Abstract
In this issue of Development Cell, Tan et al. (2018) show how a novel player in myonuclear positioning, the ubiquitin ligase Ari-1 regulates levels of Koi, a member of the LINC mechanosensing complex, and affects nuclear morphology and positioning in both Drosophila muscles as well as human vascular smooth muscle cells.
Main text
In a typical diagram of a cell, the nucleus is situated in the center, where it responds to external cues through different signaling cascades. While this model holds true for most cell types, one cell type that doesn’t conform to this conventional cellular arrangement is the skeletal muscle cell or myofiber. The mammalian myofiber is a syncytium containing hundreds of nuclei. These myonuclei are evenly spaced along the length of the fiber at the periphery, an arrangement that maximizes the intranuclear distances (Folker and Baylies, 2013). Not only is the presence of the myonuclei critical, but also their localization, structure, shape, and size are important for muscle function (Schreiber and Kennedy, 2013). Changes in any of these nuclear parameters have been linked to numerous diseases. For example, mispositioned nuclei are implicated in numerous muscle diseases such as Centronuclear Myopathies (CNMs), Emery-Dreifuss muscular dystrophy (EDMD), and Duchenne Muscular Dystrophy (DMD) (Folker and Baylies, 2013). Moreover, mutations in genes responsible for maintaining nuclear shape and structure have been implicated in muscle diseases with mispositioned nuclei, suggesting a strong correlation between myonuclear positioning and muscle disease (Romero, 2010). But how these mispositioned nuclei contribute to disease pathogenesis is not well understood. The Drosophila larval musculature offers a promising tool to investigate this dynamic, offering a simple yet versatile system that enables relatively quick and easy genetic manipulation, tissue specific expression of transgenes, and high-resolution imaging. Moreover, genes responsible for myonuclear architecture are conserved between mammals and Drosophila, which permits probing of mechanisms that result in disease (Folker and Baylies, 2013). In this issue of Developmental Cell, Tan et al. (2018), use the Drosophila larval musculature to understand how Ariadne-1 (Ari-1), a member of the Ring-between-Ring (RBR) E3 Ubiquitin ligase family of proteins regulates myonuclear organization and structure through its interactions with Parkin, another member of the same family.
Using the CRISPR-Cas9 system, Tan et al. (2018) generated an ari-1 null mutant, protein trap, and Gal4 lines, to probe Ari-1’s function in muscle. They found that ari-1 null mutant larval muscles have clustered myonuclei; interestingly, this phenotype was not as severe as the phenotype in larvae carrying ari-1 point mutations (Figure 1). These data suggested that proteins encoded by the ari-1 point mutants acted as dominant negatives, resulting in perturbed myonuclear position and morphology. Examination of myonuclear morphology in ari-1 mutants through Transmission Electron Microscopy (TEM) revealed that the myonuclei had aberrant morphologies: both the distance of the myonuclei from the cell membrane as well as myonuclear aspect ratio (ratio of nuclear length to nuclear width) were perturbed, indicating a role for ari-1 in anchoring myonuclei to the cell membrane and in regulating myonuclear shape. Defects in myonuclear positioning seen in ari-1 mutant larvae could be rescued by expression of ari-1wt as well as human ARIH1, suggesting that Ari-1’s function is conserved from human to Drosophila. To understand the mechanism behind myonuclear clustering in ari-1 mutants, Tan and colleagues (2018) examined the LINC (LInker of Nucleoskeleton and Cytoskeleton) complex, which has been implicated in muscle diseases with aberrant nuclear positioning. The LINC complex consists of KASH and SUN homology domain proteins that span the outer and inner nuclear membranes, respectively, as well as Lamins, which line the inside of inner nuclear membrane. One of the main roles of the LINC complex is to provide structural stability to the nuclear envelope by linking the cytoskeleton to the nuclear cytoskeleton, thereby ensuring that the nucleus is correctly positioned. (Lee and Burke, 2017). Tan and colleagues (2018) noted that in ari-1 mutant larvae, the levels of the SUN protein Koi were increased. Upregulating Koi levels genetically in larval muscles reproduced the ari-1 mutant phenotype, generating myonuclear mispositioning. Through a series of elegant biochemical and genetic experiments, the authors discovered that Ari-1 regulates degradation and turnover of Koi by binding to and then mono-ubiquitinating Koi.
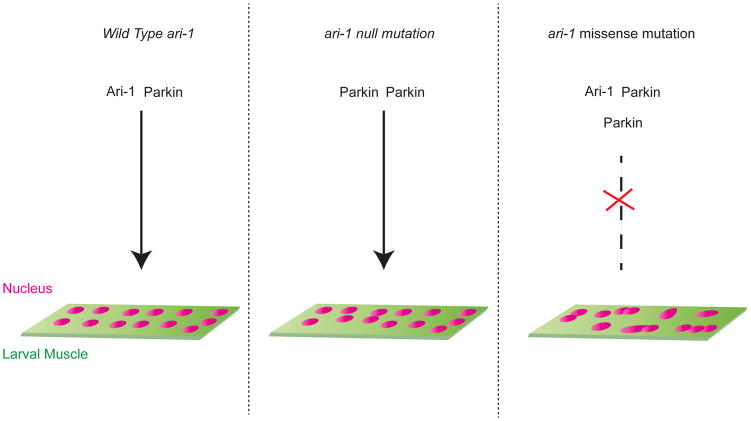
Figure 1. Ari-1 interacts with Parkin to facilitate myonuclear positioning.
(A) In a wildtype larval muscle myonuclear positioning is facilitated by the interaction of Ari-1 and Parkin, members of E3 ubiquitin ligase family, which regulate the turnover of a component of the LINC complex, Koi (Tan et al., Dev Cell) by ubquitinating Koi. (B) In an ari-1 null larval muscle, in the absence of Ari-1, Parkin forms a dimer and is able to partially regulate myonuclear positioning. (C) In larvae with a missense mutation in ari-1, mutant Ari-1 binds strongly to Parkin and perhaps to more than one Parkin, hindering the ability of both Ari-1 and Parkin to ubiquitinate Koi, which results in aberrant myonuclear positioning, nuclear clustering and defective nuclear morphology.
Because ari-1 missense mutants had a higher level of lethality and showed increased levels of aberrant myonuclear positioning compared to ari-1 null mutants, the authors hypothesized that Ari-1 formed a complex with other proteins with similar functions. The missense mutation in Ari-1 could interfere with the function of the other members of the complex, resulting in a more severe phenotype than the ari-1 null mutant. The most prominent member of the RBR family is the E3 ubiquitin ligase Parkin, homozygous mutations in which cause Parkinson’s disease (Lücking et al., 2000). Parkin’s primary role is to facilitate turnover of mitochondria (mitophagy) (Rodger et al., 2017). Since Ari-1 and Parkin were known to interact in cell culture (Parelkar et al., 2012), their interaction in the larval muscle was examined through co-IP and genetic experiments. Tan and colleagues (2018) observed that Ari-1 and Parkin physically and genetically interact. Double heterozygous mutants of ari-1 and parkin showed a more severe myonuclear positioning phenotype than either of the single heterozygous mutants. Moreover, Ari-1 missense mutants bound more strongly to Parkin than wild-type Ari-1, suggesting that the missense mutant Ari-1 function as dominant negatives. Overexpression of Parkin in Ari-1 mutant backgrounds could rescue the defects in myonuclear positioning and morphology. Similarly overexpression of human ARIH1 could rescue mitochondrial defects seen on parkin depletion suggesting that ari-1 and parkin are partially redundant. Interestingly, overexpression of ari-2, another member of RBR family, could not suppress ari-1 mutant phenotypes such as myonuclear clustering and lethality.
Tan and colleagues (2018) next extended their studies by examining human ARIH1 and its links to disease. By posting ARIH1 on GeneMatcher (Sobreira et al., 2015), the authors determined that ARIH1 mutations were seen in 3 different patients suffering from thoracic aortic and cerebrovascular disease. All 3 patients had point mutations in ARIH1, and examination of patient aortic tissue, particularly the smooth muscle cells (SMCs), revealed aberrant nuclear morphology, as seen in the Drosophila ari-1 mutants. Upon engineering each of these patient point mutations in human ARIH1 and introducing them into ari-1 mutant flies, they observed that none of these individual point mutations could rescue the lethality and myonuclear positioning defects found in ari-1 mutant flies, suggesting that the point mutations affected protein function. For the SMCs to contract and function, their contractile machinery sense mechanical forces and transduce signals to the Extracellular Matrix (ECM) through focal adhesions. Moreover, mutations in the contractile machinery that is connected to the nuclear membrane of SMCs have been implicated in aneurysms. These data suggested that impaired mechanotransduction between the SMCs and its ECM contributes to the pathogenesis of aneurysms. Based on the above findings, Tan et al. (2018) hypothesized that individual ARIH1 mutations contribute to aortic aneurysms via defects in mechanotransduction from the ECM to the nucleus in the smooth muscle cells.
Previous studies on myonuclear positioning have highlighted roles of the LINC complex, microtubules, and their associated proteins in regulating myonuclear morphology and their link to muscle development and disease (Folker and Baylies, 2013). While nuclei are known to act as mechanosensors (Kirby and Lammerding, 2018), how they sense mechanical stimuli remains unknown. While Ari-1 is involved in mechanosensing by the nuclei, several questions remain unanswered: how does upregulation of Koi lead to aberrant nuclear morphology and myonuclear clustering? What are the other targets of Ari-1? How do the myonuclei directly sense mechanical stress, and what are the pathways involved? Answering these questions will help shed further light onto nuclear- positioning, mechanosensing and their link to disease.
References
- Folker ES, Baylies MK. Nuclear positioning in muscle development and disease. Front Physiol. 2013;4:363. doi: 10.3389/fphys.2013.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby TJ, Lammerding J. Emerging views of the nucleus as a cellular mechanosensor. Nature Cell Biology. 2018;103:177. doi: 10.1038/s41556-018-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YL, Burke B. LINC complexes and nuclear positioning. Semin Cell Dev Biol. 2017 doi: 10.1016/j.semcdb.2017.11.008. [DOI] [PubMed] [Google Scholar]
- Lücking CB, Dürr A, Bonifati V, Vaughan J, De Michele G, Gasser T, Harhangi BS, Meco G, Denèfle P, Wood NW, Agid Y, Brice A French Parkinson’s Disease Genetics Study Group, European Consortium on Genetic Susceptibility in Parkinson’s Disease. Association between early-onset Parkinson’s disease and mutations in the parkin gene. N Engl J Med. 2000;342:1560–1567. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- Parelkar SS, Cadena JG, Kim C, Wang Z, Sugal R, Bentley B, Moral L, Ardley HC, Schwartz LM. The parkin-like human homolog of Drosophila ariadne-1 (HHARI) can induce aggresome formation in mammalian cells and is immunologically detectable in Lewy bodies. J Mol Neurosci. 2012;46:109–121. doi: 10.1007/s12031-011-9535-1. [DOI] [PubMed] [Google Scholar]
- Rodger CE, McWilliams TG, Ganley IG. Mammalian mitophagy - from in vitro molecules to in vivo models. FEBS J. 2017;33:2142. doi: 10.1111/febs.14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero NB. Centronuclear myopathies: a widening concept. Neuromuscul Disord. 2010;20:223–228. doi: 10.1016/j.nmd.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Schreiber KH, Kennedy BK. When lamins go bad: nuclear structure and disease. Cell. 2013;152:1365–1375. doi: 10.1016/j.cell.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobreira N, Schiettecatte F, Valle D, Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Human Mutation. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LT, Haelterman N, Kwartler C, Regalado ES, Lee P-T, Nagarkar-Jaiswal S, Guo D-C, Duraine L, Wangler MF, Bamshed MJ, Nickerson DA, Lin G, Milewicz DM, Bellen H University of Washington Center for Mendelian Genomics. Ari-1 regulates myonuclear organization together with Parkin and is associated with aortic aneuryms. Dev Cell. 2018 doi: 10.1016/j.devcel.2018.03.020. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]