Abstract
AIM
To investigate the antitumor activity of α-hederin in hepatocellular carcinoma (HCC) cells and its underlying mechanisms in vitro and in vivo.
METHODS
SMMC-7721, HepG-2 and Huh-7 HCC cells were cultured in vitro and treated with α-hederin (0, 5 μmol/L, 10 μmol/L, 15 μmol/L, 20 μmol/L, 25 μmol/L, 30 μmol/L, 35 μmol/L, 40 μmol/L, 45 μmol/L, 50 μmol/L, 55 μmol/L, or 60 μmol/L) for 12 h, 24 h, or 36 h, and cell viability was then detected by the Cell Counting Kit-8. SMMC-7721 cells were treated with 0, 5 μmol/L, 10 μmol/L, or 20 μmol/L α-hederin for 24 h with or without DL-buthionine-S,R-sulfoximine (2 mmol/L) or N-acetylcysteine (5 mmol/L) pretreatment for 2 h, and additional assays were subsequently performed. Apoptosis was observed after Hoechst staining. Glutathione (GSH) and adenosine triphosphate (ATP) levels were measured using GSH and ATP Assay Kits. Intracellular reactive oxygen species (ROS) levels were determined by measuring the oxidative conversion of 2’,7’-dichlorofluorescin diacetate. Disruption of the mitochondrial membrane potential was evaluated using JC-1 staining. The protein levels of Bax, Bcl-2, cleaved caspase-3, cleaved caspase-9, apoptosis-inducing factor and cytochrome C were detected by western blotting. The antitumor efficacy of α-hederin in vivo was evaluated in a xenograft tumor model.
RESULTS
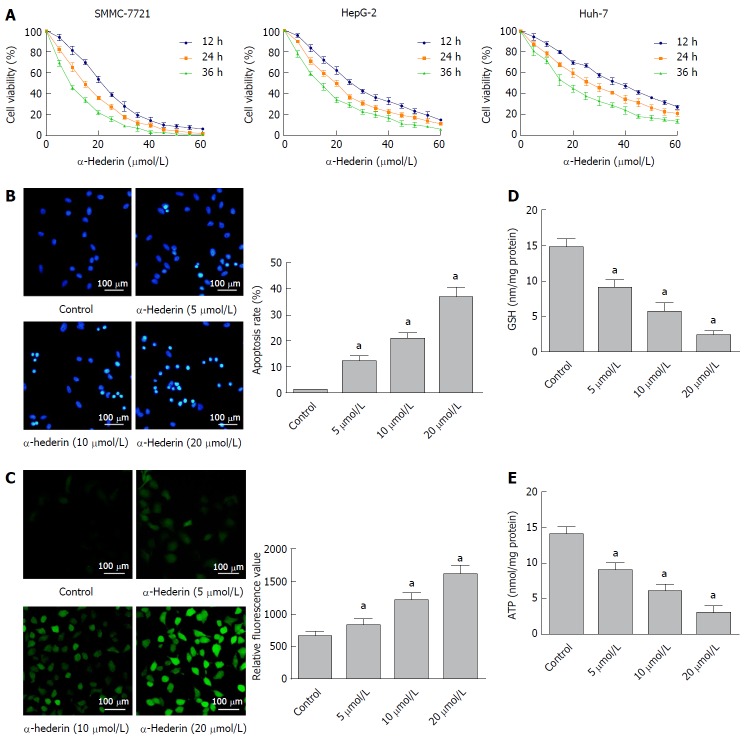
The α-hederin treatment induced apoptosis of HCC cells. The apoptosis rates in the control, low-dose α-hederin (5 μmol/L), mid-dose α-hederin (10 μmol/L) and high-dose α-hederin (20 μmol/L) groups were 0.90% ± 0.26%, 12% ± 2.0%, 21% ± 2.1% and 37% ± 3.8%, respectively (P < 0.05). The α-hederin treatment reduced intracellular GSH and ATP levels, induced ROS, disrupted the mitochondrial membrane potential, increased the protein levels of Bax, cleaved caspase-3, cleaved caspase-9, apoptosis-inducing factor and cytochrome C, and decreased Bcl-2 expression. The α-hederin treatment also inhibited xenograft tumor growth in vivo.
CONCLUSION
The α-hederin saponin induces apoptosis of HCC cells via the mitochondrial pathway mediated by increased intracellular ROS and may be an effective treatment for human HCC.
Keywords: Hepatic carcinoma, α-hederin, Apoptosis, Reactive oxygen species, Mitochondria
Core tip: The α-hederin saponin induces apoptosis of hepatocellular carcinoma cells in vitro and in vivo. We found that reactive oxygen species and the mitochondrial pathway play a vital role in α-hederin-induced apoptosis.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a highly prevalent disease worldwide, particularly in many Asian countries, with a very high incidence of over 20 cases/100000 individuals[1]. It is the fifth most common malignancy and the second most common cause of cancer-related death, and related deaths increased from 600000 in 2008 to 746000 in 2012[1,2]. It is also recognized as the main cause of death in patients with cirrhosis[3]. HCC treatment mainly includes systemic chemotherapy, radiofrequency ablation, transarterial chemoembolization, ethanol or acetic acid injection, surgical resection, and, in rare cases, liver transplantation[4]. Although resection is the most common therapy, most patients are not eligible for this treatment because of tumor extent or poor hepatic condition[4,5]. Systemic chemotherapy is another possible treatment option, but it often has a low response rate and severe side effects. Multidrug resistance occurs frequently in patients treated with chemotherapy, leading to recurrence and poor survival[6]. The poor general prognosis is related to a low overall survival rate after 5 years, ranging from 24% to 41%[7]. Therefore, it is important to develop highly effective natural treatments with limited toxicity for HCC.
Triterpene saponins are natural amphiphilic compounds that have the potential to induce cancer cell death and increase the activity of chemotherapeutic agents or radiotherapy[8,9]. The α-hederin is a secondary saponin isolated from Hedera or Nigella species. It is the major active component of various traditional medicinal herbs and shows promising activity against colon and lung cancers. The α-hederin also has biological activities, such as antioxidant activity, antiinflammatory activity, and effects on smooth muscle contraction[10-14]. It is thought to promote cell apoptosis and/or membrane alterations[15], and excess reactive oxygen species (ROS) have been reported to be involved in these processes[16]. Excess ROS can cause oxidative damage to the mitochondrial membrane and trigger apoptosis through downstream signal transduction[17,18].
Reports on the anti-HCC activity of α-hederin are limited. In this study, we evaluated the effects of α-hederin on HCC cells both in vitro and in vivo and explored the underlying mechanisms.
MATERIALS AND METHODS
Cell lines and culture
The human SMMC-7721, HepG-2 and Huh-7 HCC cell lines were purchased from the Shanghai Cell Collection (Shanghai, China). HCC cells were cultured in DMEM (Gibco, Grand Island, NY, United States) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin. All cells were cultured in a 5% CO2 humidified incubator at 37 °C. The α-hederin was purchased from Sigma-Aldrich (St. Louis, MO, United States), dissolved in 100% dimethyl sulfoxide and stored at 5 °C.
Cell proliferation assays
Cells were seeded at a density of 5 × 103 cells per well in 96-well plates and then treated with 0, 5 μmol/L, 10 μmol/L, 15 μmol/L, 20 μmol/L, 25 μmol/L, 30 μmol/L, 35 μmol/L, 40 μmol/L, 45 μmol/L, 50 μmol/L, 55 μmol/L, or 60 μmol/L α-hederin for 12 h, 24 h, or 36 h. Cell proliferation was assessed at different times using Cell Counting Kit-8 (Beyotime, Shanghai, China) according to the manufacturer's protocol. Ten microliters of CCK-8 solution was added to each well for 1 h; the absorbance was then measured at 450 nm with a microplate reader (Victor31420 Multilabel Counter; PerkinElmer, Waltham, MA, United States) to calculate the cell viability in different groups.
Cell apoptosis assays
Apoptotic cells were examined using the Hoechst 33258 staining kit (Beyotime). SMMC-7721 cells were treated with 0, 5 μmol/L, 10 μmol/L, or 20 μmol/L α-hederin for 24 h with or without pretreatment with 2 mmol/L DL-buthionine-S,R-sulfoximine (BSO) (Sigma-Aldrich) or 5 mmol/L N-acetylcysteine (NAC) (Sigma-Aldrich) for 2 h and then fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min. After staining with 20 μmol/L Hoechst 33258 for 20 min, the cells were observed under a fluorescence microscope (Olympus, Tokyo, Japan), and apoptotic cells were identified by fragmented and condensed nuclei.
Measurement of intracellular glutathione and adenosine triphosphate
Glutathione (GSH) and adenosine triphosphate (ATP) levels were measured using a GSH Assay Kit (Beyotime) and an ATP Assay Kit (Beyotime). SMMC-7721 cells were treated with 0, 5 μmol/L, 10 μmol/L, or 20 μmol/L α-hederin for 24 h with or without pretreatment with BSO (2 mmol/L) or NAC (5 mmol/L) for 2 h, and the subsequent procedures were performed according to the manufacturers’ instructions. The experimental data were obtained with a microplate reader.
ROS detection
Intracellular ROS levels were determined by measuring the oxidative conversion of 2′,7′-dichlorofluorescin diacetate (DCFH-DA) to the fluorescent compound dichlorofluorescin (DCF) using a ROS Assay Kit (Beyotime). After treatment with 0, 5 μmol/L, 10 μmol/L, or 20 μmol/L α-hederin for 24 h with or without pretreatment with BSO (2 mmol/L) or NAC (5 mmol/L) for 2 h, SMMC-7721 cells cultured in 6- and 96-well plates were incubated with 10 μmol/L DCF-DA for 20 min at 37 °C. Cells cultured in 6-well plates were observed under an upright fluorescence microscope, while cells in 96-well plates were evaluated with a microplate reader.
Mitochondrial membrane potential (ΔΨm)
Changes in the ΔΨm were identified using JC-1 dye according to the manufacturer's specifications. SMMC-7721 cells were pretreated with BSO (2 mmol/L) or NAC (5 mmol/L) for 2 h, treated with 0 or 10 μmol/L α-hederin for 24 h, and then incubated with 1 mL of the JC-1 dye for 30 min in a 37 °C incubator. The cells were washed twice with PBS and then evaluated with a confocal laser scanning microscope (Olympus). JC-1 forms a red fluorescent aggregate at hyperpolarized membrane potentials, whereas it remains in the green fluorescent monomeric form at depolarized membrane potentials.
Western blot analysis
Total cellular protein was extracted on ice using RIPA lysis buffer containing protease inhibitors (Beyotime). Proteins were separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes. Membranes were blocked with 5% nonfat dry milk and incubated overnight with various primary antibodies at 4 °C. Next, antirabbit secondary antibodies were added for 1 h at room temperature. Band intensity was measured using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, United States).
Xenograft tumor model
All animal experiments were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Wuhan University. The animal protocol was designed to minimize animal pain and discomfort. The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark cycle, 50% humidity, and ad libitum access to food and water) for 1 wk prior to experimentation. All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) after being fasted overnight, and tissues were collected.
The antitumor efficacy of α-hederin in vivo was evaluated using a xenograft tumor model. Male BALB/c-nu/nu nude mice (4-6 wk old) were purchased from HFK Experimental Animal Center (Beijing, China). HCC cells (5.0 × 106) suspended in 100 μL of PBS were subcutaneously inoculated into the right dorsal flank of nude mice. When the tumors reached 100-150 mm3, the mice were randomly divided into four groups (n = 6 per group): control group, low-dose group (2.5 mg/kg), mid-dose group (5 mg/kg), and high-dose group (10 mg/kg). The α-hederin was administered via intraperitoneal injection every 3 d.
To create the tumor growth curve, the diameter of each xenograft tumor was measured with a caliper. The mice were weighed every 3 d. At the end of the experiment, xenotransplanted tumors, livers, lungs and brains were harvested for additional analysis. Mouse blood was collected for hepatic and renal function tests.
Hematoxylin and eosin and TUNEL staining
To further evaluate treatment efficiency, the tumors were dissected and fixed in 4% formaldehyde. Next, tumors were sectioned into slices and stained with hematoxylin and eosin (HE) for histological analysis. We performed TUNEL staining to detect apoptotic cells. Positive cells were identified, counted (eight random fields per slide), and analyzed by light microscopy (Olympus).
Statistical analysis
All data were collected from at least three independent experiments. One-way analysis of variance (ANOVA) and t-tests were performed to analyze all the data (SPSS 20.0 software; IBM Corp., Armonk, NY, United States). P < 0.05 indicated statistical significance.
RESULTS
α-hederin reduces HCC cell viability and induces apoptosis of HCC cells via GSH depletion and ROS accumulation
To investigate the effects of α-hederin on HCC cell growth, we treated HCC cells with different concentrations of α-hederin for 0, 12 h, 24 h, and 36 h. As shown in Figure 1A, α-hederin significantly reduced HCC cell viability in a dose- and time-dependent manner, with IC50 values at 24 h for SMMC-7721, HepG-2 and Huh-7 cells being 13.880 μmol/L, 18.450 μmol/L and 25.520 μmol/L, respectively. We further use the one-way ANOVA to analyze the IC50 values for each time period with SMMC-7721, HepG-2 and Huh-7 cells; there was statistical significance among the IC50 value of three time periods (P < 0.05).
Figure 1.
α-hederin reduces hepatocellular carcinoma cell viability and induces the apoptosis of hepatocellular carcinoma cells through GSH depletion and reactive oxygen species accumulation. A: Cell Counting Kit-8 assays showed that α-hederin inhibits the viability of hepatocellular carcinoma cells (SMMC-7721, HepG-2, and Huh-7) in a dose- and time-dependent manner; B: SMMC-7721 cells were incubated with α-hederin (0, 5 μmol/L, 10 μmol/L, or 20 μmol/L) and stained with Hoechst 33258. Apoptotic cells were identified by fragmented and condensed nuclei under a fluorescence microscope. The percentage of apoptotic cells was calculated, P for trend < 0.01; C: SMMC-7721 cells were incubated with α-hederin (0, 5 μmol/L, 10 μmol/L, or 20 μmol/L), followed by incubation with DCFH-DA and observation under a fluorescence microscope or measurement using a microplate reader, P for trend < 0.01; D and E: SMMC-7721 cells were treated with α-hederin (0, 5 μmol/L, 10 μmol/L, or 20 μmol/L). GSH and ATP levels were measured using GSH and ATP Assay Kits and a microplate reader, P for trend < 0.01. aP < 0.05 vs control. ATP: adenosine triphosphate; GSH: Glutathione; ROS: Reactive oxygen species.
The Hoechst 33258 staining results are shown in Figure 1B; α-hederin induced the apoptosis of HCC cells in a dose-dependent manner. The apoptosis rates in the control, low-dose α-hederin (5 μmol/L), mid-dose α-hederin (10 μmol/L) and high-dose α-hederin (20 μmol/L) groups were 0.90% ± 0.26%, 12% ± 2.0%, 21% ± 2.1% and 37% ± 3.8%, respectively (P < 0.05).
To determine whether α-hederin affected the intracellular ROS generation, SMMC cells were treated with α-hederin for 24 h. As shown in Figure 1C, the relative DCFH-DA fluorescence significantly increased in a dose-dependent manner. The α-hederin significantly reduced cellular GSH (Figure 1D) and ATP levels (Figure 1E) (P < 0.05). These results show that α-hederin may reduce HCC cell viability and induce the apoptosis of HCC cells via GSH depletion and ROS accumulation.
BSO and NAC influence α-hederin-induced apoptosis of SMMC-7721 cells
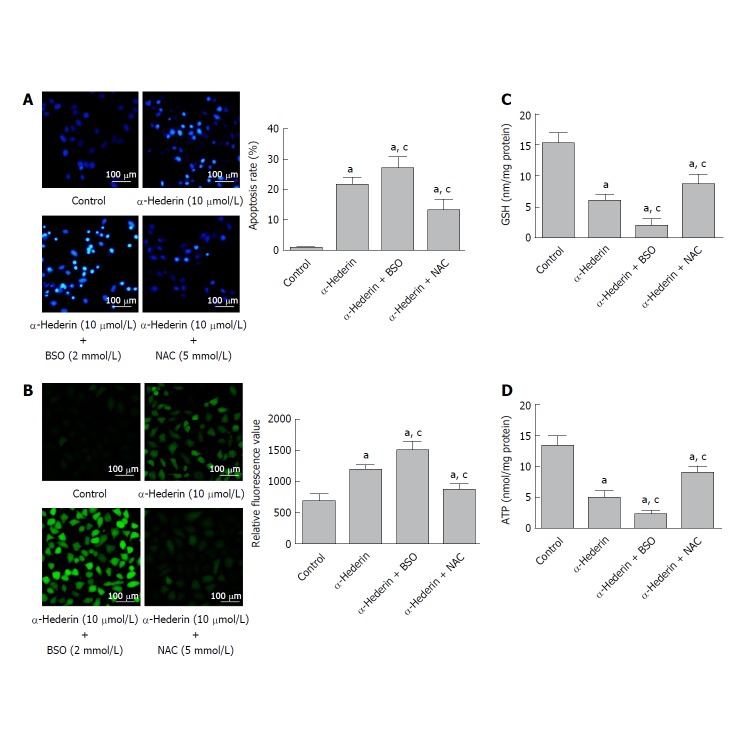
To further determine whether α-hederin induces the apoptosis of HCC cells via GSH depletion and ROS accumulation, SMMC cells were treated with 10 μmol/L α-hederin for 24 h with or without BSO (2 mmol/L) or NAC (5 mmol/L) pretreatment for 2 h. As shown in Figure 2A, the apoptosis rate varied as expected: 0.94% ± 0.25% in the control group, 22% ± 2.4% in the α-hederin group, 27% ± 3.5% in the α-hederin and BSO group, and 13% ± 3.3% in the α-hederin and NAC group (P < 0.05). Intracellular ROS levels are shown in Figure 2B.
Figure 2.
BSO and NAC influence the α-hederin-induced apoptosis of SMMC-7721 cells. SMMC-7721 cells were incubated with α-hederin (10 μmol/L) with or without BSO (2 mmol/L) or NAC (5 mmol/L) pretreatment. A: Cell apoptosis was determined by Hoechst 33258 staining; B: ROS levels in SMMC-7721 cells; C and D: Effect of α-hederin on intracellular GSH and ATP levels. aP < 0.05 vs control; cP < 0.05 vs α-hederin (10 μmol/L). ATP: adenosine triphosphate; BSO: DL-buthionine-S,R-sulfoximine; GSH: Glutathione; NAC: N-acetylcysteine.
Relative DCFH-DA fluorescence was significantly increased in the α-hederin (10 μmol/L) group compared to the control group (P < 0.05), and this increase was enhanced in the α-hederin and BSO group but reduced in the α-hederin and NAC group (P < 0.05). As shown in Figure 2C and D, intracellular GSH and ATP levels were significantly decreased in the α-hederin (10 μmol/L) group compared to the control group (P < 0.05), and these decreases were enhanced in the α-hederin and BSO group but reduced in the α-hederin and NAC group (P < 0.05). This result suggested that α-hederin induced apoptosis of HCC cells in an indirect way which is closely related to GSH and ROS.
α-hederin induces apoptosis through activation of the mitochondria-mediated pathway
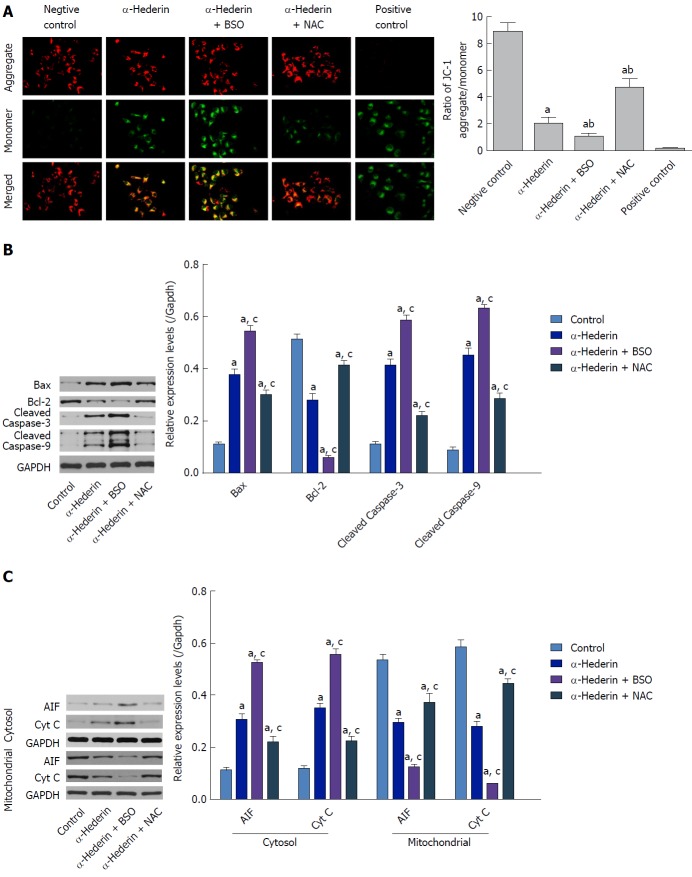
To investigate the underlying mechanism of apoptosis induced by α-hederin, we ascertained the effect of α-hederin on mitochondrial membrane depolarization using the JC-1 cationic dye. Compared to the control group, the ratio of aggregate-to-monomer fluorescence in the α-hederin (10 μmol/L) group was decreased (P < 0.05), as JC-1 fluorescence changed from red (aggregate) to green (monomer) (Figure 3A). Compared to that in the α-hederin group, the aggregate-to-monomer fluorescence ratio was decreased in the α-hederin and BSO group and increased in the α-hederin and NAC group (P < 0.05).
Figure 3.
α-hederin induces apoptosis through activation of the mitochondria-mediated pathway. A: Mitochondrial membrane potential was detected with JC-1. JC-1 aggregates (red fluorescence) under conditions of a normal mitochondrial membrane and forms a monomer (green fluorescence) under depolarizing conditions. Fluorescence was detected by a confocal laser scanning microscope (400 ×); B and C: Western blots showing the expression of mitochondrial pathway-related proteins in vitro. SMMC-7721 cells were treated with α-hederin (0 or 10 μmol/L) with or without BSO (2 mmol/L) or NAC (5 mmol/L) pretreatment, and the protein levels of Bcl-2, Bax, caspase-9, caspase-3, AIF, and Cyt C in SMMC-7721 cells were then detected by western blotting. GAPDH expression was used as an internal control. The relative expression levels of these proteins in SMMC-7721 cells in different groups were compared. aP < 0.05 vs control; cP < 0.05 vs α-hederin (10 μmol/L). AIF: Apoptosis-inducing factor; ATP: adenosine triphosphate; BSO: DL-buthionine-S,R-sulfoximine; Cyt C: Cytochrome C; NAC: N-acetylcysteine.
Then, we conducted western blotting to examine the effect of α-hederin on the levels of mitochondrial pathway-related proteins. As shown in Figure 3B, α-hederin increased the levels of Bax, cleaved caspase-3 and cleaved caspase-9, and decreased Bcl-2 expression levels. Meanwhile, the mitochondria-mediated apoptosis-related proteins apoptosis-inducing factor (AIF) and cytochrome C (Cyt C) in cytoplasm were increased by α-hederin, but AIF and Cyt C in mitochondria were decreased (Figure 3C). Pretreatment with BSO augmented the α-hederin-induced changes in protein levels, whereas pretreatment with NAC weakened these effects of α-hederin.
α-hederin inhibits tumor growth in vivo
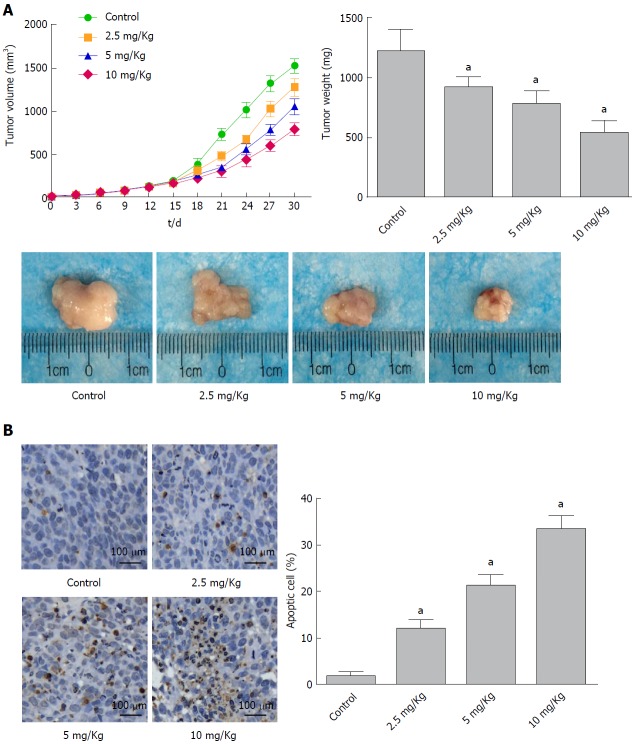
The anticancer effects of α-hederin in vivo were analyzed in a human xenograft tumor model. As shown in Figure 4A, the transplanted tumor volume increased more slowly with increasing α-hederin concentration, and the final tumor weight was lower in the α-hederin-treated groups. At the end of the experiment, the tumor weights in the control and 2.5 mg/kg, 5 mg/kg and 10 mg/kg α-hederin groups were 1217 mg ± 177 mg, 917 mg ± 84 mg, 778 mg ± 105 mg and 539 mg ± 96 mg, respectively. Tumor growth was significantly suppressed in the α-hederin groups in a dose-dependent manner (P < 0.05). TUNEL staining of the tumors is shown in Figure 4B, and cells stained brown are apoptotic. Compared to the control group, the α-hederin groups showed a gradual increase in the proportion of apoptotic cells with increasing drug concentration (P < 0.05).
Figure 4.
α-Hederin inhibits tumor growth in vivo. Mice with xenograft tumors were divided into four groups (Control and 2.5 mg/kg, 5 mg/kg and 10 mg/kg α-Hederin, n = 6 mice per group). A: Mean tumor volume at each time point and final tumor weight, P for trend < 0.05; B: TUNEL assays detected apoptotic cells in xenograft tumor tissue, as evidenced by the presence of nut-brown nuclei under a fluorescence microscope. The percentage of apoptotic cells was calculated, P for trend < 0.05. aP < 0.05 vs control.
Liver, lung and brain tissue from each group was stained with HE, and no tumor metastases were observed. We assayed the hepatic and renal functions of nude mice treated with control or α-hederin and found that alanine aminotransferase, aspartate aminotransferase, urea and creatine levels were not significantly different.
DISCUSSION
The α-hederin saponin has various biological activities, including anticancer activity in some cancer cells. However, its effects on HCC have not been clarified. In the present study, to investigate the effects of α-hederin on HCC cells, we performed the following: Cell proliferation and apoptosis assays; detected ROS, GSH and ATP levels and the mitochondrial membrane potential; conducted Western blotting analysis to examine related proteins; and generated a xenograft tumor model to evaluate the antitumor efficacy of α-hederin in vivo. Our results show that α-hederin induces the apoptosis of HCC cells in vitro and in vivo and suggest that the mechanism involves the mitochondrial pathway mediated by increased intracellular ROS.
In this study, we found that α-hederin significantly inhibited the proliferation of HCC cells and induced their apoptosis in a dose- and time-dependent manner. We also found that α-hederin decreased GSH and ATP levels and increased ROS levels in a concentration-dependent manner. These results are consistent with those of Swamy et al[16], who reported that α-hederin increased the apoptosis of murine P388 leukemia cells and increased the production of ROS in a dose- and time-dependent manner. It has been reported that cancer cells have increased ROS production compared to normal cells. ROS is generated through a variety of extracellular and intracellular actions. Severe accumulation of cellular ROS may induce lethal damage in cells.
GSH is one of the most common intracellular compounds that plays a vital role in the cellular defense against ROS damage. GSH clears intracellular ROS by nonenzymatic and enzymatic catalysis. The nonenzymatic process involves GSH acting directly. The enzyme catalyzed process is based on GSH as the substrate, and induces the clearance of ROS in cells under the catalysis of GSH-peroxidase or GSH S transferase[19,20]. During intracellular GSH synthesis, two ATP-dependent enzyme catalyzes are required: Glutamate cysteine ligase and glutathione synthetase[21].
Our study shows α-hederin significantly reduced cellular ATP levels. Therefore, a reduction in intracellular ATP contributes to a decrease in GSH, leading to ROS accumulation and cellular damage. To determine whether the apoptotic effect of α-hederin on HCC cells is associated with the generation of intracellular ROS, we pretreated SMMC-7721 cells with BSO or NAC, which improved/decreased the levels of intracellular GSH and ROS. The results showed that the apoptotic effect of α-hederin was greater after pretreatment with BSO but was ameliorated by NAC. These data indicate that the apoptosis-inducing potential of α-hederin is related to intracellular ROS production.
Mitochondria play an important role in cancer cell survival[22], as they are major sources of cellular bioenergetics and the target of ROS. ROS can induce oxidative damage that affects mitochondrial function, and a decrease in ΔΨm indicates damage to mitochondrial function. Cheng et al[23] reported on the mitochondrial apoptotic activity of α-hederin in breast cancer cells. A previous study showed that ROS causes the mitochondrial permeability transition pore (mPTP) to open in HepG-2 cells[24]. We next evaluated whether ROS induced this mitochondria-mediated apoptotic mechanism in HCC cells treated with α-hederin. Similar to breast cancer cells, SMMC-7721 cells treated with α-hederin showed a clear decrease in ΔΨm compared to untreated cells. Additionally, the ΔΨm decrease was aggravated by BSO but relieved by NAC.
To further investigate whether the ROS increase and ΔΨm loss induced by α-hederin led to HCC cell apoptosis, we detected the levels of related proteins. We found that α-hederin increased the protein levels of Bax, cleaved caspase-3 and cleaved caspase-9 but decreased Bcl-2 levels. Thus, the antiapoptotic/proapoptotic (Bcl-2/Bax) protein ratio decreased. AIF and Cyt C protein levels were increased by α-hederin. Although the α-hederin-induced changes in the above proteins were enhanced by pretreatment with BSO, they were weakened by NAC pretreatment. Bcl-2 family proteins are reported to be key factors in regulating the mitochondrial apoptosis pathway[25]. Disruption of the Bcl-2/Bax protein balance induces apoptosis.
Bcl-2 family proteins are also components of the mPTP. A decrease in Bcl-2 levels alters the mPTP structure and the ΔΨm, increasing mitochondrial membrane permeability[26]. Additionally, excess ROS can trigger opening of the mPTP[27]. As a result, AIF and Cyt C proteins are released to activate procaspase-9, which activates the caspase cascade that ultimately generates caspase-3 to induce apoptosis. On the other hand, AIF can mediate apoptosis directly in a caspase-independent way[28]. These data indicate that the mechanism by which α-hederin induces HCC cell apoptosis involves the mitochondrial pathway mediated by increased intracellular ROS.
In human xenograft tumor models in nude mice, α-hederin significantly inhibited tumor growth without causing liver and kidney damage, indicating the efficacy and safety of α-hederin for the treatment of HCC in vivo.
In conclusion, we show herein that α-hederin induces the apoptosis of HCC cells via the mitochondrial pathway mediated by increased intracellular ROS in vitro and in vivo. These findings identify α-hederin as a potential highly effective natural medicine with limited toxicity for HCC treatment. However, α-hederin has been reported to have other effects, such as membrane permeabilizing activity, which can directly induce cell death[29]. This study is not sufficient to clarify the antitumor effects of α-hederin. Further studies should focus on the detailed mechanism.
ARTICLE HIGHLIGHTS
Research background
Hepatocellular carcinoma (HCC) is a highly prevalent disease worldwide, with poor general prognosis. To develop highly effective natural treatments with limited toxicity for HCC is important. The α-hederin saponin is reported to have antitumor activity. However, the effect of α-hederin on HCC remains to be examined. We evaluated the effect and possible mechanism of α-hederin on HCC cells both in vitro and in vivo.
Research motivation
Developing new, effective and nontoxic chemotherapeutic drugs will contribute to the treatment and prognosis for HCC patients in clinic.
Research objectives
To investigate the antitumor activity of α-hederin in HCC cells and its underlying mechanisms in vitro and in vivo.
Research methods
Three HCC cells lines (SMMC-7721, HepG-2 and Huh-7 HCC cells) were used to detect the effect of α-hederin on HCC. Cell viability was detected by Cell Counting Kit-8 assay after cells were treated with α-hederin. (BSO) N-acetylcysteine (NAC) and DL-buthionine-S,R-sulfoximine (BSO) were used to interfere with the synthesis of glutathione (GSH) in the SMMC-7721 cells, then, the effects of α-hederin on cell proliferation, cell apoptosis, adenosine triphosphate (ATP) and reactive oxygen species (ROS) and mitochondrial membrane potential were detected. The protein levels of Bax, Bcl-2, cleaved caspase-3, cleaved caspase-9, apoptosis-inducing factor (AIF) and cytochrome C (Cyt C) were detected by western blotting. The antitumor efficacy of α-hederin on HCC was also evaluated in nude mice with xenograft tumor. The apoptosis of cancer cells in xenograft tumor were examined by TUNEL staining. In this research, as we used NAC and BSO to interfere with the synthesis of GSH, the mechanism we explored was more persuasive.
Research results
The α-hederin treatment inhibited cell growth of the three cell lines in a dose- and time-dependent manner. The IC50 values at 24 h for SMMC-7721, HepG-2 and Huh-7 cells were 13.88, 18.45 and 25.52 μmol/L, respectively, so we used SMMC-7721 cells for the on-going experiments. The results showed that the apoptosis rates in the control, low-dose α-hederin (5 μmol/L), mid-dose α-hederin (10 μmol/L) and high-dose α-hederin (20 μmol/L) groups were 0.90% ± 0.26%, 12% ± 2.05, 21% ± 2.15 and 37% ± 3.8%, respectively. In comparison to the control, after treatment with α-hederin, ROS increased significantly, while the ATP levels decreased. When SMMC-7721 cells were pretreated with BSO (2 mmol/L), compared with the mid-dose α-hederin group, the apoptosis rate increased to 27% ± 3.5% (P < 0.05); what’s more, the increase of ROS and the decrease of ATP were both enhanced. However, NAC pretreatment had a protective effect on SMMC-7721 cells and could alleviate the change of ROS and ATP. The proteins involving in the mitochondria-mediated pathway were detected by western blotting. The results showed α-hederin increased the levels of Bax, cleaved caspase-3 and cleaved caspase-9, and decreased Bcl-2 expression levels. Meanwhile, AIF and Cyt C in cytoplasm were up-regulated, but AIF and Cyt C in mitochondria were down-regulated. Subcutaneous xenografts were successfully constructed in 24 nude mice. After treatment with α-hederin for 3 wk, the weight of xenograft tumor was significantly reduced (P < 0.05). Compared to the control group, TUNEL staining showed a gradual increase in the proportion of apoptotic cells with the increase of α-hederin concentration (P < 0.05). There was no difference between the control mice and α-hederin-treated mic for the hepatic and renal functions. This research indicated that α-hederin could induce HCC cell apoptosis via mitochondria-mediated pathway by depleting GSH and accumulating ROS. But it did not explain how α-hederin changed the expression of GSH and ROS, and the effect of α-hederin on HCC cell invasion was not studied either. In addition, apoptosis involves multiple factors and multiple links, making it necessary to conduct in-depth research to clarify the specific mechanism.
Research conclusions
The α-hederin saponin induces apoptosis of HCC cells via the mitochondrial pathway mediated by increased intracellular ROS and may be an effective treatment for human HCC.
Research perspectives
It is of great value to discover natural anticancer compounds which have high efficacy and low toxicity in the treatment of HCC. In our study, we show that α-hederin could induce HCC cell apoptosis via mitochondria-mediated pathway by depleting GSH and accumulating ROS, which identifies α-hederin as a potential highly effective natural medicine with limited toxicity for HCC treatment. But some points remain unclear. How does α-hederin change the expression of ATP? The effect of α-hederin on HCC cell migration and invasion was not studied, either. In addition, apoptosis involves multiple factors and multiple links, and it’s necessary to conduct in-depth research to clarify specific mechanism. These results will facilitate the development of treatment for HCC.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Supported by the National Natural Science Foundation of China, No. 81572426; and the Natural Science Foundation of Hubei Province, No. 2015CKB755.
Institutional animal care and use committee statement: All animal experiments were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Wuhan University.
Conflict-of-interest statement: The authors declare that there are no conflicts-of-interest regarding the publication of this paper.
Data sharing statement: No additional data are available.
ARRIVE guidelines statement: The ARRIVE guidelines have been adopted.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 2, 2018
First decision: March 30, 2018
Article in press: April 9, 2018
P- Reviewer: Cheng TH, Sun CK S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Huang Y
Contributor Information
Jiao Li, Department of Gastroenterology, Renmin Hospital of Wuhan University, Central Laboratory of Renmin Hospital, Wuhan 430060, Hubei Province, China.
Dan-Dan Wu, Department of Gastroenterology, Renmin Hospital of Wuhan University, Central Laboratory of Renmin Hospital, Wuhan 430060, Hubei Province, China.
Ji-Xiang Zhang, Department of Gastroenterology, Renmin Hospital of Wuhan University, Central Laboratory of Renmin Hospital, Wuhan 430060, Hubei Province, China.
Jing Wang, Department of Gastroenterology, Beijing Shijitan Hospital of Capital Medical University, Beijing 100038, China.
Jing-Jing Ma, Department of Gastroenterology, Renmin Hospital of Wuhan University, Central Laboratory of Renmin Hospital, Wuhan 430060, Hubei Province, China.
Xue Hu, Department of Gastroenterology, Renmin Hospital of Wuhan University, Central Laboratory of Renmin Hospital, Wuhan 430060, Hubei Province, China.
Wei-Guo Dong, Department of Gastroenterology, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei Province, China. dongweiguo@whu.edu.cn.
References
- 1.Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14:4300–4308. doi: 10.3748/wjg.14.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303–1310. doi: 10.1002/hep.21176. [DOI] [PubMed] [Google Scholar]
- 4.Rahbari NN, Mehrabi A, Mollberg NM, Müller SA, Koch M, Büchler MW, Weitz J. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253:453–469. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- 5.Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010-2016. Clin Mol Hepatol. 2016;22:7–17. doi: 10.3350/cmh.2016.22.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Ng HLH, Lu A, Lin C, Zhou L, Lin G, Zhang Y, Yang Z, Zhang H. Drug delivery system targeting advanced hepatocellular carcinoma: Current and future. Nanomedicine. 2016;12:853–869. doi: 10.1016/j.nano.2015.12.381. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt S, Follmann M, Malek N, Manns MP, Greten TF. Critical appraisal of clinical practice guidelines for diagnosis and treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:1779–1786. doi: 10.1111/j.1440-1746.2011.06891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SJ, Sung JH, Lee SJ, Moon CK, Lee BH. Antitumor activity of a novel ginseng saponin metabolite in human pulmonary adenocarcinoma cells resistant to cisplatin. Cancer Lett. 1999;144:39–43. doi: 10.1016/s0304-3835(99)00188-3. [DOI] [PubMed] [Google Scholar]
- 9.Jiang H, Zhao P, Feng J, Su D, Ma S. Effect of Paris saponin I on radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell line. Oncol Lett. 2014;7:2059–2064. doi: 10.3892/ol.2014.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park HJ, Kwon SH, Lee JH, Lee KH, Miyamoto K, Lee KT. Kalopanaxsaponin A is a basic saponin structure for the anti-tumor activity of hederagenin monodesmosides. Planta Med. 2001;67:118–121. doi: 10.1055/s-2001-11516. [DOI] [PubMed] [Google Scholar]
- 11.Gepdiremen A, Mshvildadze V, Süleyman H, Elias R. Acute anti-inflammatory activity of four saponins isolated from ivy: alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F in carrageenan-induced rat paw edema. Phytomedicine. 2005;12:440–444. doi: 10.1016/j.phymed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Gülçin I, Mshvildadze V, Gepdiremen A, Elias R. Antioxidant activity of saponins isolated from ivy: alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F. Planta Med. 2004;70:561–563. doi: 10.1055/s-2004-827158. [DOI] [PubMed] [Google Scholar]
- 13.Mendel M, Chłopecka M, Dziekan N, Karlik W, Wiechetek M. Participation of cholinergic pathways in α-hederin-induced contraction of rat isolated stomach strips. Phytomedicine. 2012;19:591–595. doi: 10.1016/j.phymed.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Wolf A, Gosens R, Meurs H, Häberlein H. Pre-treatment with α-hederin increases β-adrenoceptor mediated relaxation of airway smooth muscle. Phytomedicine. 2011;18:214–218. doi: 10.1016/j.phymed.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Rooney S, Ryan MF. Modes of action of alpha-hederin and thymoquinone, active constituents of Nigella sativa, against HEp-2 cancer cells. Anticancer Res. 2005;25:4255–4259. [PubMed] [Google Scholar]
- 16.Swamy SM, Huat BT. Intracellular glutathione depletion and reactive oxygen species generation are important in alpha-hederin-induced apoptosis of P388 cells. Mol Cell Biochem. 2003;245:127–139. doi: 10.1023/a:1022807207948. [DOI] [PubMed] [Google Scholar]
- 17.Lee HH, Park C, Jeong JW, Kim MJ, Seo MJ, Kang BW, Park JU, Kim GY, Choi BT, Choi YH, et al. Apoptosis induction of human prostate carcinoma cells by cordycepin through reactive oxygen speciesmediated mitochondrial death pathway. Int J Oncol. 2013;42:1036–1044. doi: 10.3892/ijo.2013.1762. [DOI] [PubMed] [Google Scholar]
- 18.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 19.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutteridge JM, Halliwell B. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann N Y Acad Sci. 2000;899:136–147. doi: 10.1111/j.1749-6632.2000.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, Tao R, Shen Z, Sun L, Zhu F, Yang S. Enzymatic Production of Glutathione by Bifunctional γ-Glutamylcysteine Synthetase/Glutathione Synthetase Coupled with In Vitro Acetate Kinase-Based ATP Generation. Appl Biochem Biotechnol. 2016;180:1446–1455. doi: 10.1007/s12010-016-2178-5. [DOI] [PubMed] [Google Scholar]
- 22.Dias N, Bailly C. Drugs targeting mitochondrial functions to control tumor cell growth. Biochem Pharmacol. 2005;70:1–12. doi: 10.1016/j.bcp.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Cheng L, Xia TS, Wang YF, Zhou W, Liang XQ, Xue JQ, Shi L, Wang Y, Ding Q, Wang M. The anticancer effect and mechanism of α-hederin on breast cancer cells. Int J Oncol. 2014;45:757–763. doi: 10.3892/ijo.2014.2449. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Han L, Qi W, Cheng D, Ma X, Hou L, Cao X, Wang C. Eicosapentaenoic acid (EPA) induced apoptosis in HepG2 cells through ROS-Ca(2+)-JNK mitochondrial pathways. Biochem Biophys Res Commun. 2015;456:926–932. doi: 10.1016/j.bbrc.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 25.Llambi F, Green DR. Apoptosis and oncogenesis: give and take in the BCL-2 family. Curr Opin Genet Dev. 2011;21:12–20. doi: 10.1016/j.gde.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Q, Lesnefsky EJ. Blockade of electron transport during ischemia preserves bcl-2 and inhibits opening of the mitochondrial permeability transition pore. FEBS Lett. 2011;585:921–926. doi: 10.1016/j.febslet.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voronina S, Okeke E, Parker T, Tepikin A. How to win ATP and influence Ca(2+) signaling. Cell Calcium. 2014;55:131–138. doi: 10.1016/j.ceca.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Delavallée L, Cabon L, Galán-Malo P, Lorenzo HK, Susin SA. AIF-mediated caspase-independent necroptosis: a new chance for targeted therapeutics. IUBMB Life. 2011;63:221–232. doi: 10.1002/iub.432. [DOI] [PubMed] [Google Scholar]
- 29.Lorent JH, Léonard C, Abouzi M, Akabi F, Quetin-Leclercq J, Mingeot-Leclercq MP. α-Hederin Induces Apoptosis, Membrane Permeabilization and Morphologic Changes in Two Cancer Cell Lines Through a Cholesterol-Dependent Mechanism. Planta Med. 2016;82:1532–1539. doi: 10.1055/s-0042-114780. [DOI] [PubMed] [Google Scholar]