Abstract
Tenofovir disoproxil fumarate (TDF) is a potent nucleotide analogue that is recommended as first-line therapy for patients with chronic hepatitis B. The results of a longitudinal study of TDF treatment demonstrated no development of resistance. We observed one treatment-naïve chronic hepatitis B (CHB) patient who developed TDF resistance after complete viral suppression during long-term TDF treatment. A 37-year-old HBeAg-positive man received TDF 300 mg/d for 43 mo. The hepatitis B virus (HBV) DNA titer was 8 log10 copies/mL at baseline and became undetectable at 16 mo after treatment. However, the HBV DNA titer rebounded to 7.5 log10 copies/mL at 43 mo after treatment. We performed full sequencing to find mutation sites associated with virologic breakthrough. The results showed 9 mutation sites, most of which had not been well-known as mutation sites. We changed the therapy from tenofovir to entecavir with a regimen of 0.5 mg once daily. After 4 mo, the HBV DNA titer decreased to 267 copies/mL, and the liver enzyme levels were normalized.
Keywords: Chronic hepatitis B, Tenofovir, Resistance, Mutation
Core tip: The results of many clinical longitudinal studies of Tenofovir disoproxil fumarate (TDF) treatment have demonstrated no development of resistance until now. Recently, a few cases of resistance to TDF have been reported. However, the mutation site had not been clearly revealed and confirmed because of the rarity of resistant cases. In the present case, TDF resistance developed following the complete suppression of HBV DNA in a treatment-naïve patient. We detected 9 mutation sites, including some that have been unknown until now. We believe that the present study is helpful in revealing the exact mutation sites associated with TDF resistance.
INTRODUCTION
Chronic hepatitis B (CHB) affects approximately 250 million people worldwide and can lead to liver cirrhosis, liver failure, hepatocellular carcinoma (HCC), and death[1-3]. Globally, approximately 30% of cirrhosis cases and 53% of HCC cases have been attributed to CHB[2]. Antiviral therapy for hepatitis B virus (HBV) infection can suppress viral replication and halt disease progression[4,5]. The reduction of HBV DNA concentrations to very low or undetectable levels through antiviral therapy is associated with a reduced risk of mortality and HCC[6-8]. However, the therapeutic benefits are diminished because of the emergence of drug-resistant viruses.
Entecavir (ETC) and tenofovir disoproxil fumarate (TDF) are the two first-line therapies recommended for the treatment of CHB because they have a more potent antiviral effect and higher genetic barriers against resistance than other antiviral agents. The rate of antiviral resistance in previously untreated patients has been reported for ETC, i.e., 1.2% of patients develop resistance in 5 years[9,10]. The development of resistance to TDF has not been reported in treatment-naïve patients until now[11].
The rtA194T polymerase mutation combined with the rtL180M and rtM204V polymerase mutations is reported to be associated with TDF resistance in HIV/HBV co-infected patients[12]. However, TDF resistance-related mutations in patients infected only with HBV were unknown until now. Recently, Lee et al[13] reported two TDF resistance mutations in CHB patients during “The Liver Week 2017” symposium in the United States. The patients harbored CHB mutants with three new substitutions, namely, rtS106C, rtH126Y and rtD134E. These patients had previously been treated with various therapies, including lamivudine (LAM), adefovir (ADV) and ETC. We observed the development of TDF resistance in a patient who had no treatment history. This patient showed virologic and biochemical breakthroughs after he achieved a complete virologic response. In this study, we report the first case of TDF resistance in a treatment-naïve patient and review the pertinent literature.
CASE REPORT
The patient was a 37-year-old Korean male who visited the clinic because of elevated liver enzymes. He was first diagnosed as having chronic hepatitis B at the age of 20 and was followed up regularly in the family medicine department of Dankook University Hospital. Until his visit to the clinic, he had no history of liver enzyme elevation. His mother was also diagnosed with chronic hepatitis B but did not receive antiviral treatment. The patient’s laboratory examination showed that he was positive for HBsAg and HBeAg. His aspartate transaminase (AST) and alanine aminotransferase (ALT) levels were high, at 51 IU/L and 80 IU/L, respectively. His HBV DNA titer was greater than 8.99 log10 copies/mL, as measured by the Amplicor™ Monitor PCR assay (lower limit of detection, 116 copies/mL; Roche Diagnostics, Basel, Switzerland). Abdominal sonography revealed a diffuse mild fatty liver. No evidence of cirrhosis, such as splenomegaly, thrombocytopenia, or esophageal varices, was observed. The patient was started on Tenofovir disoproxil fumarate (TDF) 300 mg, one tablet daily. After 16 mo, the HBV DNA level was undetectable. The AST and ALT levels had also normalized to 27 IU/L and 35 IU/L, respectively. The patient continued the same treatment with complete adherence, but HBeAg was not converted. However, after 43 mo of continuous treatment, HBV DNA had increased to 7.5 log10 copies/mL. The levels of AST and ALT were also increased to 61 IU/L and 109 IU/L, respectively. The patient’s history showed that he took TDF regularly every day, and there was no history of the use of any other medicine that could either decrease the efficacy of TDF or increase its rate of metabolism. Tests for HIV and anti-HCV antibodies were negative. We performed mutation testing on the rtL80, rtI169, rtL180, rtA181, rtT184, rtA194, rtS202, rtM204, rtL220, rtN236, and rtM250 sites, all of which were previously known to be mutation sites, but all of these sites were identified as wild type. We performed further examinations on the rtS106, rtH126, rtD134, and rtL269 sites, which have been revealed as mutation sites associated with tenofovir resistance, and only the rtS106C mutation was detected. We performed full genome sequencing to find other mutation sites associated with virologic breakthrough because the rtS106C mutation alone was not sufficient to cause tenofovir resistance (Figure 1). The results showed mutations at 9 sites, namely, rtY9H, rtL91I, rtS106C, rtS106G, rtT118C, rtT118G, rtQ267L, rtI269L, rtA317S, rtK333Q, and rtN337H. Both the rtS106 and rtT118 sites demonstrated a variable nucleotide substitution of 50% C and 50% G on a chromatogram (Figure 2). Although we observed the patient for a few additional weeks, the AST and ALT levels increased to 202 IU/L and 539 IU/L, respectively. We changed the therapy from tenofovir to entecavir with a regimen of 0.5 mg once daily. After 4 mo, the HBV DNA titer decreased to 267 copies/mL. The AST and ALT levels normalized to 32 IU/L and 26 IU/L, respectively (Figure 3). HBeAg seroconversion had not yet occurred.
Figure 1.
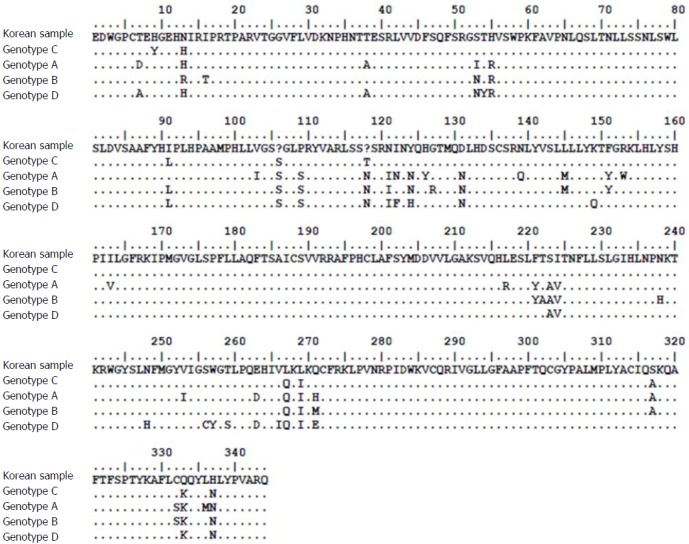
Full sequencing analysis of the hepatitis B virus reverse transcriptase gene from the patient (Korean sample). The sequence analysis shows that mutations occurred at 9 sites compared to the wild-type genotype C (the patient was infected with genotype C). The rt106 and rt118 sites are expressed as “?” because the sites contained a substitution by 2 different nucleotides.
Figure 2.
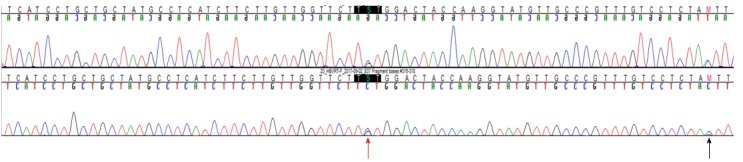
Chromatogram of the hepatitis B virus reverse transcriptase gene from the patient. The rt101 (red arrow) and rt118 (arrow) sites are shown as a double line because the site contained a substitution by 2 different nucleotides (cytosine and guanine).
Figure 3.
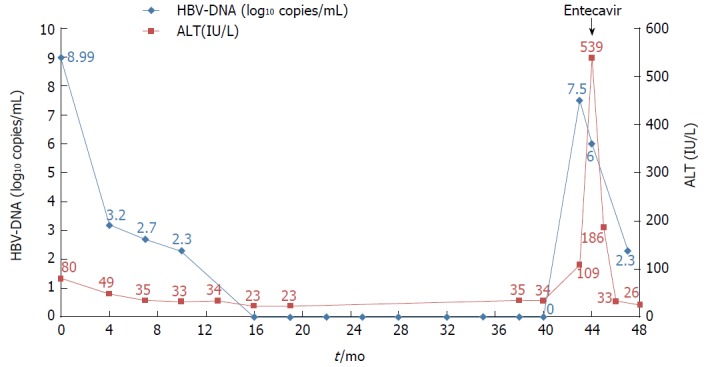
Clinical course of the patient. Hepatitis B virus DNA became undetectable after 16 mo of antiviral treatment with TDF. Virologic and biochemical breakthroughs occurred at 43 mo after treatment initiation.
DISCUSSION
The treatment of chronic hepatitis B has improved in the last decade primarily because of the availability of oral nucleos(t)ide analogue antiviral agents, such as LAM, telbivudine (LdT), ADV, ETC, and TDF. These agents are well tolerated and very effective in suppressing viral replication, and they appear to be safe, to the best of our knowledge and experience. The major limitation of long-term antiviral therapy for chronic hepatitis B is the emergence of drug resistance followed initially by an increase in HBV DNA level (virologic breakthrough) and then by an increase in serum aminotransferase level (biochemical breakthrough)[14].
Antiviral resistance is likely to develop primarily because the mutation rate during HBV replication is high and viral replication is increased in response to selection pressure[4,5]. Mutation and resistance are determined by three factors: viral fitness, nucleos(t)ide analogue potency, and the genetic barrier to resistance[15]. Viral fitness refers to the ability for viral replication in a defined environment. The potency of a nucleos(t)ide analogue describes its ability to inhibit HBV replication by acting as a substrate. The genetic barrier to resistance refers to the number of substitutions in the HBV polymerase reverse transcriptase (RT) domain required for the development of resistance[14]. Of the above three factors, nucleos(t)ide analogue potency and the genetic barrier to resistance are properties of antiviral agents. A higher nucleos(t)ide analogue potency and genetic barrier corresponds to a lower mutation rate[16].
The results of longitudinal study of TDF therapy demonstrated no resistance development throughout 8 years of treatment[17]. This result is possible because TDF provides the combination of a high genetic barrier, potent viral suppression, and reduced fitness of resistant viruses. Thus, TDF has been one of the drugs recommended as a first-line therapy for CHB patients[18,19]. TDF is also recommended for patients who have developed resistance to LAM, ETC, or LdT[20]. Several case reports and retrospective cohort studies also demonstrated the clinical efficacy of TDF in ETC-resistant or ETC-refractory patients[12,21]. In one study, the HBV DNA in some patients who experienced a virologic breakthrough while on TDF therapy contained an RT mutation site such as rtL101L/F, rtA307A/T, rtV173L + rtL180M + rtM204V, or rtA181T. However, these substitutions did not result in reduced susceptibility to TDF, and most of the patients did not adhere to treatment[11]. The rtA194T HBV polymerase mutation that was recently identified in HIV/HBV-coinfected patients treated with TDF did not confer resistance to TDF as the sole mutation in vitro[22]. In another study, phenotypic analyses revealed that the presence of the rtA194T mutation combined with the rtL180M and rtM204V mutations resulted in a greater than 10-fold increase in the IC50 for TDF compared to the wild type[23]. However, those sites have not been confirmed as mutation sites affecting TDF resistance because TDF resistance was not reported. Further studies are needed to assess the extent to which these mutations are associated with TDF resistance in HBV infection.
Reports on TDF resistance are difficult to find because TDF resistance is rare. A few years ago, a case of TDF resistance was reported in a chronic hepatitis B (CHB) patient who received sequential nucleos(t)ide therapy[24]. TDF resistance with virologic and biochemical breakthroughs had occurred during TDF rescue therapy after consecutive LAM, ETC, and LAM+ADV treatment failures. The identified HBV DNA mutation sites were rtL80M, rtL180M, rtM204V/I, rtA200V, rtF221Y, rtS223A, rtT184A/L, rtR153Q, and rtV191I, which were previously known as mutation sites related to LAM, ETC and ADV resistance. Recently, Lee et al[13] reported two TDF-resistant patients during “The Liver Week 2017” symposium in the United States. The patients described in this report had also previously taken other antiviral drugs and demonstrated multidrug resistance. However, the authors detected seven mutations in the HBV DNA, including three new substitutions, namely, rtS106C, rtH126Y, and rtD134E, which were collectively termed CYE. The TDF IC50 values for wild-type HBV and the CYE mutant were 3.8 ± 0.6 μmol/L and 14.1 ± 1.8 μmol/L, respectively. However, the CYE mutation site was not definitively identified as the site related to TDF resistance, although the TDF IC50 was higher in the CYE mutant than in the wild type. The TDF resistance described in the previous two reports developed after the failure of treatment with other nucleos(t)ide analogues. The patient in the present study had no history of nucleos(t)ide analogue treatment and showed complete viral suppression before the development of TDF resistance. It is not clear whether all 9 HBV RT mutation sites identified in the current patient were associated with TDF resistance. However, the accumulation of this mutational data is helpful for confirming the sites associated with TDF resistance. Further in vitro study is needed to reveal whether the 9 mutation sites are associated with an increased TDF IC50.
ARTICLE HIGHLIGHTS
Case characteristics
The patient had not complained of any specific symptoms.
Clinical diagnosis
The hepatitis B virus DNA titer rebounded to 7.5 log10 copies/mL at 43 mo after TDF treatment in a treatment-naive patient.
Differential diagnosis
We performed full genome sequencing to find other mutation sites to know it is associated with tenofovir disoproxil fumarate (TDF) resistance.
Laboratory diagnosis
We performed full genome sequencing to find TDF mutation sites and the results showed mutations at 9 sites, namely, rtY9H, rtL91I, rtS106C, rtS106G, rtT118C, rtT118G, rtQ267L, rtI269L, rtA317S, rtK333Q, and rtN337H.
Treatment
We changed the therapy from tenofovir to entecavir with a regimen of 0.5 mg once daily.
Experiences and lessons
We have to consider possibility of TDF resistance although its rarity.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Informed consent statement: The patient was not required to give informed consent for this study because this study used clinical data that were obtained after this patient agreed to treatment but before treatment initiation.
Conflict-of-interest statement: All authors declare no conflicts of interest related to this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 21, 2018
First decision: March 30, 2018
Article in press: April 15, 2018
P- Reviewer: Cheung R, Gunal O, Osna NA S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
Contributor Information
Woo Hee Cho, Department of Internal Medicine, Dankook University College of Medicine, Cheonan 31116, South Korea.
Hyun Jae Lee, Department of Internal Medicine, Dankook University College of Medicine, Cheonan 31116, South Korea.
Ki Bae Bang, Department of Internal Medicine, Dankook University College of Medicine, Cheonan 31116, South Korea.
Seok Bae Kim, Department of Internal Medicine, Dankook University College of Medicine, Cheonan 31116, South Korea. 11961019@dankook.ac.kr.
Il Han Song, Department of Internal Medicine, Dankook University College of Medicine, Cheonan 31116, South Korea.
References
- 1.Lok AS. Chronic hepatitis B. N Engl J Med. 2002;346:1682–1683. doi: 10.1056/NEJM200205303462202. [DOI] [PubMed] [Google Scholar]
- 2.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 4.Pawlotsky JM, Dusheiko G, Hatzakis A, Lau D, Lau G, Liang TJ, Locarnini S, Martin P, Richman DD, Zoulim F. Virologic monitoring of hepatitis B virus therapy in clinical trials and practice: recommendations for a standardized approach. Gastroenterology. 2008;134:405–415. doi: 10.1053/j.gastro.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 6.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 7.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 8.Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714–1722. doi: 10.1053/j.gastro.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 10.Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J, Wilber RB, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 11.Kitrinos KM, Corsa A, Liu Y, Flaherty J, Snow-Lampart A, Marcellin P, Borroto-Esoda K, Miller MD. No detectable resistance to tenofovir disoproxil fumarate after 6 years of therapy in patients with chronic hepatitis B. Hepatology. 2014;59:434–442. doi: 10.1002/hep.26686. [DOI] [PubMed] [Google Scholar]
- 12.Yip B, Chaung K, Wong CR, Trinh HN, Nguyen HA, Ahmed A, Cheung R, Nguyen MH. Tenofovir monotherapy and tenofovir plus entecavir combination as rescue therapy for entecavir partial responders. Dig Dis Sci. 2012;57:3011–3016. doi: 10.1007/s10620-012-2402-2. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Lee YB, Cho HK, Cho YY, Cho EJ, Kim YJ, Yoon JH, Zoulim F, Kim KH; The Seoul Liver Group. Identification of a Triple Mutation that Confers Tenofovir Resistance in Chronic Hepatitis B Patients. Hepatology. 2017;66 Suppl 1:S69–S70. [Google Scholar]
- 14.Hulgan T, Haas DW. Toward a pharmacogenetic understanding of nucleotide and nucleoside analogue toxicity. J Infect Dis. 2006;194:1471–1474. doi: 10.1086/508550. [DOI] [PubMed] [Google Scholar]
- 15.Richman DD. The impact of drug resistance on the effectiveness of chemotherapy for chronic hepatitis B. Hepatology. 2000;32:866–867. doi: 10.1053/jhep.2000.18194. [DOI] [PubMed] [Google Scholar]
- 16.Ghany MG, Doo EC. Antiviral resistance and hepatitis B therapy. Hepatology. 2009;49:S174–S184. doi: 10.1002/hep.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Corsa AC, Buti M, Cathcart AL, Flaherty JF, Miller MD, Kitrinos KM, Marcellin P, Gane EJ. No detectable resistance to tenofovir disoproxil fumarate in HBeAg+ and HBeAg- patients with chronic hepatitis B after 8 years of treatment. J Viral Hepat. 2017;24:68–74. doi: 10.1111/jvh.12613. [DOI] [PubMed] [Google Scholar]
- 18.European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 20.Villet S, Ollivet A, Pichoud C, Barraud L, Villeneuve JP, Trépo C, Zoulim F. Stepwise process for the development of entecavir resistance in a chronic hepatitis B virus infected patient. J Hepatol. 2007;46:531–538. doi: 10.1016/j.jhep.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Kim YJ, Sinn DH, Gwak GY, Choi MS, Koh KC, Paik SW, Yoo BC, Lee JH. Tenofovir rescue therapy for chronic hepatitis B patients after multiple treatment failures. World J Gastroenterol. 2012;18:6996–7002. doi: 10.3748/wjg.v18.i47.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delaney WE 4th, Ray AS, Yang H, Qi X, Xiong S, Zhu Y, Miller MD. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob Agents Chemother. 2006;50:2471–2477. doi: 10.1128/AAC.00138-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheldon J, Camino N, Rodés B, Bartholomeusz A, Kuiper M, Tacke F, Núñez M, Mauss S, Lutz T, Klausen G, et al. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antivir Ther. 2005;10:727–734. [PubMed] [Google Scholar]
- 24.Lee HW, Chang HY, Yang SY, Kim HJ. Viral evolutionary changes during tenofovir treatment in a chronic hepatitis B patient with sequential nucleos(t)ide therapy. J Clin Virol. 2014;60:313–316. doi: 10.1016/j.jcv.2014.03.018. [DOI] [PubMed] [Google Scholar]


