Abstract
Biomedical properties of hydrogen water have been extensively investigated, but the effect of hydrogen on good healthy subjects remains unclear. This study was designed to explore the hygiene improvement by electrolytically generated hydrogen warm water (40°C) on capillary blood streams, skin moisture, and keratin plugs in skin pores in normal good healthy subjects with their informed consents. Fingertip-capillary blood stream was estimated after hand-immersing in hydrogen warm water by videography using a CCD-based microscope, and the blood flow levels increased to about 120% versus normal warm water, after 60 minutes of the hand-immersing termination. Skin moisture of subjects was assessed using an electro-conductivity-based skin moisture meter. Immediately after taking a bath filled with hydrogen warm water, the skin moisture increased by 5–10% as compared to before bathing, which was kept on for the 7-day test, but indistinct, because of lower solubility of hydrogen in “warm” water than in room-temperature water. Cleansing of keratin plugs in skin-pores was assessed by stereoscopic microscopy and scanning electron microscopy. After hydrogen warm water bathing, the numbers of cleansed keratin plugs also increased on cheek of subjects 2.30- to 4.47-fold as many as the control for normal warm water. And areas of cleansed keratin plugs in the cheeks increased about 1.3-fold as much as the control. More marked improvements were observed on cheeks than on nostrils. Hydrogen warm water may thoroughly cleanse even keratin-plugs of residual amounts that could not be cleansed by normal warm water, through its permeability into wide-ranged portions of hair-pores, and promote the fingertip blood streams more markedly than merely through warmness due to normal warm water.
Keywords: hydrogen, hydrogen warm water, normal warm water, healthy subjects, blood stream, skin moisture, skin-pore, hair-pore, keratin plug cleansing, antioxidant ability
INTRODUCTION
Every aerobic organism consumes oxygen in a physiological process, concurrently with generating the potentially deleterious reactive oxygen species.1 And a chronic state of oxidative stress is defined as a quantitative imbalance between pro-oxidants and antioxidants and is widely considered as a cause of disease and aging.2 It is recognized that reactive oxygen species and iron have a dangerous partnership in inflammation.3 Traditional antioxidants including vitamin C, vitamin E, and β-carotene have relatively weak effects, and poor penetration of lipid bilayers and cell membranes results in inadequate clinical effects. Novel types of antioxidants that are effective and safe are eagerly awaited, and mild antioxidants with strong targeting tendencies have been developed. Purified nanomaterials with antioxidant properties have been developed, including hydrogen water,4,5 Platinum nanocolloid,5 H2-silica,4,5,6 and fullerene C60.7
Recently, a hydrogen molecule that has a very low molecular weight and diffuses readily, without any toxicity8 has been received much attention as a novel antioxidant in preventive and therapeutic medical applications.4,5 Multiple pharmacological effects of hydrogen molecules have been reported including antioxidant activities.4,5,6,8,9,10,11 Drinking hydrogen water for 72 weeks significantly improved the total Unified Parkinson's Disease Rating Scale (UPDRS) score of Parkinson's disease patients.12 Han et al.13 reported that hydrogen water inhibited the accumulation of the β-galactosidase, an indicator of aging, in the cytoplasm and abnormal nuclei appearance in murine embryonic fibroblasts. Although most drugs specifically act to their targets, a hydrogen molecule seems to differ from conventional pharmaceutical drugs, because a hydrogen molecule rapidly diffuses and militates moderately into tissues and cells.14 By these reasons, we have been performed the application of nanomaterials with antioxidant properties on the anti-aging field because of their excellent activities to anti-inflammatory and healing effects13.
Based on these findings, hydrogen water may be potentially applied as preventive medicines for oxidative pathway control, which is important in an aging society. However, health promotion effects of hydrogen water have not been investigated sufficiently on normal subjects. In the present study, we aimed to explore the hygiene improvement by electrolytically generated hydrogen-containing warm water on fingertip-capillary blood streams, skin moisture, and cleansing of cheek-keratin plugs. We examined them on normal subjects in terms of tissue permeability and antioxidant ability of hydrogen as compared with normal warm water.
SUBJECTS AND METHODS
Preparation of hydrogen warm water
Electrolytically hydrogen warm water was prepared using the electrolysis device, Spahare (Flax Co., Ltd., Yokohama, Japan). In the apparatus, the gaseous hydrogen was produced by the electrolysis of warm water in a bathtub. The warm water without electrolysis was applied as normal warm tap water for the control.
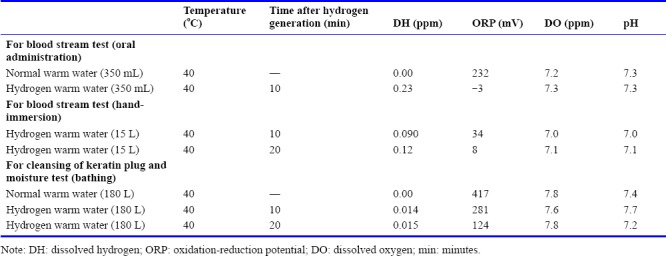
Hydrogen warm water, which was applied to fingertip-capillary blood stream test by 350 mL as oral administration and 15 L as hand-immersion, contained 0.23 and 0.09 ppm, respectively, of dissolved hydrogen (DH) as measured with a DH meter (DH-35A, DKK-TOA Corp., Tokyo, Japan) at 10 minutes after administration. Dissolved hydrogen content was also measured at 20 minute-operation by 15 L of hydrogen warm water, and the DH was 0.12 ppm. The pH and dissolved oxygen (DO) were measured with a pH/DO meter (D-55, Horiba Inc., Kyoto, Japan), and oxidation-reduction potential (ORP) was measured using an ORP meter (RM-20P, DKK-TOA Corp.). Then, hydrogen warm water of 180 L, which was applied in a bathtub for cleansing of keratin plug test and skin moisture test, contained dissolved hydrogen at 0.014 ppm after 10 minutes operation. Dissolved hydrogen content was also measured after 20 minutes operation by 180 mL of hydrogen warm water, and the DH was 0.015 ppm.
These parameters, temperatures, DH concentrations, oxidation-reduction potential (ORP) and DH concentrations of hydrogen warm water and normal warm water are shown in Table 1. All other reagents were commercial products of the reagent grade.
Table 1.
Parameters of hydrogen warm water and normal warm water which were applied to a fingertip-capillary blood stream test and a bathing test
Promoting of fingertip-capillary blood stream by oral- or immersed-hand-mediated administration of hydrogen warm water
Promoting of fingertip-capillary blood streams by hydrogen warm water was assessed on subjects with comparing oral administration and immersed-hand-mediated administration of hydrogen warm water. Two females at the ages of 51 and 43 years in normal good health state with their informed consents were enrolled in these tests. At first, subjects drank hydrogen warm water of 350 mL at 40°C for 10 minutes as an oral administration test. Subsequently, capillary blood streams on several capillary-loop points of fingertip were observed by videography using a CCD (charge coupled device)-based microscope (Bscan-Pro, Goko International Co., Ltd., Nagano, Japan; Figure 1) at each time point for 120 minutes. Then, subjects immersed their hands into hydrogen warm water of 15 L at 40°C for 10 minutes as an immersed-hand-mediated administration test, and capillary blood streams on fingertip were assessed in the same way as mentioned above. The blood flow level was assessed semi-quantitatively as % of the control for three typical microphotographic areas of the ring fingertip using Image J software (National Institute of Health, Bethesda, MD, USA).
Figure 1.
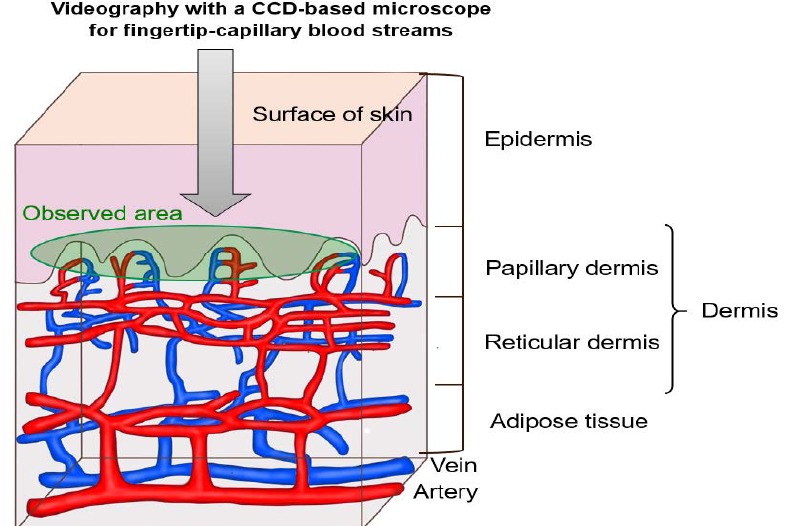
Observation of the fingertip-capillary blood streams by videography using a charge coupled device-based microscope.
The blood-flow fluctuation was so large as not necessarily significant, and therefore was measured for neither hydrogen room-temperature water nor normal warm water.
Skin moisture improvement by hydrogen warm water bathing
Skin moisture improvement on several skin loci such as cheek, neck, upper arm inside part, elbow, lower arm inside part, and back of hand by hydrogen warm water bathing was assessed on subjects. Two females at the age of 18 and 43 years in normal good health state with their informed consents were enrolled in these tests. At first, subjects took a bath filled with hydrogen warm water of 180 L for 10 minutes at 40°C. In bathing, their faces were covered with a hydrogen warm water-dampened towel, and the towel was exchanged every minute to keep the skin wet. Immediately before and immediately after and 60 minutes after bathing, skin moisture was measured three times using an electro-conductivity-based skin moisture meter DM-R2 (Panasonic Corp., Osaka, Japan). These experiments were performed every day for seven days. And before those seven days, experiments by normal warm water bathing were conducted in the same way as mentioned above.
Cleansing of keratin plugs on nostril and cheek by hydrogen warm water assessed by stereoscopic microscopy and scanning electron microscopy
Cleansing of keratin plugs on nostril and cheek, and sebum-filled hair follicles by hydrogen warm water were assessed on subjects. Female and male at the ages of 36 and 40 years, respectively, in normal good health state with their informed consents were enrolled in these tests. Keratin plugs on nostril and cheek were observed with a stereoscopic microscope (Z16 APO, Leica Microsystems GmbH, Wetzlar, Germany) and a scanning electron microscope (VE-9800, Keyence Corp., Osaka, Japan) for each skin loci and their silicon-plasticizer-replicas (Raptor Survey Institute, Ltd., Shizuoka, Japan), respectively.
Ethical approval
The present study was officially approved by Research Ethic Committee of NPO (Non-Profitable Organization) Corporation, Japanese Center for Anti-Aging Med-Sciences authentificated by Hiroshima Prefectural Government.
Statistical analysis
Results were expressed as the mean ± SD, for n = 3 or 4, and evaluated by the Student's t-test. The P-values that is at least smaller than 0.05 were regarded as to be statistically significant. The T. TEST function of Excel 2016 (Microsoft) was used for these analyses.
RESULTS
Promoting of fingertip-capillary blood streams by oral- or immersed-hand-mediated administration of hydrogen warm water assessed by videography
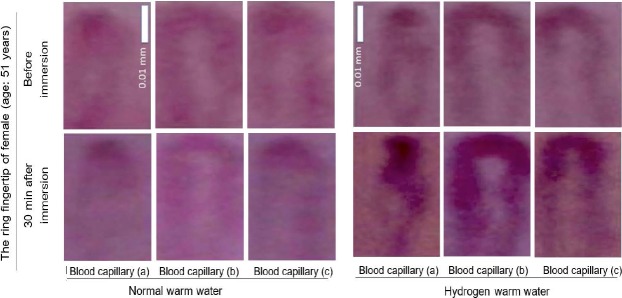
We observed fingertip-capillary blood streams by videography using a CCD-based microscope (Figure 1). Images of the ring fingertip-capillary blood streams of female at the age of 51 years are shown in Figure 2. The subject immersed her hands in hydrogen warm water or normal warm water (Table 1). After 30 minutes from termination of 10-minute immersion in hydrogen warm water, capillary blood streams obviously increased on every observed point of the ring fingertip with compared to before immersion (Figure 2). On the contrary, normal warm water did not exhibit any increase in capillary blood streams in the same points, because of post-bathing getting-cold. These effects were observed similarly on the other subject female at the age of 43 years (data not shown). These results suggest that the sustained promotion of blood streams by hydrogen warm water is not attributed to “warmness” of the hydrogen warm water because of no effect by normal warm water, but attributed to antioxidant ability of “hydrogen” dissolved in warm water.
Figure 2.
Promotion of the ring fingertip-capillary blood streams by immersed-hand-mediated administration of hydrogen warm water in female at the age of 51 years with compared to normal warm water.
Note: Images were shown for three typical capillary-loop points on the ring fingertip of the subject before immersion and at 30 minutes (min) after immersion of hands. Scale bars: 0.01 mm in the vertical length, but enlarged 2.6-folds in the lateral direction.
Figure 3 shows promotion of capillary blood streams on the ring fingertip of female at the age of 51 years by comparing oral administration with immersed-hand-mediated-administration of hydrogen warm water. When oral administration of hydrogen warm water, the blood flow level decreased initially, assumedly through warmness-caused transiently extended diameter of the blood vessels, and then restored to about 100% at 60 minutes (Figure 3). The promotion of blood streams was markedly caused by immersed-hand-mediated-administration of hydrogen warm water, the blood flow level (%) gradually decreased initially, and then increased to about 120% at 60 minutes (Figure 3). It was suggested in the case of immersed-hand-mediated administration of hydrogen warm water that dissolved hydrogen directly permeates into skin and thereby promotes capillary blood streams, which was more effective than oral administration.
Figure 3.
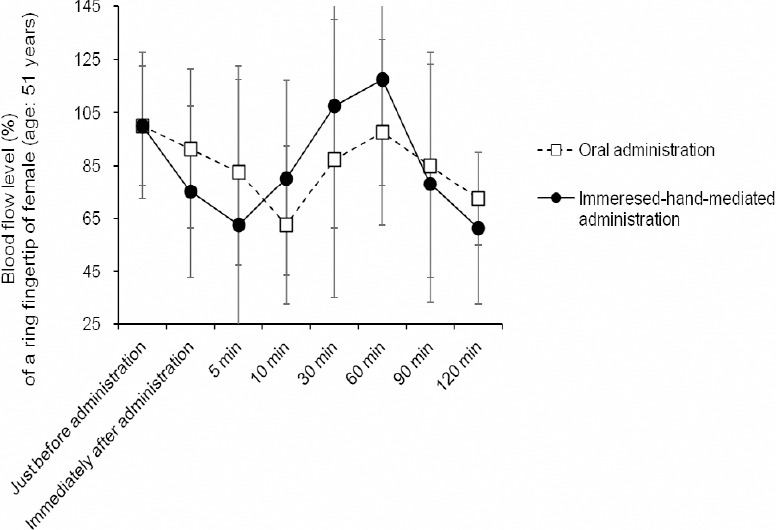
Promotion of capillary blood streams at the ring fingertip of the left hand of female at the age of 51 years by comparing oral administration with immersed-hand-mediated administration of hydrogen warm water.
Note: The charge coupled device-based microscopic photographs were expanded for 2.3-fold width direction, with unification for brightness and color tone of the background. The blood flow level (%) was assessed semi-quantitatively as % of the control for three micrographic areas of fingertips using an Image J software. Date are expressed as the mean ± SD, n = 3. Each administration was executed three times. min: Minutes.
Skin moisture improvement by hydrogen warm water bathing
Immediately after taking a bath filled with hydrogen warm water (Table 1), the skin moisture (%) on five parts of six parts of female at the age of 43 years increased by 5–10% as compared to before bathing, which was kept on for the 7-day test. On female at the age of 18 years, skin moisture (%) at immediately after bathing filled with hydrogen warm water increased in two parts of six parts by 5–10% as compared to before bathing. Figure 4 shows skin moisture (%) on backs of hands for the 7-day test. However, skin moisture (%) increased to near the level as those by normal water bathing (Figure 4). Cleansing of keratin plugs on nostril and cheek by hydrogen warm water assessed by stereoscopic microscopy and scanning electron microscopy.
Figure 4.
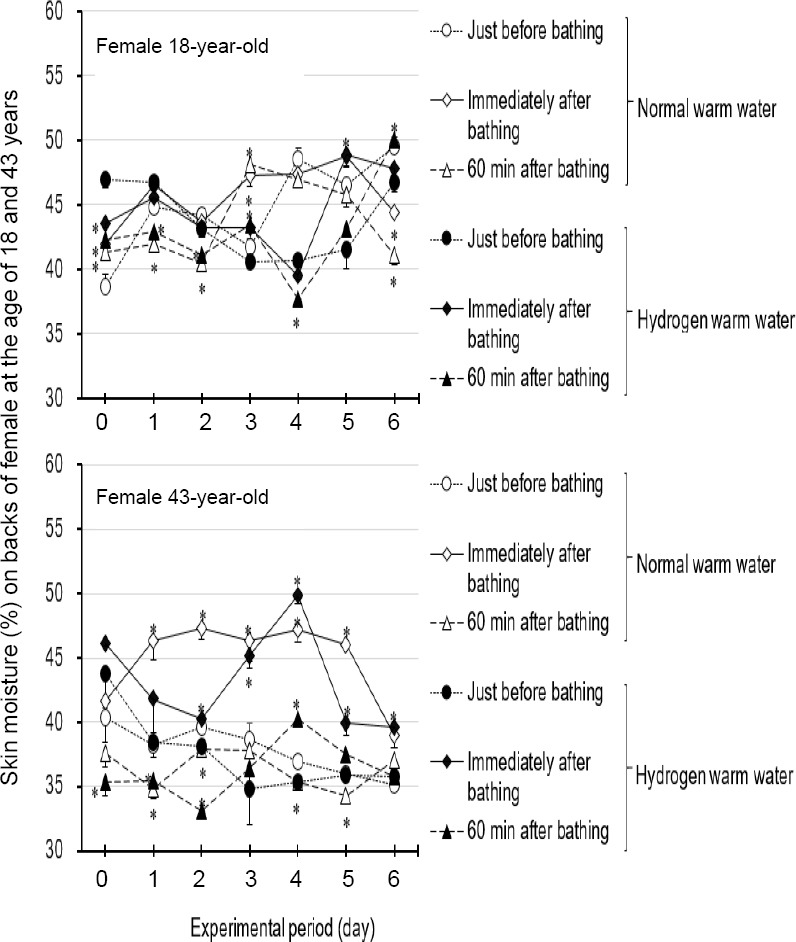
Skin moisture (%) improvement for seven days by hydrogen warm water bathing or normal warm water bathing on backs of hands of two female subjects at the ages of 18 and 43 years.
Note: Skin moisture values were evaluated with an electro-conductivity-based skin moisture meter. Data are expressed as the mean ± SD, n = 3. *P < 0.05, vs. just before bathing. Min: Minute(s).
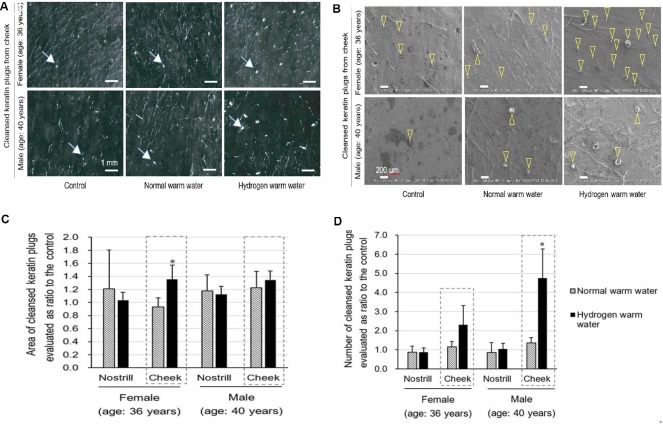
We verified cleansing of keratin plugs on nostril and cheek by hydrogen warm water bathing (Table 1). Images of cleansed keratin plugs from cheek on subjects were shown in stereoscopic micrographs of Figure 5A and in scanning electron micrographs of Figure 5B. After hydrogen warm water bathing, area of cleansed keratin plugs (% of the control) in cheek of 36 years old female increased about 1.3-fold as much as the control. And number of cleansed keratin plugs also increased 2.30- and 4.47-fold as many as the control on cheek of female at the age of 36 years and male at the age of 40 years, respectively (Figure 5C), similar on nostrils. Thus, the cleansing effects by hydrogen warm water were superior to normal warm water (Figure 5C).
Figure 5.
Effect of cleansing of keratin plugs on nostril and cheek by hydrogen warm water.
Note: (A) Stereoscopic images of cleansed and transferred keratin plugs on cheek by hydrogen warm water bathing with compared to normal warm water bathing. Representative plugs are shown by arrows. This experiment was performed on female at the age of 36 years and male at the age of 40 years. Scale bars: 1 mm. (B) Scanning electron microscopic images of cleansed and transferred keratin plugs (indicated by arrowheads) on cheek by hydrogen warm water bathing with compared to normal warm water bathing. This experiment was performed on female at the age of 36 years and male at the age of 40 years. Scale bars: 200 μm. (C, D) Cleansing of keratin plugs on nostril and cheek by hydrogen warm water bathing with compared to normal warm water bathing. Cleansing effects were assessed as the area (C) and number (D) of cleansed keratin plugs by stereoscopic microscopy shown in A and scanning electron microscopy shown in B. These experiments were performed in female at the age of 36 years and male at the age of 40 years. Date are expressed as the mean ± SD, n = 4. *P < 0.05, vs. hydrogen warm water.
DISCUSSION
We aimed to explore the hygiene improvement by electrolytically generated hydrogen warm water on fingertip-capillary blood streams in the dermis located more deeply than 0.1 mm long from the skin surface, skin moisture, and cleansing of cheek-keratin plugs in the present study.
The fingertip-capillary blood streams observed by videography show that the promotion of blood streams were caused by immersed-hand-mediated-administration of hydrogen warm water more appreciably than oral administration, suggesting that, upon the immersed-hand-mediated administration of hydrogen warm water, the dissolved hydrogen directly permeates into the depth of skin and thereby promotes capillary blood streams in the dermis located as deep as 0.1 mm long from the skin surface. This effect persisted approximately 60 minutes, supposed to be enough time to exclude waste into the blood effectively, thus helpful to anti-aging. It was reported by our research team that rosary-like aggregation of erythrocytes was caused by oxidative stress, together with disappearance of the surface dent structure being necessary for passage of erythrocytes within the inside of a capillary vessel being more narrow than a diameter of erythrocytes, but prevented by hydrogen water, as observed by scanning electron microscopy.14 As another mechanism that underlies the hydrogen-promoted blood streams, oxidative stress-induced delay of blood streams passing through narrow channels in a blood-streaming rheology comb-shaped apparatus was observed to be restored by hydrogen water.14
In our experiments, the inhibitory effects of hydrogen to prevent either agglutination or capillary-clogging of erythrocytes might cause the promotion of blood streams.
Rheology of blood streams is closely related to oxidative stress. It is reported that oxidative stress can lead to damages of both endothelial cells and circulating blood cells, but erythrocyte filterability was unaltered in the vitamin E-administered group in mountaineers.15 Erythrocytes are the first target to free radical oxidation, but alpha-tocopherol increases the functional resistance of erythrocytes maybe by the protection of protein components of the plasma against damaging action by free radicals.16 In accordance, we hypothesize that dissolved hydrogen bubbles contained in hydrogen warm water permeates and scavenges reactive oxygen species in erythrocytes and inhibits coagulation of erythrocytes, resulting in blood stream promotion in the present study (Figure 6).
Figure 6.
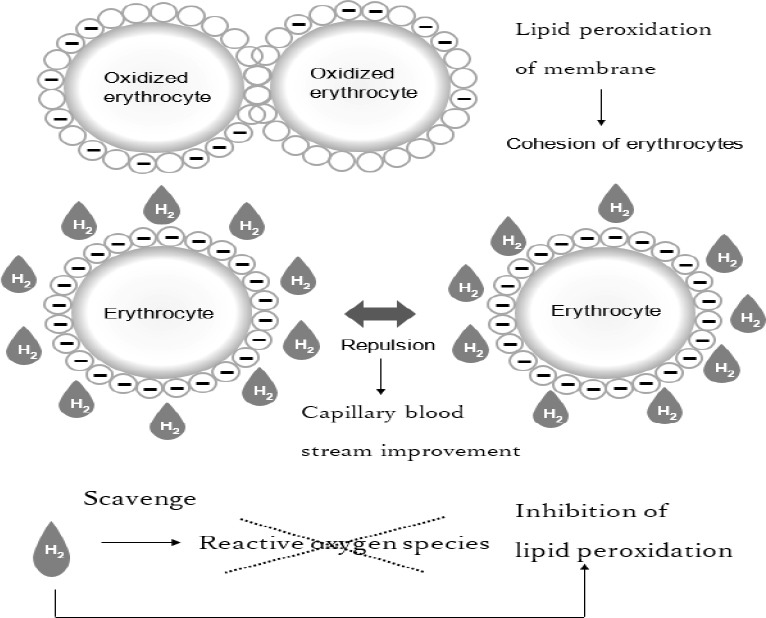
A possible mechanism whereby hydrogen prevents erythrocyte cohesion, and increases capillary blood streams via oxidative stress repression due to negatively charged surface lipid membrane of erythrocytes.
Immediately after taking a bath filled with hydrogen warm water, the skin moisture (%) on subjects increased by 5–10% as compared to before bathing, although for the stable effects by hydrogen warm water at least 6 days treatment is necessary because at some days up to 5 days, normal water seems to be more effective than hydrogen water (Figure 4). These results suggest that both hydrogen warm water bathing and normal warm water bathing serve for skin moisture, but a test period longer than 7 days might be necessary for analysis to elucidate the relation of dissolved hydrogen to skin moisture improvement. The physiological effects of hydrogen water depend on dissolved hydrogen concentration. It is reported that hydrogen water containing about 0.5 ppm of dissolved hydrogen affects allergic contact dermatitis in mice.17 In our previous study, hydrogen water containing 0.8–1.3 ppm of dissolved hydrogen intake via catheter-feeding via the nose directly to the stomach was demonstrated, for severely hospitalized elderly patients with pressure ulcer, to execute wound size reduction and early recovery.18 Sakai et al.19 suggest that the daily consumption of water containing a high concentration of hydrogen (over 7 ppm or 3.5 mg in 500 mL of water) may aid in maintaining functional vasculature via neutralization of detrimental reactive oxygen species or suppression of the inflammatory events. Results in the present study may be owing to dissolved hydrogen contents of 0.014–0.015 ppm in hydrogen warm water bathing (Table 1), which was not enough high to exhibit physiological effects. Generally, the saturated concentration of hydrogen in water is 1.6 ppm at normal room temperatures, but it is more difficult to achieve 1.6 ppm in “warm” water at 40°C in a bathtub. Though the technical issue how to dissolve higher concentration of hydrogen in “warm” water remains to be solved, hydrogen warm water bathing is expected to promote skin moisture.
We verified cleansing of keratin plugs on nostril and cheek by hydrogen warm water bathing. Results obtained indicate the cleansing effects by hydrogen warm water was superior to normal warm water (Figure 5C, D). Hydrogen molecules were supposed to be dispersed in water mostly as several hundred nanometer scale bubbles18 and thereby can easily permeate into small openings of skin. It is reported that hydrogen inhibited cellular damages through prevention of lipid peroxidation and DNA oxidation by selective-scavenging of hydroxyl radicals but not disturbing superoxide anion radicals, hydrogen peroxide, or nitric oxide in cells.20 The production of malondialdehyde, an indicator of lipid peroxidation, is markedly reduced by hydrogen-rich saline in rat skin flap.21 Our results suggest that hydrogen permeates deeply into skin and scavenges reactive oxygen species, resulting in inhibition lipid peroxidation in sebum-filled hair follicles (Figure 7).
Figure 7.
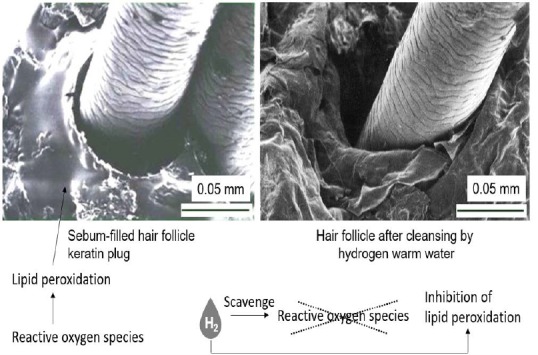
A possible mechanism whereby hydrogen warm water prevents sebum-filled hair follicles, and keratin plugs via the oxidative stress-repressing ability.
Note: Two biopsies of forehead skin were provided based on an informed consent from a subject of 54-years-old female. Scale bars: 0.05 mm.
The similar hydrogen concentration improved the fingertip blood flow and cleansing keratin plugs, however for the skin moisture, instable effects of increase was observed. The mechanism underlying protection provided by hydrogen warm water might be different between these measurements, depending on the penetration ability of skin surface (keratin layer), epidermal cells, dermal cells and blood vessels. In spite of the facts, these beneficial effects of hydrogen warm water would helpful to the anti-aging medicine and cosmetic.
Recently, hydrogen water is extensively investigated, but the effect of hydrogen on good-healthy subjects remains unclear. In the present study, hydrogen warm water was employed to characterize physiological effects of hydrogen in the alleviation of three following different aspects. Fingertip-capillary blood streams were promoted by immersed-hand-mediated-administration of hydrogen warm water, but not by normal warm water, showing the responsibility of hydrogen, for the blood-stream promotion. Hydrogen warm water bathing increased skin moisture stably after 6 days treatment with fluctuating small effects sometimes.
Furthermore, hydrogen warm water exhibited the cleansing effect of keratin plugs on nostril and cheek more markedly than normal warm water, showing the thorough cleansing by hydrogen warm water, keratin-plugs of residual amounts that could not be cleansed by normal warm water could be removed effectively. These beneficial effects could be attributed to the permeability into skin or lipid membrane, and the antioxidant ability of hydrogen.
Acknowledgement
We acknowledged to Ms. Haruko Mimura for her technical assistance and Dr. Shinya Kato of Mie University for his assistance of manuscript preparation in this study.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
Financial support
This work was in part supported by a Grant-in-Aid #1704 for Anti-Aging Research from Japanese Center for Anti-Aging MedSciences, Hiroshima, Japan. Funders had no involvement in the study design; data collection, management, analysis, and interpretation; paper writing; or decision to submit the paper for publication.
Research ethics
The present study was officially approved by Research Ethic Committee of NPO (Non-Profitable Organization) Corporation Japanese Center for AntiAging MedSciences authentificated by Hiroshima Prefectural Government. The study was performed in accordance with the Declaration of Helsinki and relevant ethical principles.
Declaration of patient consent
The authors certify that they have obtained all appropriate participant consent forms. In the form the participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open peer reviewers
Xue-jun Sun, Second Military Medical University, China; Lei Huang, Loma Linda University, USA.
Funding: This work was in part supported by a Grant-in-Aid #1704 for Anti-Aging Research from Japanese Center for Anti-Aging Med-Sciences, Hiroshima, Japan.
REFERENCES
- 1.Pruchniak MP, Aražna M, Demkow U. Biochemistry of oxidative stress. Adv Exp Med Biol. 2016;878:9–19. doi: 10.1007/5584_2015_161. [DOI] [PubMed] [Google Scholar]
- 2.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris CJ, Earl JR, Trenam CW, Blake DR. Reactive oxygen species and iron--a dangerous partnership in inflammation. Int J Biochem Cell Biol. 1995;27:109–122. doi: 10.1016/1357-2725(94)00084-o. [DOI] [PubMed] [Google Scholar]
- 4.Saitoh Y, Okayasu H, Xiao L, Harata Y, Miwa N. Neutral pH hydrogen-enriched electrolyzed water achieves tumor-preferential clonal growth inhibition over normal cells and tumor invasion inhibition concurrently with intracellular oxidant repression. Oncol Res. 2008;17:247–255. doi: 10.3727/096504008786991620. [DOI] [PubMed] [Google Scholar]
- 5.Asada R, Kageyama K, Tanaka H, et al. Antitumor effects of nano-bubble hydrogen-dissolved water are enhanced by coexistent platinum colloid and the combined hyperthermia with apoptosis-like cell death. Oncol Rep. 2010;24:1463–1470. doi: 10.3892/or_00001006. [DOI] [PubMed] [Google Scholar]
- 6.Kato S, Saitoh Y, Miwa N. Inhibitions by hydrogen-occluding silica microcluster to melanogenesis in human pigment cells and tyrosinase reaction. J Nanosci Nanotechnol. 2013;13:52–59. doi: 10.1166/jnn.2013.6848. [DOI] [PubMed] [Google Scholar]
- 7.Sera N, Tokiwa H, Miyata N. Mutagenicity of the fullerene C60-generated singlet oxygen dependent formation of lipid peroxides. Carcinogenesis. 1996;17:2163–2169. doi: 10.1093/carcin/17.10.2163. [DOI] [PubMed] [Google Scholar]
- 8.Saitoh Y, Harata Y, Mizuhashi F, Nakajima M, Miwa N. Biological safety of neutral-pH hydrogen-enriched electrolyzed water upon mutagenicity, genotoxicity and subchronic oral toxicity. Toxicol Ind Health. 2010;26:203–216. doi: 10.1177/0748233710362989. [DOI] [PubMed] [Google Scholar]
- 9.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 10.Ohta S. Hydrogen gas and hydrogen water act as a therapeutic and preventive antioxidant with a novel concept. Nihon Ronen Igakkai Zasshi. 2008;45:355–362. [PubMed] [Google Scholar]
- 11.Zheng J, Liu K, Kang Z, et al. Saturated hydrogen saline protects the lung against oxygen toxicity. Undersea Hyperb Med. 2010;37:185–192. [PubMed] [Google Scholar]
- 12.Yoritaka A, Takanashi M, Hirayama M, Nakahara T, Ohta S, Hattori N. Pilot study of H therapy in Parkinson's disease: a randomized double-blind placebo-controlled trial. Mov Disord. 2013;28:836–839. doi: 10.1002/mds.25375. [DOI] [PubMed] [Google Scholar]
- 13.Han AL, Park SH, Park MS. Hydrogen treatment protects against cell death and senescence induced by oxidative damage. J Microbiol Biotechnol. 2017;27:365–371. doi: 10.4014/jmb.1608.08011. [DOI] [PubMed] [Google Scholar]
- 14.Kato S, Hokama R, Okayasu H, Saitoh Y, Iwai K, Miwa N. Colloidal platinum in hydrogen-rich water exhibits radical-scavenging activity and improves blood fluidity. J Nanosci Nanotechnol. 2012;12:4019–4027. doi: 10.1166/jnn.2012.6163. [DOI] [PubMed] [Google Scholar]
- 15.Simon-Schnass I, Korniszewski L. The influence of vitamin E on rheological parameters in high altitude mountaineers. Int J Vitam Nutr Res. 1990;60:26–34. [PubMed] [Google Scholar]
- 16.Roĭtman EV, Dement'eva II, Azizova OA, Nikitina NA, Gagaeva EV, Lopukhin IuM. Changes in blood rheological properties and erythrocyte osmotic resistance in activation of free radical processes. Klin Lab Diagn. 2001:42–43. [PubMed] [Google Scholar]
- 17.Ignacio RM, Kwak HS, Yun YU, et al. The drinking effect of hydrogen water on atopic dermatitis induced by dermatophagoides farinae allergen in NC/Nga mice. Evid Based Complement Alternat Med. 2013;2013:538673. doi: 10.1155/2013/538673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Kato S, Matsuoka D, Tanaka H, Miwa N. Hydrogen water intake via tube-feeding for patients with pressure ulcer and its reconstructive effects on normal human skin cells in vitro. Med Gas Res. 2013;3:20. doi: 10.1186/2045-9912-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai T, Sato B, Hara K, et al. Consumption of water containing over 35 mg of dissolved hydrogen could improve vascular endothelial function. Vasc Health Risk Manag. 2014;10:591–597. doi: 10.2147/VHRM.S68844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Yu Q, Liu Y, Zhang R, Xue L. Hydrogen gas alleviates oxygen toxicity by reducing hydroxyl radical levels in PC12 cells. PLoS One. 2017;12:e0173645. doi: 10.1371/journal.pone.0173645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao L, Wang YB, Qin SR, et al. Protective effect of hydrogen-rich saline on ischemia/reperfusion injury in rat skin flap. J Zhejiang Univ Sci B. 2013;14:382–391. doi: 10.1631/jzus.B1200317. [DOI] [PMC free article] [PubMed] [Google Scholar]