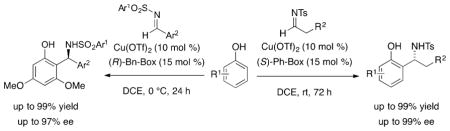
Abstract
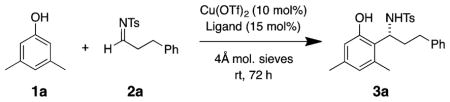
A new copper-catalyzed enantioselective aza-Freidel-Crafts reaction between phenols and N-sulfonyl aldimines that provides chiral secondary benzylamines in good to excellent yields and excellent enantioselectivities (up to 99% ee) is disclosed. In particular, excellent scope with alkylimines was observed for the first time. The synthetic utility of the products was demonstrated in the first enantioselective synthesis of a dual orexin receptor antagonist, a compound that contains an amine-bearing stereocenter adjacent to a bis-ortho-functionalized arene.
Graphical abstract
Chiral benzyl amines are ubiquitous in many biologically active compounds, including the anticancer and antimalarial natural product strychnopentamine,1,2 a class of synthetic dual orexin receptor antagonists,3,4 and a designed inhibitor of the frataxin/ubiquitin interaction.5 The field of asymmetric aza-Friedel–Crafts phenol addition to ketimines6–8 and to aryl9–15 and propargyl aldimines16 to produce chiral benzylamines has been vigorously explored in recent years. Conversely, examples of highly enantioselective additions of phenols to alkyl aldimines are sparse. Most aza-Friedel–Crafts examples have focused on phenol or naphthol additions to aryl aldimines,17–20 while the few alkyl aldimine examples have generated corresponding benzylamines in moderate yields and ee’s.18
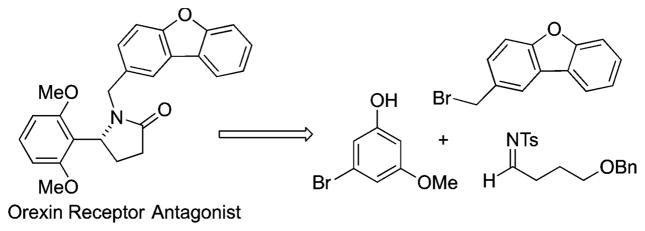
Analogous additions of indoles to imines have also been reported.21 In particular, Zhou developed an enantioselective indole addition to aryl imines using a commercially available Cu(OTf)2 and 4-benzyl bis(oxazoline) catalyst system.22 Herein, we report our exploration of enantioselective phenol additions to alkyl aldimines, which has resulted in the identification of a catalytic enantioselective protocol employing chiral copper(II) catalysts. Synthetically, these new reactions are particularly effective at installing an enantioenriched chiral secondary amine-bearing carbon in between (ortho to) two functional groups of an arene, such as those found in the above-mentioned bioactive compounds (e.g., Scheme 1).1–5 Such a transformation could be challenging due to the steric hindrance associated with the product and potential precursors.
Scheme 1.
Retrosynthetic Analysis of a Bioactive Benzylic Amine
To scope out the feasibility, several catalyst systems were tried to promote enantioselective addition of the mildly activated 3,5-dimethylphenol 1a to N-tosyl-hydrocinnamaldehyde imine 2a. Superior reactivity was observed with Cu(OTf)2 and bis-(oxazoline) ligands, compared to reactions with alternate catalysts (see Supporting Information (SI) for details). Thus, Cu(OTf)2 complexes with various chiral bis(oxazoline) ligands were screened for reactivity and enantioselectivity. The (S)-Ph-Box ligand afforded the best reactivity and enantiomeric excess of the ligands tested (Table 1, entry 4, 54% yield, 84% ee). In an attempt to increase the atom-economy of the reaction, the ratio of phenol to imine used was reduced from 5 to 1.5 equiv. This led to a reduction in isolated yield from 54% to 38% (compare Table 1 entries 4 to 9). Fortunately, when the solvent was changed from CH2Cl2 to 1,2-dichloroethane (DCE), a 53% yield of 3a was obtained in 94% enantiomeric excess (Table 1, entry 13, 1.5 equiv of phenol used). Room temperature was found to be the best temperature for high enantioselectivity while maintaining synthetically useful yields. A reaction time of 72 h was chosen, as the reactions at 24 and 48 h provided lower isolated yields (compare Table 1, entries 13 to 18 and 19). No product was obtained when Cu(OTf)2 was omitted (Table 1, entry 20).
Table 1.
Aza-Friedel–Crafts Alkyl Imine Optimizationa
| |||||
|---|---|---|---|---|---|
| entry | solvent | ligand | phenol (equiv) | yield (%) | ee (%) |
| 1 | CH2Cl2 | (R)-Bn-Box | 5 | 19 | −43 |
| 2 | CH2Cl2 | (S)-i-Pr-Box | 5 | 23 | 69 |
| 3 | CH2Cl2 | (S)-t-Bu-Box | 5 | 5 | 37 |
| 4 | CH2Cl2 | (S)-Ph-Box | 5 | 54 | 84 |
| 5 | CH2Cl2 |
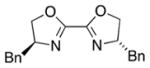
|
5 | 28 | <5 |
| 6 | CH2Cl2 |
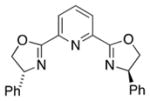
|
5 | 5 | −5 |
| 7 | CH2Cl2 | (4R,5S)-bis-Ph-Box | 5 | 7 | 91 |
| 8 | DCE | (S)-i-Pr-Quinox | 1.5 | 57 | <5 |
| 9 | CH2Cl2 | (S)-Ph-Box | 1.5 | 38 | 84 |
| 10 | xylenes | (S)-Ph-Box | 1.5 | 24 | 66 |
| 11 | THF | (S)-Ph-Box | 1.5 | 11 | 94 |
| 12 | toluene | (S)-Ph-Box | 1.5 | 16 | 75 |
| 13 | DCE | (S)-Ph-Box | 1.5 | 53 | 94 |
| 14 | PhCF3 | (S)-Ph-Box | 1.5 | 6 | 63 |
| 15 | EtOAc | (S)-Ph-Box | 1.5 | 36 | 94 |
| 16b | DCE | (S)-Ph-Box | 1.5 | 66 | 76 |
| 17c | DCE | (S)-Ph-Box | 1.5 | 49 | 92 |
| 18d | DCE | (S)-Ph-Box | 1.5 | 26 | 83 |
| 19e | DCE | (S)-Ph-Box | 1.5 | 39 | 87 |
| 20f | DCE | (S)-Ph-Box | 1.5 | 0 | — |
Reaction conditions: imine 2a (0.20 mmol) and phenol 1a (0.30 mmol) and flame-dried 4 Å mol. sieves were added to a solution of Cu(OTf)2 (0.020 mmol) and ligand (0.030 mmol) in 1 mL of solvent, and the mixture was stirred at rt for 72 h unless otherwise noted.
Reaction run at 40 °C.
Reaction performed at 0.3 M with respect to imine.
Reaction performed for 24 h.
Reaction performed for 48 h.
Reaction performed without Cu(OTf)2.
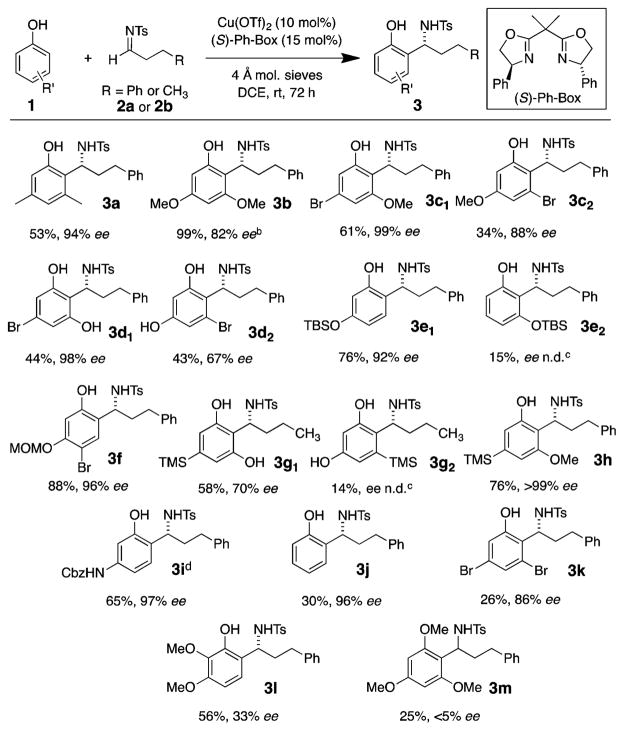
With the optimal conditions for the aza-Friedel–Crafts reaction of alkyl aldimines in hand (Table 1, entry 13), the scope of phenols viable in the additions to the N-tosyl-hydrocinnamaldehyde imine 2a and the N-tosyl-n-butyraldehyde imine 2b was investigated (Scheme 2). 3,5-Dimethoxyphenol, an electron-rich nucleophile, required reduced temperature to provide benzylamine 3b selectively and to avoid uncatalyzed background reactions (both ortho- and para-addition). Using 3-bromo-5-methoxyphenol and 5-bromobenzene-1,3-diol gave high overall yields; however, both reactions afforded two products each. In both cases, excellent ee’s were found for the addition between the oxygens of the phenols (products 3c1 and 3d1), and more modest ee’s were found for each regioisomer (3c2 and 3d2). Mono-TBS-protected resorcinol also gave excellent overall yield and a ~5:1 ratio of regioisomers 3e. Product 3f derived from MOM-protected-4-bromoresorcinol was formed in excellent yield and ee. Additionally, 3-trimethylsilylresorcinols were effective nucleophiles for providing benzylamines 3g1, 3g2, and 3h in excellent yields. Silylresorcinol adduct 3h was formed as a single regioisomer. N-Cbz-3-aminophenol also reacted with imine 2a to form product 3i in good yield and excellent enantioselectivity.
Scheme 2. Phenol Scopea.
aReaction conditions: imine 2 (0.20 mmol) and phenol 1 (0.30 mmol) and flame-dried 4 Å mol. sieves were added to a solution of Cu(OTf)2 (0.020 mmol) and (S)-Ph-Box (0.03 mmol) in 1 mL of DCE, and the reaction was allowed to stir at rt for 72 h, unless otherwise noted. bReaction performed at 0 °C for 24 h. cn.d. = not determined. dAbsolute configuration determined by X-ray crystallography (see SI). All others were assigned by analogy.
A crystal structure of benzylamine 3h enabled assignment of the absolute configuration of the chiral center as the (S)-enantiomer (see SI). The configurations of the other products were assigned S by analogy to 3h. Unsubstituted phenol displayed modest reactivity and gave adduct 3j in 30% yield in excellent ee (96%). Even electron-deficient 3,5-dibromophenol gave adduct 3k in a modest yield (26%) but good enantioselectivity. 2,3-Dimethoxyphenol, an ortho-substituted phenol, gave benzyl amine 3l in good yield but poor enantioselectivity, implying sensitivity of the catalyst for 1,2-disubstituted phenols. 1,3,5-Trimethoxybenzene reacted with the imine to give 25% yield of the racemic adduct 3m, suggesting the phenol likely binds to the copper catalyst in the enantioselective transition state (vide infra, Figure 1).
Figure 1.
Proposed major transition states.
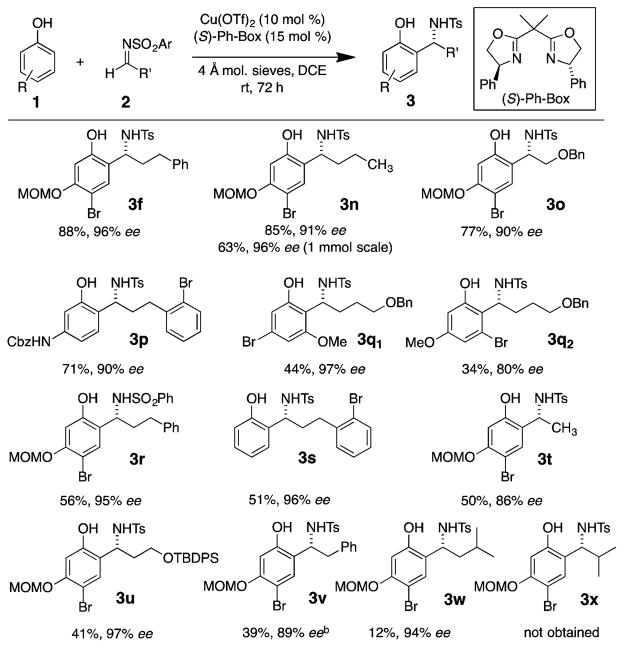
An expanded scope of alkyl aldimines was next investigated in the aza-Friedel–Crafts reaction (Scheme 3). Products 3n, 3o, and 3r were all formed smoothly and with high ee’s. The reaction to form 3n was run on 1 mmol scale of 2b. Benzylic amine 3n was formed in lower but acceptable yield (63%), and no reduction in enantioselectivity was observed. The 2-bromo-hydrocinnamal-dehyde imine from products 3p and 3s gave good yields for both nucleophiles, as well as excellent ee’s. A reaction of 3-bromo-5-methoxyphenol and 4-N-tosyl-benzyloxybutyl imine provided two regioisomeric products 3q; the product of imine addition between the oxygens of the nucleophile was formed in excellent enantioselectivity, while the other regioisomer suffered slightly in the same respect. Even N-tosyl acetaldehyde imine gave moderate reactivity (50% yield) and a good ee (86%), implying an extended chain off the aldimine is not necessary to achieve high enantioselectivity. TBDPS-protected N-tosyl-3-hydroxy-propylimine underwent reaction to give product 3u in moderate yield and excellent enantioselectivity. Results of aza-Freidel–Crafts to form products 3v, 3w, and 3x indicated the effect of alkyl imine sterics profoundly affected reactivity. Reaction of a phenyl acetaldehyde imine to give benzylamine 3v using the standard conditions occurred in good yield, but poor enantioselectivity (6%, see SI). Changing the ligand from (S)-Ph-Box to (S)-iPr-Box afforded adduct 3v in 39% yield and 89% ee. Adding a methyl group to the β-position to the imine significantly reduced the reactivity, yet excellent product enantioselectivity was maintained (3w, 12% yield, 94% ee). The isobutyraldehyde imine was unreactive (3x was not obtained).
Scheme 3. Alkyl Imine Scopea.
aReaction conditions: imine 2 (0.20 mol) and phenol 1 (0.03 mmol) and flame-dried 4 Å mol. sieves were added to a solution of Cu(OTf)2 (0.020 mmol) and (S)-Ph-Box (0.030 mmol) in 1 mL of DCE and allowed to stir at rt for 72 h unless otherwise noted. b(S)-iPr-Box used instead of (S)-Ph-Box.
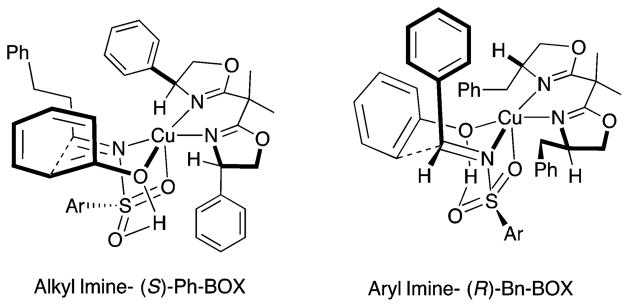
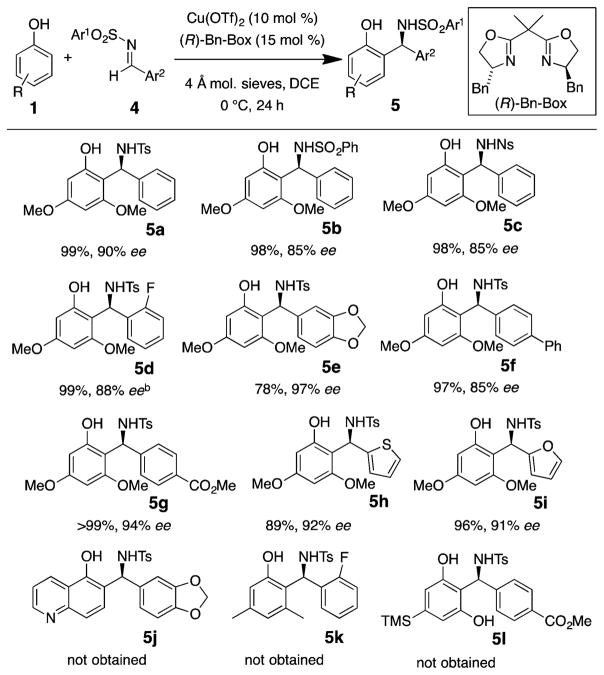
The enantioselective aza-Friedel–Crafts reactions of aryl aldimines were next investigated. 3,5-Dimethoxyphenol was found to be the only phenol sufficiently reactive in this transformation, unfortunately. While the (S)-Ph-Box-derived catalyst demonstrated poor regioselectivity and enantioselectivity with N-tosyl aryl imines, the (R)-Bn-Box-derived catalyst provided higher regioselectivity (see SI for optimization table), and the reaction temperature was reduced to 0 °C to obtain optimal enantioselectivity. A range of N-sulfonyl aryl imines functionalized with both electron-donating and electron-withdrawing groups underwent reaction with 3,5-dimethoxyphenol (Scheme 4). N-Tosyl, N-benzenesulfonyl, and N-nosyl benzylimines all underwent the reaction efficiently and selectively to give products 5a–c (Scheme 4). A crystal structure of the bis(aryl)methylsulfonamide 5d was obtained, and the absolute configuration was established as R (see SI). The absolute configurations of all other benzylamine adducts 5 were assigned by analogy.
Scheme 4. Aryl Imine Aza-Friedel–Crafts Scopea.
aReaction conditions: imine (0.20 mmol) and phenol (0.30 mmol) and flame-dried 4 Å mol. sieves were added to a solution of Cu(OTf)2 (0.020 mmol) and (R)-Bn-Box (0.030 mmol) in 1 mL of DCE at 0 °C, and the mixture was stirred 24 h. bAbsolute configuration determined by X-ray crystallography (see SI).
In order to rationalize the observed enantioselectivity of the transformation, we invoked transition states where the N-sulfonyl aldimine is bound to the [Cu(II)]-bis(oxazoline)] complex via a 1,3-binding mode at the nitrogen and an oxygen of the sulfonyl (Figure 1).26 The oxygen of the phenol is bound directly to the metal, and due to the sterics of the ligand, ortho-selective phenol addition is preferred on the Re-face for alkyl aldimine addition using (S)-Ph-Box and the Si-face using (R)-Bn-Box for aryl aldimine addition. A steric match between aryl imine/alkyl-Box or alkyl imine/aryl-Box appeared optimal from the ligand screening data (Table 1 and optimization table in SI). The imine sulfonyl group was expected to be very sterically demanding; thus, its placement on the same face as the closest (cis) chiral ligand substituent was avoided. Hydrogen bonding between the phenol hydrogen and the other sulfonyl oxygen as drawn (Figure 1) could add additional activation and structure at the transition state.
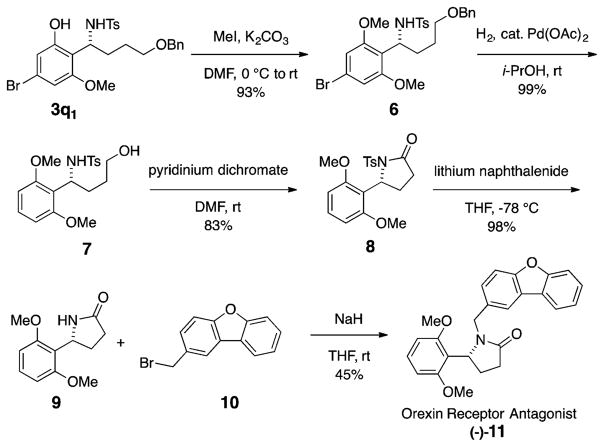
The synthetic utility of the alkyl aldimine aza-Friedel–Crafts reaction was demonstrated by synthesizing a dual orexin receptor antagonist in its enantioenriched form for the first time (Scheme 5).3,4 Phenolic benzyl amine 3q1 was O-methylated, followed by hydrogenation to both hydrodebrominate the aromatic ring and cleave the benzyl ether, affording alcohol 7.23 Oxidative cyclization utilizing pyridinium dichromate gave the 5-aryl-N-tosyl amide 8.24 Tosyl deprotection using lithium-naphthalenide25 and N-alkylation provided the dual orexin receptor antagonist (–)-11.
Scheme 5.
Enantioselective Synthesis of an Orexin Receptor Antagonist
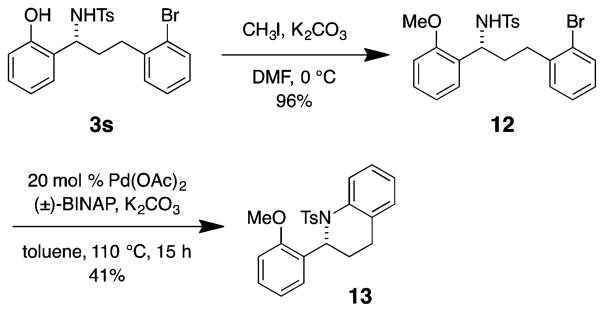
Additionally, benzylamine 3s was converted to 2-aryltetrahydroquinoline 13 (Scheme 6). O-Methylation of phenolic benzylamine 3s provided 12. Palladium-catalyzed intramolecular cyclization/coupling26 of the pendant amine to the aryl bromide of 12 then efficiently formed the N-tosyl-2-aryl-tetrahydroquinoline 13.
Scheme 6.
Synthesis of a Tetrahydroquinoline
In conclusion, we have developed a catalytic enantioselective aza-Friedel–Crafts reaction between phenols and aldimines using a copper(II)-bis(oxazoline) catalyst. Good to excellent ee’s were observed for alkyl aldimines and aryl aldimines. The synthetic utility of the products was displayed by the enantioselective synthesis of a known dual orexin receptor antagonist, as well as a chiral 2-aryltetrahydroquinoline.
Supplementary Material
Acknowledgments
We gratefully acknowledge the National Institutes of Health (GM078383) for financial support of this work. We acknowledge Dr. William W. Brennessel and the Crystallography Facility at the University of Rochester for obtaining the X-ray structures of 3i (CCDC 1588079) and 5d (CCDC 1588080). We thank Dr. Valerie Frerichs (UB instrument center) for help with the chiral HPLC.
Footnotes
Accession Codes
CCDC 1588079–1588080 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.8b00282.
Experimental procedures and chiral HPLC traces of all new compounds (PDF)
NMR spectra of all new compounds (PDF)
References
- 1.Frederich M, Tits M, Goffin E, Philippe G, Grellier P, De Mol P, Hayette MP, Angenot L. Planta Med. 2004;70:520. doi: 10.1055/s-2004-827151. [DOI] [PubMed] [Google Scholar]
- 2.Frederich M, Bentires-Alj M, Tits M, Angenot L, Greimers R, Gielen J, Bours V, Merville MP. J Pharmacol Exp Ther. 2003;304:1103. doi: 10.1124/jpet.102.044867. [DOI] [PubMed] [Google Scholar]
- 3.Sifferlen T, Boller A, Chardonneau A, Cottreel E, Hoecker J, Aissaoui H, Williams JT, Brotschi C, Heidmann B, Siegrist R, Gatfield J, Treiber A, Brisbare-Roch C, Jenck F, Boss C. Bioorg Med Chem Lett. 2014;24:1201. doi: 10.1016/j.bmcl.2013.12.092. [DOI] [PubMed] [Google Scholar]
- 4.Sifferlen T, Boller A, Chardonneau A, Cottreel E, Gatfield J, Treiber A, Roch C, Jenck F, Aissaoui H, Williams JT, Brotschi C, Heidmann B, Siegrist R, Boss C. Bioorg Med Chem Lett. 2015;25:1884. doi: 10.1016/j.bmcl.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 5.Lavecchia A, Di Giovanni C, Cerchia C, Russo A, Russo G, Novellino E. J Med Chem. 2013;56:2861. doi: 10.1021/jm3017199. [DOI] [PubMed] [Google Scholar]
- 6.Montesinos-Magraner M, Vila C, Cantón R, Blay G, Fernandez I, Muñoz MC, Pedro JR. Angew Chem, Int Ed. 2015;54:6320. doi: 10.1002/anie.201501273. [DOI] [PubMed] [Google Scholar]
- 7.Zhou D, Huang Z, Yu X, Wang Y, Li J, Wang W, Xie H. Org Lett. 2015;17:5554. doi: 10.1021/acs.orglett.5b02668. [DOI] [PubMed] [Google Scholar]
- 8.Kumari P, Barik S, Khan NH, Ganguly B, Kureshy RI, Abdi SHR, Bajaj HC. RSC Adv. 2015;5:69493. [Google Scholar]
- 9.Chauhan P, Chimni SS. Eur J Org Chem. 2011;2011:1636. [Google Scholar]
- 10.Takizawa S, Arteaga FA, Yoshida Y, Kodera J, Nagata Y, Sasai H. Dalton Trans. 2013;42:11787. doi: 10.1039/c2dt32202a. [DOI] [PubMed] [Google Scholar]
- 11.Chauhan P, Chimni SS. Tetrahedron Lett. 2013;54:4613. [Google Scholar]
- 12.Takizawa S, Hirata S, Murai K, Fujioka H, Sasai H. Org Biomol Chem. 2014;12:5827. doi: 10.1039/c4ob00925h. [DOI] [PubMed] [Google Scholar]
- 13.Montesinos-Magraner M, Canton R, Vila C, Blay G, Fernandez I, Munoz MC, Pedro JR. RSC Adv. 2015;5:60101. doi: 10.1002/anie.201501273. [DOI] [PubMed] [Google Scholar]
- 14.Bai S, Liao Y, Lin L, Luo W, Liu X, Feng X. J Org Chem. 2014;79:10662. doi: 10.1021/jo5020036. [DOI] [PubMed] [Google Scholar]
- 15.Kato M, Hirao S, Nakano K, Sato M, Yamanaka M, Sohtome Y, Nagasawa K. Chem - Eur J. 2015;21:18606. doi: 10.1002/chem.201503280. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Jiang L, Li L, Dai J, Xiong D, Shao Z. Angew Chem, Int Ed. 2016;55:15142. doi: 10.1002/anie.201608918. [DOI] [PubMed] [Google Scholar]
- 17.Liu G, Zhang S, Li H, Zhang T, Wang W. Org Lett. 2011;13:828. doi: 10.1021/ol102987n. [DOI] [PubMed] [Google Scholar]
- 18.Li GX, Qu J. Chem Commun. 2012;48:5518. doi: 10.1039/c2cc31735d. [DOI] [PubMed] [Google Scholar]
- 19.Kumari P, Jakhar A, Khan NH, Tak R, Kureshy RI, Abdi SHR, Bajaj HC. Catal Commun. 2015;69:138. [Google Scholar]
- 20.Niu LF, Xin YC, Wang RL, Jiang F, Xu PF, Hui XP. Synlett. 2010;2010:765. [Google Scholar]
- 21.Reviews: Poulsen TB, Jorgensen KA. Chem Rev. 2008;108:2903. doi: 10.1021/cr078372e.Kobayashi S, Mori Y, Fossey JS, Salter MM. Chem Rev. 2011;111:2626. doi: 10.1021/cr100204f.
- 22.Select examples: Jia YX, Xie JH, Duan HF, Wang LX, Zhou WL. Org Lett. 2006;8:1621. doi: 10.1021/ol0602001.Wang YQ, Song J, Hong R, Li H, Deng L. J Am Chem Soc. 2006;128:8156. doi: 10.1021/ja062700v.
- 23.Gao X, Woo SK, Krische MJ. J Am Chem Soc. 2013;135:4223. doi: 10.1021/ja4008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tejo C, See YFA, Mathiew M, Chan PWH. Org Biomol Chem. 2016;14:844. doi: 10.1039/c5ob02302e. [DOI] [PubMed] [Google Scholar]
- 25.Shu C, Liu MQ, Wang SS, Li L, Ye LW. J Org Chem. 2013;78:3292. doi: 10.1021/jo400127x. [DOI] [PubMed] [Google Scholar]
- 26.Ghorai MK, Nanaji Y, Yadav AK. Org Lett. 2011;13:4256. doi: 10.1021/ol2016077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.