Abstract
Background:
The relationship between obstructive sleep apnea (OSA) and platelet reactivity in patients undergoing percutaneous coronary intervention (PCI) has not been defined. The present prospective, single-center study explored the relationship between platelet reactivity and OSA in patients with PCI.
Methods:
A total of 242 patients were finally included in the study. OSA was screened overnight by polysomnography. Platelet reactivity was assessed with a sequential platelet counting method, and the platelet maximum aggregation ratio (MAR) and average aggregation ratio were calculated. All patients were assigned per apnea-hypopnea index (AHI) to non-OSA (n = 128) and OSA (n = 114) groups. The receiver operating characteristic curve analysis was used to evaluate the accuracy of AHI for high platelet reactivity (HPR) on aspirin and clopidogrel, and multivariable logistic regression was used to determine the independent predictors of HPR on aspirin and clopidogrel.
Results:
Median AHI was significantly higher in the OSA group than in the non-OSA group (34.5 events/h vs. 8.1 events/h, Z = −13.422, P < 0.001). Likewise, median arachidonic acid- and adenosine diphosphate-induced maximum aggregation rate (MAR) in the OSA group was significantly higher than those in the non-OSA group (21.1% vs. 17.7%, Z = −3.525, P < 0.001 and 45.8% vs. 32.2%, Z = −5.708, P < 0.001, respectively). Multivariable logistic regression showed that OSA was the only independent predictor for HPR on aspirin (odds ratio [OR]: 1.055, 95% confidence interval [CI]: 1.033–1.077, P < 0.001) and clopidogrel (OR: 1.036, 95% CI: 1.017–1.056, P < 0.001). The cutoff value of AHI for HPR on aspirin was 45.2 events/h (sensitivity 47.1% and specificity 91.3%), whereas cutoff value of AHI for HPR on clopidogrel was 21.3 events/h (sensitivity 68.3% and specificity 67.7%).
Conclusion:
Platelet reactivity appeared to be higher in OSA patients with PCI despite having received a loading dose of aspirin and clopidogrel, and OSA might be an independent predictor of HPR on aspirin and clopidogrel.
Keywords: Antiplatelet Drugs, Maximum Aggregation Rate, Obstructive Sleep Apnea, Percutaneous Coronary Intervention, Platelet Reactivity
摘要
背景:
目前阻塞性睡眠呼吸暂停与经皮冠脉支架介入治疗(PCI)患者血小板活性之间的关系尚未明确报道。本前瞻性单中心 研究主要探究这两者之间的潜在联系。
方法:
本研究最终纳入242名患者。利用多导睡眠监测仪检测睡眠呼吸暂停现象,并使用连续血小板检测的方法评估血小板功 能,计算最大血小板聚集率(MAR)和平均血小板聚集率(AAR)。按照呼吸暂停低通气指数(AHI)将患者分为睡眠呼吸 暂停(OSA)组(n=114)和非睡眠呼吸暂停(non-OSA)组(n=128)。使用ROC曲线评估AHI对阿司匹林和氯吡格雷高血 小板反应性的诊断准确度,运用多元回归分析来确定阿司匹林和氯吡格雷高血小板反应性的独立预测因子。
结果:
在OSA组中,AHI的中位数显著高于non-OSA组(34.5 events/h vs. 8.1 events/h, Z=-13.422, P<0.001)。而且,OSA组的 AA和ADP诱导的最大血小板聚集率同样显著高于non-OSA组(分别为21.1% vs. 17.7%, Z=-3.525, P<0.001; 以及45.8% vs. 32.2%, Z=-5.708, P<0.001)。多元logistic回归分析显示OSA为阿司匹林和氯吡格雷高血小板反应性的独立预测因子(OR: 1.055, 95% CI: 1.033-1.077, P<0.001;以及OR: 1.036, 95% CI: 1.017-1.056, P<0.001)。预测阿司匹林高血小板反应性的AHI截断值为45.2 events/h (敏感性 47.1%, 特异性 91.3%),而预测氯吡格雷高血小板反应性的AHI截断值为21.3 events/h (敏感性 68.3%, 特异性 67.7%)。
结论:
PCI患者虽然在术前接受了负荷剂量的阿司匹林和氯吡格雷,但OSA患者的血小板活性仍处于较高水平。OSA可能是阿 司匹林和氯吡格雷高血小板反应性的独立预测因子。
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by recurrent complete or partial upper airway obstruction, resulting in intermittent hypoxemia and arousals from sleep.[1] OSA has been associated with increased risk for cardiovascular disease including atherothrombotic diseases such as stroke and coronary artery disease (CAD).[2] Moreover, our previous study documented a high prevalence of moderate-to-severe OSA among patients undergoing coronary stent implantation, and OSA was associated with increased 2-year composite major adverse cardiovascular events (MACEs) rate.[3]
The pathophysiological mechanisms underlying the association between OSA and cardiovascular disease remain to be elucidated. Some have proposed that the repeated cycles of intermittent hypoxemia, sympathetic activation, and sleep disruption caused by OSA may lead to vascular abnormalities including endothelial dysfunction, vascular inflammation, and increased platelet activation and aggregation in the general population.[4] Higher mean platelet volume (MPV)[5] and decreased platelet-to-lymphocyte ratio[6] also have been documented among patients with OSA, indicating the important role of platelet activation and aggregation.
Patients undergoing percutaneous coronary intervention (PCI) are a special population receiving a loading dose of antiplatelet agents (such as aspirin and clopidogrel) before the index procedure as well as a long-term maintenance dose to avoid stent thrombosis, especially for drug-eluting stents (DESs).[7] Although the antiplatelet effect of the latter drugs might be decreased in PCI patients with OSA in possible association with higher risk of stent thrombosis, the relationship between OSA and platelet reactivity in patients undergoing DES implantation with loading doses of antiplatelet agents has not been defined and is the subject of the present study.
METHODS
Ethical approval
The procedure was approved by the Institutional Ethics Committee of Nanjing First Hospital, and in accordance with the Helsinki Declaration of 1975, as revised in 2000. The written informed consent was obtained from all patients.
Study population
A total of 340 patients in Nanjing First hospital were enrolled in this study from October 2012 to April 2014 within the framework of a previously reported trial.[3] All enrolled individuals were treated with DES implantation in at least one major epicardial coronary artery, and OSA was assessed by polysomnography. The detailed exclusion criteria were as follows: intra-aortic balloon pump or other hemodynamic support devices; known OSA on continuous positive airway pressure (CPAP) treatment; intubation for mechanical ventilation; sedation given before overnight sleep study; cardiogenic shock with systolic blood pressure <90 mmHg (1 mmHg = 0.133 kPa); clinical heart failure requiring oxygen supplement; perceived high risk of malignant ventricular arrhythmia; pregnancy; history of malignancy; the inability to provide informed consent; and expected lifespan <12 months. Ninety-eight candidates were excluded from the present study due to receiving ticagrelor or disagreement of platelet function test. Hence, a total of 242 patients undergoing DES implantation were included in the subgroup analysis of association between platelet reactivity and OSA.
Percutaneous coronary intervention and polysomnography
All interventional procedures were performed in accordance with current guidelines. A loading dose of clopidogrel (300 mg) was administered before the index procedure. Use of glycoprotein IIb/IIIa inhibitors was at operator's discretion. Routine blood tests, hepatorenal function, lipid profile, and ultrasonic cardiogram were examined before coronary angiography. After intervention, all patients received 100 mg/d aspirin indefinitely and 75 mg/d clopidogrel for at least 12 months.
All data from overnight polysomnography screening, which is the gold standard for OSA diagnosis,[8] were collected and stored on the Embletta Gold standardized level-3 portable diagnostic system (Natus Medical Inc., Ontario, Canada). Recorded polysomnographic signals included nasal airflow, thoracoabdominal excursions, arterial oxygen saturation (SpO2), snoring episodes, limb movement, electrocardiogram, and body position. All sleep studies were manually analyzed by two independent sleep technologists with registered polysomnographic technologist credentials, and disagreement was resolved by a third technologist. The primary measure of the sleep study was the apnea-hypopnea index (AHI), quantified as the total number of apnea and hypopnea episodes per hour of sleep. Apnea was defined as a ≥90% decrease in airflow from baseline value for ≥10 s. Hypopnea was defined as a 30–90% reduction in airflow from baseline value lasting ≥10 s, in conjunction with ≥3% oxygen desaturation. The respiratory events of sleep study were performed per standard criteria, and all enrolled patients had moderate-to-severe OSA (AHI >15 events/h) and none mild OSA (AHI ≤15 events/h).
Blood collection and platelet function test
Antecubital vein blood samples were collected in 3.2% sodium citrate tubes (Becton-Dickinson, Franklin Lakes, New Jersey, USA) before intraprocedural heparin administration at the catheterization laboratory. Blood samples were stored at room temperature and tested within 2 h after collection.
Platelet reactivity was assessed with a sequential platelet counting method by PL-11 (SINNOWA Medical Science & Technology Co., Nanjing, China).[9] The whole procedure was automatically performed within 15 min after transferring 500 μl citrated blood sample into a polycarbonate tube and inserting it into the detection position. Arachidonic acid (AA, 2 mg/ml) and adenosine diphosphate (ADP, 50 μmol/L) were automatically trickled into the blood sample as aggregation inducers after second detection time. PL-11 counted platelet several times until it detected the lowest level. The maximum aggregation ratio (MAR) was calculated according to the following formula: MAR = 100 − ([1st platelet count − 2nd platelet count]/2 − lowest platelet count) × 100%. The average aggregation ratio (AAR) was calculated as follows: AAR = (max MAR + min MAR)/2. In addition, high platelet reactivity (HPR) on aspirin was defined as MAR% ≥30%, while HPR on clopidogrel was defined as MAR% ≥55% according to the preclinical data.
Statistical analysis
Continuous variables are presented as means ± standard deviation (SD) or median (Q1, Q3) and were compared using the Student's t-test (for normal distribution) and Mann-Whitney U-test (for nonnormal distribution). Categorical variables are expressed as frequencies or percentages and were compared by Chi-square statistics or Fisher's exact test. Diagnostic accuracy of AHI for HPR on aspirin and clopidogrel was evaluated using receiver operating characteristic curve analysis, with optimal cutoff values of AHI identified by the Youden's index (sensitivity + specificity−1). Multivariable logistic regression was used to determine the independent predictors of HPR on aspirin and clopidogrel with purposeful selection of covariates. Variables associated with univariate analysis (all with P ≤0.1) and those judged to be of clinical importance from previously published reports were eligible for inclusion in the multivariable model-building process. All tests were two sided. Data were analyzed using SPSS software (version 22.0, SPSS Institute Inc., Chicago, USA) and a P < 0.05 was considered statistically significant.
RESULTS
Baseline clinical, laboratory, and angiographic characteristics
A total of 242 patients (aged 41–89 years) were finally enrolled in this study, and their baseline clinical characteristics are shown in Table 1. Patients were assigned based on AHI to the non-OSA (n = 128) and OSA (n = 114) groups. Baseline clinical characteristics between these two groups were well matched, except for higher body mass index (25.4 ± 3.0 kg/m2 vs. 24.5 ± 3.3 kg/m2, t = 4.342, P = 0.038) and higher white blood cell count (7.0 ± 1.9 × 109/L vs. 6.5 ± 1.7 × 109/L, t = 2.329, P = 0.020) in the OSA versus non-OSA groups. In Table 1, it showed that more patients in the OSA group had multivessel disease (68.4% vs. 53.1%, χ2 = 5.992, P = 0.015) than non-OSA group.
Table 1.
Baseline clinical and laboratory characteristics of percutaneous coronary intervention patients
| Characteristics | Non-OSA (n = 128) | OSA (n = 114) | t/χ2 | P |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years) , mean ± SD | 63.1 ± 10.1 | 65.4 ± 10.2 | 2.981* | 0.086 |
| Male, n (%) | 93 (72.7) | 92 (80.7) | 2.169† | 0.142 |
| BMI (kg/m2), mean ± SD | 24.5 ± 3.3 | 25.4 ± 3.0 | 4.342* | 0.038 |
| Smoking, n (%) | 62 (46.9) | 53 (46.5) | 0.921† | 0.583 |
| Hypertension, n (%) | 91 (71.1) | 85 (74.6) | 0.363† | 0.546 |
| SBP (mmHg), mean ± SD | 134.4 ± 19.9 | 132.6 ± 17.4 | 0.540* | 0.463 |
| DBP (mmHg), mean ± SD | 81.2 ± 9.6 | 80.5 ± 9.3 | 0.255* | 0.614 |
| Hyperlipidemia, n (%) | 80 (62.5) | 79 (69.9) | 1.465† | 0.227 |
| Diabetes, n (%) | 33 (25.8) | 37 (32.5) | 1.303† | 0.254 |
| LVEF (%) | 61.2 ± 7.8 | 59.6 ± 10.2 | 1.426* | 0.866 |
| Previous MI, n (%) | 12 (9.4) | 15 (13.2) | 0.866† | 0.352 |
| Previous PCI, n (%) | 20 (15.6) | 24 (21.1) | 1.190† | 0.276 |
| Previous CABG, n (%) | 1 (0.8) | 1 (0.9) | 0.007† | 0.935 |
| Stroke, n (%) | 16 (12.5) | 12 (10.5) | 0.228† | 0.633 |
| PAD, n (%) | 1 (0.8) | 4 (3.5) | 1.974† | 0.161 |
| eGFR <60 ml·min−1·1.73 m−2, n (%) | 26 (20.3) | 26 (23.0) | 0.014† | 0.612 |
| Laboratory characteristics | ||||
| WBC (×109/L), mean ± SD | 6.5 ± 1.7 | 7.0 ± 1.9 | 2.329* | 0.020 |
| RBC (×1012/L), mean ± SD | 4.2 ± 0.5 | 4.4 ± 0.5 | 1.739* | 0.082 |
| Hemoglobin (g/L), mean ± SD | 130.9 ± 16.7 | 135.9 ± 16.0 | 1.730* | 0.084 |
| Platelet (×109/L), mean ± SD | 186.4 ± 58.1 | 188.7 ± 52.8 | 0.347* | 0.729 |
| Serum creatinine (µmol/L), mean ± SD | 81.0 ± 30.2 | 83.0 ± 28.1 | 1.168* | 0.243 |
| Glucose (mmol/L), mean ± SD | 5.9 ± 1.8 | 6.2 ± 2.3 | 0.584* | 0.559 |
| Cholesterol (mmol/L), mean ± SD | 4.0 ± 1.0 | 3.9 ± 1.0 | 0.638* | 0.523 |
| Triglyceride (mmol/L), mean ± SD | 1.7 ± 0.9 | 1.7 ± 1.1 | 0.549* | 0.583 |
| HDL-C (mmol/L), mean ± SD | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.361* | 0.174 |
| LDL-C (mmol/L), mean ± SD | 2.4 ± 0.7 | 2.3 ± 0.8 | 0.756* | 0.450 |
| hsCRP (µg/ml), mean ± SD | 32.9 ± 9.5 | 31.6 ± 9.7 | 0.825* | 0.409 |
| IL-6 (pg/ml), mean ± SD | 10.7 ± 3.0 | 11.0 ± 2.9 | 0.645* | 0.519 |
| Angiographic and procedural characteristics | ||||
| Transradial, n (%) | 124 (96.9) | 111 (97.4) | 0.052† | 0.820 |
| Multivessel disease, n (%) | 68 (53.1) | 78 (68.4) | 5.992† | 0.015 |
| DES used, n (%) | 128 (100) | 114 (100) | NA | NA |
| Total implanted stents number, mean ± SD | 1.8 ± 1.1 | 2.0 ± 1.0 | 0.970* | 0.326 |
| Mean stent diameter (mm), mean ± SD | 2.9 ± 0.7 | 3.0 ± 0.6 | 0.187* | 0.666 |
| Total stent length (mm), mean ± SD | 48.7 ± 29.5 | 51.0 ± 28.4 | 0.378* | 0.539 |
| Complete revascularization, n (%) | 93 (72.7) | 72 (63.2) | 2.513† | 0.114 |
| Final TIMI Grade 3, n (%) | 128 (100) | 112 (98.2) | 2.267† | 0.133 |
1 mmHg = 0.133 kPa.*t values; † χ2 values. SD: Standard deviation; BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; LVEF: Left ventricular ejection fraction; MI: Myocardial infarction; PCI: Percutaneous coronary intervention; CABG: Coronary artery bypass grafting; PAD: Peripheral artery disease; eGFR: Estimated glomerular filtration rate; WBC: White blood cell; RBC: Red blood cell; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; hsCRP: High-sensitivity C-reactive protein; IL-6: Interleukin-6; DES: Drug-eluting stent; TIMI: Thrombolysis in myocardial infarction; OSA: Obstructive sleep apnea; NA: Not applicable.
Sleep study
The sleep characteristics are shown in Table 2. All enrolled patients received an overnight sleep study for about 409 min (Z = −0.672, P = 0.501 for two groups). The median AHI in the OSA group was 34.5 events/h, significantly higher than the non-OSA group (8.1 events/h, Z = −13.422, P < 0.001). Patients in the OSA group had significantly lower average and lowest arterial oxygen saturation (SpO2; average SpO2: 93.2% [92.1%, 94.2%] vs. 93.9% [93.0%, 94.8%], Z = −3.264, P = 0.001; lowest SpO2: 82.0% [79.0%, 85.0%] vs. 87.0% [82.0%, 89.0%], Z = −5.720, P < 0.001) and higher total percentage of time SpO2 <90% (16.5% vs. 2.7%, Z = −5.825, P < 0.001), compared to those in the non-OSA group.
Table 2.
Sleep characteristics of percutaneous coronary intervention patients
| Characteristics | Non-OSA (n = 128) | OSA (n = 114) | Z | P |
|---|---|---|---|---|
| AHI | 8.1 (3.9, 11.6) | 34.5 (23.2, 47.7) | −13.422 | <0.001 |
| Index time (min) | 409.2 (398.7, 414.2) | 408.8 (388.1, 414.3) | −0.672 | 0.501 |
| SpO2 (%) | 93.9 (93.0, 94.8) | 93.2 (92.1, 94.2) | −3.264 | 0.001 |
| OD (events/h) | 3.2 (1.0, 6.1) | 19.0 (9.7, 35.2) | −10.549 | <0.001 |
| Snore time (min) | 2.4 (0.5, 11.7) | 5.3 (0.8, 18.1) | −0.905 | 0.365 |
| Snore episodes, n | 27.0 (8.0, 109.0) | 69.0 (11.0, 167.0) | −1.818 | 0.069 |
| Total percentage of time SpO2 <90% | 2.7 (0.4, 17.3) | 16.5 (7.2, 50.7) | −5.825 | <0.001 |
| Lowest SpO2 (%) | 87.0 (82.0, 89.0) | 82.0 (79.0, 85.0) | −5.720 | <0.001 |
| Snoring time (min) | 8.5 (1.5, 52.4) | 21.6 (2.8, 65.1) | −0.965 | 0.335 |
| Number of snoring | 26.0 (0.7, 109.0) | 69.0 (11.0, 167.0) | −1.901 | 0.057 |
| Longest snoring (min) | 1.2 (0.5, 3.9) | 1.7 (0.7, 4.7) | −1.227 | 0.220 |
Data are shown as median (Q1, Q3). AHI: Apnea-hypopnea index; OD: Obstructive desaturation; SpO2: Arterial oxygen saturation; OSA: Obstructive sleep apnea.
Obstructive sleep apnea and platelet reactivity
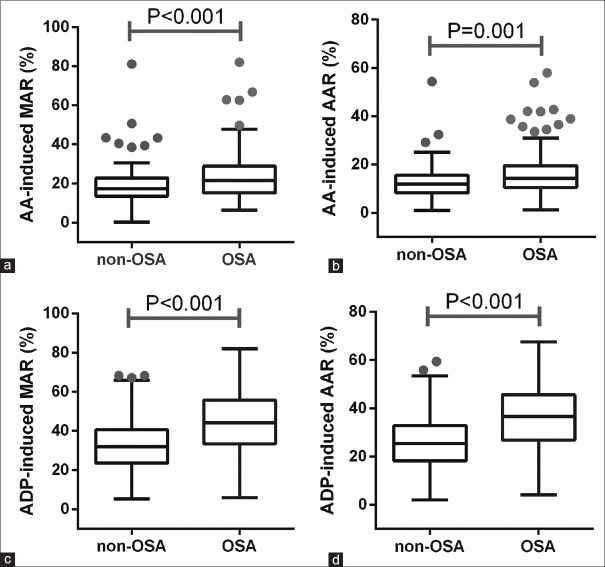
As shown in Figure 1, the median AA-induced MAR of 21.1% (15.1%, 28.7%) and ADP-induced MAR of 45.8% (34.4%, 56.5%) in the OSA group were significantly higher than those in the non-OSA group (AA-MAR: 17.7% [13.4%, 22.6%], Z = −3.525, P < 0.001; ADP-MAR: 32.2% [23.6%, 40.3%], Z = −5.708, P < 0.001). Moreover, the median AA-induced AAR of 14.2% (10.5%, 19.4%) and ADP-induced AAR of 36.6% (26.8%, 45.6%) and 25.4% (18.5%, 32.6%) in the OSA group were higher than those in the non-OSA group identically (ADP-AAR: 12.5% [8.7%, 15.5%], Z = −3.355, P = 0.001 and ADP-AAR: 25.4% [18.5%, 32.6%], Z = −5.693, P < 0.001). There was a moderate correlation (r = 0.412, P < 0.001) between ADP-induced MAR and AHI and a weak correlation (r = 0.251, P < 0.001) between AA-induced MAR and AHI. By multivariable logistic regression, OSA, as represented by AHI, appeared as the only independent predictor for HPR on aspirin (odds ratio [OR]: 1.055, 95% confidence interval [CI]: 1.033–1.077, P < 0.001; Table 3) and HPR on clopidogrel (OR: 1.036, 95% CI: 1.017–1.056, P < 0.001).
Figure 1.
Platelet activation test parameters from PL-11 in OSA (n = 114) and non-OSA (n = 128) groups. (a) AA-induced MAR in non-OSA and OSA groups. (b) AA-induced AAR in non-OSA and OSA groups. (c) ADP-induced MAR in non-OSA and OSA groups. (d) ADP-induced AAR in non-OSA and OSA groups. OSA: Obstructive sleep apnea; AA: Arachidonic acid; MAR: Maximum aggregation rate; AAR: Average aggregation rate; ADP: Adenosine diphosphate.
Table 3.
Multivariate analyses of high platelet reactivity on aspirin and clopidogrel of percutaneous coronary intervention patients
| Factors | OR (95% CI) | P |
|---|---|---|
| High platelet reactivity on aspirin | ||
| Age | 0.987 (0.945–1.030) | 0.540 |
| Smoking | 1.038 (0.562–1.916) | 0.906 |
| Hyperlipidemia | 0.975 (0.403–2.359) | 0.955 |
| Diabetes | 0.893 (0.361–2.209) | 0.806 |
| eGFR | 1.000 (0.985–1.015) | 0.991 |
| OSA | 1.055 (1.033–1.077) | <0.001 |
| High platelet reactivity on clopidogrel | ||
| Age | 0.982 (0.945–1.020) | 0.342 |
| Smoking | 0.957 (0.543–1.686) | 0.879 |
| Hyperlipidemia | 1.331 (0.595–2.978) | 0.486 |
| Diabetes | 2.012 (0.933–4.337) | 0.074 |
| eGFR | 1.010 (0.998–1.022) | 0.106 |
| OSA | 1.036 (1.017–1.056) | <0.001 |
OR: Odds ratio; CI: Confidence interval; eGFR: Estimated glomerular filtration rate; OSA: Obstructive sleep apnea.
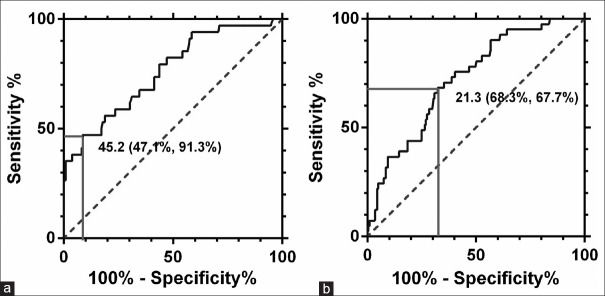
As shown in Figure 2, the area under the curve (AUC) of AHI for HPR on aspirin was 0.760 (95% CI: 0.670–0.850, P < 0.001), with cutoff value of 45.2 events/h (sensitivity 47.1% and specificity 91.3%), whereas the AUC of AHI for HPR on clopidogrel was 0.724 (95% CI: 0.645–0.803, P < 0.001), with cutoff value of 21.3 events/h (sensitivity 68.3% and specificity 67.7%).
Figure 2.
Receiver operating characteristic curve analyses of platelet function parameters for diagnosis of antiplatelet treatment. (a) Receiver operating characteristic analysis for aspirin resistance. (b) Receiver operating characteristic analysis for clopidogrel resistance.
DISCUSSION
To the best of our knowledge, few studies have explored the association between OSA and platelet reactivity in patients undergoing DES implantation. The major novel findings from the present evaluation were: (1) platelet aggregation was activated in OSA patients with DES implantation, despite the loading dose of aspirin and clopidogrel, which might underlie the increased risk of stent thrombosis in OSA patients after coronary intervention; (2) OSA might be an independent predictor of HPR on aspirin and clopidogrel; and (3) the cutoff value of AHI for HPR on aspirin was 45.2 events/h (sensitivity 47.1% and specificity 91.3%) and the cutoff value of AHI for HPR on clopidogrel was 21.3 events/h (sensitivity 68.3% and specificity 67.7%).
OSA is a common disease worldwide, with prevalence among adults ranging from 5% to 14%, which is often underestimated due to a lack of awareness and limited access to polysomnography.[10] Chronic intermittent hypoxia is the significant feature in OSA, which is associated with increased risk of secondary cardiovascular disease. Moreover, OSA is also an independent risk factor for hypertension, CAD, atrial fibrillation, cardiac failure, and stroke. In our previous study,[3] about 45% of patients with DES implantation had moderate-to-severe OSA, and OSA was associated with significantly increased 2-year composite MACE rate. However, the underlying mechanisms of OSA-induced atherosclerosis remain unclear, and platelet aggregation and activation underlie occurrence and development of atherosclerosis. The previous clinical study showed that platelet activation was associated with greater levels of oxygen desaturation in obese patients with OSA.[4] Another clinical study also indicated that the severity of OSA significantly contributed to platelet aggregability, which was partially improved by CPAP treatment at 3 months.[11] The review by Liak and Fitzpatrick[12] documented that hematocrit, blood viscosity, certain clotting factors, tissue factor, platelet activity, and whole blood coagulability were increased in patients with OSA, while fibrinolysis was impaired. In addition, it has been reported that elevated epinephrine and fibrinogen levels in OSA induced binding to the glycoprotein IIb/IIIa complex on the platelet surface, leading to platelet aggregation and activation.[13] In summary, there is considerable evidence on the association between OSA and a procoagulant state, and OSA patients have abnormal platelet aggregation and activation.
Despite the aforementioned observations, there has been a paucity of evidence on the association between OSA and platelet activity in patients with DES implantation. As is well known, platelets take part in the occurrence and development of atherosclerosis and thrombosis, especially promoting the instability and rupture of atheromatous plaque in acute coronary syndrome. Hence, platelet inhibitors, for example, aspirin and clopidogrel,[14] are the cornerstones of treatment of CAD, particularly with coronary stent implantation. Despite the well-documented efficacy of antiplatelet agents, recurrent thrombotic events, particularly stent thrombosis, have been repeatedly demonstrated in stented patients. One of the most important reasons underlying stent thrombosis might be inadequate platelet aggregation and activation inhibition. The present clinical study demonstrated that platelet aggregation was activated in OSA patients with DES implantation despite the routine use of aspirin and clopidogrel, and OSA might be a potential predictor for HPR on aspirin and clopidogrel, which has been confirmed to be associated with increased risk of stent thrombosis.[15] The latter observation might explain why OSA has been independently associated with subsequent stent thrombosis and other adverse cardiac and cerebrovascular events in patients undergoing PCI.[3,16] To this end, the type of antiplatelet agents could be switched, or dosage doubled in OSA patients to achieve a similar antiplatelet effect, which should be verified by further well-designed clinical studies.
There were several limitations in this study. First, none of the 242 patients had received treatments for OSA, and it was not possible to evaluate the effects of CPAP treatment on platelet reactivity. Second, patients were not retested for sleep data and platelet reactivity during follow-up. Third, platelet reactivity was assessed with a sequential platelet counting method by PL-11, which has been demonstrated to be correlated with light transmittance aggregometry and VerifyNow.[9] Fourth, apart from platelet reactivity, other parameters such as MPV, platelet distribution width as well as platelet-large cell rate were not assessed in the present study. Fifth, the present study was the cross-sectional design, which limited the clinical importance of this study.
In conclusion, despite a loading dose of aspirin and clopidogrel, platelet aggregation was activated in OSA patients undergoing DES implantation, and OSA might be an independent predictor of HPR on aspirin and clopidogrel. Appropriately powered clinical trials are necessary to confirm the findings from this study.
Financial support and sponsorship
The present trial was supported by grants from the National Science Foundation of China (No. NSFC 81770342), Nanjing Health and Family Planning Commission (No. YKK16124), and Nanjing Municipal Commission of Science and Technology (No. 201715026).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank the dedicated volunteers participating in this study and co-workers who made this possible including Hai-Mei Xu (performed platelet functional tests) and Sheng-Xia Zhang (data registration).
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc. 2011;86:549–54. doi: 10.4065/mcp.2010.0810. doi: 10.4065/mcp.2010.0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monahan K, Redline S. Role of obstructive sleep apnea in cardiovascular disease. Curr Opin Cardiol. 2011;26:541–7. doi: 10.1097/HCO.0b013e32834b806a. doi: 10.1097/HCO.0b013e32834b806a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang JJ, Gao XF, Ge Z, Jiang XM, Xiao PX, Tian NL, et al. Obstructive sleep apnea affects the clinical outcomes of patients undergoing percutaneous coronary intervention. Patient Prefer Adherence. 2016;10:871–8. doi: 10.2147/PPA.S104100. doi: 10.2147/PPA.S104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahangdale S, Yeh SY, Novack V, Stevenson K, Barnard MR, Furman MI, et al. The influence of intermittent hypoxemia on platelet activation in obese patients with obstructive sleep apnea. J Clin Sleep Med. 2011;7:172–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Karakaş MS, Altekin RE, Baktır AO, Küçük M, Cilli A, Yalçınkaya S, et al. Association between mean platelet volume and severity of disease in patients with obstructive sleep apnea syndrome without risk factors for cardiovascular disease. Turk Kardiyol Dern Ars. 2013;41:14–20. doi: 10.5543/tkda.2013.42948. doi: 10.5543/tkda.2013.42948. [DOI] [PubMed] [Google Scholar]
- 6.Koseoglu HI, Altunkas F, Kanbay A, Doruk S, Etikan I, Demir O, et al. Platelet-lymphocyte ratio is an independent predictor for cardiovascular disease in obstructive sleep apnea syndrome. J Thromb Thrombolysis. 2015;39:179–85. doi: 10.1007/s11239-014-1103-4. doi: 10.1007/s11239-014-1103-4. [DOI] [PubMed] [Google Scholar]
- 7.Mauri L, Kereiakes DJ, Normand SL, Wiviott SD, Cohen DJ, Holmes DR, et al. Rationale and design of the dual antiplatelet therapy study, a prospective, multicenter, randomized, double-blind trial to assess the effectiveness and safety of 12 versus 30 months of dual antiplatelet therapy in subjects undergoing percutaneous coronary intervention with either drug-eluting stent or bare metal stent placement for the treatment of coronary artery lesions. Am Heart J. 2010;160:1035–41. doi: 10.1016/j.ahj.2010.07.038. 1041.e1. doi: 10.1016/j.ahj.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 8.El Shayeb M, Topfer LA, Stafinski T, Pawluk L, Menon D. Diagnostic accuracy of level 3 portable sleep tests versus level 1 polysomnography for sleep-disordered breathing: A systematic review and meta-analysis. CMAJ. 2014;186:E25–51. doi: 10.1503/cmaj.130952. doi: 10.1503/cmaj.130952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan J, Cong Y, Ren J, Zhu Y, Li L, Deng X, et al. Comparison between a new platelet count drop method PL-11, light transmission aggregometry, VerifyNow aspirin system and thromboelastography for monitoring short-term aspirin effects in healthy individuals. Platelets. 2015;26:25–30. doi: 10.3109/09537104.2013.865835. doi: 10.3109/09537104.2013.865835. [DOI] [PubMed] [Google Scholar]
- 10.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oga T, Chin K, Tabuchi A, Kawato M, Morimoto T, Takahashi K, et al. Effects of obstructive sleep apnea with intermittent hypoxia on platelet aggregability. J Atheroscler Thromb. 2009;16:862–9. doi: 10.5551/jat.2188. doi: 10.5551/jat.2188. [DOI] [PubMed] [Google Scholar]
- 12.Liak C, Fitzpatrick M. Coagulability in obstructive sleep apnea. Can Respir J. 2011;18:338–48. doi: 10.1155/2011/924629. doi: 10.1155/2011/924629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamarrón C, Valdés Cuadrado L, Alvarez-Sala R. Pathophysiologic mechanisms of cardiovascular disease in obstructive sleep apnea syndrome. Pulm Med. 2013;2013:521087. doi: 10.1155/2013/521087. doi: 10.1155/2013/521087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spectre G, Varon D. New antiplatelet agents. Curr Opin Hematol. 2009;16:365–70. doi: 10.1097/MOH.0b013e32832ec222. doi: 10.1097/MOH.0b013e32832ec222. [DOI] [PubMed] [Google Scholar]
- 15.Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ, Metzger DC, et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): A prospective multicentre registry study. Lancet. 2013;382:614–23. doi: 10.1016/S0140-6736(13)61170-8. doi: 10.1016/S0140-6736(13)61170-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee CH, Sethi R, Li R, Ho HH, Hein T, Jim MH, et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation. 2016;133:2008–17. doi: 10.1161/CIRCULATIONAHA.115.019392. doi: 10.1161/CIRCULATIONAHA.115.019392. [DOI] [PubMed] [Google Scholar]