Abstract
Background:
Research on the changes to knee structures in asymptomatic amateur ice hockey players (AAIHPs) has been limited. We aimed to assess the performance of the knees in AAIHPs using 3.0-T magnetic resonance imaging (MRI).
Methods:
A total of 71 asymptomatic knees (32 AAIHPs and 39 age- and sex-matched controls) were imaged using a 3.0-T MRI scanner at the Affiliated Zhongshan Hospital of Dalian University in April 2017. Two experienced musculoskeletal radiologists were blinded to assess all MRI findings, including bursae around the knee, bone marrow edema (BME), meniscal signal changes, and articular cartilage and ligament damage. Any disagreements were resolved by a third professor of musculoskeletal radiology. Categorical variables were compared using the Chi-square test and continuous variables using the Student's t-test or Mann-Whitney U-test.
Results:
The most common finding was fluid-filled bursae surrounding the knee. In the AAIHP group, which totaled 32 knees and 416 bursae, 155 (37%) fluid-filled bursae were present. In the control group, there were a total of 39 knees and 507 bursae, and 91 (18%) fluid-filled bursae were present. There was a significant difference in the number of fluid-filled bursae between the two groups (P < 0.05). However, in AAIHPs, the prevalence of meniscal signal changes (16 knees, 50%) was higher than in the control group (2 knees, 5%; P < 0.001). Importantly, 15 of the 19 were grade II signals. Other changes were only found in AAIHPs. Articular cartilage lesions were detected in 47% of their knees, predominantly at the patellofemoral joint, and BME was found in 34% of their knees.
Conclusion:
The MRI findings of knees in AAIHPs mainly manifested as self-protection reaction, and proper ice hockey exercise could be advocated.
Keywords: Articular, Cartilage, Hockey, Knee, Magnetic Resonance Imaging, Meniscus
摘要
背景:
关于无症状业余冰球运动员(asymptomatic amateur ice hockey player,AAIHP)膝关节结构变化的研究较少,本研究的 目的是应用3.0-T磁共振成像仪(Magnetic Resonance Imaging, MRI)评估AAIHP膝关节MRI的表现。
方法:
2017年4月于大连大学附属中山医院共收集71个无症状膝关节(32个业余冰球运动员膝和39个年龄性别匹配的对照组 膝)纳入研究。两名经验丰富的骨肌放射科医师采用盲法评估膝周滑囊积液、骨髓水肿(bone marrow edema, BME)、半月 板信号改变、关节软骨及韧带损伤情况,意见不一致时由第三位骨肌放射学教授决定。分类变量间的比较运用卡方检验,连 续变量间的比较运用Student's t检验或Mann-Whitney U检验行统计学分析。
结果:
膝周滑囊积液为最常见的MRI表现,AAIHP组32膝共416个膝周滑囊中共观察到155个(37%)膝周滑囊积液;对照组 39膝共507个膝周滑囊中共观察到91个(18%)膝周滑囊积液,两组间差异有统计学意义(P<0.05)。AAIHP组半月板信号改 变(16膝,50%)高于对照组(2膝,5%), (P<0.001)。最重要的是19个膝关节半月板信号改变中有15个是II级信号改变。其 他改变仅在AAIHP组中发现,膝关节关节软骨损伤占47%,且主要表现为髌股关节的软骨损伤;34%的膝盖中检测到BME。
结论:
无症状业余冰球运动膝关节主要表现为自我保护反应,冰球运动值得提倡。
INTRODUCTION
Ice hockey is a fast-speed, heavy-intensity, and high-impact sport, with increasing participation rates across all genders, ages, and levels around the world.[1,2] Approximately 76 countries have ice hockey teams that are recognized by the International Ice Hockey Federation.[3] However, common concerns include injuries to the head, shoulder, hip, knee, and ankle because of the unique technical and physical nature of the sport. The knee was found to be the most frequently injured part of the lower body.[4]
Magnetic resonance imaging (MRI) of the knee has become the most commonly performed musculoskeletal MRI examination, and is a valuable tool for evaluating the presence of joint effusion and meniscal signal changes, confirming articular cartilage lesions, examining bone marrow edema (BME), and diagnosing ligamentous injuries.[5,6,7,8] Moreover, the high-field-strength 3.0-T MRI can create images of anatomical and pathological structures with higher spatial resolution and thinner section thickness than a 1.5-T MRI without increasing acquisition time or sacrificing signal-to-noise ratio.[7] Studies have shown that 3.0-T MRI is more sensitive and specific than 1.5-T MRI for the evaluation of cartilage changes, meniscal signal changes, and ligament tears.[6,7]
Much of the previous research related to the use of MRI in the evaluation of ice hockey injuries has been based on concussion.[9,10] Research on the changes to knee structures, particularly in amateur ice hockey players, has been limited. Thus, the purpose of this study was to assess whether high-performance ice hockey leads to changes to knee structures detectable by 3.0-T MRI and compare them to age- and gender-matched controls.
METHODS
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of Affiliated Zhongshan Hospital of Dalian University (No. 2017-163). Informed written consent was obtained from all patients before their enrollment in this study.
Participants
This case-control study was conducted in April 2017 with two groups of men. One of the groups comprised 17 amateur ice hockey players who were free of knee-related symptoms and were between the ages of 36 and 59 years (mean age 49.5 ± 5.8 years). Total training length ranged from 6 to 43 years (mean 15.8 ± 10.2 years) and involved training once to twice every week. The control group comprised volunteers who did not play ice hockey but participated in other regular sporting activities, who were also free of any knee-related symptoms and were between the ages of 37 and 59 years (mean age 48.6 ± 5.8 years). Each participant underwent a 3.0-T MRI examination, with a total of 34 knees included in the group of athletes and 39 knees in the control group. Exclusion criteria were as follows: a history of knee injection or knee surgery, chronic disease of the knee, and knee discomfort leading to functional limitations at the time of the study.
Magnetic resonance imaging acquisition
Imaging was performed using a 3.0-T MRI scanner (MAGNETOM Verio, Siemens Healthcare, Germany) equipped with a dedicated 8-channel knee coil. All participants were scanned in the supine position with the knee in full extension and kept as straight as possible. The positioning line was centered on the lower edge of the patella. The scanning range included the distal femur and the proximal tibia and fibula. For all scans, we performed a sagittal, coronal, and axial proton density (PD)-weighted sequence with fat suppression and a sagittal T1-weighted turbo spin-echo sequence. The image sequence included the following: (1) a sagittal and axial PD-weighted turbo spin-echo sequence with fat suppression, a flip angle of 90°, a repetition time of 3000 ms, an echo time of 41 ms, a field of view (FOV) of 170 mm, 20 slices, a 480 × 640-pixel matrix, and a slice thickness of 3 mm; (2) a coronal PD-weighted sequence with fat suppression, a flip angle of 150°, a repetition time of 3500 ms, an echo time of 41 ms, a FOV of 170 mm, 20 slices, a 217 × 320-pixel matrix, and a slice thickness of 3 mm; and (3) a sagittal T1-weighted turbo spin-echo with a repetition time of 579 ms, an echo time of 11 ms, a flip angle of 90°, a FOV of 170 mm, 20 slices, a 480 × 640-pixel matrix, and a slice thickness of 3 mm. The total acquisition time was 6 min and 38 s. All images were transferred to a picture archiving communications system (PACS, Carestream Health, Inc., Canada) station.
Image analysis
Two experienced musculoskeletal radiologists who were blinded to the analysis recorded and evaluated all MRI scans. Any disagreements were resolved by a third professor of musculoskeletal radiology. Joint effusions, meniscal lesions, articular cartilage lesions, BME, and ligament damage were included in the assessment. All images were evaluated side by side on PACS.
A bursa around the knee was classified as present when there was more than 5 mm of synovial liquid in the suprapatellar bursa and as absent when there was less than 5 mm.[11] Other fluid-filled bursae were considered present or absent and described based on their locations.
The lateral and medial menisci were graded separately according to the methods described by Stoller et al.[12] (Grade 0 = normal signal; Grade I = one or several punctate signal intensities that do not reach the surface of the meniscus; Grade II = linear signal intensity that does not reach the surface of the meniscus; and Grade III = signal intensity that reaches the surface of the meniscus).
Articular cartilage was graded based on a modification of the Noyes and Stabler[13] classification system (Grade 0 = normal thickness and signal; Grade I = normal thickness but an altered signal; Grade II = superficial partial-thickness cartilage defect less than 50% of the total cartilage thickness; Grade III = deep partial-thickness cartilage defect more than 50% of the total cartilage thickness; and Grade IV = full-thickness chondral defect with exposure of subchondral bone). A total of six articular surfaces were evaluated, including those of the patella, trochlea, lateral femoral condyle, medial femoral condyle, lateral tibial plateau, and medial tibial plateau.
BME was described as present or not based on a low signal on T1-weighted images and a high signal intensity on PD-weighted images.[14]
Ligaments (patellar ligament, anterior cruciate ligament, posterior cruciate ligament, medial collateral ligament, and lateral collateral ligament) were considered abnormal if they were not consecutive, were abnormal in size, or if they had increased signal intensity on T2-weighted images.[15]
Statistical analysis
Data were entered into Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA) and statistical analyses were performed latter using Statistical Package for the Social Sciences 19.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables with symmetrical distribution were reported as mean ± standard deviation. Categorical variables were expressed as frequencies and percentages. For continuous variables, the Student's t-test was used. Categorical variables were compared using the Chi-square test. P < 0.05 was considered statistically significant.
RESULTS
Subjects' characteristics
No significant difference (P = 0.537, t = 0.621) was observed between amateur ice hockey players and the control group in mean age (amateur ice hockey players, 49.5 ± 5.8 years; controls, 48.6 ± 5.8 years).
Knee magnetic resonance imaging findings
The results for 71 knees were analyzed. Two knees in the asymptomatic amateur ice hockey players (AAIHPs) were excluded from the evaluation due to a history of medial collateral ligament (MCL) reconstruction. As shown in Table 1, the most common finding was a fluid-filled bursae surrounding the knee. In the group of AAIHPs, totaling 32 knees and 416 bursae, 155 (37%) fluid-filled bursae were present. In the controls, there were a total of 39 knees and 507 bursae, and 91 (18%) fluid-filled bursae were present. There was a significant difference in the number of bursae surrounding the knee between the two groups (P < 0.001). Between the AAIHP and the control groups, there was a statistically significant difference in the number of deep infrapatellar bursae, popliteal bursae, pes anserine bursae, semimembranosus-tibial collateral ligament (SM-TCL) bursae, medial gastrocnemius bursae, fibular collateral ligament (FCL)-popliteus bursae, popliteus bursae, and iliotibial bursae (P < 0.05; Figures 1–4). No significant difference was found in the number of suprapatellar bursae, prepatellar bursae, deep infrapatellar bursae, MCL bursae, FCL-biceps femoris bursae, and lateral gastrocnemius bursae (P > 0.05).
Table 1.
The prevalence of MRI findings in the knees of AAIHP and controls, n (%)
| Items | Players (n = 32) | Controls (n = 39) | χ2 | P |
|---|---|---|---|---|
| Bursae around the knee | 18 (56) | 20 (51) | 0.174 | 0.676 |
| Meniscus | 16 (50) | 2 (5) | 18.701 | 0.000 |
| Articular cartilage | 15 (47) | 0 | – | – |
| BME | 11 (34) | 0 | – | – |
| Ligament | 3 (9) | 0 | – | – |
MRI: Magnetic resonance imaging; AAIHP: Asymptomatic amateur ice hockey players; BME: Bone marrow edema. –: Not applicable.
Figure 1.
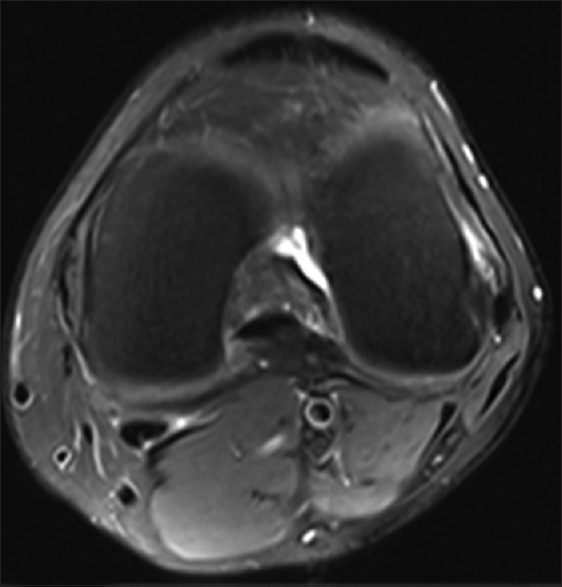
A 47-year-old healthy control. Axial PD-weighted MRI image with fat suppression of the left knee. MRI: Magnetic resonance imaging; PD: Proton density.
Figure 4.
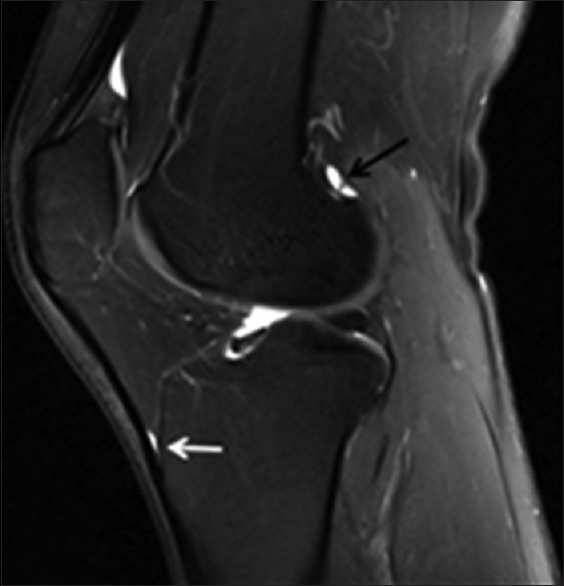
A 54-year-old AAIHP. Sagittal PD-weighted MRI image with fat suppression, showing a fluid-filled deep infrapatellar bursa (white solid arrow) and lateral gastrocnemius bursa (black solid arrow). PD: Proton density; AAIHP: Asymptomatic amateur ice hockey players; MRI: Magnetic resonance imaging.
Figure 2.
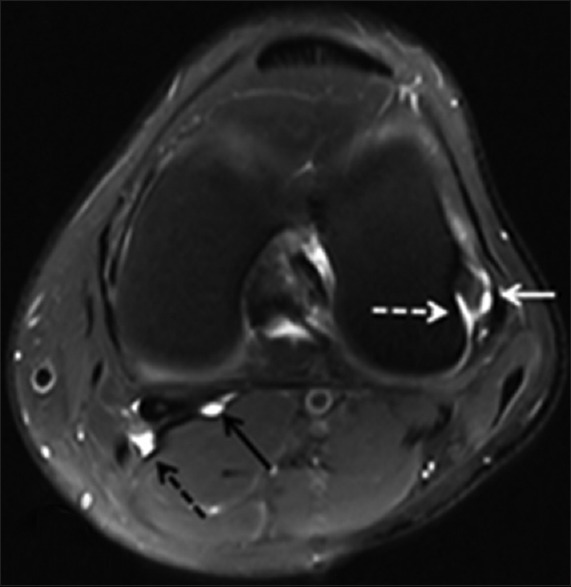
A 59-year-old AAIHP. Axial PD-weighted MRI image with fat suppression, showing a fluid-filled FCL-popliteus bursa (white solid arrow), popliteus bursa (white dashed arrow), medial gastrocnemius bursa (black solid arrow), and popliteal bursa (black dashed arrow) surrounding the left knee. PD: Proton density; AAIHP: Asymptomatic amateur ice hockey players; MRI: Magnetic resonance imaging; FCL: Fibular collateral ligament; PD: Proton density.
Figure 3.
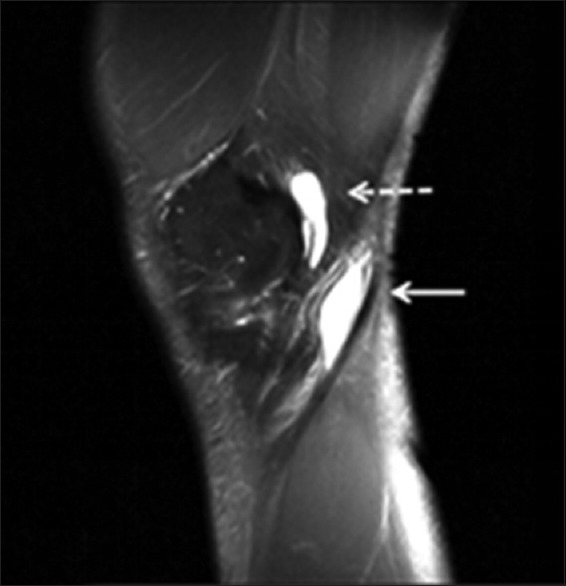
A 50-year-old AAIHP. Sagittal PD-weighted MRI image with fat suppression, showing a fluid-filled SM-TCL bursa (white dashed arrow) and popliteal bursa (white solid arrow). PD: Proton density; AAIHP: Asymptomatic amateur ice hockey players; MRI: Magnetic resonance imaging; SM-TCL: Semimembranosus-tibial collateral ligament.
The second most prevalent finding in the group of AAIHP was meniscal signal changes. Sixteen (50%) of 32 knees in that group and only 2 (5%) of the 39 knees in the control group had meniscal signal changes [Table 2], with the difference between the groups being statistically significant (P = 0.000). In the AAIHP group, medial meniscal signal changes were observed in 14 (44%) of the 32 knees and lateral meniscal signal changes were observed in 5 (16%) of the 32 knees, while 15 of the 19 were Grade II signals [Figure 5]. The difference between medial and lateral was statistically significant (P = 0.014). In the control group, medial meniscal signal changes were observed in 2 (5%) of the 39 knees and no abnormalities were observed in the lateral meniscus, and this difference in incidence between the medial and lateral sides was not statistically significant (P = 0.608). No complete meniscal tears were detected in either group.
Table 2.
Fluid-filled bursae detected in the knees of AAIHP and controls, n (%)
| Variables | Players (n = 32) | Controls (n = 39) | χ2 | P |
|---|---|---|---|---|
| Anterior | ||||
| Prepatellar bursa | 1 (3) | 0 | 0.027 | 0.868 |
| Superficial infrapatellar bursa | 0 | 0 | – | – |
| Deep infrapatellar bursa | 21 (66) | 16 (41) | 4.262 | 0.039 |
| Medial | ||||
| Popliteal bursa | 7 (22) | 0 | 5.959 | 0.015 |
| Pes anserine bursa | 7 (22) | 0 | 5.959 | 0.015 |
| SM-TCL bursa | 11 (34) | 1 (3) | 12.665 | 0.000 |
| MCL bursa | 2 (6) | 0 | 0.502 | 0.478 |
| Medial gastrocnemius bursa | 21 (66) | 11 (28) | 9.942 | 0.002 |
| Lateral | ||||
| FCL-biceps femoris bursa | 3 (9) | 0 | 1.315 | 0.251 |
| FCL-popliteus bursa | 13 (41) | 5 (13) | 7.180 | 0.007 |
| Popliteus bursa | 29 (91) | 27 (69) | 4.828 | 0.028 |
| Iliotibial bursa | 26 (81) | 17 (44) | 10.438 | 0.001 |
| Lateral gastrocnemius bursa | 14 (44) | 14 (36) | 0.454 | 0.501 |
| Total | 155 (37) | 91 (18) | 43.590 | 0.000 |
AAIHP: Asymptomatic amateur ice hockey players; SM-TCL: Semimembranosus-tibial collateral ligament; MCL: Medial collateral ligament; FCL: Fibular collateral ligament. –: Not applicable.
Figure 5.
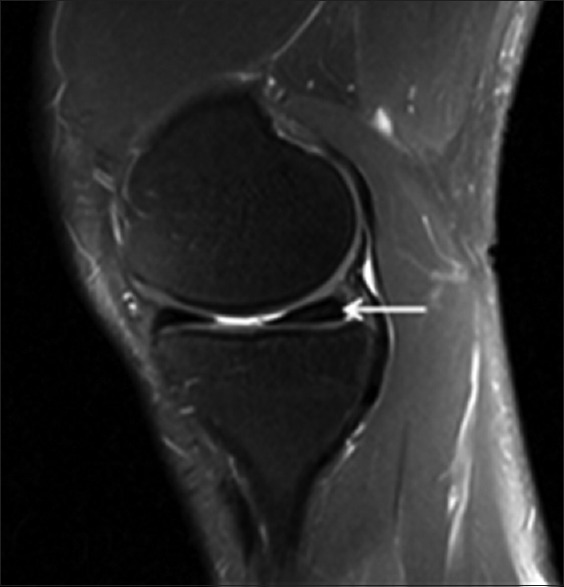
A 55-year-old AAIHP. Sagittal PD-weighted MRI image with fat suppression, showing meniscal grade II signals (white solid arrow). PD: Proton density; AAIHP: Asymptomatic amateur ice hockey players; MRI: Magnetic resonance imaging.
Additional changes were only observed in the group of AAIHP [Table 1]. First, 15 (47%) of the 32 knees had articular cartilage changes [Figure 6]. Twenty-two lesions were also found in this group in the following locations: 8 in the patella, 10 in the trochlea, 1 in the lateral femoral condyle, 2 in the medial femoral condyle, and 1 in the medial tibial plateau [Table 3]. Second, BME was present in 11 (34%) of 32 knees, with a total of 17 of these lesions observed. Third, signal alterations were detected only in the anterior cruciate ligament (ACL) in 3 (9%) of the 32 knees. No signal alterations were present in the MCL or other ligaments.
Figure 6.
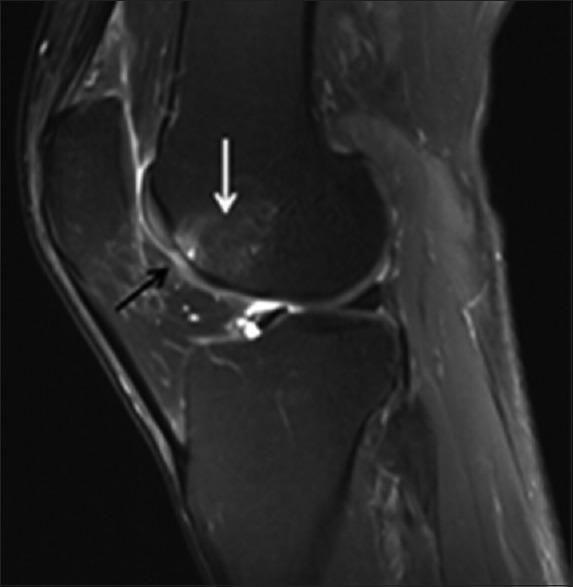
A 55-year-old AAIHP. Sagittal PD-weighted MRI image with fat suppression, showing Grade III changes of the articular cartilage (black solid arrow) and BME (white solid arrow). PD: Proton density; AAIHP: Asymptomatic amateur ice hockey players; MRI: Magnetic resonance imaging; BME: Bone marrow edema.
Table 3.
Distribution of articular cartilage and meniscal findings on MRI
| Variables | Players (n = 32) | Controls (n = 39) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | I | II | III | IV | 0 | I | II | III | IV | |
| Articular cartilage | ||||||||||
| Patella | 24 | 5 | 3 | 0 | 0 | 39 | 0 | 0 | 0 | 0 |
| Trochlea | 22 | 6 | 2 | 0 | 2 | 39 | 0 | 0 | 0 | 0 |
| Lateral femoral condyle | 31 | 1 | 0 | 0 | 0 | 39 | 0 | 0 | 0 | 0 |
| Medial femoral condyle | 30 | 1 | 1 | 0 | 0 | 39 | 0 | 0 | 0 | 0 |
| Lateral tibial plateaus | 32 | 0 | 0 | 0 | 0 | 39 | 0 | 0 | 0 | 0 |
| Medial tibial plateaus | 31 | 1 | 0 | 0 | 0 | 39 | 0 | 0 | 0 | 0 |
| Meniscus | ||||||||||
| Medial | 18 | 1 | 12 | 1 | – | 37 | 0 | 2 | 0 | – |
| Lateral | 27 | 0 | 3 | 2 | – | 39 | 0 | 0 | 0 | – |
| P | 0.014 | 0.608 | ||||||||
| χ2 | 6.063 | 0.263 | ||||||||
MRI: Magnetic resonance imaging. –: Not applicable.
DISCUSSION
The purpose of this study was to compare the prevalence of knee structural changes on 3.0-T MRI in a group of AAIHP versus healthy controls. A compelling observation from this study was that AAIHPs do not have more knee joint structural changes than healthy controls.
The most common finding was fluid-filled bursae surrounding the knee, and mature ice hockey players had significantly more fluid-filled bursae than controls, including deep infrapatellar bursae, popliteal bursae, pes anserine bursae, SM-TCL bursae, medial gastrocnemius bursae, FCL-popliteus bursae, popliteus bursae, and iliotibial bursae, indicating that the presence of bursae around the knee was directly associated with ice hockey activity. This was in agreement with previous studies that reported that joint effusion was seen in more than 50% of nonprofessional runners before the marathon with a mild increase after running.[16] Research has shown that visualization of pes anserine bursae, SM-TCL bursae, and medial gastrocnemius bursae may be caused by direct trauma or pressure.[17,18] Although the prevalence of fluid-filled popliteal bursae varies, this bursa generally occurred in association with other intra-articular changes, such as medial meniscal signal changes, an ACL tear, and/or chondral lesions.[19] The deep infrapatellar bursa, FCL-popliteus bursa, popliteus bursa, and iliotibial bursa may fill with fluid due to varus stresses on the knee while playing ice hockey.[20] In the player group, middle-aged players had spent enough time exercising to develop fluid-filled bursae resulting from long-term repetitive loads placed on the knee. The presence of fluid in the bursae was likely transient in nature and probably decreases after a certain recovery time. Bursae surrounding the knee may lead to resistance to major permanent damage after long periods of exercise, which may be a form of self-protection for the knee.
We also observed that meniscal signal changes were detected in 50% of AAIHPs (the medial and lateral meniscus were involved in 44% and 16% of knees, respectively) compared with only 5% of controls. Notably, 79% of the meniscal signal changes in players were grade II signals, providing support for the idea that the meniscal changes were directly associated with their ice hockey activity. The menisci are fibrocartilaginous tissues whose main function is to transmit and distribute knee loads. It has been clearly shown that mechanical factors play a vital role in the development, growth, maintenance, and repair of the meniscus.[21] Stehling et al.[22] evaluated changes in T2 relaxation time in the meniscus using 3.0-T MRI in the asymptomatic knees of marathon runners before, 48–72 h after, and 3 months after a marathon. They found that the marathon runners had a significant increase in mean T2 values within the 24–48 h following the competition of the race, suggesting that there was a significantly higher water content in the meniscus after the marathon. However, the mean T2 values dropped back to the initial value after 3 months, a sign of decreased water content back to the normal range. The increase in signal alterations was transient. Promising evidence has demonstrated that the meniscus may have the ability to recover from heavy loads, such as long-distance running, and maintain its structure and functional characteristics.[23] Most meniscal abnormalities were localized to the medial meniscus as compared with the lateral meniscus in these asymptomatic populations. Meniscal injury tends to be a result of a combined axial and rotational load at the tibiofemoral joint due to movements during sporting events, such as player-to-player contact as well as twisting with the knee in flexion. The shear stresses exerted on the medial meniscus, especially given its size, shape, and relative immobility, may account for these findings. The menisci likely respond to ice hockey movements in a more dynamic way than previously realized, and ice hockey may even enhance the self-protection of the knee.
Articular cartilage injuries were present in 47% of the AAIHP, but in none of the controls. This finding is similar to previous MRI studies of athletes that have shown a 41% prevalence of articular cartilage abnormalities.[4,15] However, Boeth et al.[24] found that cartilage lesions were present in 56% of athletes, which was more frequent than in our study. One reason for this may be that 44% of their cases had a history of knee surgery, which increases the risk of cartilage damage.[25] A surprising finding was that the articular cartilage of the patellofemoral joint was frequently affected (47% knees, 82% of the affected cartilage) in amateur ice hockey players. There were 8 and 10 knees (25% and 31%) with lesions of the patella and trochlea, respectively. Kaplan et al.[26] described a high percentage of patellofemoral changes, with patella changes in 35% and trochlea changes in 25% of their study population. One possible explanation for these findings is that the patellofemoral joint frequently sustains intense stress in amateur ice hockey players. These applied loads affect the functionality of the patellofemoral joint. Unlike other self-repairing tissues such as bone, articular cartilage is prone to damage and has a limited ability to repair itself. Its lacks vascular, neural, and lymphatic networks.[27] It is therefore reasonable that heavy-intensity, high-impact ice hockey may result in acute traumatic cartilage injuries and chronic joint stress, with potentially long-term effects.
Eleven (34%) of the 32 knees in AAIHP had BME in at least one location, but none of the controls had BME. In sports, BME is generally the result of acute or chronic trauma.[28] The cause of BME is unknown in asymptomatic athletes. It has been hypothesized that during sports, bone responds to mechanical stress with trabecular remodeling and subsequent microtrauma and edema, which may correspond to the bone bruise detected on MRI. Kornaat and Van de Velde[29] studied BME lesions on MRI in asymptomatic athletes. They argued that BME lesions were detected in almost all cases and more than half of the lesions came and went, with new lesions occurring and others disappearing over a 7-month follow-up period. None of the BME lesions were associated with clinical complaints. This demonstrates that these changes are likely to be related to the bone's physiological response to sports.
Our study identified the prevalence of ACL damage to be 9% in AAIHP and 0% in the controls, which was similar to the finding of Tuominen et al.,[4] who found ACL ruptures in 10.5% of male international ice hockey players. They also found that MCL sprains were the most common ligament injuries. We were surprised to see no MCL injuries in our study. However, 2 of the 34 knees were excluded from our study due to MCL surgery. Contact is the most common mechanism of ACL injury in ice hockey players, and this injury is usually caused by a sudden change in direction or a deceleration, causing overextension and a shearing force that involves valgus or varus stress to the knee resulting from the collision.[30] Moreover, Granan et al.[31] showed that injury patterns were related to the nature of the sport. Compared with other athletes, older basketball players may take longer to develop intra-articular damage. Some of the middle-aged individuals included in our study played basketball or football, or were skiers, which may increase the prevalence of ACL injury.
The high prevalence of MRI findings in our study was likely due to the use of high-field 3.0-T MR, which has a high sensitivity for detecting and evaluating abnormalities of the knee in asymptomatic patients. There were several additional strengths of our study. This study compared knee MRI findings in amateur ice hockey players versus age- and gender-matched healthy individuals. Furthermore, the knee MRIs were reviewed by two completely blinded, experienced musculoskeletal radiologists.
However, our study had several limitations. First, the statistical power was limited by the small sample size. Second, even though we applied strict inclusion/exclusion criteria, the potential for participant bias could not be entirely eliminated. Some ice hockey players take part in other activities involving knee rotation and loading, including running, football, and skiing, which we did not consider. However, because we included participants from the same area and population, we assumed they participated equally in such sports. Third, the results of this study may not be applicable to female amateur ice hockey players because men's ice hockey allows body checking while women's ice hockey prohibits it.
In conclusion, MRI findings suggest that knee changes, including fluid in bursae, meniscal grade II signals, BME, articular cartilage (predominantly at the patellofemoral joint), and ACL damage, are more common in AAIHP than in asymptomatic healthy individuals. Knee joint structures, especially the meniscus, respond to ice hockey in a more dynamic way than previously thought. Other knee structures, such as bursae surrounding the knee and bone marrow, seem to be resistant to significant permanent damage after long periods of exercise. Although fast-speed, heavy-intensity, and high-impact sports such as ice hockey may have potentially deleterious effects on the patellofemoral articular cartilage, other structures of the knee may adapt to provide a protective effect.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Castanares-Zapatero D, Gillard N, Capron A, Haufroid V, Hantson P. Reversible cardiac dysfunction after venlafaxine overdose and possible influence of genotype and metabolism. Forensic Sci Int. 2016;266:e48–51. doi: 10.1016/j.forsciint.2016.05.030. doi: 10.1016/j.forsciint.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn AW, Noonan BC, Kelly BT, Larson CM, Bedi A. The hip in ice hockey: A Current concepts review. Arthroscopy. 2016;32:1928–38. doi: 10.1016/j.arthro.2016.04.029. doi: 10.1016/j.arthro.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Popkin CA, Schulz BM, Park CN, Bottiglieri TS, Lynch TS. Evaluation, management and prevention of lower extremity youth ice hockey injuries. Open Access J Sports Med. 2016;7:167–76. doi: 10.2147/OAJSM.S118595. doi: 10.2147/OAJSM.S118595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuominen M, Stuart MJ, Aubry M, Kannus P, Parkkari J. Injuries in men's international ice hockey: A 7-year study of the international ice hockey federation adult world championship tournaments and olympic winter games. Br J Sports Med. 2015;49:30–6. doi: 10.1136/bjsports-2014-093688. doi: 10.1136/bjsports-2014-093688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Cicuttini F, Jin X, Wluka AE, Han W, Zhu Z, et al. Knee effusion-synovitis volume measurement and effects of Vitamin D supplementation in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2017;25:1304–12. doi: 10.1016/j.joca.2017.02.804. doi: 10.1016/j.joca.2017.02.804. [DOI] [PubMed] [Google Scholar]
- 6.Van Dyck P, Vanhoenacker FM, Lambrecht V, Wouters K, Gielen JL, Dossche L, et al. Prospective comparison of 1.5 and 3.0-T MRI for evaluating the knee menisci and ACL. J Bone Joint Surg Am. 2013;95:916–24. doi: 10.2106/JBJS.L.01195. doi: 10.2106/JBJS.L.01195. [DOI] [PubMed] [Google Scholar]
- 7.Wong S, Steinbach L, Zhao J, Stehling C, Ma CB, Link TM, et al. Comparative study of imaging at 3.0 T versus 1.5 T of the knee. Skeletal Radiol. 2009;38:761–9. doi: 10.1007/s00256-009-0683-0. doi: 10.1007/s00256-009-0683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toslak IE, Cekic B, Turk A, Eraslan A, Parlak AE. Evaluation of diffusion-weighted MR imaging as a technique for detecting bone marrow edema in patients with osteitis pubis. Magn Reson Med Sci. 2017;16:317–24. doi: 10.2463/mrms.mp.2016-0104. doi: 10.2463/mrms.mp.2016-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki T, Pasternak O, Mayinger M, Muehlmann M, Savadjiev P, Bouix S, et al. Hockey concussion education project, part 3. White matter microstructure in ice hockey players with a history of concussion: A diffusion tensor imaging study. J Neurosurg. 2014;120:882–90. doi: 10.3171/2013.12.JNS132092. doi: 10.3171/2013.12.JNS132092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamard E, Théoret H, Skopelja EN, Forwell LA, Johnson AM, Echlin PS, et al. A prospective study of physician-observed concussion during a varsity university hockey season: Metabolic changes in ice hockey players. Part 4 of 4. Neurosurg Focus. 2012;33:E4:1–7. doi: 10.3171/2012.10.FOCUS12305. doi: 10.3171/2012.10.FOCUS12305. [DOI] [PubMed] [Google Scholar]
- 11.Kolman BH, Daffner RH, Sciulli RL, Soehnlen MW. Correlation of joint fluid and internal derangement on knee MRI. Skeletal Radiol. 2004;33:91–5. doi: 10.1007/s00256-003-0707-0. doi: 10.1007/s00256-003-0707-0. [DOI] [PubMed] [Google Scholar]
- 12.Stoller DW, Martin C, Crues JV, 3rd, Kaplan L, Mink JH. Meniscal tears: Pathologic correlation with MR imaging. Radiology. 1987;163:731–5. doi: 10.1148/radiology.163.3.3575724. doi: 10.1148/radiology.163.3.3575724. [DOI] [PubMed] [Google Scholar]
- 13.Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17:505–13. doi: 10.1177/036354658901700410. doi: 10.1177/036354658901700410. [DOI] [PubMed] [Google Scholar]
- 14.Behzadi C, Welsch GH, Laqmani A, Henes FO, Kaul MG, Schoen G, et al. Comparison of T2* relaxation times of articular cartilage of the knee in elite professional football players and age-and BMI-matched amateur athletes. Eur J Radiol. 2017;86:105–11. doi: 10.1016/j.ejrad.2016.10.028. doi: 10.1016/j.ejrad.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Major NM, Helms CA. MR imaging of the knee: Findings in asymptomatic collegiate basketball players. AJR Am J Roentgenol. 2002;179:641–4. doi: 10.2214/ajr.179.3.1790641. doi: 10.2214/ajr.179.3.1790641. [DOI] [PubMed] [Google Scholar]
- 16.Schueller-Weidekamm C, Schueller G, Uffmann M, Bader TR. Does marathon running cause acute lesions of the knee. Evaluation with magnetic resonance imaging. Eur Radiol. 2006;16:2179–85. doi: 10.1007/s00330-005-0132-y. doi: 10.1007/s00330-005-0132-y. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy CL, McNally EG. The MRI appearance of cystic lesions around the knee. Skeletal Radiol. 2004;33:187–209. doi: 10.1007/s00256-003-0741-y. doi: 10.1007/s00256-003-0741-y. [DOI] [PubMed] [Google Scholar]
- 18.Chatra PS. Bursae around the knee joints. Indian J Radiol Imaging. 2012;22:27–30. doi: 10.4103/0971-3026.95400. doi: 10.4103/0971-3026.95400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman AM, Marzo JM. Popliteal cysts: A current review. Orthopedics. 2014;37:e678–84. doi: 10.3928/01477447-20140728-52. doi: 10.3928/01477447-20140728-52. [DOI] [PubMed] [Google Scholar]
- 20.Steinbach LS, Stevens KJ. Imaging of cysts and bursae about the knee. Radiol Clin North Am. 2013;51:433–54. doi: 10.1016/j.rcl.2012.10.005. doi: 10.1016/j.rcl.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 21.McNulty AL, Guilak F. Mechanobiology of the meniscus. J Biomech. 2015;48:1469–78. doi: 10.1016/j.jbiomech.2015.02.008. doi: 10.1016/j.jbiomech.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stehling C, Luke A, Stahl R, Baum T, Joseph G, Pan J, et al. Meniscal T1rho and T2 measured with 3.0T MRI increases directly after running a marathon. Skeletal Radiol. 2011;40:725–35. doi: 10.1007/s00256-010-1058-2. doi: 10.1007/s00256-010-1058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoessly ML, Wildi LM. Magnetic resonance imaging findings in the knee before and after long-distance running-documentation of irreversible structural damage? A Systematic review. Am J Sports Med. 2017;45:1206–17. doi: 10.1177/0363546516656180. doi: 10.1177/0363546516656180. [DOI] [PubMed] [Google Scholar]
- 24.Boeth H, MacMahon A, Eckstein F, Diederichs G, Schlausch A, Wirth W, et al. MRI findings of knee abnormalities in adolescent and adult volleyball players. J Exp Orthop. 2017;4:6. doi: 10.1186/s40634-017-0080-x. doi: 10.1186/s40634-017-0080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nepple JJ, Wright RW, Matava MJ, Brophy RH. Full-thickness knee articular cartilage defects in national football league combine athletes undergoing magnetic resonance imaging: Prevalence, location, and association with previous surgery. Arthroscopy. 2012;28:798–806. doi: 10.1016/j.arthro.2011.11.010. doi: 10.1016/j.arthro.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan LD, Schurhoff MR, Selesnick H, Thorpe M, Uribe JW. Magnetic resonance imaging of the knee in asymptomatic professional basketball players. Arthroscopy. 2005;21:557–61. doi: 10.1016/j.arthro.2005.01.009. doi: 10.1016/j.arthro.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21–34. doi: 10.1038/nrrheum.2014.157. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanhoenacker FM, Snoeckx A. Bone marrow edema in sports: General concepts. Eur J Radiol. 2007;62:6–15. doi: 10.1016/j.ejrad.2007.01.013. doi: 10.1016/j.ejrad.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Kornaat PR, Van de Velde SK. Bone marrow edema lesions in the professional runner. Am J Sports Med. 2014;42:1242–6. doi: 10.1177/0363546514521990. doi: 10.1177/0363546514521990. [DOI] [PubMed] [Google Scholar]
- 30.Kluczynski MA, Kang JV, Marzo JM, Bisson LJ. Magnetic resonance imaging and intra-articular findings after anterior cruciate ligament injuries in ice hockey versus other sports. Orthop J Sports Med. 2016;4:2325967116646534. doi: 10.1177/2325967116646534. doi: 10.1177/2325967116646534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granan LP, Inacio MC, Maletis GB, Funahashi TT, Engebretsen L. Sport-specific injury pattern recorded during anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41:2814–8. doi: 10.1177/0363546513501791. doi: 10.1177/0363546513501791. [DOI] [PubMed] [Google Scholar]


