Abstract
AIM
To investigate the effects of plecanatide and dolcanatide on maintenance of paracellular permeability, integrity of tight junctions and on suppression of visceral hypersensitivity.
METHODS
Transport of fluorescein isothiocyanate (FITC)-dextran was measured to assess permeability across cell monolayers and rat colon tissues. Effects of plecanatide and dolcanatide on the integrity of tight junctions in Caco-2 and T84 monolayers and on the expression and localization of occludin and zonula occludens-1 (ZO-1) were examined by immunofluorescence microscopy. Anti-nociceptive activity of these agonists was evaluated in trinitrobenzene sulfonic acid (TNBS)-induced inflammatory as well as in non-inflammatory partial restraint stress (PRS) rat models. Statistical significance between the treatment groups in the permeability studies were evaluated using unpaired t-tests.
RESULTS
Treatment of T84 and Caco-2 monolayers with lipopolysaccharide (LPS) rapidly increased permeability, which was effectively suppressed when monolayers were also treated with plecanatide or dolcanatide. Similarly, when T84 and Caco-2 monolayers were treated with LPS, cell surface localization of tight junction proteins occludin and ZO-1 was severely disrupted. When cell monolayers were treated with LPS in the presence of plecanatide or dolcanatide, occludin and ZO-1 were localized at the cell surface of adjoining cells, similar to that observed for vehicle treated cells. Treatment of cell monolayers with plecanatide or dolcanatide without LPS did not alter permeability, integrity of tight junctions and cell surface localization of either of the tight junction proteins. In rat visceral hypersensitivity models, both agonists suppressed the TNBS-induced increase in abdominal contractions in response to colorectal distension without affecting the colonic wall elasticity, and both agonists also reduced colonic hypersensitivity in the PRS model.
CONCLUSION
Our results suggest that activation of GC-C signaling might be involved in maintenance of barrier function, possibly through regulating normal localization of tight junction proteins. Consistent with these findings, plecanatide and dolcanatide showed potent anti-nociceptive activity in rat visceral hypersensitivity models. These results imply that activation of GC-C signaling may be an attractive therapeutic approach to treat functional constipation disorders and inflammatory gastrointestinal conditions.
Keywords: Plecanatide, Guanylyl cyclase-C agonists, Dolcanatide, Uroguanylin, Preclinical, Cyclic guanosine monophosphate, Constipation, Inflammatory bowel diseases
Core tip: Our results indicate that plecanatide and dolcanatide, guanylate cyclase-C receptor agonists designed to replicate the activity of the human intestinal peptide uroguanylin, maintain intestinal barrier function and exhibit potent anti-nociceptive activity in animal models of visceral hypersensitivity, suggesting a novel mechanism, beyond the well described secretory function, for these agonists in the treatment of functional constipation disorders and inflammatory bowel disease.
INTRODUCTION
Chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C), affecting approximately 20% of the United States population[1,2], are characterized by abnormalities in motility and visceral hypersensitivity with overlapping symptoms such as abdominal pain and discomfort, bloating, incomplete bowel movements, straining, or hard and lumpy stools[3,4].
Therapeutic targets for CIC and IBS-C have focused primarily on promotion of gastrointestinal (GI) fluid secretion through activation of chloride channels such as chloride channel type 2 and the cystic fibrosis transmembrane conductance regulator (CFTR)[5]. Additionally, inhibition of sodium-hydrogen exchanger 3 is being explored for treating IBS-C[6]. Drugs approved by the United States. Food and Drug Administration (FDA) for treating IBS-C include Amitiza® (lubiprostone) for adult women and Linzess® (linaclotide)[7,8] for adults. Lubiprostone, a bicyclic fatty acid metabolite of prostaglandin E1, specifically stimulates chloride channel type 2 causing an efflux of chloride into the lumen of the GI tract, which promotes fluid secretion, facilitating bowel movement[9]. Linaclotide, an analog of the heat-stable enterotoxin of Escherichia coli (E. coli), binds and activates guanylate cyclase-C (GC-C) to stimulate production of cyclic guanosine monophosphate (cGMP), which enhances secretion of electrolytes and fluid into the GI lumen to promote bowel movement and ameliorate abdominal pain[10]. Plecanatide (Trulance®) is an FDA-approved drug for treatment of adults with CIC[11,12] and the drug was recently approved for treatment of adults with IBS-C.
Recent studies suggest that the immune system is dysregulated in irritable bowel syndrome (IBS), leading to increased total counts of innate immune cells, including mast cells, monocytes and macrophages in IBS patients[13-15]. Mast cell mediators and cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6) and IL-1α, modulate colorectal afferent excitability and disrupt intestinal barrier function. The paracellular permeability of the intestinal epithelial barrier is regulated by a tight junction (TJ) protein complex composed of transmembrane proteins such as occludin, claudin and zonula occludens (ZO), which bind to the actin cytoskeleton[16]. Alterations in the structure and/or function of the TJ protein complexes are associated with epithelial barrier disruption and increased permeability of the mucosa, allowing entry of inflammatory mediators to promote low-grade inflammation and visceral hypersensitivity[16-19]. Studies have correlated down-regulation of TJ proteins with the severity of visceral hypersensitivity in IBS[19,20].
There is considerable overlap in clinical symptoms between IBS and inflammatory bowel disease (IBD)[21-23]. Pro-inflammatory cytokines, such as TNF-α and interferon-γ released during GI inflammation, activate myosin light chain kinase (MLCK) responsible for phosphorylation of the myosin II regulatory light chain, resulting in contraction of actomyosin and dysfunction of the intestinal barrier[20]. Activation of GC-C signaling protects intestinal barrier function by regulating MLCK activity. In this regard, loss of GC-C signaling in GC-C-/- mice leads to a dysfunctional intestinal barrier and increased paracellular permeability[24]. Coincidently, the loss in expression of uroguanylin, the endogenous agonist of GC-C receptors, is also associated with colon cancer[25] and IBD[26,27]. Thus, activation of GC-C signaling is an attractive strategy for the treatment of GI disorders and inflammatory diseases.
Plecanatide is structurally identical to uroguanylin, differing only in the substitution of Asp with Glu at the 3-position at the N-terminus for greater binding affinity. Dolcanatide is similar to plecanatide in structure except that L-Asn1 and L-Leu16 are replaced by D-Asn1 and D-Leu16 at the N- and C-termini, respectively, which is thought to provide enhanced biostability. In this study, we provide the first evidence that plecanatide and dolcanatide, both analogs of uroguanylin, suppress lipopolysaccharide (LPS)-mediated increase in permeability in epithelial cell models and reduce visceral hypersensitivity in trinitrobenzene sulfonic acid (TNBS) and partial restraint stress (PRS) animal models.
MATERIALS AND METHODS
Ethical approval
Animal care and handling procedures for ex vivo studies performed in the United States were as per the approved protocol by the Institutional Animal Care and Use Committee of Lampire Biologicals (Pipersville, PA, United States). Animal handling procedures for in vivo studies conducted in France were approved by the Institutional Animal Care and Use Local Committee (Toulouse, France). The investigators affirm that all appropriate measures were taken to minimize pain or discomfort of the animals used in this study.
Test peptides, chemicals and reagents
Plecanatide (CAS: 467426-54-6) and dolcanatide (CAS: 1092457-65-2) were synthesized by AmbioPharm, Inc. (Augusta, SC, United States). For all in vitro experiments with cell lines and colon tissues, optimal concentrations derived from dose response curves were used.
Fluorescein isothiocyanate (FITC)-dextran (approximate molecular weight, 4 kD) and E. coli LPS were purchased from Sigma (St Louis, MO, United States). Trypsin, GlutaMax, and Pen Strep were procured from Life Technologies (Grand Island, NY, United States). Rabbit anti-occludin antibody, rabbit anti-ZO-1 antibody, DAPI (4’, 6’-diamidino-2-phenylindole, dihydrochloride), and Alexa Fluor 488 conjugated goat anti-rabbit secondary antibodies were from Thermo Fisher Scientific (Waltham, MA, United States). Ussing chamber and its accessories were purchased from Physiologic Instruments (San Diego, CA, United States). All other chemicals and reagents were obtained from Sigma-Aldrich Corp. (St Louis, MO, United States) or Fisher Scientific (Pittsburgh, PA, United States).
Measurement of epithelial cell paracellular permeability
Human colon carcinoma cell lines T84 and Caco-2, obtained from ATCC (Manassas, VA, United States), were cultured by procedures as previously described[25]. Paracellular permeability was determined by calculating the flux of FITC-dextran across epithelial cell monolayers. T84 (1.5 × 105) and Caco-2 (8 × 103) cells were cultured on 12 mm Transwell® permeable polyester membrane inserts (pore size, 0.4 μm) until the transepithelial resistance reached > 1000 Ω cm2 for T84 cells or > 400 Ω.cm2 for Caco-2. Cell monolayers were treated overnight with 100 μg/mL LPS in the presence or absence of 1 μmol/L plecanatide or 1 μmol/L dolcanatide. Subsequently, the media was aspirated and 1 mg/mL FITC-dextran dissolved in Krebs Ringer buffer solution was added to the apical chamber and cells were incubated for an additional one h at 37 °C. Fluorescence in 100 μL of basolateral buffer solution was measured in a Tecan M-1000 plate reader. The excitation and emission wavelengths for FITC were 494 nm and 518 nm respectively. Data represent mean relative fluorescence ± standard error of the mean from at least two biological replicates, each analyzed in triplicate.
Measurement of permeability across colon tissues from rats
Adult Sprague Dawley male and female rats [Crl:CD(SD)], aged seven to eight wk, weighing 170-210 g were purchased from Charles River Laboratories (Shrewsbury, MA, United States) and allowed to acclimate for a minimum of one wk. Animals were maintained on a 12 h light-dark cycle and were fasted overnight with free access to water prior to tissue harvest. The next morning the animals were euthanized by CO2 inhalation. Following a midline abdominal incision, the entire colon segment was removed for permeability studies.
Freshly harvested tissue from proximal to mid-colon (approximately 2 cm pieces) were randomly selected and transferred to RPMI media in 24-well tissue culture plates containing vehicle, 10 μmol/L plecanatide or 10 μmol/L dolcanatide in the presence or absence of 100 μg/mL LPS. The plates were placed in a humidified incubator (5% CO2) at 37 °C. The next day, each tissue piece was mounted on an Ussing chamber slider (0.5 cm2). Apical and basolateral chambers were bathed in Krebs Ringer buffer solution and gassed with 95% O2 and 5% CO2. The temperature was maintained at 37 °C with a water-jacketed system. To measure permeability, 2 mg/mL of FITC-dextran dissolved in Krebs Ringer buffer solution (pH 7.4) was added to the apical chamber. Fluorescence was measured every 15 min (for two h) in samples (100 μL of buffer) from the basolateral chamber using a Tecan M-1000 plate reader. The excitation and emission wavelengths used were 494 nm and 518 nm respectively. Data values represent mean relative fluorescence ± standard error of the mean recorded 75 min after the addition of FITC-dextran from multiple independent treatments.
Immunofluorescence microscopy
T84 and Caco-2 monolayers in 24-well plates were treated overnight with 100 μg/mL of LPS in the presence or absence of 1 μmol/L plecanatide or 1 μmol/L dolcanatide. Following the treatment, cells were washed three times with chilled phosphate buffer saline (PBS), fixed with 4% formaldehyde in PBS for 15 min, blocked and permeabilized in PBS containing 3% bovine serum albumin (BSA) and 0.3% Triton X-100 at room temperature for 30 min. Subsequently, monolayers were incubated with PBS containing 0.1% Tween-20, 2% BSA, rabbit antioccludin (1:150) or rabbit antiZO-1 (1:25) antibodies and incubated overnight at 4 °C followed by three washes in chilled PBS and incubation for one h at room temperature in Alexa Fluor 488 labeled secondary antibody (1:500) and counterstained with DAPI. Occludin and ZO-1 were visualized with an Olympus IX81 microscope and images were obtained using SlideBook 5.0 software. Two independent experiments were conducted and approxiamately 30 fields examined for each treatment. Images were acquired at 40 × resolution.
Surgical procedures in rats used in the visceral hypersensitivity studies
Wistar male rats (n = 8/dosage group) weighing 220 -250 g (Janvier SA, Le Genest St Isle, France) were used in the visceral hypersensitivity experiments. Rats were housed individually in a temperature controlled (approxiamately 25 °C) room with relative humidity (50%) with food and water ad libitum. The evening prior to the procedure, food and water were removed from the cages. Rats were sedated with acepromazine (0.5 mg/kg i.p.) and ketamine (100 mg/kg i.m.) (Imalgène; Rhône Mérieux, Toulouse, France). Pairs of nichrome wire electrodes (60 cm in length and 80 μM in diameter) were implanted bilaterally in the abdominal external oblique musculature, just superior to the inguinal ligament, 2 cm laterally from the midline. The free ends of electrodes were exteriorized on the back of the neck and protected by a plastic tube attached to the skin. Baseline electromyographic recordings began eight to nine days after surgical implantation of electrodes. Electrical activity of abdominal striated muscle was recorded with an electroencephalograph (Mini VIII, Alvar, Paris, France) using a short time constant (0.03 s) to remove low frequency signals (< 3 Hz) and to selectively record spike bursts corresponding to abdominal contractions.
Colorectal distention
The colorectal distension (CRD) procedure was based on methods previously described[28]. During the acclimation sessions, rats were placed in plastic tunnels where they could move but not escape. Prior to the CRD procedure, a balloon (latex condom) was inserted into the rectum of conscious rats until the base of the balloon was at the anus (4 cm insertion). The tube was fixed at the base of the tail and animals were allowed to recover for 30 min. The balloon was then connected to a barostat and inflated progressively from 0-60 mmHg in 15 mmHg steps. Each step of inflation lasted five min. Responses to applied CRD pressure levels were measured with electromyographic recordings during the five-min interval and data are expressed as contractions/five min. Colonic volume adaptation to increasing pressures (compliance) was also measured using a potentiometric recorder (Linseis, Germany).
TNBS-induced visceral hypersensitivity in rats
The effect of plecanatide or dolcanatide on CRD was evaluated in a basal condition prior to TNBS exposure, as well as after treatment with TNBS in rats (n = 8/dose group). Plecanatide or dolcanatide were formulated in PBS to deliver via oral gavage doses of 0.01 or 0.05 mg/kg in 1.5 mL. Following the basal test, after a 12 h fasting period, rats were treated with 0.3 mL of TNBS (80 mg/kg in 50% ethanol), intrarectally through a silicone rubber catheter introduced to a depth of 6 cm into the anus under light anesthesia as previously described[29]. Following administration of TNBS, the animals were routinely evaluated for changes in physical appearance or behavior. Four days after the TNBS treatment, the oral administration of plecanatide or dolcanatide and the CRD testing was repeated.
Partial restraint stress-induced colorectal hypersensitivity
PRS was performed as described by Williams et al[30]. Rats were lightly anesthetized with ethyl-ether and foreshoulders, upper forelimbs and thoracic trunk were wrapped in a confining harness of paper tape to restrict, but not prevent, body movements. Rats (n = 8/dose group) were then placed in their home cage for two h. For the control condition, rats were anesthetized but not wrapped. Subsequently the rats were administered 1.5 mL of vehicle (phosphate-buffered saline), plecanatide or dolcanatide formulated to deliver doses of 0.01 and 0.05 mg/kg by oral gavage 30 min before the end of the PRS session. Thirty min following the stress procedure, rats underwent the CRD testing.
Statistical analysis
GraphPad Prism (Version 6.05) was used to calculate descriptive statistics and inferential tests. Differences between the treatment groups in the permeability studies were evaluated using unpaired t-tests. Two-way analyses of variance were used to evaluate differences between the vehicle control and the plecanatide or dolcanatide dose groups followed by comparisons at each pressure level using Dunnett’s or Sidak’s multiple comparison tests.
RESULTS
Plecanatide and dolcanatide suppressed LPS-induced paracellular permeability
Treatment with LPS reportedly can disrupt the TJ complex by down-regulating junctional protein expression. Additionally, LPS is known to augment mucosal hypersensitivity through secretion of inflammatory cytokines and other mediators[31]. Treatment with LPS (100 μg/mL) resulted in a statistically significant increase in paracellular permeability of FITC-dextran across Caco-2 (Figure 1A and B) and T84 (Figure 1C and D) cell monolayers. Importantly, the LPS-induced increase in the permeability of FITCdextran was completely suppressed in both cell monolayers treated with plecanatide (Figure 1A and C) or dolcanatide (Figure 1B and D). No appreciable effect on paracellular permeability was observed when monolayers were treated with either GC-C agonist alone.
Figure 1.
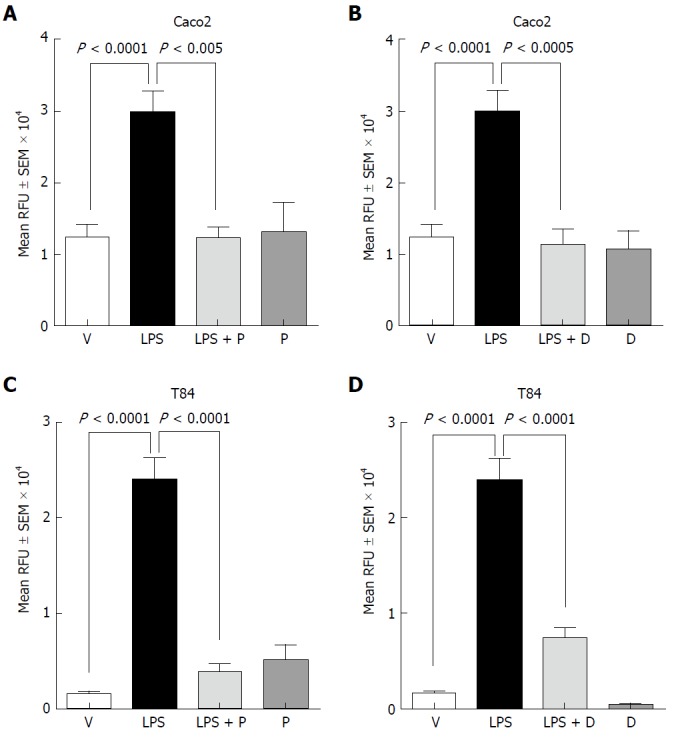
Effect of plecanatide and dolcanatide on lipopolysaccharide-induced increase in permeability of 4 kDa fluorescein isothiocyanate-dextran across Caco-2 and T84 cell monolayers. Caco-2 (A and B) and T84 (C and D) cells cultured on snap well inserts were treated with vehicle or 1 μmol/L of plecanatide (A and C) or dolcanatide (B and D) in the presence or absence of 100 μg/mL of LPS for 16 h. Subsequently, 1 mg/mL of FITC-dextran was added to the apical compartment. Paracellular permeability was determined by measuring the amount of FITC-dextran present in the basal compartment. Data represent mean ± SEM analyzed in triplicates. D: Dolcanatide; FITC: Fluorescein isothiocyanate; LPS: Lipopolysaccharide; P: Plecanatide; RFU: Relative fluorescence units; SEM: Standard error of the mean; V: Vehicle.
Next, we examined the effects of plecanatide or dolcanatide on the LPS-mediated increase in permeability across freshly harvested rat colon tissues. Consistent with the results presented in Figure 1, LPS treatment resulted in a statistically significant increase in the paracellular permeability of FITC-dextran across rat colon tissues, which was effectively suppressed by treatment with plecanatide or dolcanatide (Figure 2). These results indicate that activation of GC-C signaling may be suppressing the deleterious permeability effects of caused by LPS treatment.
Figure 2.
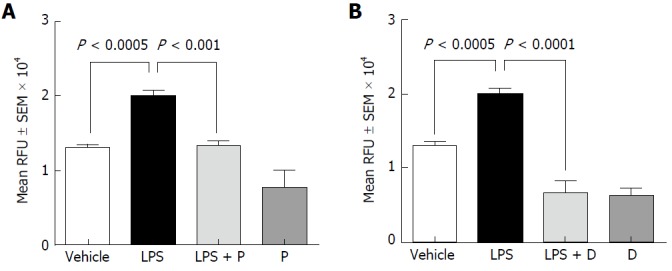
Effect of plecanatide (A) and dolcanatide (B) on lipopolysaccharide-induced increased permeability of 4 kD fluorescein isothiocyanate-dextran across rat colon tissues. Rat colon tissues (2 cm pieces) were incubated overnight with vehicle, 10 μM plecanatide or dolcanatide in the presence or absence of 100 μg/mL LPS. Data represent mean fluorescence ± SEM recorded 75 min after the addition of FITC-dextran. D: Dolcanatide; FITC: Fluorescein isothiocyanate; LPS: Lipopolysaccharide; P: Plecanatide; RFU: Relative fluorescence units; SEM: Standard error of the mean.
Plecanatide and dolcanatide maintain TJ integrity
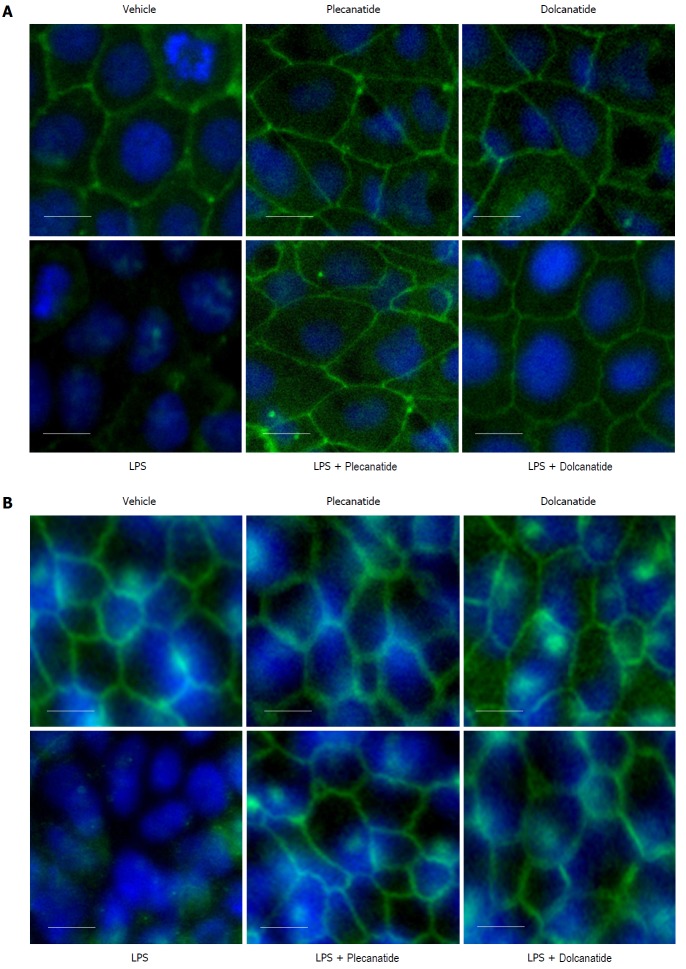
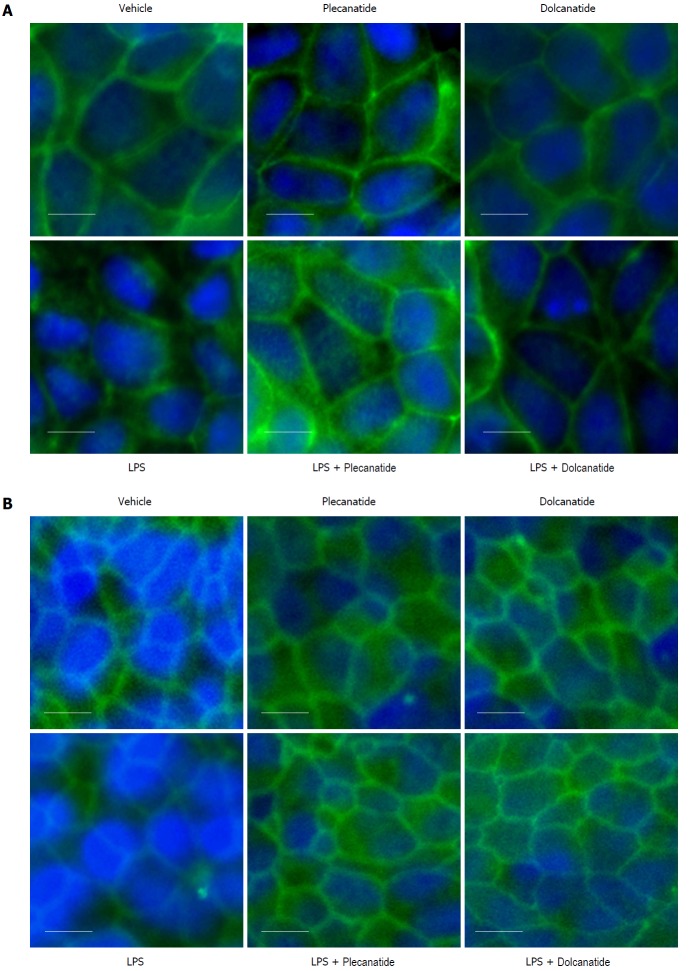
Since LPS treatment consistently increased paracellular permeability in both cell lines and colonic tissue, we decided to examine the effects of LPS on the expression and localization of TJ proteins in these epithelial cell monolayers by immunofluorescence microscopy. Treatment with LPS severely disrupted localization of occludin and ZO-1 proteins at TJs in Caco-2 (Figures 3A and 4A) and T84 cells (Figures 3B and 4B), respectively. Importantly, when cell monolayers were treated with LPS in the presence of plecanatide or dolcanatide, expression of occludin and ZO-1 were normalized and localized at the cell surface of adjoining cells, similar to that observed for vehicle treated cells (Figures 3 and 4). Notably, treatment of cell monolayers with plecanatide or dolcanatide without LPS did not alter expression and localization of either of the TJ proteins. These results indicate that treatment with plecanatide or dolcanatide suppress LPS-mediated disruption in expression/localization of TJs in Caco-2 and T84 monolayers.
Figure 3.
Effect of plecanatide and dolcanatide on localization of occludin in epithelial cells. Caco-2 (A) and T84 (B) cell monolayers were treated with 1 μmol/L plecanatide or dolcanatide in the presence or absence of 100 μg/mL of LPS for 16 h followed by immunofluorescence imaging for occludin. Representative microscopic fields demonstrate disruption of occludin localization by LPS. Co-treatment of LPS with plecanatide or dolcanatide preserved occludin localization around the cell membrane, as was observed for vehicle treated cells. Images taken at 40 × resolution. Blue fluorescence corresponds to DAPI stained nucleus. DAPI: 4’, 6’-diamidino-2-phenylindole; LPS: lipopolysaccharide.
Figure 4.
Effect of plecanatide and dolcanatide on localization of ZO-1 in epithelial cells Caco-2 (A) and T84 (B) cell monolayers were treated with 1 μmol/L plecanatide or dolcanatide in the presence of 100 μg/mL of LPS for 16 h followed by immunofluorescence imaging for ZO-1. Representative microscopic fields depicted above demonstrate disruption of ZO-1 localization by LPS. In Caco-2 cells, LPS treatment appears to cause accumulation of ZO-1 in the cytoplasm. Co-treatment of LPS with plecanatide or dolcanatide preserved ZO-1 localization around the cell membrane as observed for vehicle treated cells. Images taken at 40× resolution. Blue fluorescence corresponds to DAPI stained nucleus. DAPI: 4’, 6’-Diamidino-2-phenylindole; LPS: Lipopolysaccharide; ZO-1: Zonula occludens-1.
Basal colorectal sensitivity in rats
Initially, we conducted several pilot experiments to optimize experimental conditions and dose range of plecanatide and dolcanatide for evaluation. Under basal conditions, with no CRD pressure in vehicle treated rats, abdominal contractions occurred at approximately 4.1 contractions/5 min. As expected, increasing CRD pressure (0-60 mmHg) led to a linear increase in the number of abdominal contractions reaching approximately 6-fold higher than without any pressure. Oral treatment with plecanatide or dolcanatide without CRD pressure did not alter the rate of abdominal contractions (data not shown).
Effect of plecanatide and dolcanatide in TNBS-induced rectal allodynia in rats
Consistent with our prior experience in this model, the number of abdominal contractions, as an index of inflammation-induced visceral pain, four days after TNBS treatment (Figure 5), gradually increased in a pressure-dependent manner as compared to the number of abdominal contractions under basal conditions without TNBS treatment[32]. As expected, TNBS treatment resulted in increased abdominal contractions even in the absence of distending pressure. Oral treatment with plecanatide or dolcanatide at the lower doses (0.01 and 0.05 mg/kg) considerably attenuated (P ≤ 0.001) the TNBS-induced increase in the number of abdominal contractions with increasing distending pressures up to 60 mmHg (Figure 5B and C). However, higher doses (> 0.1 mg/kg) had no significant effect on reduction in abdominal contractions at any pressure of distention (data not shown).
Figure 5.
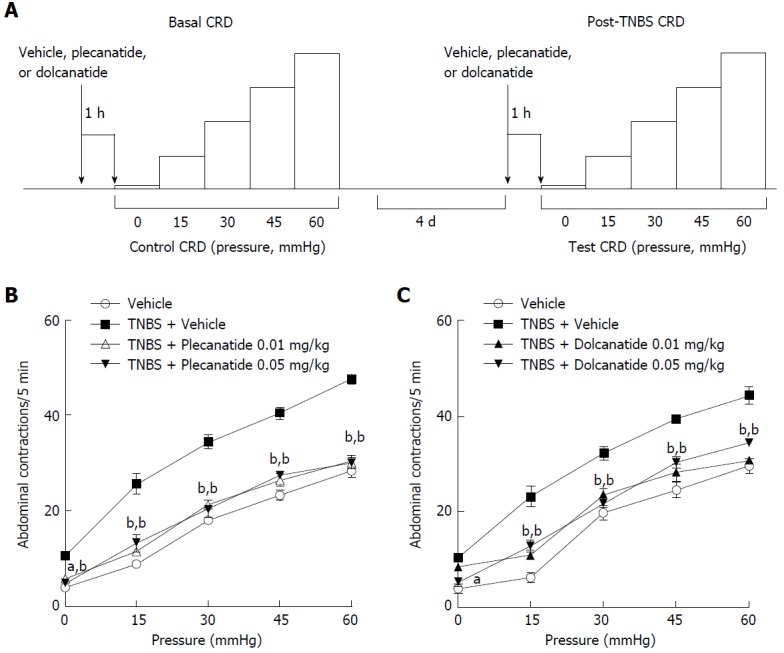
Design and results of the TNBS-induced visceral hypersensitivity models. A: Schematic depicting the sequence of test sessions and treatments to evaluate visceral hypersensitivity induced by TNBS rat models. Effects of oral administration of plecanatide or dolcanatide as compared with vehicle on the increase in abdominal contractions to colorectal distention (CRD) during testing conducted four days after intrarectal administration of TNBS. Doses of 0.01 and 0.05 mg/kg of plecanatide (B) or dolcanatide (C) reduced the rate of muscular contractions toward levels observed in the vehicle group prior to TNBS administration. Data are the mean ± SEM (n = 8 rats/group). aP < 0.05, bP < 0.01 as compared to the values for the post-trinitrobenzene sulfonic acid vehicle group. SEM: Standard error of the mean; TNBS: Trinitrobenzene sulfonic acid.
Effect of plecanatide and dolcanatide on stress-induced colorectal hypersensitivity
To investigate the anti-nociceptive effect of plecanatide or dolcanatide under non-inflammatory conditions, we utilized a wrap restraint model of stress-induced visceral hypersensitivity in Wistar rats, a strain with high stress responsiveness (Figure 6A). In vehicle treated rats, the number of abdominal contractions increased after the PRS session with increasing CRD pressures up to 60 mmHg. Oral treatment with plecanatide (Figure 6B and C) or dolcanatide (Figure 6D and E) resulted in a significant reduction in the rate of PRS-induced abdominal contractions with increasing CRD pressures. Both GC-C agonists exhibited no effect on colorectal volume at the doses tested (data not shown).
Figure 6.
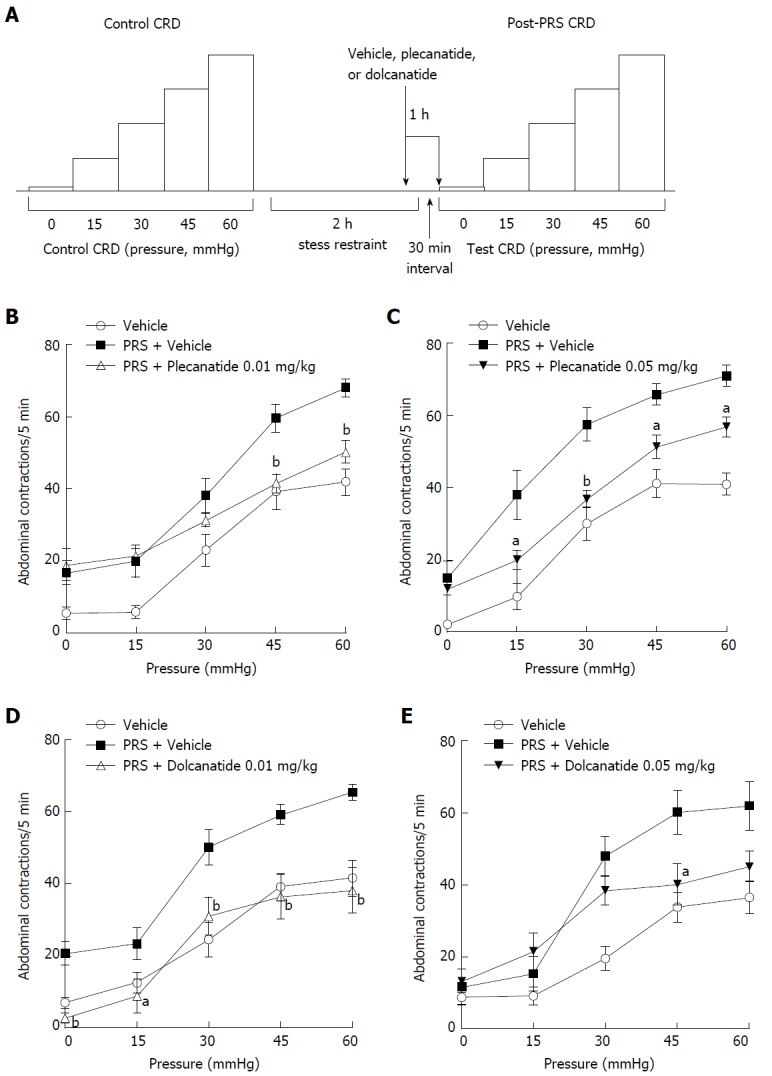
Design and results of the partial restraint stress-induced visceral hypersensitivity models. A: Schematic depicting the sequence of test sessions and treatments to evaluate visceral hypersensitivity induced by PRS in rat models. Effects of oral administration of plecanatide, dolcanatide or vehicle on the increase in abdominal contractions to CRD when tested 30 min after a two h period of partial restraint. Doses of 0.01 and 0.05 mg/kg of plecanatide (B and C) or dolcanatide (D and E) 30 min before completion of the restraint session reduced the rate of muscular contractions toward the levels observed in a previous test session without exposure to partial restraint. Data are the mean ± SEM (n = 8 rats/group). aP < 0.05, bP < 0.01 as compared to the values for PRS + vehicle control. CRD: Colorectal distention; PRS: Partial restraint stress; SEM: Standard error of the mean.
DISCUSSION
The role of GC-C signaling in the regulation of ion and fluid homeostasis in the GI tract is well established[33,34] and affirmed clinically with the approval of plecanatide for the treatment of adults with CIC in the United States. However, recent advances have expanded our understanding of the involvement of GC-C signaling cascade in additional physiological activities such as in the maintenance of intestinal barrier function[33,35] and in the protection against GI inflammation and colorectal carcinogenesis[25,26,34]. Dysregulation of GC-C signaling either due to familial mutations in GC-C gene or loss of its endogenous ligands has further underscored the pathophysiological importance of GC-C signaling in GI indications[36]. In this study, we present in vitro and in vivo data with plecanatide and dolcanatide, two GC-C agonists, to demonstrate the physiological role GC-C signaling plays in the maintenance of intestinal barrier function and in suppression of visceral hypersensitivity in inflammatory and non-inflammatory rat models.
Dysregulation of the intestinal epithelial barrier function, known to be associated with several gut disorders, can be elicited by a number of agents, including luminal bacterial antigens eliciting activation of immune system and pro-inflammatory cytokines[20,37,38]. Our results demonstrate that LPS treatment considerably increased paracellular permeability in cell monolayers (Caco-2 and T84) and in rat colon tissues. The concentrations of plecanatide and dolcanatide used in these experiments were based on the dose-response curves established with these cell lines and rat tissues as reported earlier[25,39-41]. These deleterious effects of LPS were completely suppressed by treatment with plecanatide or dolcanatide. The fluorescence microscopy data with monolayers of Caco-2 and T84 indicate that LPS treatment also severely disrupted the localization of TJ proteins such as occludin and ZO-1. Importantly, treatment with either agonist effectively suppressed LPS-mediated disruption in localization of occludin and ZO-1 at the TJ surrounding the cells. These data are consistent with the recent findings that GC-C signaling plays a critical role in the maintenance of intestinal barrier function[24,35]. As expected from analogs of uroguanylin, plecanatide and dolcanatide are likely to exert their pharmacological activities through activation of GC-C signaling in the GI tract. In this context, we recently reported that oral treatment with plecanatide or dolcanatide ameliorated GI inflammation through activation of GC-C signaling in the distal large intestine[39]. Subsequently, we reported that oral treatment with plecanatide delayed the onset of inflammation-driven colitis to colorectal carcinogenesis in ApcMin/+-FCCC mice[40]. The emerging paradigm suggests that normal function of GC-C signaling may include host defense by limiting systemic dissemination of luminal antigens through maintenance of mucosal barrier function.
Results presented in this study further demonstrate that oral treatment with plecanatide or dolcanatide reduced TNBS- and PRS-induced visceral hypersensitivity, as assessed by reductions in CRD-induced abdominal contractions in rats. However, treatment with either of the GC-C agonists did not alter the colonic compliance produced by either of the methods to induce visceral hypersensitivity. Only the lower doses (0.01 and 0.05 mg/kg) showed the most significant inhibition of visceral hypersensitivity in both models and that higher doses (> 0.5 mg/kg) of either GC-C agonist were not effective in these models. These results are consistent with the results obtained with orally administered linaclotide, also a GC-C agonist, in the same rat models showing that only the lower doses of linaclotide (0.01 to 0.3 mg/kg) were effective in the TNBS model, whereas the higher doses (3 and 30 mg/kg) were completely ineffective[32]. Although the precise explanation for the discrepancy in dose response remains to be determined, it is possible that the loss of anti-hyperalgesic effect at higher doses is associated with the loss of pharmacological specificity of the treatment. It is known that high levels of cGMP also down-regulate cAMP-specific phosphodiesterases resulting in increased levels of cAMP and activation of cAMP-dependent signaling pathways[42,43]. In this context, elevated levels of cAMP are known to be associated with hyperalgesia. We have also observed a similar bell-shaped response in animal models of colitis, inflammation-driven colorectal carcinogenesis, and polyp formation in ApcMin/+ mice[39,40].
Although the molecular mechanisms by which plecanatide or dolcanatide reduce visceral hypersensitivity still remain to be fully elucidated, these drug candidates seem to mimic the physiological function of uroguanylin in activating GC-C, resulting in increased fluid secretion to promote bowel movement. Consistent with this notion, we reported earlier that oral treatment with plecanatide promotes normal bowel movement in adult patients with CIC[11,12]. Based on the data presented herein, it is conceivable that activation of GC-C signaling and the initiation of downstream events may ameliorate the low-grade inflammation and thereby suppress activation of visceral nociceptive sensory pathways in the gut. The unique combination of regulation of ion/fluid secretion and anti-inflammatory activities of plecanatide makes this drug suitable to treat CIC and IBS-C. Oral treatment with plecanatide also demonstrated efficacy and a well-tolerated safety profile in two phase III clinical trials in patients with CIC[11,12]. Notably, the efficacy of plecanatide in reducing visceral hypersensitivity in animal models is consistent with the reduction in abdominal pain observed in two large phase III IBS-C clinical studies[44]. Thus, GC-C agonists are emerging as promising drug candidates for the treatment of functional GI disorders and IBD[8,11,12,44].
ARTICLE HIGHLIGHTS
Research background
Activation of guanylate cyclase-C (GC-C) signaling is an emerging therapeutic target for the treatment of gastrointestinal disorders and inflammatory diseases. Loss of GC-C signaling may disrupt intestinal water and ion secretion, resulting in chronic idiopathic constipation or irritable bowel syndrome with constipation (IBS-C). In addition, reduced GC-C signaling may also disrupt intestinal barrier function due to increased paracellular permeability, allowing entry of inflammatory mediators to promote low-grade inflammation and visceral hypersensitivity associated with abdominal pain in IBS-C patients.
Research motivation
Plecanatide and dolcanatide are analogs of human uroguanylin, the endogenous agonist of GC-C receptors, and are targeted at the treatment of functional constipation disorders and inflammatory bowel disease (IBD), respectively; therefore we sought to further characterize the mechanisms of these peptides using in vitro and in vivo models of these diseases.
Research objectives
To discern the role of plecanatide and dolcanatide in the maintenance of mucosal membrane integrity and in the reduction of visceral hypersensitivity in inflammatory and non-inflammatory animal models.
Research methods
Maintenance of epithelial cell integrity by plecanatide or dolcanatide in response to chemical challenge by lipopolysaccharide (LPS) was assessed using cell lines, as well as tissue harvested from rat intestines. Paracellular permeability was determined by calculating the flux of fluorescein isothiocyanate (FITC)-dextran using immunofluorescence microscopy. Electromyographic recordings were used to assess suppression of visceral hypersensitivity by plecanatide or dolcanatide in rat models of inflammatory and non-inflammatory visceral pain.
Research results
Plecanatide or dolcanatide effectively suppressed LPS-induced paracellular permeability. Oral treatment with plecanatide or dolcanatide considerably attenuated visceral hypersensitivity in inflammatory and non-inflammatory models of visceral pain.
Research conclusions
The data presented suggest further mechanisms, in addition to their better known secretory effects, whereby plecanatide or dolcanatide treatment, through activation of the GC-C receptor, may protect the epithelial barrier from increased paracellular permeability and provide anti-nociceptive activity, which may ultimately benefit patients with functional constipation disorders and IBD.
Research perspectives
Plecanatide is a secretagogue approved in the US for the treatment of adults with chronic idiopathic constipation or IBS-C. Dolcanatide is under evaluation for the treatment of opioid-induced constipation and ulcerative colitis. This study provides preclinical evidence that plecanatide and dolcanatide may act to preserve the integrity of the intestinal epithelium and to provide anti-nociceptive activity, supporting ongoing investigations of these peptides in functional constipation disorders and IBD.
ACKNOWLEDGMENTS
All in vivo animal studies presented in this manuscript were conducted under direct supervision of the late Dr. Lionel Bueno, to whom this manuscript is dedicated. Editorial and manuscript submission support were provided by The Medicine Group (New Hope, PA, United States).
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Supported by Synergy Pharmaceuticals Inc., which provided the funding and the plecanatide, dolcanatide and placebo used for these studies.
Institutional animal care and use committee statement: Animal care and handling procedures for ex vivo studies performed in the United States were as per the approved protocol by the Institutional Animal Care and Use Committee of Lampire Biologicals (Pipersville, PA, United States). Animal handling procedures for in vivo studies conducted in France were approved by the Institutional Animal Care and Use Local Committee (Toulouse, France).
Conflict-of-interest statement: Foss JA, Eddy EP, Palejwala VA and Shailubhai K are employees and/or stockholders of Synergy Pharmaceuticals Inc. All other authors have no conflicts to declare.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 26, 2018
First decision: February 5, 2018
Article in press: March 18, 2018
P- Reviewer: Luo HS, Luthin DR S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
Contributor Information
Illona-Marie Boulete, UMR 1331 Toxalim INRA/INPT, Toulouse 31555, France.
Anusha Thadi, Baruch S. Blumberg Institute, Doylestown, PA 18902, United States.
Catherine Beaufrand, UMR 1331 Toxalim INRA/INPT, Toulouse 31555, France.
Viren Patwa, Baruch S. Blumberg Institute, Doylestown, PA 18902, United States.
Apoorva Joshi, Baruch S. Blumberg Institute, Doylestown, PA 18902, United States.
John A Foss, Synergy Pharmaceuticals Inc., 420 Lexington Avenue, New York, NY 10170, United States.
E Priya Eddy, Synergy Pharmaceuticals Inc., 420 Lexington Avenue, New York, NY 10170, United States.
Helene Eutamene, UMR 1331 Toxalim INRA/INPT, Toulouse 31555, France.
Vaseem A Palejwala, Synergy Pharmaceuticals Inc., 420 Lexington Avenue, New York, NY 10170, United States.
Vassilia Theodorou, UMR 1331 Toxalim INRA/INPT, Toulouse 31555, France.
Kunwar Shailubhai, Baruch S. Blumberg Institute, Doylestown, PA 18902, United States. shailubhai@gmail.com; Synergy Pharmaceuticals Inc., 420 Lexington Avenue, New York, NY 10170, United States.
References
- 1.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:1582–1591; quiz 1581, 1592. doi: 10.1038/ajg.2011.164. [DOI] [PubMed] [Google Scholar]
- 3.Heidelbaugh JJ, Stelwagon M, Miller SA, Shea EP, Chey WD. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol. 2015;110:580–587. doi: 10.1038/ajg.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drossman DA, Morris CB, Schneck S, Hu YJ, Norton NJ, Norton WF, Weinland SR, Dalton C, Leserman J, Bangdiwala SI. International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. J Clin Gastroenterol. 2009;43:541–550. doi: 10.1097/MCG.0b013e318189a7f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilleri M. Pharmacological agents currently in clinical trials for disorders in neurogastroenterology. J Clin Invest. 2013;123:4111–4120. doi: 10.1172/JCI70837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zielińska M, Wasilewski A, Fichna J. Tenapanor hydrochloride for the treatment of constipation-predominant irritable bowel syndrome. Expert Opin Investig Drugs. 2015;24:1093–1099. doi: 10.1517/13543784.2015.1054480. [DOI] [PubMed] [Google Scholar]
- 7.Li F, Fu T, Tong WD, Liu BH, Li CX, Gao Y, Wu JS, Wang XF, Zhang AP. Lubiprostone Is Effective in the Treatment of Chronic Idiopathic Constipation and Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Mayo Clin Proc. 2016;91:456–468. doi: 10.1016/j.mayocp.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Layer P, Stanghellini V. Review article: Linaclotide for the management of irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2014;39:371–384. doi: 10.1111/apt.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacy BE, Levy LC. Lubiprostone: a novel treatment for chronic constipation. Clin Interv Aging. 2008;3:357–364. doi: 10.2147/cia.s2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu SW, Rao SS. Advances in the management of constipation-predominant irritable bowel syndrome: the role of linaclotide. Therap Adv Gastroenterol. 2014;7:193–205. doi: 10.1177/1756283X14537882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeMicco M, Barrow L, Hickey B, Shailubhai K, Griffin P. Randomized clinical trial: efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation. Therap Adv Gastroenterol. 2017;10:837–851. doi: 10.1177/1756283X17734697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miner PB Jr, Koltun WD, Wiener GJ, De La Portilla M, Prieto B, Shailubhai K, Layton MB, Barrow L, Magnus L, Griffin PH. A Randomized Phase III Clinical Trial of Plecanatide, a Uroguanylin Analog, in Patients With Chronic Idiopathic Constipation. Am J Gastroenterol. 2017;112:613–621. doi: 10.1038/ajg.2016.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez C, González-Castro A, Vicario M, Santos J. Cellular and molecular basis of intestinal barrier dysfunction in the irritable bowel syndrome. Gut Liver. 2012;6:305–315. doi: 10.5009/gnl.2012.6.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 15.Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 16.Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010;20:142–149. doi: 10.1016/j.tcb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Piche T. Tight junctions and IBS--the link between epithelial permeability, low-grade inflammation, and symptom generation? Neurogastroenterol Motil. 2014;26:296–302. doi: 10.1111/nmo.12315. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertiaux-Vandaële N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, Leroi AM, Déchelotte P, Ménard JF, Ducrotté P, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. 2011;106:2165–2173. doi: 10.1038/ajg.2011.257. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol. 2011;46:421–431. doi: 10.1007/s00535-011-0379-9. [DOI] [PubMed] [Google Scholar]
- 22.Collins SM, Piche T, Rampal P. The putative role of inflammation in the irritable bowel syndrome. Gut. 2001;49:743–745. doi: 10.1136/gut.49.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bercik P, Verdu EF, Collins SM. Is irritable bowel syndrome a low-grade inflammatory bowel disease? Gastroenterol Clin North Am. 2005;34:235–245, vi-vii. doi: 10.1016/j.gtc.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Han X, Mann E, Gilbert S, Guan Y, Steinbrecher KA, Montrose MH, Cohen MB. Loss of guanylyl cyclase C (GCC) signaling leads to dysfunctional intestinal barrier. PLoS One. 2011;6:e16139. doi: 10.1371/journal.pone.0016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shailubhai K, Yu HH, Karunanandaa K, Wang JY, Eber SL, Wang Y, Joo NS, Kim HD, Miedema BW, Abbas SZ, et al. Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res. 2000;60:5151–5157. [PubMed] [Google Scholar]
- 26.Brenna Ø, Bruland T, Furnes MW, Granlund Av, Drozdov I, Emgård J, Brønstad G, Kidd M, Sandvik AK, Gustafsson BI. The guanylate cyclase-C signaling pathway is down-regulated in inflammatory bowel disease. Scand J Gastroenterol. 2015;50:1241–1252. doi: 10.3109/00365521.2015.1038849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lan D, Niu J, Miao J, Dong X, Wang H, Yang G, Wang K, Miao Y. Expression of guanylate cyclase-C, guanylin, and uroguanylin is downregulated proportionally to the ulcerative colitis disease activity index. Sci Rep. 2016;6:25034. doi: 10.1038/srep25034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gué M, Del Rio-Lacheze C, Eutamene H, Théodorou V, Fioramonti J, Buéno L. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil. 1997;9:271–279. doi: 10.1046/j.1365-2982.1997.d01-63.x. [DOI] [PubMed] [Google Scholar]
- 29.Morteau O, Hachet T, Caussette M, Bueno L. Experimental colitis alters visceromotor response to colorectal distension in awake rats. Dig Dis Sci. 1994;39:1239–1248. doi: 10.1007/BF02093789. [DOI] [PubMed] [Google Scholar]
- 30.Williams CL, Villar RG, Peterson JM, Burks TF. Stress-induced changes in intestinal transit in the rat: a model for irritable bowel syndrome. Gastroenterology. 1988;94:611–621. doi: 10.1016/0016-5085(88)90231-4. [DOI] [PubMed] [Google Scholar]
- 31.Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol. 2009;9:715–720. doi: 10.1016/j.coph.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eutamene H, Bradesi S, Larauche M, Theodorou V, Beaufrand C, Ohning G, Fioramonti J, Cohen M, Bryant AP, Kurtz C, et al. Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain. Neurogastroenterol Motil. 2010;22:312–e84. doi: 10.1111/j.1365-2982.2009.01385.x. [DOI] [PubMed] [Google Scholar]
- 33.Steinbrecher KA. The multiple roles of guanylate cyclase C, a heat stable enterotoxin receptor. Curr Opin Gastroenterol. 2014;30:1–6. doi: 10.1097/MOG.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 34.Shailubhai K. Therapeutic applications of guanylate cyclase-C receptor agonists. Curr Opin Drug Discov Devel. 2002;5:261–268. [PubMed] [Google Scholar]
- 35.Mann EA, Harmel-Laws E, Cohen MB, Steinbrecher KA. Guanylate cyclase C limits systemic dissemination of a murine enteric pathogen. BMC Gastroenterol. 2013;13:135. doi: 10.1186/1471-230X-13-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhn M. Molecular Physiology of Membrane Guanylyl Cyclase Receptors. Physiol Rev. 2016;96:751–804. doi: 10.1152/physrev.00022.2015. [DOI] [PubMed] [Google Scholar]
- 37.Ma TY. Intestinal epithelial barrier dysfunction in Crohn’s disease. Proc Soc Exp Biol Med. 1997;214:318–327. doi: 10.3181/00379727-214-44099. [DOI] [PubMed] [Google Scholar]
- 38.Hollander D. Intestinal permeability, leaky gut, and intestinal disorders. Curr Gastroenterol Rep. 1999;1:410–416. doi: 10.1007/s11894-999-0023-5. [DOI] [PubMed] [Google Scholar]
- 39.Shailubhai K, Palejwala V, Arjunan KP, Saykhedkar S, Nefsky B, Foss JA, Comiskey S, Jacob GS, Plevy SE. Plecanatide and dolcanatide, novel guanylate cyclase-C agonists, ameliorate gastrointestinal inflammation in experimental models of murine colitis. World J Gastrointest Pharmacol Ther. 2015;6:213–222. doi: 10.4292/wjgpt.v6.i4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang WL, Masih S, Thadi A, Patwa V, Joshi A, Cooper HS, Palejwala VA, Clapper ML, Shailubhai K. Plecanatide-mediated activation of guanylate cyclase-C suppresses inflammation-induced colorectal carcinogenesis in Apc+/Min-FCCC mice. World J Gastrointest Pharmacol Ther. 2017;8:47–59. doi: 10.4292/wjgpt.v8.i1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patwa V, Joshi A, Thadi A, Eddy EP, Palejwala VA, Jacob GS, Shailubhai K. 967 Plecanatide, like uroguanylin, activates guanylate cyclase-C signaling in a pH-dependent manner in T84 cells, and in murine intestinal epithelial cells and tissues (abstract) Gastroenterology. 2016;150:S193–S194. [Google Scholar]
- 42.Forte LR, Thorne PK, Eber SL, Krause WJ, Freeman RH, Francis SH, Corbin JD. Stimulation of intestinal Cl- transport by heat-stable enterotoxin: activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1992;263:C607–C615. doi: 10.1152/ajpcell.1992.263.3.C607. [DOI] [PubMed] [Google Scholar]
- 43.Cunha FQ, Teixeira MM, Ferreira SH. Pharmacological modulation of secondary mediator systems--cyclic AMP and cyclic GMP--on inflammatory hyperalgesia. Br J Pharmacol. 1999;127:671–678. doi: 10.1038/sj.bjp.0702601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fogel R, Dorn SD, Krause R, Eng P, Kirshoff R, Nguyen A, Griffin P. Efficacy and safety of plecanatide in patients with irritable bowel syndrome with constipation: results from 2 randomized, double-blind, placebo-controlled clinical trials. Gastroenterology. 2017;152:S1309–S1310. doi: 10.1038/s41395-018-0026-7. [DOI] [PubMed] [Google Scholar]