 1, lane 2), whereas translocation requires higher levels (Fig. 1, lane 3) (Olsen and Keegstra, 1992).
To examine whether GTP could effect early-import intermediate formation prior to ATP addition, chloroplasts were incubated with GTP-γS for 5 min before the addition of precursor and 100 μm ATP. GTP-γS dramatically reduced the level of early-import intermediates formed (Fig. 1A, compare lanes 2 and 5). Quantitation of the inhibitory effect of GTP-γS showed that the amount of intermediate bound was reduced by 90% (Fig. 1B, compare lanes 2 and 5) compared with control. Additional purification of GTP-γS neither enhanced nor diminished its inhibitory effect on intermediate formation (Fig. 1, A and B, compare lane 2 with lanes 5 and 8). Even the addition of levels of ATP that support translocation (1 mm) could not overcome the inhibitory effect of GTP-γS pretreatment (Fig. 1, A and C, compare lane 3 with lanes 6 and 9). However, when assays were performed with the nonhydrolyzable analog GMP-PNP, the generation of early-import intermediates was not significantly reduced (Fig. 1, A and B, compare lanes 2 and 11). This result was indeed surprising, since it was predicted that GMP-PNP, like GTP-γS, should behave in a similar fashion (Olsen and Keegstra, 1992).
We suspected that this variability might be related to the purity of the commercially available GMP-PNP used in our studies. Therefore, GMP-PNP was purified by anion-exchange chromatography (data not shown). Purified GMP-PNP was incubated with chloroplasts prior to the addition of ATP, and reduced but did not completely abolish the formation of early-import intermediates (Fig. 1A, compare lane 2 with 14). Quantitation revealed that purified GMP-PNP reduced early-import intermediate formation by 40% compared with control (Fig. 1B, compare lane 2 with 14). However, when chloroplasts were incubated with 5 mm purified GMP-PNP (Fig. 1, lane 16) before the addition of 1 mm ATP, translocation levels of precursor were reduced significantly (Fig. 1, A and C, compare lanes 12, 15, and 16). We conclude from these studies that GTP has a role that is distinct from the ATP requirement for the formation of early-import intermediates.
Could the reduced amount of early-import intermediates associated with chloroplastic envelope simply have been the result of competition between GTP and ATP for the same nucleotide-binding site? Based on the following observations, we conclude that this was not the case. Figure 2 shows that 100 μm ATP alone supported early-import intermediate formation (Fig. 2A, lane 2). Likewise, high levels of GTP (1 mm) alone also supported early-import intermediate formation, but to a significantly lesser degree compared with 100 μm ATP (Fig. 2, A and B, compare lanes 2 and 4). Even when 100 μm ATP and 1 mm GTP were incubated together, the formation of early-import intermediates remained high (Fig. 2, A and B, compare lanes 2 and 5). However, when GTP analogs were incubated with ATP, intermediate formation was inhibited (Figs. 1 and 2). From these observations, we conclude that GTP and ATP do not compete for the same binding site but, rather, that GTP has a different function than ATP during the events leading to the formation of early-import intermediates.
The Generation of Early-Import Intermediates Is Reduced When GTP/GDP Exchange Is Inhibited
Proteins that bind and hydrolyze GTP regulate a wide variety of cellular processes (Bourne et al., 1991; Powers and Walter, 1995; Bacher et al., 1996; Millman and Andrews, 1997; Rapiejko and Gilmore, 1997; for review, see Walter and Johnson, 1994). It has been demonstrated that these GTPases can act as “molecular switches” that can be turned on by GTP binding and turned off by hydrolyzing GTP to GDP (Bourne et al., 1991). Therefore, numerous cellular functions are regulated via a cycle of GTP binding, hydrolysis, and exchange. Does a GTP/GDP-exchange cycle regulate the association of early-import intermediates with the chloroplastic envelope membrane? To investigate this question, a GTP/GDP competition assay was performed (see Fig. 2, lanes 8 and 9).
In this experiment, chloroplasts were incubated with equal concentrations of GTP and GDP for 5 min before the addition of sufficient ATP to support early-import intermediate formation or translocation. It has been demonstrated that the rate of GTP hydrolysis and GDP exchange by GTPases is generally slow (Bourne et al., 1991). We therefore anticipated that by allowing GDP to compete with GTP for the same GTP-binding site, the levels of early-import intermediates generated would be reduced due to the disruption of a GTP-binding and hydrolysis cycle. Surprisingly, however, when chloroplasts were incubated with a GTP/GDP mixture, the amount of early-import intermediates generated was not significantly affected compared with a control assay pre-incubated with GTP alone (Fig. 2, compare lanes 5 and 8 and 6 and 9). However, the failure of GDP to exert an effect on the second stage of import may have been the result of GDP undergoing a conversion to GTP by a chloroplast-associated diphosphokinase (Chen and Douglas, 1987). This conversion would supply sufficient GTP to the assay to support early-import intermediate formation and subsequent translocation of precursor proteins. To address this possibility, we utilized the analog GDP-βS, since it has been shown that it cannot serve as a substrate for nucleoside diphosphate kinase (Chen and Douglas, 1987).
As shown in Figure 2, we investigated whether GDP-βS could reduce the formation of early-import intermediates. Initially, the effects of both GDP and GDP-βS on the early stages of import were examined (see Fig. 2A, lanes 11, 12, 14, and 15). When chloroplasts were incubated with GDP, the levels of early-import intermediates were reduced by only 10% compared with controls (Fig. 2, A and B, compare lanes 2 and 11). Likewise, GDP had no dramatic effect on the translocation step of import (Fig. 2C, compare lanes 3 and 12). However, when chloroplasts were incubated with GDP-βS before the addition of ATP (0.1 mm), early-import intermediate formation was reduced by approximately 40% compared with controls (Fig. 2, A and B, compare lane 2 and 14). Even when higher levels of ATP (1 mm) were added to GDP-βS-pretreated chloroplasts, translocation of precursor was reduced by 40% compared with controls (Fig. 2, A and C, compare lanes 3 and 15). Because GDP-βS is not a substrate for nucleoside diphosphate kinase, the simplest explanation is that inhibition involved the disruption of a GTP/GDP-exchange process that may regulate the early stages of import. However, because GDP-βS is structurally similar to GDP and mimics GDP binding, its rate of exchange may be slower than that of GDP, thus inhibiting GTP/GDP exchange. Additional experiments using other approaches will be needed to examine the details of this postulated GTP/GDP-exchange cycle.
GTP and GTP Analogs Have No Effect on the Translocation Step of Import
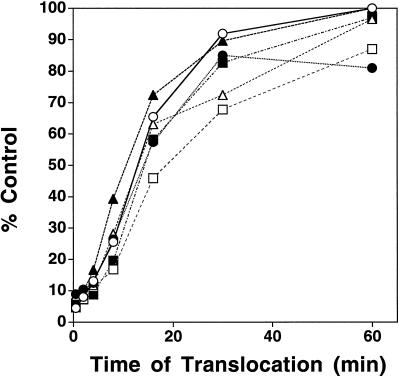
Although considerable evidence supports a role for GTP in the early stages of precursor import, its role during the translocation stage has not been directly measured. To address the impact of GTP and GTP analogs on the translocation step of import, we set up assays to examine the translocation once early-import intermediates had been formed. To perform these assays, early-import intermediates were first formed by incubating chloroplasts with precursor in the presence of low levels of ATP. These intermediates were then inserted into the import apparatus and were ready to begin the translocation process. Time-course experiments were performed to address whether GTP plays a role during the translocation stage of import (see Fig. 3). Intact chloroplasts containing early-import intermediates were recovered by sedimentation through a 40% (v/v) Percoll cushion. Recovered chloroplasts were extensively washed and then incubated with GTP or a GTP analog for 5 min. Translocation was initiated by the addition of 1 mm ATP. The kinetics of translocation was studied by the removal of aliquots at the times indicated. The results are presented in Figure 3. The data illustrate that none of the GTP analogs tested reduced the rate of translocation of prebound precursor relative to an ATP control containing no GTP analogs (Fig. 3). We conclude from these results that GTP hydrolysis and/or exchange influences the early stages of import, not the translocation stage.
Figure 3.
ATP hydrolysis rather than GTP hydrolysis mediates the translocation step of import. The effects of GTP analogs on the translocation of prSS into chloroplasts was examined by a time-course experiment. Precursor proteins were incubated with nigericin-treated chloroplasts for 10 min in the dark at room temperature to generate early-import intermediates. Chloroplasts were recovered by sedimentation through a 40% (v/v) Percoll cushion and incubated with 5 mm GTP analog for 5 min before 1 mm ATP was added. Aliquots were removed at the times indicated. Samples were analyzed by SDS-PAGE and fluorography and quantitated with a phosphor imager. The control for quantitation was prSS translocated in the presence of 1 mm ATP alone (○). Results shown are from one of three separate experiments. •, GTP; ▵, GMP-PNP; ▴, GTP-γS; □, GDP; ▪, GDP-βS.
DISCUSSION
We used chromatographically purified GTP analogs to arrest import. Because the energetics of import is complex, involving both ATP and GTP utilization, we compared the role of GTP before and after the ATP-stimulated formation of an early-import intermediate. Our results indicate that GTP has a separate role that is distinct from the ATP needed for the formation of an early-import intermediate (Fig. 1). We also show that GTP has no effect on the translocation steps that occur after the early-import intermediate has been formed (Fig. 3). We conclude that once the early-import intermediates are formed, ATP hydrolysis rather than GTP hydrolysis mediates translocation. This ATP is most probably utilized by various molecular chaperones thought to be involved in transport across the chloroplastic envelope membranes (Schnell et al., 1994; Akita et al., 1997; Kourtz and Ko, 1997; Nielsen et al., 1997).
From this study and others (Olsen et al., 1989; Olsen and Keegstra, 1992; Kessler et al., 1994; Kouranov and Schnell, 1997), we can begin to formulate models for import that incorporate the results listed above. Previously we divided the import pathway into the following stages: energy-independent binding, energy-dependent formation of an early-import intermediate, and translocation. Certainly any chloroplastic import model is complicated by the following points: (a) at least two components of the import apparatus, Toc34 and Toc86, bind and hydrolyze GTP; and (b) the overall import process utilizes both ATP and GTP. Regardless of this complex energy picture, both the nucleotide requirements for each stage of import and the protein components involved are slowly being revealed. For instance, recent experiments by Kouranov and Schnell (1997) provided partial characterization of the energy-independent binding step. Using a cross-linking approach, they showed that precursor proteins could interact with both Toc34 and Toc86 during the energy-independent binding stage of import. Furthermore, they observed that the contact between Toc34 and precursors was disrupted by the presence of nucleotide triphosphate. They proposed that Toc34 might regulate the transition from energy-independent binding to energy-dependent formation of import intermediates by utilizing a cycle of GTP binding and hydrolysis. Our investigation likewise supports the idea that GTP hydrolysis influences the second stage of import, the formation of import intermediates, and has no role during the later stages of protein import.
Interestingly, GTP may have another role during the formation of import intermediates: it may also regulate the association/dissociation of Toc34 with translocation components of the inner envelope membrane (Kouranov and Schnell, 1997). While this postulated role for GTP is intriguing, the evidence to support it is conflicting. For example, a stable translocation complex containing Toc34 and other translocation components of the outer and inner envelope membrane in the absence of bound precursor and nucleotides has been demonstrated (Akita et al., 1997; Nielsen et al., 1997). However, we cannot exclude the possibility that in these experiments (Akita et al., 1997; Nielsen et al., 1997) both Toc34 and Toc86 have sufficient endogenous levels of bound GTP to promote the formation of a stable translocation complex even in the absence of precursor protein. This complex may become unstable upon precursor binding and/or GTP hydrolysis. Further investigation is needed regarding the nature of the interaction between various import components of the outer and inner envelope membrane and how these interactions may be regulated by GTP.
Finally, we propose that the formation of early-import intermediates may be achieved through a cycle of GTP binding, hydrolysis, and GDP exchange. The evidence supporting the possible involvement of a GTP/GDP cycle on intermediate formation is based on the following observations: First, when chloroplasts are incubated with the analogs GTP-γS or GMP-PNP, intermediate formation, and therefore subsequent translocation, are inhibited. Structurally, GTP-γS and GMP-PNP are similar to GTP; however, GTP-γS is slowly hydrolyzable and GMP-PNP is non-hydrolyzable. Given these observations, it seems very unlikely that Toc34 and Toc86 can hydrolyze these analogs very efficiently (if at all). As a result, these analogs can be used to trap Toc34 and Toc86 in a conformation that mimics a permanent GTP-bound state.
When Toc34 and Toc86 become locked, the GTP/GDP-exchange cycle is halted, resulting in a reduction in intermediate formation and subsequent translocation. This result was observed in the present study (Fig. 1). In addition to GTP hydrolysis, however, GDP exchange is also critical in rejuvenating the GTP/GDP cycle and promoting the formation of early-import intermediates. We utilized the GTP analog GDP-βS to examine GDP exchange. This analog is structurally similar to GDP and can mimic GDP binding (Bourne et al., 1991). Figure 2 shows that intermediate formation, and therefore subsequent translocation, were inhibited by GDP-βS. One mechanism that could explain this result is that GDP-βS exchange is extremely slow, resulting in efficiently blocking a GTP/GDP exchange cycle that is necessary for the formation of early-import intermediates.
Many questions remain regarding the mechanism of how GTP is utilized by the translocation apparatus during the import process. For instance, do both Toc34 and Toc86 operate by using a GTP/GDP exchange cycle? Is the formation of early-import intermediates more stable when Toc34 or Toc86 are in a GTP-bound state? Sufficient answers to these and other questions will require other novel approaches. One possible approach would entail simplifying the import assay by using purified outer membrane vesicles to analyze precursor interaction in the presence of various GTP analogs. Another viable alternative would be to employ a genetic approach that would modify the GTP-binding sites of Toc34 and Toc86. These modifications may reveal how GTP is utilized by both Toc34 and Toc86 in order to generate early-import intermediates. Other genetic strategies also have the potential to determine the function of other components of the import apparatus. Indeed, a genetic approach has already been used to analyze the function of a putative component of the import apparatus in Arabidopsis (Jarvis et al., 1998). Therefore, genetic and other approaches should provide researchers with valuable tools with which to dissect the dynamic import process of chloroplasts.
ACKNOWLEDGMENTS
We thank Drs. Sigrun Reumann and Tony Sanderfoot for critical reading of the manuscript. We also thank Marlene Cameron and Kurt Stepnitz for preparation of the figures.
Footnotes
This research was supported by the Cell Biology Program of the National Science Foundation and by the Division of Energy Biosciences at the U.S. Department of Energy.
LITERATURE CITED
- Akita M, Nielsen E, Keegstra K. Identification of protein transport complexes in the chloroplastic envelope membrane via chemical cross-linking. J Cell Biol. 1997;136:983–994. doi: 10.1083/jcb.136.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher G, Lutcke H, Jungnickel B, Rapoport TA, Dobberstein B. Regulation by the ribosome of the GTPase of the signal-recognition particle during protein targeting. Nature. 1996;381:248–251. doi: 10.1038/381248a0. [DOI] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Bruce BD. The role of lipids in plastid protein transport. Plant Mol Biol. 1998;38:223–246. [PubMed] [Google Scholar]
- Bruce BD, Perry S, Froehlich J, Keegstra K. In vitro import of protein into chloroplasts. In: Gelvin SB, Schilperoort RB, editors. Plant Molecular Biology Manual, Vol J1. Boston: Kluwer Academic Publishers; 1994. pp. 1–15. [Google Scholar]
- Chen W-J, Douglas MG. Phosphodiester bond cleavage outside mitochondria is required for the completion of protein import into the mitochondrial matrix. Cell. 1987;49:651–658. doi: 10.1016/0092-8674(87)90541-1. [DOI] [PubMed] [Google Scholar]
- Chua NH, Schmidt GW. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci USA. 1978;75:6110–6114. doi: 10.1073/pnas.75.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highfield PE, Ellis RJ. Synthesis and transport of the small subunit of chloroplastic ribulose bisphosphate carboxylase. Nature. 1978;271:420–424. [Google Scholar]
- Hinnah SC, Hill K, Wagner R, Schlicher T, Soll J. Reconstitution of a chloroplast protein import channel. EMBO J. 1997;16:7351–7360. doi: 10.1093/emboj/16.24.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch S, Muckel E, Heemeyer F, von Heijne G, Soll J. A receptor component of the chloroplast protein translocation machinery. Science. 1994;266:1989–1992. doi: 10.1126/science.7801125. [DOI] [PubMed] [Google Scholar]
- Horst M, Oppliger W, Feifel B, Schatz G, Glick BS. The mitochondrial protein import motor: dissociation of mitochondrial hsp70 from its membrane anchor requires ATP binding rather than ATP hydrolysis. Protein Sci. 1996;5:759–767. doi: 10.1002/pro.5560050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis P, Chen L-J, Li H-M, Peto C, Fankhauser C, Chory J. An Arabidopsis mutant defective in the plastid general protein import apparatus. Science. 1998;282:100–103. doi: 10.1126/science.282.5386.100. [DOI] [PubMed] [Google Scholar]
- Keegstra K, Cline K. Protein import and routing systems of chloroplasts. Plant Cell. 1999;11:557–570. doi: 10.1105/tpc.11.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel FG, Patel H, Kessler A, Schnell DJ. Identification of two GTP-binding proteins in the chloroplast protein import machinery. Science. 1994;266:1035–1039. doi: 10.1126/science.7973656. [DOI] [PubMed] [Google Scholar]
- Kouranov A, Schnell DJ. Analysis of the interactions of preproteins with the import machinery over the course of protein import into chloroplasts. J Cell Biol. 1997;139:1677–1685. doi: 10.1083/jcb.139.7.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtz L, Ko K. The early stage of chloroplast protein import involves Com70. J Biol Chem. 1997;272:2808–2813. doi: 10.1074/jbc.272.5.2808. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ma Y, Kouranov A, LaSala S, Schnell DJ. Two components of the chloroplast protein import apparatus, IAP86 and IAP75, interact with the transit sequence during the recognition and translocation of precursor proteins at the outer envelope. J Cell Biol. 1996;134:1–13. doi: 10.1083/jcb.134.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman JS, Andrews DW. Switching the model: a concerted mechanism for GTPases in protein targeting. Cell. 1997;89:673–676. doi: 10.1016/s0092-8674(00)80248-2. [DOI] [PubMed] [Google Scholar]
- Nielsen E, Akita M, Davila-Aponte J, Keegstra K. Stable association of chloroplastic precursors with protein translocation complexes that contain proteins from both envelope membranes and a stromal Hsp100 molecular chaperone. EMBO J. 1997;16:935–946. doi: 10.1093/emboj/16.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblong JE, Lamppa GK. Identification of two structurally related proteins involved in proteolytic processing of precursors targeted to the chloroplast. EMBO J. 1992;11:4401–4409. doi: 10.1002/j.1460-2075.1992.tb05540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen LJ, Keegstra K. The binding of precursor proteins to chloroplast requires nucleoside triphosphates in the intermembrane space. J Biol Chem. 1992;267:433–439. [PubMed] [Google Scholar]
- Olsen LJ, Theg SM, Selman BR, Keegstra K. ATP is required for the binding of precursor proteins to chloroplasts. J Biol Chem. 1989;264:6724–6729. [PubMed] [Google Scholar]
- Pain D, Blobel G. Protein import into chloroplasts requires a chloroplast ATPase. Proc Natl Acad Sci USA. 1987;84:3288–3292. doi: 10.1073/pnas.84.10.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SE, Keegstra K. Envelope membrane proteins that interact with chloroplastic precursor proteins. Plant Cell. 1994;6:93–105. doi: 10.1105/tpc.6.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N, Neupert W. Transport of F1-ATPase subunit β into mitochondria depends on both a membrane potential and nucleoside triphosphates. FEBS Lett. 1986;209:152–156. doi: 10.1016/0014-5793(86)81101-2. [DOI] [PubMed] [Google Scholar]
- Powers T, Walter P. Two directly interacting GTPases as GTP-regulated GTPase stimulating proteins for each other. Science. 1995;269:1422–1424. doi: 10.1126/science.7660124. [DOI] [PubMed] [Google Scholar]
- Rapiejko PJ, Gilmore R (1997) Empty site forms of the SRP54 and SRα GTPases mediate targeting of ribosome-nascent chain complexes to the endoplasmic reticulum. 89: 703–713 [DOI] [PubMed]
- Reed JE, Cline K, Stephens LC, Bacot KO, Vitanen PV. Early events in the import/assembly pathway of an integral thylakoid protein. Eur J Biochem. 1990;194:33–42. doi: 10.1111/j.1432-1033.1990.tb19423.x. [DOI] [PubMed] [Google Scholar]
- Richter S, Lamppa GK. A chloroplastic processing enzyme functions as the general stromal processing peptidase. Proc Natl Acad Sci USA. 1998;95:7463–7468. doi: 10.1073/pnas.95.13.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell DJ. Protein targeting to the thylakoid membrane. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:97–126. doi: 10.1146/annurev.arplant.49.1.97. [DOI] [PubMed] [Google Scholar]
- Schnell DJ, Kessler F, Blobel G. Isolation of components of the chloroplast protein import machinery. Science. 1994;266:1007–1012. doi: 10.1126/science.7973649. [DOI] [PubMed] [Google Scholar]
- Seedorf M, Waegemann K, Soll J. A constituent of the chloroplast import complex represents a new type of GTP-binding protein. Plant J. 1995;7:401–411. doi: 10.1046/j.1365-313x.1995.7030401.x. [DOI] [PubMed] [Google Scholar]
- Theg SM, Bauerle C, Olsen LJ, Selman BR, Keegstra K. Internal ATP is the only energy requirement for the translocation of precursor proteins across chloroplastic membranes. J Biol Chem. 1989;264:6730–6736. [PubMed] [Google Scholar]
- Tranel PJ, Froehlich J, Goyal A, Keegstra K. A component of the chloroplastic protein import apparatus is targeted to the outer envelope membrane via a novel pathway. EMBO J. 1995;14:2436–2446. doi: 10.1002/j.1460-2075.1995.tb07241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderVere PS, Bennett TM, Oblong JE, Lamppa GK. A chloroplast processing enzyme involved in precursor maturation shares a zinc-binding motif with a recently recognized family of metalloendopeptidases. Proc Natl Acad Sci USA. 1995;92:7177–7181. doi: 10.1073/pnas.92.16.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van't Hof R, de Kruijff B. Characterization of the import process of a transit peptide into chloroplasts. J Biol Chem. 1995;270:22368–22373. doi: 10.1074/jbc.270.38.22368. [DOI] [PubMed] [Google Scholar]
- Walter P, Johnson AE. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1994;10:87–119. doi: 10.1146/annurev.cb.10.110194.000511. [DOI] [PubMed] [Google Scholar]