Abstract
Ventilator-associated-pneumonia (VAP) is characterized by morbidity, mortality, and prolonged length of stay in intensive care unit (ICU). The present study aimed to examine the effect of N-acetyl-cysteine (NAC) in preventing VAP in patients hospitalized in ICU. We performed a prospective, randomized, double-blind, placebo-controlled trial of 60 mechanically ventilated patients at high risk of developing VAP. NAC (600 mg/twice daily) and placebo (twice daily) were administered to NAC group (n = 30) and control group (n = 30), respectively, through the nasogastric tube in addition to routine care. The clinical response was considered as primary (incidence of VAP) and secondary outcomes. Twenty-two (36.6%) patients developed VAP. Patients treated with NAC were significantly less likely to develop clinically confirmed VAP compared with patients treated with placebo (26.6% vs. 46.6%; P = 0.032). Patients treated with NAC had significantly less ICU length of stay (14.36 ± 4.69 days vs. 17.81 ± 6.37 days, P = 0.028) and less hospital stay (19.23 ± 5.54 days vs. 24.61 ± 6.81 days; P = 0.03) than patients treated with placebo. Time to VAP was significantly longer in the NAC group (9.42 ± 1.9 days vs. 6.46 ± 2.53 days; P = 0.002). The incidence of complete recovery was significantly higher in the NAC group (56.6% vs. 30%; P = 0.006). No adverse events related to NAC were identified. NAC is safe and effective to prevent and delay VAP, and improve its complete recovery rate in a selected, high-risk ICU population.
Keywords: acetylcysteine, pneumonia, ventilator-associated, prevention, control, prophylaxis, intratracheal intubation
INTRODUCTION
Ventilator-associated-pneumonia (VAP) is a complication developed in about 30% of mechanically ventilated patients.1,2,3,4 Patients with VAP have higher morbidity and mortality rates, and faced with prolonged intensive care unit (ICU) and hospital lengths of stay, and are consequently imposed greater hospital costs.1,5,6,7 The pathogenesis of VAP is complex; however, bacterial colonization of respiratory and digestive tracts, biofilm formation, and micro aspiration of contaminated secretions are the most important pathogenic factors involved.5,8 The current preventive strategies for VAP are mainly directed at colonization and aspiration modification. These strategies include elevation of the head of the bed, intensive oral care, subglottic secretion draining or silver-coated endotracheal tubes, and reducing the duration of mechanical ventilation using regular sedation vacations and weaning protocols.3,8,9,10,11,12 Underlying disorders, prolonged hospital stay, and high prevalence of antibiotic-resistant pathogens obstruct the treatment of VAP.13 Respiratory support dysfunctions such as mucociliary dysfunction and damage caused by oxidative stress are predisposing factors of VAP.13 N-acetyl-cysteine (NAC) is a mucolytic drug with anti-inflammatory,14 antioxidant15,16 and immunomodulating17 properties. Given the pathogenesis of VAP and the functions of NAC, it appears that NAC can be effective in preventing VAP as a non-antibiotic strategy. Therefore, we conducted a study aimed to examine the effect of NAC in preventing of VAP in patients hospitalized in ICU.
SUBJECTS AND METHODS
Study design
This was a randomized, double blind, placebo-controlled clinical trial conducted from March 2014 until June 2016 in an academic infectious department of Vali-asr Hospital, Arak, Iran. Written informed consent was obtained from patients'legal guardians. The investigators were committed to the principles of the Declaration of Helsinki throughout the study. The protocol of the study was approved by the Ethical Committee of Arak University of Medical Sciences (approved No: 4-144-92).
Subjects
Adult ICU admitted patients undergoing endotracheal intubation and mechanical ventilation were eligible. All eligible patients who referred to ICU were selected for study and recruitment after signing the informed consent. Our exclusion criteria during the follow-up duration were: 1) Less than 72-hour intubation, 2) death within 72 hours after intubation, 3) transference to other hospitals, and 4) termination of NAC administration: withdrawal with the consent of the patients'legal guardians; judgment if the physician in charge due to adverse events and safety concerns; and difficult administration due to GI problems and other reasons. Therefore, patients with pregnancy, recent gastrointestinal tract injury, oropharyngeal mucosal injury, tracheostomy, presence of pneumonia at the beginning of hospitalization, history of antibiotic consumption within the last 4 weeks prior to ICU hospitalization, and those disconnected from ventilator or died within 72 hours after intubation were excluded. Patients were also excluded if the investigators were unable to obtain informed written consent and administer the first dose of the study drug within 12 hours of intubation.
Randomization and blinding
Simple randomization using computer-assisted randomization table was considered for current study. NAC and placebo were provided by a pharmaceutical company (Avesina, Tehran, Iran). Two preparations were delivered to the nurses in glass containers with lid of similar shape and size, without a name and with a code. Investigators, primary care clinicians, and bedside nurses were blinded to group assignments.
Intervention
Demographic and baseline information collected using a checklist including questions regarding age, gender, VAP risk factors,18 reason for ICU admission, Acute Physiology and Chronic Health Evaluation II (APACHE II) score (< 18, 18–24, > 24), oral prosthodontics, and oral hygiene condition (poor, good).
Patients were randomly assigned into NAC or placebo groups. NAC (600 mg; water-soluble tablets) was administered to the NAC group twice daily through the nasogastric tube. Placebo (water-soluble vitamin tablets) was administered to the control group through the nasogastric tube twice daily. Drug administration was started at the beginning of hospitalization within the first 12 hours of mechanical ventilation, and continued until performing extubation, tracheostomy, discharge, or death.
Patients received all routine care, including VAP-preventive measures as per hospital protocols and antibiotic therapy as deemed necessary, under the direction of their admitting physicians throughout the study.18,19 Institutional VAP-prevention measures remained unchanged throughout the study period.
Outcome assessment
The clinical response was considered as primary and secondary outcomes. The primary outcome was the incidence of VAP. The secondary outcome included: 1) Time to VAP, 2) duration of mechanical ventilation, 3) ICU stay, 4) hospital stay, 5) VAP complications, and 6) recovery rate.
VAP was diagnosed based on clinical examinations and daily chest X-ray (CXR) results according to the American College of Chest Physicians (ACCP) criteria18,19 as follows: The presence of new and continuous infiltrations (for over 24 hours) in CXR results accompanied by 2–3 of the findings bellow: 1) Fever (< 38.5°C or < 35°C); 2) leukocytosis (WBC > 10,000/mm3 or WBC < 3,000/mm3); 3) purulent sputum.
The recovery rate was defined as follows: 1) Complete recovery: termination of fever after 48 hours, termination of initial physical-pulmonary examination results after 1 week, leukocytosis improvement after 4 days, and improved CXR results within 4–12 weeks. 2) Modest recovery: termination of fever after 4–7 days and improved examination results after over 10 days. 3) Lack of recovery: continuation of symptoms or the development of complications. 4) Mortality: death during hospitalization in ICU.
Patients who were excluded were replaced by the other eligible patients. Notably, the patients excluded were not analyzed and only the patients that completed the study were subjected to further analysis.
Statistical analysis
The sample size was determined based on alpha (0.05) and beta (0.2) values, i.e. type I and type II errors. The collected data were analyzed with SPSS 18.0 software (SPSS Inc, Chicago, IL, USA) and descriptive statistics methods for frequency determination. Groups were compared with t-test; continuous variables with abnormal distribution were compared by Mann-Whitney U-test and categorical variables with abnormal distribution were compared using chi-square test. P-values less than 0.05 were considered statistically significant.
RESULTS
Demographic and baseline findings
A total of 174 patients admitted to the ICU required endotracheal intubation, but 114 were not enrolled because informed consent could not be obtained during the first 12 hours of mechanical ventilation, or patients were unlikely to require intubation for at least 72 hours. Finally, 60 patients were randomly assigned into NAC (n = 30) group and placebo group (n = 30). Figure 1 illustrates the study recruitment process (CONSORT flowchart).
Figure 1.
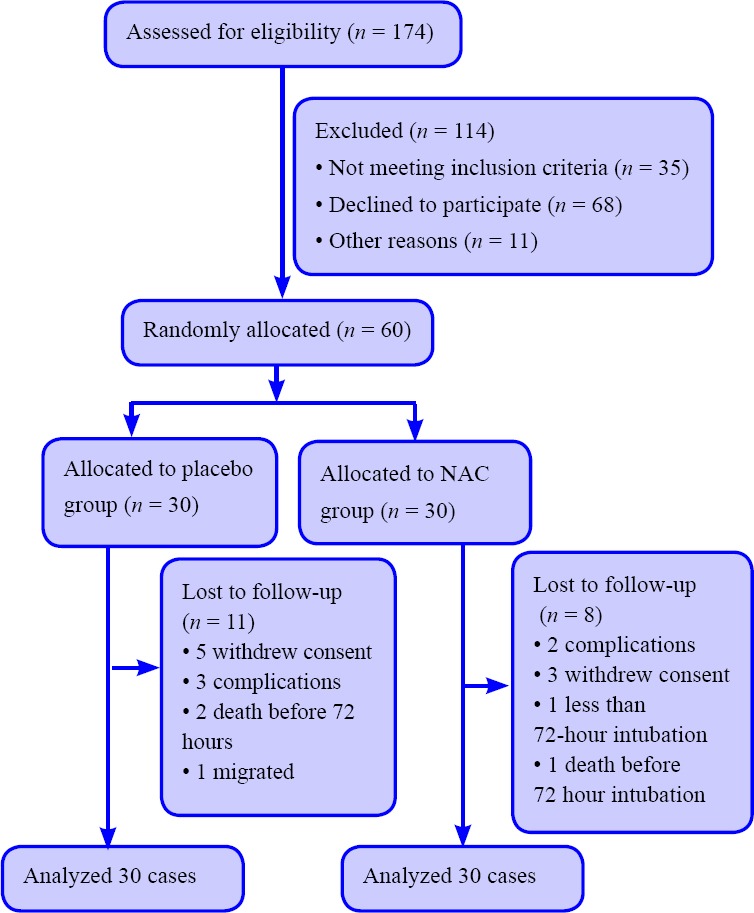
CONSORT flowchart of patients undergoing endotracheal intubation and mechanical ventilation.
Note: NAC: N-acetyl-cysteine.
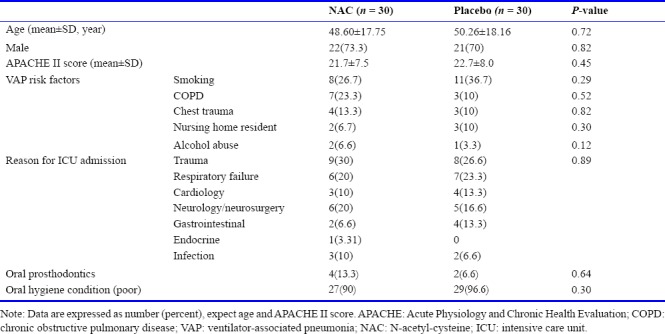
Patients were evenly distributed between groups based on demographic and other baseline characteristics (Table 1). The mean age was 49.43 ± 18.7 year and 71.6% were male. The mean APACHE II score was 22.2 ± 4.5. The most common VAP risk factor was smoking (NAC: 8 [26.7%], placebo: 11 [36.7%]; P = 0.29). The most common reason for ICU admission was trauma (NAC: 9 [30%], placebo: 8 [26.6%]; P = 0.89).
Table 1.
Demographic and baseline characteristics of patients undergoing endotracheal intubation and mechanical ventilation
Primary outcome
Twenty-two (36.6%) patients developed VAP. The incidence of VAP was significantly lower in the NAC group (n = 8, 26.6%) than in the placebo group (n = 14, 46.6%) (P = 0.032).
Secondary outcomes
ICU stay was 16.08 ± 5.9 days. Hospital stay and time to VAP in all patients were 21.92 ± 6.3 days and 7.94 ± 3.01 days, respectively. Mechanical ventilation duration was 9.16 ± 8.2 days. Complete recovery and mortality were observed in 26 (43.3%) and 5 (8.3%) patients, respectively. Secondary outcome information is summarized in Table 2.
Table 2.
Secondary outcomes of patients undergoing endotracheal intubation and mechanical ventilation with NAC and placebo intervention
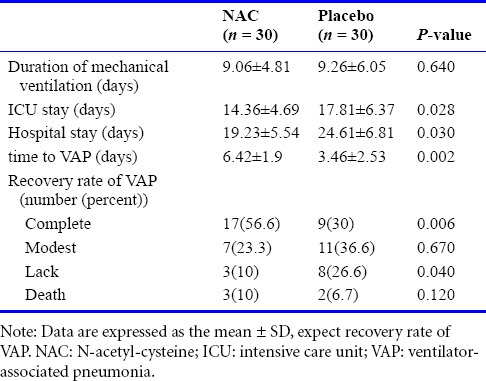
Patients treated with NAC had significantly less ICU stay (14.36 ± 4.69 days vs. 17.81 ± 6.37 days, P = 0.028) and less hospital stay (19.23 ± 5.54 days vs. 24.61 ± 6.81 days; P = 0.03) than patients treated with placebo. Time to VAP was significantly longer in the NAC group (6.42 ± 1.9 days vs. 3.46 ± 2.53 days, P = 0.002). Mechanical ventilation duration was not significantly different between two groups (9.06 ± 4.81 days vs. 9.26 ± 6.05 days, P = 0.64).
The incidence of complete recovery was significantly higher in the NAC group (56.6%, vs. 30%, P = 0.006). The incidence of lack of recovery was significantly lower in the NAC group (10% vs. 26.6%, P = 0.04). Mortality within 72 hours after intubation was not significantly different between two groups (2 [6.6%] in placebo group vs. 3 [10%] in NAC group).
Five (16.6%) of 30 patients in the NAC group and 7 (23.3%) patients in the control group had complications (P = 0.58). Two patients in NAC group and three patients in placebo had serious complications that who excluded from study. Complications were related to underlying diseases. No complications were thought to be drug-related.
DISCUSSION
The current study suggests that NAC can be effective to prevent and delay VAP and improve its complete recovery rate in a selected, high-risk ICU population.
The effectiveness of NAC has been shown in patients with some lung diseases such as acute respiratory distress syndrome (ARDS),20,21 idiopathic pulmonary fibrosis (IPF),22,23 chronic obstructive pulmonary disease (COPD),14,24 influenza17,25 specially influenza A (H5N1),26 cystic fibrosis27 and smoking-related damage.28 However, studies on the efficacy of NAC on pneumonia, especially the VAP remain limited.
In 2010, Zhao et al.29 showed that NAC has anti-bacterial properties against P. aeruginosa and may detach P. aeruginosa biofilms in chronic respiratory tract infections. Based on this study, NAC at 0.5 mg/mL could detach mature P. aeruginosa biofilms. Disruption was proportional to NAC concentrations, and biofilms were completely disrupted at 10 mg/mL NAC. Extracellular polysaccharides (EPS) production by P. aeruginosa were also decreased by 27.64% and 44.59% at NAC concentrations of 0.5 mg/mL and 1 mg/mL, respectively.
Qu et al.30 in 2016, evaluated NAC inhalation on VAP caused by biofilm in endotracheal tubes. They selected 117 cases tracheally intubated and undergoing mechanical ventilation for ≥ 48 hours in ICU. All the cases were randomly divided into control group (n = 60) and study group (n = 57). The patients in the study group were treated with different doses of aerosolized NAC according to different ages, once every 8 hours, until stopping mechanical ventilation. Electron microscopy showed that biofilm had formed in the endotracheal tube inner wall in early period of mechanical ventilation. With prolonged mechanical ventilation, biofilm structure improved. During the mechanical ventilation, the thickness of biofilm in the study group decreased compared with control group. Biofilm culture positive rate and incidence of VAP decreased in the study group compared with the control group (65% [37/57] vs. 80% [48/60], P < 0.05; 11% [6/57] vs. 32% [19/60], P < 0.01). The most similar study to our study was a clinical trial reported by Qu et al.30 Although studies method was notable differences, however, the results were consistent about the effectiveness of NAC in VAP.
In a systematic review, Chalumeau et al.31 investigated 6 trials with 497 participants for the evaluation of the efficacy of NAC in the treatment of acute lower and upper respiratory tract infections in children without chronic pulmonary diseases. NAC seemed to have a moderate efficacy in children older than 2 years, and NAC administration was safe in these children.
The majority of studies on the efficacy of NAC in pulmonary diseases have been conducted based on functions defined for NAC, i.e. mucociliary, antioxidant and anti-inflammatory functions.13,16,21,22 Our study was also designed based on these functions: 1) The mechanical mucociliary function is a defense mechanism of the respiratory system directed at preventing the deposition of its contaminated secretions in respiratory tracts and subsequent contaminations.21 The mucociliary system consists of respiratory cilia that keep the respiratory system clear of secretions through their beating motion.13 Diminished respiratory support functions such as mucociliary function against respiratory infections and stress induced by underlying diseases are among disorders that leave ICU patients susceptible to pneumonia.13 NAC is a mucolytic drug that facilitates mucus flow and discharge through breaking disulfide bonds of thick bronchial system secretions, reducing the development and prolongation risks for infectious respiratory system diseases.16 2) The second significant function of NAC is its antioxidant role fulfilled via two pathways: a) the direct pathway (reaction with free radicals), and b) the indirect pathway (glutathione synthesis precursors as a major body antioxidant).16,32 Dekhuijzen15 has interpreted the NAC antioxidant effect well.
Oxidative agents play a significant destructive role in the course of VAP development. The prevention of oxidative degradation cycle through antioxidant agents may reduce the incidence or severity of VAP.33,34 Duflo et al.33 examined alveolar and serum oxidative stress in 78 and 10 patients with and without VAP, respectively. Serum oxidative stress markers and patients'bronchoalveolar lavage (BAL) samples were evaluated for oxidative activity. VAP group exhibited significantly greater oxidative activity compared with the non-VAP group. Manzanares et al.34 examined the efficacy of selenium, as an antioxidative agent, in VAP patients through a clinical study. Results revealed that not only could selenium reduce the incidence of VAP in ICU patients, but also reduce its severity. In addition to the positive effects of NAC in preventing VAP and facilitating clinical recovery, the results of our study also demonstrated the lower ICU and hospital length of stay, in the NAC group compared with the placebo. Complications of VAP were not different between the two groups. No adverse events related to NAC administration were identified and NAC was well tolerated. NAC also was well tolerated in other studies in patients with30 and without14,15 endotracheal intubation.
Limitations
The small sample size in this study, compared with other clinical trials on VAP, stemmed from the insufficient number of ICU beds and lack of consent on the part of a number of patients'legal guardians for their participation in the study (due to their critical condition), with the latter resulting in the exclusion of 114 eligible patients from the onset of study.
The high mean of APACHE II score (22.2) in our patients revealed that the ICU patients in the study exhibited high risks for VAP. One of the inclusion criteria in the study included an extremely high prognosis for undergoing at least 72 hours of mechanical ventilation, meaning that the included patients were basically critically ill. Of course, this was inevitable, due to patients requiring less than 48-hour ventilation could not have been defined as cases of VAP.35 This issue demonstrates that the results of this study do not encompass all ICU patients and can only be attributed to those with high risks for VAP.
We did not investigate the VAP microbiology and the effect of NAC on the VAP microbiological pattern. According to Morrow et al.,18 evaluation of the effect of adjunct treatment on the VAP microbiology can help in the selection of its treatments.
We did not review hospital costs in the groups. The assessment of hospital costs along with the impact of adjunct treatment can be decisive in choosing the appropriate treatment for VAP.
Conclusion
These data suggest that NAC is safe and effective to prevent and delay VAP and improve its complete recovery rate in a selected, high-risk ICU population. Future studies are needed to explore efficacy and safety of different doses of NAC in VAP patients with different clinical conditions in ICU.
Acknowledgments
The research team wishes to thank vice chancellor of research of Arak University of Medical Sciences for the financial support and also patients who contributed to this research.
Footnotes
Conflicts of interest
All authors declared that they had no conflict of interest.
Financial support
This study was financially supported by Arak University of Medical Sciences, Iran. Funders had no involvement in the study design; data collection, management, analysis, and interpretation; paper writing; or decision to submit the paper for publication.
Research ethics
The investigators were committed to the relevant principles and laws of the Declaration of Helsinki throughout the study. The study protocol was approved by the Ethical Committee of Arak University of Medical Sciences (4-144-92).
Declaration of patient consent
The authors certify that they have obtained all appropriate patients' legal guardians consent forms. In the form the patients' legal guardians have given their consent for the patients' images and other clinical information to be reported in the journal. The patients' legal guardians understand that the patients' names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open peer reviewer
Wen-Wu Liu, Second Military Medical University, China.
Funding: This study was financially supported by Arak University of Medical Sciences, Iran.
REFERENCES
- 1.Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 2.Blot S, Koulenti D, Dimopoulos G, et al. Prevalence, risk factors, and mortality for ventilator-associated pneumonia in middle-aged, old, and very old critically ill patients. Crit Care Med. 2014;42:601–609. doi: 10.1097/01.ccm.0000435665.07446.50. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim EH, Tracy L, Hill C, Fraser VJ, Kollef MH. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest. 2001;120:555–561. doi: 10.1378/chest.120.2.555. [DOI] [PubMed] [Google Scholar]
- 5.Kollef MH. What is ventilator-associated pneumonia and why is it important? Respir Care. 2005;50:714. [PubMed] [Google Scholar]
- 6.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 7.Zarinfar N, Sharafkhah M, Amiri M, Rafeie M. Probiotic effects in prevention from ventilator-associated pneumonia. Koomesh. 2016;17:803–813. [Google Scholar]
- 8.Kollef MH. Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit Care Med. 2004;32:1396–1405. doi: 10.1097/01.ccm.0000128569.09113.fb. [DOI] [PubMed] [Google Scholar]
- 9.Craven DE. Preventing ventilator-associated pneumonia in adults: sowing seeds of change. Chest. 2006;130:251–260. doi: 10.1378/chest.130.1.251. [DOI] [PubMed] [Google Scholar]
- 10.Cook D. Ventilator associated pneumonia: perspectives on the burden of illness. Intensive Care Med. 2000;26(Suppl 1):S31–37. doi: 10.1007/s001340051116. [DOI] [PubMed] [Google Scholar]
- 11.Dodek P, Keenan S, Cook D, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004;141:305–313. doi: 10.7326/0003-4819-141-4-200408170-00011. [DOI] [PubMed] [Google Scholar]
- 12.Tablan OC, Anderson LJ, Besser R, et al. Guidelines for preventing health-care--associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53:1–36. [PubMed] [Google Scholar]
- 13.Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J. Harrison's Principles of Internal Medicine. 18th ed. McGraw-Hill Professional; 2011. [Google Scholar]
- 14.Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2:187–194. doi: 10.1016/S2213-2600(13)70286-8. [DOI] [PubMed] [Google Scholar]
- 15.Dekhuijzen PN. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J. 2004;23:629–636. doi: 10.1183/09031936.04.00016804. [DOI] [PubMed] [Google Scholar]
- 16.Millea PJ. N-acetylcysteine: multiple clinical applications. Am Fam Physician. 2009;80:265–269. [PubMed] [Google Scholar]
- 17.Hui DS, Lee N, Chan PK. Adjunctive therapies and immunomodulatory agents in the management of severe influenza. Antiviral Res. 2013;98:410–416. doi: 10.1016/j.antiviral.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med. 2010;182:1058–1064. doi: 10.1164/rccm.200912-1853OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight DJ, Gardiner D, Banks A, et al. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: a randomised, double-blind, placebo-controlled trial. Intensive Care Med. 2009;35:854–861. doi: 10.1007/s00134-008-1368-1. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Ding S, Li C, Wang Y, Chen Z, Wang Z. Effects of N-acetylcysteine treatment in acute respiratory distress syndrome: a meta-analysis. Exp Ther Med. 2017;14:2863–2868. doi: 10.3892/etm.2017.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren B, Royall N, Smith H, Bhullar IS. Novel treatment of acute respiratory distress syndrome after chlorine gas inhalation injury. Am Surg. 2016;82:e219–220. [PubMed] [Google Scholar]
- 22.Homma S, Azuma A, Taniguchi H, et al. Efficacy of inhaled N-acetylcysteine monotherapy in patients with early stage idiopathic pulmonary fibrosis. Respirology. 2012;17:467–477. doi: 10.1111/j.1440-1843.2012.02132.x. [DOI] [PubMed] [Google Scholar]
- 23.Bando M, Hosono T, Mato N, et al. Long-term efficacy of inhaled N-acetylcysteine in patients with idiopathic pulmonary fibrosis. Intern Med. 2010;49:2289–2296. doi: 10.2169/internalmedicine.49.4011. [DOI] [PubMed] [Google Scholar]
- 24.Tse HN, Raiteri L, Wong KY, et al. High-dose N-acetylcysteine in stable COPD: the 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest. 2013;144:106–118. doi: 10.1378/chest.12-2357. [DOI] [PubMed] [Google Scholar]
- 25.Lai KY, Ng WY, Osburga Chan PK, Wong KF, Cheng F. High-dose N-acetylcysteine therapy for novel H1N1 influenza pneumonia. Ann Intern Med. 2010;152:687–688. doi: 10.7326/0003-4819-152-10-201005180-00017. [DOI] [PubMed] [Google Scholar]
- 26.Geiler J, Michaelis M, Naczk P, et al. N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem Pharmacol. 2010;79:413–420. doi: 10.1016/j.bcp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Conrad C, Lymp J, Thompson V, et al. Long-term treatment with oral N-acetylcysteine: affects lung function but not sputum inflammation in cystic fibrosis subjects A phase II randomized placebo-controlled trial. J Cyst Fibros. 2015;14:219–227. doi: 10.1016/j.jcf.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Kashefi NS, Nathan JI, Dissanaike S. Does a nebulized heparin/N-acetylcysteine protocol improve outcomes in adult smoke inhalation? Plast Reconstr Surg Glob Open. 2014;2:e165. doi: 10.1097/GOX.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao T, Liu Y. N-acetylcysteine inhibit biofilms produced by Pseudomonas aeruginosa. BMC Microbiol. 2010;10:140. doi: 10.1186/1471-2180-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu D, Ren XX, Guo LY, et al. Effect of N-acetylcysteine inhalation on ventilator-associated pneumonia caused by biofilm in endotracheal tubes. Zhonghua Er Ke Za Zhi. 2016;54:278–282. doi: 10.3760/cma.j.issn.0578-1310.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Chalumeau M, Duijvestijn YC. Acetylcysteine and carbocysteine for acute upper and lower respiratory tract infections in paediatric patients without chronic broncho-pulmonary disease. Cochrane Database Syst Rev. 2013:CD003124. doi: 10.1002/14651858.CD003124.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther. 2014;141:150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Duflo F, Debon R, Goudable J, Chassard D, Allaouchiche B. Alveolar and serum oxidative stress in ventilator-associated pneumonia. Br J Anaesth. 2002;89:231–236. doi: 10.1093/bja/aef169. [DOI] [PubMed] [Google Scholar]
- 34.Manzanares W, Biestro A, Torre MH, Galusso F, Facchin G, Hardy G. High-dose selenium reduces ventilator-associated pneumonia and illness severity in critically ill patients with systemic inflammation. Intensive Care Med. 2011;37:1120–1127. doi: 10.1007/s00134-011-2212-6. [DOI] [PubMed] [Google Scholar]
- 35.Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med. 2001;163:540–577. doi: 10.1164/ajrccm.163.2.9906116. [DOI] [PubMed] [Google Scholar]


