Abstract
Injury or disease to the CNS results in multifaceted cellular and molecular responses. One such response, the glial scar, is a structural formation of reactive glia around an area of severe tissue damage. While traditionally viewed as a barrier to axon regeneration, beneficial functions of the glial scar have also been recently identified. In this Perspective, we discuss the divergent roles of the glial scar during CNS regeneration and explore the possibility that these disparities are due to functional heterogeneity within the cells of the glial scar—specifically, astrocytes, NG2 glia and microglia.
Regeneration of the damaged mammalian CNS continues to represent the holy grail of regenerative medicine. The CNS is a complex network of neuronal connections that are supported, refined and modified by a population of glial cells of increasingly appreciated diversity, including astrocytes, oligodendrocytes and microglia. CNS pathologies—including injury or trauma, infection and neurodegenerative and autoimmune diseases—have debilitating and costly effects on human life. Therefore, much effort has focused on understanding the molecular mechanisms underlying endogenous cellular responses to injury and disease in the mammalian CNS. One cellular response that has sparked wide debate over its conflicting and varied roles during CNS repair is the glial scar.
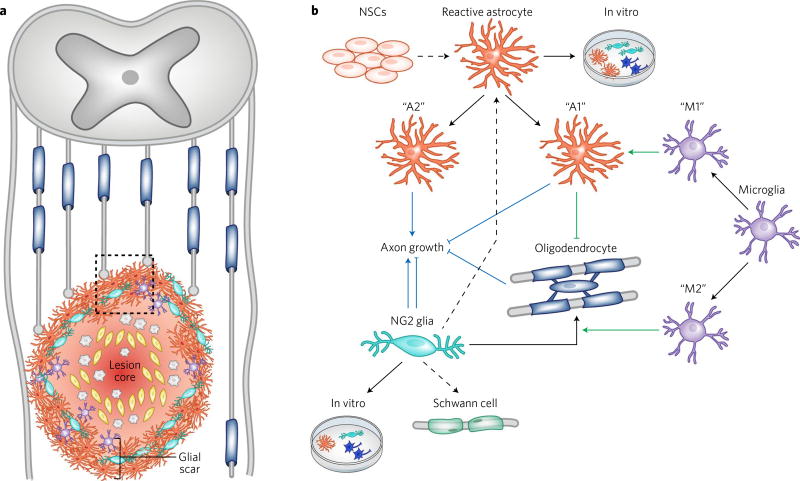
The glial scar has been widely studied in the context of spinal cord injury (SCI), but it also occurs after traumatic brain injury, after ischemic stroke and in many neurodegenerative diseases, including multiple sclerosis. Upon damage to the CNS, newly proliferated reactive astrocytes1, NG2 glia and microglia form a compact border around an area of severe tissue damage, or lesion core. The lesion core contains a mixture of perivascular-derived fibroblasts, pericytes, ependymal cells and phagocytic macrophages2. Some debate over the glial scar is likely caused by the differing and ambiguous use of the term. While multiple previous studies have referred to the entire CNS lesion as the glial scar, this can be misleading because the lesion core contains very few glial cells. Furthermore, the lesion core (also referred to as the fibrotic or mesenchymal scar) contains a rich deposit of extracellular matrix proteins that largely inhibit axonal growth and remyelination. Therefore, we will instead use the term “glial scar” to refer only to the glial cell border that surrounds the non-neural lesion core (Fig. 1).
Fig. 1. Cellular interactions in the glial scar.
a, Diagram of the glial scar after spinal cord injury. The glial scar is made up of reactive astrocytes (orange), NG2 glia (teal) and microglia (purple) that form a tight barrier around the lesion core, or area of severe tissue damage. The lesion core contains blood-borne macrophages (gray) and stromal cells (yellow). Injured axons (gray lines) fail to grow through the glial scar. b, The cellular interactions and developmental potential of heterogeneous glial cells within the glial scar (boxed region in a). Black arrows indicate the in vivo and in vitro lineage potential of each glial cell type, with black dashed arrows representing less common cell fates (that is, NG2 glial differentiation into Schwann cells or reactive astrocytes). Green lines depict cellular interactions among glial cells in the scar. Specifically, M1 microglia promote an A1 reactive astrocyte phenotype, while M2 microglia have been shown to promote differentiation of NG2 glia to oligodendrocytes. A1 reactive astrocytes secrete a toxin that kills oligodendrocytes. Blue lines depict the effect of each cell type on axonal growth (blue arrow indicates promotion of axon growth while blunt end indicates inhibition). The A1 and A2 astrocyte subtypes are based on Liddelow et al.15 while the M1 and M2 microglial subtypes are based on Miron et al.43. NSCs, neural stem cells.
Traditionally, the glial scar has been viewed as a barrier to CNS regeneration. However, over the past decade, increasing evidence has suggested that the glial scar can also support CNS repair. Simultaneously, increased evidence of the complexity and heterogeneity of glial cell physiology implies that glial cells within the scar may be more heterogeneous than previously believed. In this Perspective, we discuss functional heterogeneity of reactive astrocytes, NG2 glia and microglia—the three primary cell types that make up the glial scar. We then examine the contrasting roles of the glial scar during CNS repair in view of this cellular heterogeneity. We argue that further understanding of the distinct roles played by different glial cell populations, both within and across different injuries and diseases, is critical for developing effective future therapies.
Inherent heterogeneity of the glial scar
Damage to the mammalian CNS results in widely varied cellular, molecular and structural changes in the lesion site and nearby affected regions. This is due to (i) the myriad of CNS diseases and injuries, (ii) the variability among individuals with a specific injury or disease, (iii) the location within the brain and severity of the insult, and (iv) the heterogeneous cell populations that respond differently to injury or disease. Increased understanding of CNS cellular diversity raises the question of whether glial scar heterogeneity is fundamentally shaped by functionally diverse glial populations that make up the scar. Furthermore, are the divergent functions of the glial scar due to distinct cellular responses that vary with anatomical location and time after injury?
Is glial cell diversity preserved in the injured CNS?
Originally believed to be nothing more than support cells for neurons, glial cells are now accepted to play critical roles in CNS development, homeostasis and repair. Because of these diverse functions, the perception that astrocytes, NG2 glia and microglia are homogenous cell populations in the healthy CNS has been largely rejected. Astrocytes display distinct regional identities and functional properties in both the mouse spinal cord3,4 and adult brain5–7. A recent study by the Deneen laboratory identified five distinct astrocyte subpopulations that differentially support synaptogenesis5. Similarly, gray and white matter NG2 glia exhibit differences in proliferation and differentiation rates8,9, as well as physiological properties10. Within a given region, NG2 glia also display differences in protein expression11. Lastly, microglia from different regions of the adult mouse brain display distinct gene expression profiles12 and were recently found to differ significantly in morphology, membrane properties and lysosome content13. Overall, these studies indicate that both intrinsic factors and environmental cues are likely to direct neural-circuit-specialized or region-specific glial cells. Whether this diversity is preserved following injury or disease and whether it shapes distinct cellular responses in the damaged CNS remain critical questions to address.
Functional diversity of glial cells in the injured CNS
Reactive astrocytes
Reactive astrocytes have been traditionally identified by hypertrophy and high expression of glial fibrillary acidic protein (GFAP), but increasing evidence now indicates a more complex and heterogeneous nature. The endogenous astrocytic cellular response to CNS damage ranges from mild reactive astrogliosis following mild non-contusive trauma to formation of a compact astroglial scar, and includes a wide spectrum of changes in gene expression, proliferation, morphology and physiology2. Transcriptional profiling of reactive astrocytes isolated from ischemic stroke and neuroinflammation mouse models found that, despite a small core of shared genes, reactive astrocytes upregulate genes specific to the type of injury or disease14. Interestingly, the Barres laboratory recently characterized the functional properties of neuroinflammation-induced reactive astrocytes (termed A1 astrocytes) and found that they secrete a neurotoxin that promotes neuronal and oligodendrocyte cell death15. They identified A1 reactive astrocytes in tissue samples from patients with neurodegenerative diseases, suggesting that A1 astrocytes may represent a new common cellular target for therapies. In contrast to these results, reactive astrocytes induced by ischemia appear to acquire a more protective phenotype, increasing expression of neurotrophic factors and transferring mitochondria to injured neurons14,16. The mechanisms regulating these diverse functional properties remain unknown, but evidence suggests that environmental cues, especially microglia-derived signals15,17, are important.
Distinct subtypes of reactive astrocytes are also found in individual animal models of CNS injury. The degree of astrogliosis is highly dependent on the distance of astrocytes from lesions, with mildly reactive astrocytes found distal to the lesion site1. However, distinct reactive astrocyte populations have also been observed within the same CNS region. For example, in spinal cord glial scars, reactive astrocytes have been found to express differing levels of GFAP, nestin and brain lipid-binding protein (BLBP)18. Furthermore, only subsets of astrocytes were found to react to a cortical stab injury, either by polarization toward lesion sites or by proliferation19. These proliferative astrocytes were largely localized to juxtavascular sites, indicating that niche-specific cues may direct functional properties of reactive astrocytes (see Box 1). Interestingly, this proliferative population of reactive astrocytes was also shown by clonal analysis to be derived from distinct progenitors20, suggesting that one source of reactive astrocyte heterogeneity may be distinct cellular origins. In support of this, neural stem cell (NSC)-derived reactive astrocytes have been shown to contribute to glial scars in the brain21, while a recent study found little contribution of NSC-derived reactive astrocytes to SCI-induced glial scars22. Therefore, reactive astrocyte heterogeneity is dependent on the site of injury. Whether NSC-derived reactive astrocytes are more permissive or inhibitory for regeneration remains unknown. However, studies have found that NSC-derived reactive astrocytes are more important in restricting inflammation23 and can be converted to neurons in vivo21, indicating that they may be a useful cellular target for promoting repair.
Box 1. Reactive astrocytes as neural stem cells.
During development and in the mature CNS, a restricted number of cells maintain the ability to self-renew and differentiate into multiple lineages. These radial glia or neural stem cells (NSCs) are defined by their ability to form neurospheres and differentiate into multiple lineages (neurons, astrocytes and oligodendrocytes) in vitro, but rarely do so in vivo78. Interestingly, reactive astrocytes have been described as sharing characteristics with NSCs. Fate-mapping studies have shown that a subset of reactive astrocytes resume proliferation in vivo following traumatic or ischemic brain injury79–81, but usually undergo only one round of cell division19. In vitro, about 5% of all reactive astrocytes are able to form neurospheres with higher self-renewal capacity79. Additionally, both NSCs and reactive astrocytes display limited lineage potential in vivo but enhanced multipotency in vitro, generating both neurons and glia80,81. Lastly, transcriptomic analysis identified a group of genes activated in common between reactive astrocytes and NSCs, including genes involved in proliferation and neurogenesis78. Future studies using single-cell RNA sequencing will hopefully further explain why subtypes of reactive astrocytes respond differently to CNS damage. The ability to manipulate extrinsic signaling cues in regions of the glial scar to direct the behavior and lineage potential of reactive astrocytes would present valuable options for treating CNS injury and disease.
Taken together, these findings raise several important questions. Do specific subsets of astrocytes respond to different types of CNS damage? Is reactive astrocyte heterogeneity primarily induced by extrinsic factors (that is, context-dependent cues and non-cell-autonomous mechanisms) or intrinsic factors (cell-autonomous mechanisms)? To begin answering these questions, reactive astrocytes from different CNS regions following the same injury must be compared. With recent advances in molecular tools (Fig. 2), the ability to identify disease- or injury-specific reactive astrocytes will have important implications for developing new therapies24.
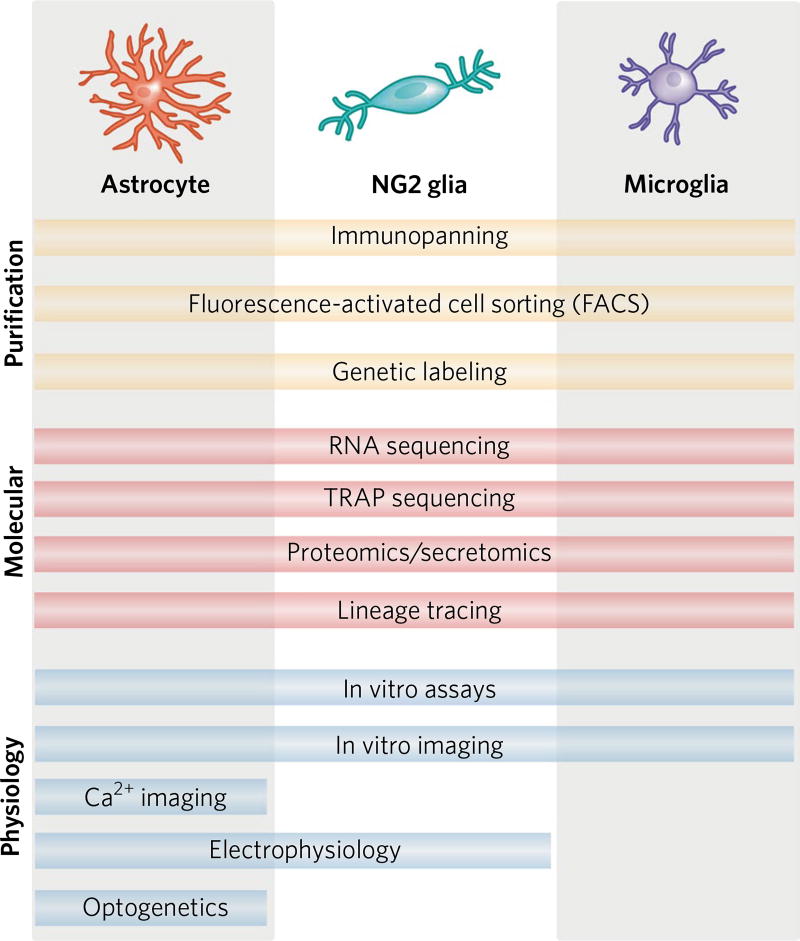
Fig. 2. Tools for assessing functional cellular diversity in glia.
Elucidating cellular diversity requires robust purification protocols that effectively isolate astrocytes, NG2 glia or microglia from surrounding CNS tissue. Once cells are purified, they can be characterized using a range of different molecular tools, including new techniques such as single-cell RNA sequencing and translating ribosome affinity purification (TRAP) sequencing. These techniques result in molecular profiles that can be used to identify new molecular markers for glial subtypes, potential physiological differences among cellular subtypes and potential therapeutic targets for promoting functional repair following CNS damage. Assessing cellular physiology is critical for understanding functional heterogeneity of astrocytes, NG2 glia and microglia. While in vitro assays (for example, cellular proliferation and synapse modulation) and in vivo imaging techniques have been used to characterize all three glial populations, there is a lack of sophisticated tools for analyzing microglial physiology. Refs. for purification protocols: Zhang et al.71, Lin et al.5, Bennett et al.44. Refs. for molecular tools: Doyle et al.72, Kim et al.73. Refs. for physiology: Nimerjahn et al.74, Perea et al.75, Larson et al.76, Gee et al.77.
NG2 glia
NG2 glia constitute approximately 4–5% of the total cells in the postnatal and adult brain25 and a large percentage of the proliferating cells in the glial scar26. Following injury and in many neurodegenerative diseases, NG2 glia in the glial scar share several characteristics with reactive astrocytes: cellular hypertrophy, increased proliferation and increased expression of proteoglycans26. However, their function remains controversial, partly because there are multiple cell types in the scar and lesion core that upregulate the proteoglycan NG2 after injury: oligodendrocyte progenitor cells, pericytes, Schwann cells and macrophages. Unfortunately, this makes it difficult to interpret studies where further qualifying characteristics were not used to specify the cell type being investigated (for example, coexpression of NG2 and Olig2 for oligodendrocyte progenitor cells). Therefore, we use the term NG2 glia to refer specifically to NG2-expressing cells that give rise to glia.
Most studies have focused on mechanisms underlying the differentiation of NG2 glia into oligodendrocytes and their contribution to remyelination. Several developmental signals have been found to be upregulated in the glial scar that promote NG2 glial proliferation and migration to trauma sites: fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), Wnts27. However, many NG2 glia in the scar do not differentiate into myelinating oligodendrocytes. Interestingly, NG2 glia have been found to also generate astrocytes and Schwann cells following injury. In NG2-Cre estrogen receptor (CreER) mice, up to 25% of reporter-labeled cells express GFAP 1 week after SCI28, while only 8% express GFAP 10 d after cortical stab injury29. Both of these numbers decrease to 10% or less at 1 month after injury. Conversely, very few (~3% of) platelet-derived growth factor receptor-α (PDGFRα)-CreER reporter-labeled cells express GFAP in the spinal cord after lysolecithin-induced demyelination30. Instead, ~20% of these reporter-labeled cells expressed the Schwann cell marker periaxin30. This is especially surprising because Schwann cells are neural crest–derived, whereas NG2 glia arise from the neural ectoderm. NG2-derived Schwann cells were also identified following contusion SCI31, but not after cortical stab wound injury29. Together these results indicate that the differentiation potential of NG2 glia greatly differs depending on the type and location of CNS injury. Whether all NG2 glia acquire the ability to transdifferentiate or if certain subsets are restricted to specific lineages remains unknown. While NG2-derived Schwann cells likely contribute to remyelination, albeit at low levels, the role of NG2-derived reactive astrocytes remains unclear. Because NG2-derived reactive astrocytes are a transient population that do not upregulate proteins expressed highly by scar-forming astrocytes (nestin and vimentin), it is possible that they resemble more ‘helpful’ reactive astrocytes32. Overall, NG2 glia participate in the formation and resolution of glial scars beyond serving simply as a reservoir for generating oligodendrocytes.
Microglia
While microglia are widely known for their role as sentinels and effectors of the CNS immune response, evidence now shows that they display an array of functions: synaptic organization33, phagocytosis of cellular debris34, trophic support for neurons35 and the regulation of neuronal excitability36. Microglia are among the first cells to respond to CNS injury or disease, proliferating and migrating to lesion sites. As with reactive astrocytes and NG2 glia, it remains uncertain to what extent microglia both promote and hinder CNS recovery and repair37. This is likely context-dependent, varying with regard to the type of injury or disease, environmental cues and phase of recovery. Recent evidence for brain-region-specific12 and neurodegeneration-specific38 microglial gene expression signatures highlights how the environment influences microglial phenotype. Interestingly, microglia from distinct regions of the adult mouse brain display differences in expression of immune regulation and activation genes12, indicating that the microglial cellular response may vary depending on the site of CNS damage.
Microglia have been largely grouped into two types: a ramified or ‘resting’ state, critical for CNS homeostasis, and a reactive or amoeboid state, induced by CNS damage. Reactive microglia are sometimes further classified into M1 (classically activated, pro-inflammatory) and M2 (alternatively activated, anti-inflammatory) subtypes, but there is debate as to whether this classification is appropriate39. Evidence has shown that M1 microglia are the dominant phenotype following SCI40, stroke41 and traumatic brain injury42. Lipopolysaccharide-induced M1 microglia promoted a toxic reactive astrocyte phenotype via secretion of interleukin-1α, tumor necrosis factor and complement component C1q15. M1 microglia were also found to promote proliferation of oligodendrocyte progenitor cells following focal demyelination, while M2 microglia promoted oligodendrocyte differentiation via secretion of activin-A43. Therefore, stimulating microglial polarization toward an M2 phenotype has been promoted as a potential therapeutic tool.
This interpretation is likely an oversimplification of a spectrum of several different microglial activation states with different functions. Because reactive microglia are largely indistinguishable from infiltrating macrophages, it has been difficult to obtain direct evidence for functionally diverse microglial subtypes in the glial scar. Recent technical advances in purification protocols, single-cell sequencing and unique cell surface markers44 will hopefully result in better insight into microglial heterogeneity and its effects on axon regeneration (Fig. 2). Determining how environmental cues differentially affect microglia and the subsequent cross-talk between glial cells and regenerating axons in the glial scar remains an important but daunting challenge for neuroscientists. Overall, the presence of functionally heterogeneous cell types in the glial scar is likely to strongly contribute to the contrasting roles of the glial scar during regeneration.
Is the glial scar inhibitory or beneficial to regeneration?
The question of whether formation of the glial scar aids CNS regeneration and functional recovery has been discussed and debated for many years. There is widespread evidence supporting the notion that compact astroglial scars prevent axon regeneration. Evolutionarily, there is a stark contrast in regenerative abilities between mammals and lower vertebrate classes (fish, reptiles and amphibians). Species such as salamanders maintain a surprisingly robust ability to regenerate the CNS throughout life and do so without formation of a glial scar45. There is also a large difference in regenerative capabilities between the mammalian CNS and peripheral nervous system (PNS). Unlike the CNS, peripheral nerves can regenerate over long distances, find their appropriate target cells and form functional synapses. This dissimilarity is believed to be due to differences in intrinsic properties of the neurons46 and in the composition of the injured CNS and PNS environmental milieu. Most strikingly, PNS axons transplanted into the injured CNS fail to regenerate, while injured CNS neurons are able to project axons within bridges of peripheral nerve tissue47. Since these pioneering studies, the repressive nature of the glial scar has been largely attributed to a high concentration of inhibitory proteins, including chondroitin sulfate proteoglycans (CSPGs) and myelin proteins.
Inhibitory environment of the glial scar
The glial scar environmental milieu likely varies across different types of CNS injury and disease. For example, lipopolysaccharide injection or optic nerve crush both result in production of inflammatory factors from reactive M1 microglia that promote an A1 reactive astrocyte phenotype15. These A1 reactive astrocytes in turn secrete an unidentified neurotoxin that kills neurons and oligodendrocytes in vitro and in vivo15. Gene expression profiling of A1 reactive astrocytes also identified strong expression of several genes of the classical complement cascade that are known to be destructive to synapses14. Therefore, injuries or neurodegenerative diseases that induce A1 reactive gliosis presumably create a highly toxic environment for regenerating axons and NG2 glia. By contrast, ischemia-induced reactive astrocytes produce several neuroprotective factors and cytokines, such as cardiotrophin-like cytokine factor 1 (CLCF1), leukemia inhibitory factor (LIF) and thrombospondins14. Within the same injury, subtypes of reactive astrocytes may also express differing levels of inhibitory proteins. Following contusion SCI, scar-forming astrocytes upregulate several genes that distinguish them from milder reactive astrocytes, including CSPGs and the repulsive axon guidance protein Slit232.
CSPGs—which include the lecticans (aggrecan, versican, neurocan and brevican), phosphacan, and NG2—have been largely credited with axon regeneration failure in the CNS48. Following SCI, CSPGs are highly upregulated by both reactive astrocytes and other cells in the glial scar49. As in their developmental role, CSPGs have been shown to efficiently repel regenerating axons in vitro50. CSPGs also directly prevent oligodendrocyte maturation and remyelination in vitro51 and in animal models of multiple sclerosis52. Degradation of CSPGs by treatment with chondroitinase ABC following SCI53 and focal ischemia54 has resulted in locomotor improvement due to sprouting of spared axons. A recent study found that modulation of the CSPG receptor protein tyrosine phosphatase-σ (PTPσ) following SCI restores serotonergic innervation to the injured mouse spinal cord, along with functional recovery of locomotor and urinary systems55. Overall, reducing CSPG signaling in the glial scar has been a major therapeutic focus, with promising but varying results. Targeted ablation of individual CSPGs from specific cell populations in the glial scar is needed to better understand the respective roles of CSPGs during axon regeneration.
In addition to axonal growth, the glial scar also presents an inhibitory environment for endogenous remyelination. Our laboratory recently characterized a protein secreted by reactive astrocytes, endothelin-1 (ET-1), as a negative regulator of NG2 glial differentiation and functional remyelination56,57. Blocking ET-1 signaling by either pharmacological or genetic approaches enhances maturation of NG2 glia into oligodendrocytes after focal demyelination of the corpus callosum. Notably, ET-1 signaling increases Jagged1 expression in reactive astrocytes, activating Notch signaling in neighboring NG2 glia and preventing their differentiation. Therefore, ET-1 modulates both the astrocytic and oligodendroglial responses to CNS damage. Other signaling proteins in the glial scar, such as bone morphogenetic proteins (BMPs), have been shown to play similar roles58. Intriguingly, gray matter tracts have been found to undergo more remyelination than white matter lesions in patients with multiple sclerosis59. This may be due to different environmental factors (for example, levels of ET-1 or differential accumulation of microglia) and/or the different proliferative states of resident NG2 glia in gray and white matter. Determining whether high ET-1 production is restricted to specific subtypes of reactive astrocytes remains an important issue to address.
Beneficial functions of the glial scar
In face of the evidence above, a logical hypothesis is that blocking formation of the glial scar—the dense glial border surrounding the lesion core—should result in increased axonal growth and remyelination. However, a series of studies by the Sofroniew laboratory over the past decade has demonstrated that preventing formation of the astroglial scar following CNS injury does not result in increased regeneration1,49,60. Recently, this was further confirmed using two different genetic methods to ablate scar-forming astrocytes following severe crush SCI49. Selectively ablating proliferating astrocytes or deleting STAT3 signaling selectively from astrocytes each results in increased axonal dieback49. One explanation for the increased dieback is an altered inflammatory response. Previous studies have reported that reactive astrocytes are important in restricting the inflammatory response to the damaged CNS region, thereby protecting healthy CNS tissue. These protective influences include the sequestration of blood-derived macrophages and repair of the blood–brain barrier1. Ablation of scar-forming astrocytes has also been shown to exacerbate neuronal cell death and demyelination following injury, as a result of an influx of blood-derived macrophages and fibrotic cells1,60. Therefore, the glial scar is important in preserving tissue integrity and mitigating further inflammatory damage.
Unresolved discrepancies regarding the glial scar
One proposed explanation for the dual nature of the glial scar is that the scar has beneficial effects during the acute phase of injury, but prevents axon growth in chronic or later stages61. In support of this theory, a recent study by Hara et al. pharmacologically blocked integrin signaling 2 weeks after SCI, thereby attenuating astrocyte scar formation and improving locomotor performance32. However, Anderson et al. ablated reactive astrocytes in chronic glial scars 5 weeks after SCI and found that it did not promote axonal growth49. Whether this remains true for even more mature glial scars (months after injury) remains to be seen. Anderson et al. interpreted their results to signify that scar-forming astrocytes aid, rather than inhibit, axonal growth following injury49. This interpretation has been challenged by others in the field62, who claim that it ignores the deleterious effects of lesion-derived macrophages on regenerating axons. So what explains these differing outcomes? Anderson et al. ablated scar-forming astrocytes using genetically targeted diphtheria toxin receptor and low doses of diphtheria toxin49, whereas Hara et al. administered an anti-β1-integrin antibody to the lesion epicenter, blocking the interaction of reactive astrocytes with collagen I32. It is possible that the latter approach preserved beneficial reactive astrocytes in the glial scar—perhaps akin to those in the ischemic glial scar. It is also likely that the anti-β1-integrin antibody affected other cellular interactions in the glial scar, in addition to reactive astrocytes. Unfortunately, neither study characterized the effects of scar ablation on other cells in the damaged CNS. It is therefore difficult to interpret whether the changes in axonal growth are due to the absence of astrocyte-derived cues or to altered cellular responses in microglia and/or NG2 glia.
In addition to reactive astrocytes, there are also conflicting reports on the effects of NG2 glia on axonal growth in the glial scar. NG2 glia express high levels of the CSPG NG2, which has been shown to inhibit neurite outgrowth in vitro63. Delivery of NG2 neutralizing antibody following SCI results in increased axonal growth and functional regeneration64,65. Furthermore, reducing proliferation of NG2 glia after optic nerve crush increases the number of axons crossing the proximal crush site66. Together, these findings suggest that NG2 glia inhibit axon regeneration. However, NG2 knockout mice display more axonal dieback from spinal cord lesions than wild-type controls67,68. NG2 null mice also exhibit less remyelination following lysolecithin-induced demyelination in the spinal cord69, likely owing to a smaller pool of NG2 glial progenitors for oligodendrocyte generation. Intriguingly, regenerating axons have been observed closely associating with NG2-expressing cells in the glial scar, forming synapse-like connections68. These synaptic connections may mirror what is seen in the developing CNS, as NG2 glia have been found to receive direct synaptic inputs from excitatory and inhibitory neurons throughout the brain70. However, while these synaptic connections may be beneficial during early phases of glial scar formation, it is hypothesized that they ultimately trap regenerating axons in a dystrophic state68. Overall, these conflicting reports show that NG2 glia are likely to have both beneficial and inhibitory roles in the glial scar. Whether these diverse functions can be attributed to distinct subtypes of NG2 glia remains to be seen. It is also important to note that because NG2 glia, pericytes and infiltrating macrophages all express NG2, it is difficult to assess the individual roles of each cell type on axonal growth in NG2 null mice. Therefore, conditional ablation of NG2 from different cell populations in the glial scar is needed to better understand the effects of NG2 glia and the NG2 protein on axonal regeneration.
Conclusions and future directions
The CNS is a complex and structured organ system, and damage or disease to this system results in equally multifaceted cellular and molecular responses. As our knowledge of cellular diversity in the normal and pathological CNS continues to increase, it becomes even more important to compare cellular responses both within and across injury and preclinical disease models. Advanced molecular and imaging tools now make these experiments possible. Over the past decade, significant advancements have been made toward understanding signaling processes that direct reactive astrogliosis. However, the full range of reactive astrocyte diversity remains to be determined. Furthermore, more attention must be directed toward molecular and physiological characterization of NG2 glia and microglia across different injury and disease models. Recognizing how different subtypes of reactive astrocytes, NG2 glia and microglia shape the environmental milieu of the glial scar is critical for correct interpretation of the glial scar’s many roles during injury and repair. More studies are needed that characterize the roles of single molecules in specific cell types using state-of-the-art genetically targeted loss-of-function techniques.
Historically, treatments for CNS damage have been largely classified according to the inducing damage or injury (for example, SCI, demyelination, stroke or Alzheimer’s disease) and the corresponding symptoms. However, with the arrival of high throughput sequencing methods, we are now close to classifying CNS pathologies according to their molecular profiles. An intriguing and perhaps more realistic possibility is classifying pathologies according to their cellular profiles—specifically, what subtypes of reactive astrocytes, NG2 glia and microglia are present in the damaged tissue. Each class of cells can be molecularly characterized and compared in different types of injury that lead to glial scar formation. Developing targeted therapies that repress or promote expression of specific gene pathways in distinct glial cell populations may provide the best approach for promoting maximal functional recovery across a broader range of CNS injury and disease.
Acknowledgments
The authors would like to kindly thank J. Triplett, E. Goldstein and T. Forbes for critically reading the manuscript. This work was supported by R01NS090383 from NINDS (V.G.), U54HD090257 from NICHD (District of Columbia Intellectual and Developmental Disabilities Research Center) (V.G.) and F32NS098647 from NINDS (K.L.A.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interests
The authors declare no competing financial interests.
References
- 1.Wanner IB, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J. Neurosci. 2013;33:12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson MA, Ao Y, Sofroniew MV. Heterogeneity of reactive astrocytes. Neurosci. Lett. 2014;565:23–29. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai HH, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molofsky AV, et al. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature. 2014;509:189–194. doi: 10.1038/nature13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.John Lin CC, et al. Identification of diverse astrocyte populations and their malignant analogs. Nat. Neurosci. 2017;20:396–405. doi: 10.1038/nn.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chai H, et al. Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron. 2017;95:531–549. doi: 10.1016/j.neuron.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben Haim L, Rowitch DH. Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci. 2017;18:31–41. doi: 10.1038/nrn.2016.159. [DOI] [PubMed] [Google Scholar]
- 8.Dimou L, Simon C, Kirchhoff F, Takebayashi H, Götz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viganò F, Möbius W, Götz M, Dimou L. Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat. Neurosci. 2013;16:1370–1372. doi: 10.1038/nn.3503. [DOI] [PubMed] [Google Scholar]
- 10.Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J. Physiol. (Lond.) 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parras CM, et al. The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes. J. Neurosci. 2007;27:4233–4242. doi: 10.1523/JNEUROSCI.0126-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grabert K, et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 2016;19:504–516. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Biase LM, et al. Local cues establish and maintain region-specific phenotypes of basal ganglia microglia. Neuron. 2017;95:341–356. doi: 10.1016/j.neuron.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamanian JL, et al. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayakawa K, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–555. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamby ME, et al. Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G-protein-coupled receptors. J. Neurosci. 2012;32:14489–14510. doi: 10.1523/JNEUROSCI.1256-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White RE, McTigue DM, Jakeman LB. Regional heterogeneity in astrocyte responses following contusive spinal cord injury in mice. J. Comp. Neurol. 2010;518:1370–1390. doi: 10.1002/cne.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bardehle S, et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat. Neurosci. 2013;16:580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- 20.Martín-López E, García-Marques J, Núñez-Llaves R, López-Mascaraque L. Clonal astrocytic response to cortical injury. PLoS One. 2013;8:e74039. doi: 10.1371/journal.pone.0074039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faiz M, et al. Adult neural stem cells from the subventricular zone give rise to reactive astrocytes in the cortex after stroke. Cell Stem Cell. 2015;17:624–634. doi: 10.1016/j.stem.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Ren Y, et al. Ependymal cell contribution to scar formation after spinal cord injury is minimal, local and dependent on direct ependymal injury. Sci. Rep. 2017;7:41122. doi: 10.1038/srep41122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabelström H, et al. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013;342:637–640. doi: 10.1126/science.1242576. [DOI] [PubMed] [Google Scholar]
- 24.Liddelow SA, Barres BA. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Dimou L, Gallo V. NG2-glia and their functions in the central nervous system. Glia. 2015;63:1429–1451. doi: 10.1002/glia.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J. Neurosci. 2001;21:3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackett AR, Lee JK. Understanding the NG2 glial scar after spinal cord injury. Front. Neurol. 2016;7:199. doi: 10.3389/fneur.2016.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hackett AR, et al. STAT3 and SOCS3 regulate NG2 cell proliferation and differentiation after contusive spinal cord injury. Neurobiol. Dis. 2016;89:10–22. doi: 10.1016/j.nbd.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komitova M, Serwanski DR, Lu QR, Nishiyama A. NG2 cells are not a major source of reactive astrocytes after neocortical stab wound injury. Glia. 2011;59:800–809. doi: 10.1002/glia.21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zawadzka M, et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Assinck P, et al. Myelinogenic plasticity of oligodendrocyte precursor cells following spinal cord contusion injury. J. Neurosci. 2017;37:8635–8654. doi: 10.1523/JNEUROSCI.2409-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hara M, et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat. Med. 2017;23:818–828. doi: 10.1038/nm.4354. [DOI] [PubMed] [Google Scholar]
- 33.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 34.Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coull JA, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 37.Kawabori M, Yenari MA. The role of the microglia in acute CNS injury. Metab. Brain Dis. 2015;30:381–392. doi: 10.1007/s11011-014-9531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu IM, et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep. 2013;4:385–401. doi: 10.1016/j.celrep.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 40.Kigerl KA, et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu X, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 42.Hsieh CL, et al. Traumatic brain injury induces macrophage subsets in the brain. Eur. J. Immunol. 2013;43:2010–2022. doi: 10.1002/eji.201243084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miron VE, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett ML, et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. USA. 2016;113:E1738–E1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zukor KA, Kent DT, Odelberg SJ. Meningeal cells and glia establish a permissive environment for axon regeneration after spinal cord injury in newts. Neural Dev. 2011;6:1. doi: 10.1186/1749-8104-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- 47.David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 48.Dyck SM, Karimi-Abdolrezaee S. Chondroitin sulfate proteoglycans: key modulators in the developing and pathologic central nervous system. Exp. Neurol. 2015;269:169–187. doi: 10.1016/j.expneurol.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Anderson MA, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pendleton JC, et al. Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPσ. Exp. Neurol. 2013;247:113–121. doi: 10.1016/j.expneurol.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Lau LW, et al. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann. Neurol. 2012;72:419–432. doi: 10.1002/ana.23599. [DOI] [PubMed] [Google Scholar]
- 53.Bradbury EJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 54.Soleman S, Yip PK, Duricki DA, Moon LD. Delayed treatment with chondroitinase ABC promotes sensorimotor recovery and plasticity after stroke in aged rats. Brain. 2012;135:1210–1223. doi: 10.1093/brain/aws027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lang BT, et al. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature. 2015;518:404–408. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hammond TR, et al. Astrocyte-derived endothelin-1 inhibits remyelination through notch activation. Neuron. 2014;81:588–602. doi: 10.1016/j.neuron.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hammond TR, et al. Endothelin-B receptor activation in astrocytes regulates the rate of oligodendrocyte regeneration during remyelination. Cell Rep. 2015;13:2090–2097. doi: 10.1016/j.celrep.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, et al. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J. Neurosci. 2011;31:6053–6058. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Albert M, Antel J, Brück W, Stadelmann C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 2007;17:129–138. doi: 10.1111/j.1750-3639.2006.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faulkner JR, et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 2009;10:235–241. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- 62.Silver J. The glial scar is more than just astrocytes. Exp. Neurol. 2016;286:147–149. doi: 10.1016/j.expneurol.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 63.Dou CL, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J. Neurosci. 1994;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan AM, Colletti M, Rorai AT, Skene JH, Levine JM. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J. Neurosci. 2006;26:4729–4739. doi: 10.1523/JNEUROSCI.3900-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petrosyan HA, et al. Neutralization of inhibitory molecule NG2 improves synaptic transmission, retrograde transport, and locomotor function after spinal cord injury in adult rats. J. Neurosci. 2013;33:4032–4043. doi: 10.1523/JNEUROSCI.4702-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodriguez JP, et al. Abrogation of β-catenin signaling in oligodendrocyte precursor cells reduces glial scarring and promotes axon regeneration after CNS injury. J. Neurosci. 2014;34:10285–10297. doi: 10.1523/JNEUROSCI.4915-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Castro R, Jr, Tajrishi R, Claros J, Stallcup WB. Differential responses of spinal axons to transection: influence of the NG2 proteoglycan. Exp. Neurol. 2005;192:299–309. doi: 10.1016/j.expneurol.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 68.Filous AR, et al. Entrapment via synaptic-like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J. Neurosci. 2014;34:16369–16384. doi: 10.1523/JNEUROSCI.1309-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kucharova K, Chang Y, Boor A, Yong VW, Stallcup WB. Reduced inflammation accompanies diminished myelin damage and repair in the NG2 null mouse spinal cord. J. Neuroinflammation. 2011;8:158. doi: 10.1186/1742-2094-8-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bergles DE, Jabs R, Steinhäuser C. Neuron-glia synapses in the brain. Brain Res. Rev. 2010;63:130–137. doi: 10.1016/j.brainresrev.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doyle JP, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim S, et al. Neurotoxicity of microglial cathepsin D revealed by secretome analysis. J. Neurochem. 2007;103:2640–2650. doi: 10.1111/j.1471-4159.2007.04995.x. [DOI] [PubMed] [Google Scholar]
- 74.Nimmerjahn A. Two-photon imaging of microglia in the mouse cortex in vivo. Cold Spring Harb. Protoc. 2012 doi: 10.1101/pdb.prot069294. https://doi.org/10.1101/pdb.prot069294. [DOI] [PubMed]
- 75.Perea G, Yang A, Boyden ES, Sur M. Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nat. Commun. 2014;5:3262. doi: 10.1038/ncomms4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larson VA, Zhang Y, Bergles DE. Electrophysiological properties of NG2+ cells: matching physiological studies with gene expression profiles. Brain Res. 2016;1638(Pt. B):138–160. doi: 10.1016/j.brainres.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gee JM, et al. Imaging activity in neurons and glia with a Polr2a-based and cre-dependent GCaMP5G-IRES-tdTomato reporter mouse. Neuron. 2014;83:1058–1072. doi: 10.1016/j.neuron.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Götz M, Sirko S, Beckers J, Irmler M. Reactive astrocytes as neural stem or progenitor cells: in vivo lineage, in vitro potential, and genome-wide expression analysis. Glia. 2015;63:1452–1468. doi: 10.1002/glia.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buffo A, et al. Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc. Natl. Acad. Sci. USA. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shimada IS, LeComte MD, Granger JC, Quinlan NJ, Spees JL. Self-renewal and differentiation of reactive astrocyte-derived neural stem/progenitor cells isolated from the cortical peri-infarct area after stroke. J. Neurosci. 2012;32:7926–7940. doi: 10.1523/JNEUROSCI.4303-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sirko S, et al. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. Cell Stem Cell. 2013;12:426–439. doi: 10.1016/j.stem.2013.01.019. [DOI] [PubMed] [Google Scholar]