Abstract
Background:
The incidence of Ebstein's anomaly is extremely low, and except for the Mayo Clinic, no cardiac center has reported on a sufficient number of patients. The aim of our study was to report the outcomes of Ebstein's anomaly patients treated with tricuspid valvuloplasty (TVP) or tricuspid valve replacement (TVR).
Methods:
TVP or TVR was performed in 245 patients from July 2006 to April 2016. We reviewed patients' records and contacted patients via outpatient service and over the telephone.
Results:
The mean follow-up time was 43.6 ± 32.6 months, and 224 (91.4%) patients underwent follow-up. The mean operative age was 31.2 ± 15.7 years. TVR was performed in 23 patients, and TVP was performed in 201 patients. The 30-day mortality rate was 1.3%, and the overall survival rate was 97.9% at 5 and 10 years. The early mortality rate of the TVP group was lower than that of the TVR group (0.5% vs. 8.7%, P = 0.028), and the overall mortality rate of the TVP group was lower than that of the TVR group, without statistical significance (1.0% vs. 8.7%). After propensity score matching, the rates of mortality and New York Heart Association class ≥ III were lower in the TVP group than those in the TVR group without statistical significance. Seven patients with Type B Wolff-Parkinson-White (WPW) syndrome underwent one-stage surgery, and arrhythmias disappeared. Six patients suffered from episodes of left ventricular outflow tract obstruction (LVOTO) during surgery. Severe LVOTO could be treated with reoperation of the atrialized right ventricle.
Conclusions:
Ebstein's anomaly patients treated with TVP or TVR can experience optimal outcomes with midterm follow-up. However, TVP should be the first-choice treatment. Optimal outcomes can be obtained from one-stage operation in patients with Type B WPW syndrome. Severe LVOTO during surgery might be related to improper operation of the atrialized right ventricle.
Keywords: Congenital Heart Disease, Right Heart Failure, Tricuspid Regurgitation, Tricuspid Valve Dysplasia
摘要
背景:
三尖瓣下移畸形的发生率极低。除梅奥诊所外,其他心脏中心报道的三尖瓣下移畸形患者数量仍较少。本研究的主要 目的是报道三尖瓣下移畸形患者行三尖瓣成形术或三尖瓣置换后的近中期结果。
方法:
从2006年7月到2016年4月,共有245例三尖瓣下移畸形患者行三尖瓣成形术或三尖瓣置换术。我们通过复习患者的病历 资料,门诊随访和电话调查的方式进行随访。
结果:
平均随访时间43.6±32.6月。共随访到224例患者,随访率为91.4%。平均手术年龄31.2±15.7岁。其中行三尖瓣置换术患 者23例,行三尖瓣成形术患者201例。30天内死亡率为1.3%,5年及10年总生存率为97.9%。三尖瓣成形术患者早期死亡率低于 三尖瓣置换术组(0.5% vs. 8.7%, Fisher's exact test, P = 0.028)。三尖瓣成形术组总死亡率低于三尖瓣置换术组(1.0% vs. 8.7% ),但并无统计学差异。经倾向性评分匹配后,三尖瓣成形组的死亡率、纽约心功能分级=III级发生率低于三尖瓣置换组,但 无统计学差异。7例患者行同期处理三尖瓣下移畸形和B型预激综合征,术后心律失常消失。6例患者于术中出现左室流出道梗 阻现象,重度的左室流出道梗阻可以通过再次处理房化右室纠正。
结论:
三尖瓣下移畸形患者行三尖瓣成形术或三尖瓣置换术后早中期效果良好,但三尖瓣成形术应为首选;三尖瓣下移畸形 合并B型预激的患者可行同期手术,效果良好;三尖瓣下移矫治术中可能出现罕见的左室流出道梗阻,重度的左室流出道梗 阻可能与房化右室处理不当相关。
INTRODUCTION
Ebstein's anomaly is a complex congenital heart disease with an extremely low incidence of 1 per 200,000 live births.[1,2] The pathological characteristics of each Ebstein's anomaly patient are different, which makes the surgical management complex and irregular, especially when it is treated with tricuspid valvuloplasty (TVP). The main characteristics of Ebstein's anomaly include: (1) displaced and dysplastic septal and posterior leaflets; (2) an elongated, fenestrated, or dysplastic anterior leaflet; (3) a markedly dilated atrialized right ventricle that becomes thin and dyskinetic; and (4) a displaced functional annulus and a markedly expanded anatomic annulus. Ebstein's anomaly might occur in combination with other cardiac anomalies or arrhythmias. Ebstein's anomaly combined with an atrial septal defect (ASD) or a patent foramen ovale (PFO) accounts for 30–70% of all cases of Ebstein's anomaly.[3] The incidence rate of Ebstein's anomaly combined with Wolff-Parkinson-White (WPW) syndrome is 10–29%, and at least 15% of the patients suffer from episodes of supraventricular tachycardia.[4,5,6] In 1988, according to degree of leaflet tethering, degree of apical displacement, and degree of dilatation of the atrialized right ventricle, Carpentier et al.[7] proposed the classification for Ebstein's anomaly. A variety of surgical methods were introduced in treatment of Ebstein's anomaly. Those treatments included TVP or tricuspid valve replacement (TVR) for the principle element in the treatment of Ebstein's anomaly and additional concomitant procedures for the correction of comorbid anomalies.
The purpose of our study was to report the early and midterm outcomes of Ebstein's anomaly patients treated with TVP or TVR and to compare the outcomes of the TVP group and the TVR group. The outcome of one-stage surgery for Type B WPW syndrome and Ebstein's anomaly would be reported, and causes of the complication of left ventricular outflow tract obstruction (LVOTO) occurring during Ebstein's anomaly corrective surgery would be analyzed.
METHODS
Ethical approval
The surgical database of Fuwai Hospital in Beijing, China, which was obtained prospectively, was studied retrospectively. The study protocol was approved by the Institutional Review Board of Fuwai Hospital. The requirement to obtain informed consent was waived because of the retrospective nature of the study.
Patient selection and data collection
From July 2006 to April 2016, TVP or TVR was performed in 245 patients. We reviewed patients' records and contacted patients via our outpatient service and over the telephone. The demographic and functional parameters evaluated included age, gender, body mass index, functional class, presence of cyanosis, preoperative hemoglobin concentration, cardiothoracic ratio, the presence of preoperative arrhythmias, prior operations, echocardiographic data, and Carpentier type and associated anomalies. Surgical outcome parameters (including mortality, cause of death, complications, and functional outcome, when available) were also evaluated.
Indications for surgery
The most important indication for surgery was severe tricuspid regurgitation (TR). The other indications for surgery included cyanosis, symptoms of dyspnea and right heart failure, progressive cardiomegaly, a cardiothoracic ratio greater than 0.65 and associated anomalies, such as an ASD, a ventricular septal defect (VSD), and pulmonary valve stenosis. Surgery was also considered when patients suffered from tachyarrhythmias.
Surgical technique
The operative management of patients with Ebstein's anomaly consisted of: (1) closure of the ASD or PFO or partial closure of the ASD or PFO when right ventricular function was poor; (2) surgical division or ablation of the accessory conduction pathways causing Type B WPW syndrome; (3) correction of any associated anomalies, such as a VSD or pulmonary stenosis; (4) consideration of the plication of the atrialized right ventricle; (5) reconstruction of the TV when feasible or valve replacement; (6) testing of the TV via bulb syringe injections of saline into the right ventricle during pulmonary artery occlusion after the completion of valve repair; (7) selective excision of the redundant right atrial wall; and (8) intraoperative assessment of the TV with routine transesophageal echocardiography after repair or replacement.
The management of Ebstein's anomaly depended on the surgeons' experience.
Modified Danielson method
A series of pledget-reinforced mattress sutures were placed at base of the septal and posterior leaflets of the TV. Then, suturing was continued to plicate atrialized right ventricle up to the anatomical valve annulus. Stitches were passed through the annuloplasty ring, and plication sutures were tied over the annuloplasty ring to remodel the annulus and obliterate the atrialized right ventricle.
Modified Carpentier's method
Three-fourths of the enlarged anterior leaflet and as much of the posterior leaflet as possible were detached from the annulus. The detached leaflets were everted to expose the support mechanisms, in which fenestrations were made to lengthen the chordae and relieve the obstruction below the leaflets. A unique aspect of the repair was the placement of pledget-reinforced plication stitches in the atrialized right ventricle to create a vertical plication. Plication suturing was continued from the base of the leaflet attachment to the ventricle to the true annulus. No stitches were placed for right atrial plication.
Cone technique
First, parts of the anterior and posterior leaflets were detached so that they could be used to form a single piece. Then, the posterior leaflet edge was rotated clockwise and sutured to the septal edge of the anterior leaflet, and the true tricuspid annulus was plicated. Finally, the completed valve was attached to the true tricuspid annulus.
Tricuspid valve replacement
The valve was excised. Then, the valve was replaced with a mechanical prosthesis or a stent-mounted xenograft. The device was attached to the annulus by pledget-reinforced mattress sutures. When feasible, the atrialized portion of the right ventricle was plicated by the sutures used to attach the prosthesis.
Surgical ablation or division
Surgical ablation or division was performed as described by Sealy et al.[8] Briefly, the location of surgical ablation or division was in the right atrium, 2 mm from the tricuspid annulus. However, the area in which our surgery was performed was slightly wider, as it extended from the internal part of the displaced anterior leaflet to the coronary sinus. SD entailed separating the atrium from the annulus after dissecting away the atrioventricular fat pad. The dissected areas were then lightly electrocoagulated and repaired by suturing.
Statistical analysis
All data were analyzed with SPSS Version 18.0 (SPSS Inc., USA). Descriptive statistics for categorical variables were described as frequencies and percentages, and continuous data were expressed as the mean ± standard deviation (SD) or as median and range. Categorical variables were compared using a Chi-square test or Fisher's exact test. Midterm survival was demonstrated by Kaplan-Meier survival curves. Binary logistic regression was carried out to analyze early (<30 days) mortality, and Cox proportional hazards models were used to identify the multivariate predictors of overall mortality. Propensity score matching was used to match the data for the TVP patients with those for the TVR patients. P < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
From July 2006 to April 2016, TVP or TVR was performed in 245 Ebstein's anomaly patients. The mean follow-up time at our center was 43.6 ± 32.6 months (range from 1 to 124 months). Twenty-one patients were lost to follow-up, and the follow-up rate was 91.4%. The mean operative age of the remaining 224 Ebstein's anomaly patients was 31.2 ± 15.7 years (range from 7 to 66 years). One hundred and thirty-four (59.8%) patients were female. Most patients were symptomatic; 3.6% (8/224) were categorized as New York Heart Association (NYHA) Class I, 42.9% (96/224) as Class II, 51.8% (116/224) as Class III, and 1.8% (4/224) as Class IV. Cyanosis occurred in 10.7% (24/224) of the patients. The mean hemoglobin concentration was 147.7 ± 22.1 g/L (range from 90 to 248 g/L), and the mean cardiothoracic ratio was 0.59 ± 0.08 (range from 0.42 to 0.81). Sixty-three (28.1%) patients had a right bundle branch block, 17 (7.6%) patients had first-degree atrioventricular block, 29 (12.9%) patients had WPW syndrome, 16 (7.1%) patients had atrial fibrillation, and 8 (3.6%) patients had paroxysmal supraventricular tachycardia (PSVT). Seven (7.6%) patients had a history of previous cardiovascular surgery, and 15 (6.7%) patients had a history of prior radiofrequency catheter ablation (3 PSVT patients and 12 WPW syndrome patients). The diagnosis was established by echocardiography. With regard to Carpentier type, 35.3% (79/224) of patients were classified as Type A, 53.6% (120/224) as Type B, and 11.2% (25/224) as Type C. The incidence rate of Ebstein's anomaly combined with ASD or PFO was 50.0% (112/224; Table 1). TVP was performed in 201 (89.7%) patients, and TVR was performed in 23 (10.3%) patients. The median cardiopulmonary bypass time was 121 min (mean 126.3 ± 47.3 min), and the median cross-clamp time was 83.0 min (mean 88.6 ± 35.1 min). The preoperative variables in the TVP and TVR group before or after matching are shown in Table 2.
Table 1.
Associated cardiac defects in Ebstein’s anomaly patients (n = 224)
| Items | Patients, n(%) |
|---|---|
| ASD/PFO | 112 (50.0) |
| VSD | 6 (2.7) |
| Coronary artery fistula | 1 (0.4) |
| Cor triatriatum | 1 (0.4) |
| PAPVC | 1 (0.4) |
| PS | 4 (1.8) |
| Persistent left superior vena cava | 1 (0.4) |
| PECD | 1 (0.4) |
ASD: Atrial septal defect; PFO: Patent foramen ovale; VSD: Ventricular septal defect; PAPVC: Partial anomalous pulmonary venous connection; PS: Pulmonary valve stenosis; PECD: Partial endocardial cushion defect.
Table 2.
Preoperative variables in the TVP and TVR groups
| Variables | All study patients | Matched groups | ||||||
|---|---|---|---|---|---|---|---|---|
| TVP (n = 201) | TVR (n = 23) | t/χ2 | P | TVP (n = 22) | TVR (n = 22) | t/χ2 | P | |
| Age (years) | 31.0 ± 15.5 | 32.8 ± 17.3 | 0.541† | 0.589 | 26.5 ± 12.1 | 33.2 ± 17.6 | 1.479† | 0.148 |
| Female, n (%) | 122 (60.7) | 12 (52.2) | 0.624 | 0.430 | 13 (59.1) | 11 (50.0) | 0.367 | 0.545 |
| Body surface area (m2) | 1.7 ± 0.3 | 1.8 ± 0.3 | 1.242† | 0.215 | 1.7 ± 0.3 | 1.8 ± 0.3 | −1.244† | 0.220 |
| Prior TV surgery | 0.5 | 26.1 | * | <0.01 | 0.0 | 13.6 | * | 0.021 |
| Cyanosis (%) | 10.4 | 13.0 | * | 0.721 | 22.7 | 13.6 | * | 0.698 |
| Preoperative AF (%) | 5.0 | 26.1 | 13.869 | <0.01 | 0.0 | 27.3 | * | 0.021 |
| Preoperative WPW syndrome (%) | 13.4 | 8.7 | * | 0.747 | 13.6 | 9.1 | * | 1.000 |
| NYHA class ≥III (%) | 51.2 | 73.9 | 4.264 | 0.039 | 72.7 | 72.7 | * | 1.000 |
| Preoperative hemoglobin (g/L) | 147.4 ± 22.3 | 150.4 ± 20.3 | 0.608† | 0.544 | 147.4 ± 19.0 | 151.3 ± 20.3 | −0.659† | 0.513 |
| Preoperative C/R | 0.59 ± 0.08 | 0.62 ± 0.10 | 2.030† | 0.044 | 0.58 ± 0.08 | 0.62 ± 0.10 | −1.439† | 0.158 |
| TR ≥ moderate (%) | 94.5 | 87.0 | * | 0.162 | 100.0 | 86.4 | * | 0.233 |
| Carpentier type (%) | ||||||||
| Type A | 36.3 | 26.1 | * | 0.001 | 18.2 | 22.7 | * | 0.068 |
| Type B | 55.7 | 34.8 | 68.2 | 36.4 | ||||
| Type C | 8.0 | 39.1 | 13.6 | 40.9 | ||||
*Fisher’s exact test. †: t value. TV: Tricuspid valve; AF: Atrial fibrillation; NYHA class: New York Heart Association class; C/R: Cardiothoracic ratio; TR: Tricuspid regurgitation; TVP: Tricuspid valvuloplasty; TVR: Tricuspid valve replacement; WPW: Wolff-Parkinson-White.
Early term survival
There were 3 (1.4%) deaths in our cohort during the first 30 days after operation. The first patient was a 13-year-old girl who underwent TVR (Hancock II 29# bioprosthetic valve) because she did not have enough leaflet for TVP. She died of right heart failure despite being treated with continuous renal replacement therapy (CRRT) and vasoactive agents in the Intensive Care Unit (ICU).
The second patient was a 33-year-old man who had cyanosis at rest. Echocardiography and magnetic resonance imaging showed a PFO with a bidirectional shunt, marked right ventricular enlargement, and decreased right ventricular systolic function, with a right ventricular ejection fraction of 15%. TVP and PFO closure were performed. The patient died of right heart failure despite the use of CRRT and extracorporeal membrane oxygenation (ECMO).
The third patient was a 36-year-old man who was diagnosed with Ebstein's anomaly, coronary heart disease, remote myocardial infarction, and a ventricular aneurysm when he was admitted. Echocardiography revealed that the right ventricle was markedly dilated; motion of the anterior wall, anterolateral wall, and apex was absent; the left ventricular ejection fraction was 62%; the left ventricular end-diastolic diameter was 49 mm; and mitral regurgitation was absent. Coronary artery bypass graft, ventricular aneurysm resection, and TVR (31# bileaflet mechanical prosthetic valve) were performed. Delayed sternal closure was performed because the heart was swollen. The patient underwent two emergency operations in the ICU, one for refractory ventricular tachycardia and one for unstable circulation. He ultimately died due to heart failure.
Associated coronary artery disease, TVR, secondary thoracotomy, the need for CRRT, and the need for ECMO postoperatively were univariate risk factors in the univariate fashion. No independent risk factors were identified in the multivariate model.
Tricuspid valvuloplasty versus tricuspid valve replacement
The early mortality rate was 0.5% in the TVP group and 8.7% in the TVR group (Fisher's exact test, P = 0.028). The patients in the TVP group had shorter intubation times (t = 3.354, P = 0.003) and lengths of ICU stay (t = 2.245, P = 0.026) than those in the TVR group. The rates of the following complications were lower in the TVP group than in the TVR group: urgent thoracotomy in the ICU (Fisher's exact test, P = 0.010), third-degree atrioventricular block (Fisher's exact test, P = 0.025), and need for CRRT (Fisher's exact test, P = 0.008). The rate of secondary thoracotomy in the TVP group was lower than that in the TVR group (Fisher's exact test, P = 0.083). There was no significant difference in the rate of need for ECMO between the two groups (Fisher's exact test, P = 1.000) [Table 3].
Table 3.
Perioperative and follow-up variables in the TVP and TVR groups
| Variables | All study patients | Matched groups | ||||||
|---|---|---|---|---|---|---|---|---|
| TVP (n = 201) | TVR (n = 23) | t | P | TVP (n = 22) | TVR (n = 22) | t | P | |
| ICU stay time (days) | 2.2 ± 2.7 | 3.5 ± 2.6 | 2.245 | 0.026 | 2.1 ± 1.3 | 3.6 ± 2.7 | −2.245 | 0.030 |
| Intubation time (h) | 17.5 ± 14.3 | 34.2 ± 23.3 | 3.354 | 0.003 | 15.3 ± 7.4 | 34.9 ± 23.6 | −3.727 | 0.001 |
| Secondary thoracotomy (%) | 1.5 | 8.7 | * | 0.083 | 0.0 | 9.1 | * | 0.488 |
| Urgent thoracotomy (%) | 0.0 | 8.7 | * | 0.010 | 0.0 | 9.1 | * | 0.488 |
| Postoperative third-degree AVB (%) | 2.0 | 13.0 | * | 0.025 | 0.0 | 9.1 | * | 0.488 |
| Postoperative CRRT (%) | 1.0 | 13.0 | * | 0.008 | 0.0 | 13.6 | * | 0.233 |
| Postoperative mechanical support (%) | 1.0 | 0.0 | * | 1.000 | 0.0 | 0.0 | * | |
| Early mortality (%) | 0.5 | 8.7 | * | 0.028 | 0.0 | 9.1 | * | 0.488 |
| Overall mortality (%) | 1.0 | 8.7 | * | 0.053 | 0.0 | 9.1 | * | 0.488 |
| Recurrent TR (%) | 16.6 | 4.8 | * | 0.212 | 18.2 | 5.0 | * | 0.346 |
| Follow-up NYHA class ≥III (%) | 3.0 | 4.8 | * | 0.510 | 0.0 | 5.0 | * | 0.476 |
*Fisher’s exact test. ICU: Intensive Care Unit; AVB: Atrioventricular block; CRRT: Continuous renal replacement therapy; TR: Tricuspid regurgitation; NYHA: New York Heart Association; TVP: Tricuspid valvuloplasty; TVR: Tricuspid valve replacement.
Overall survival
There were 3 early deaths because of heart failure, and there was 1 late death due to cardiac arrest. The overall survival rate was 97.9% at 5 and 10 years [Figure 1]. With regard to the independent predictors of overall mortality (1.8%), TVR, secondary thoracotomy, the need for ECMO, and the need for CRRT were identified by univariate analysis, but no independent predictors were identified by multivariable analysis.
Figure 1.
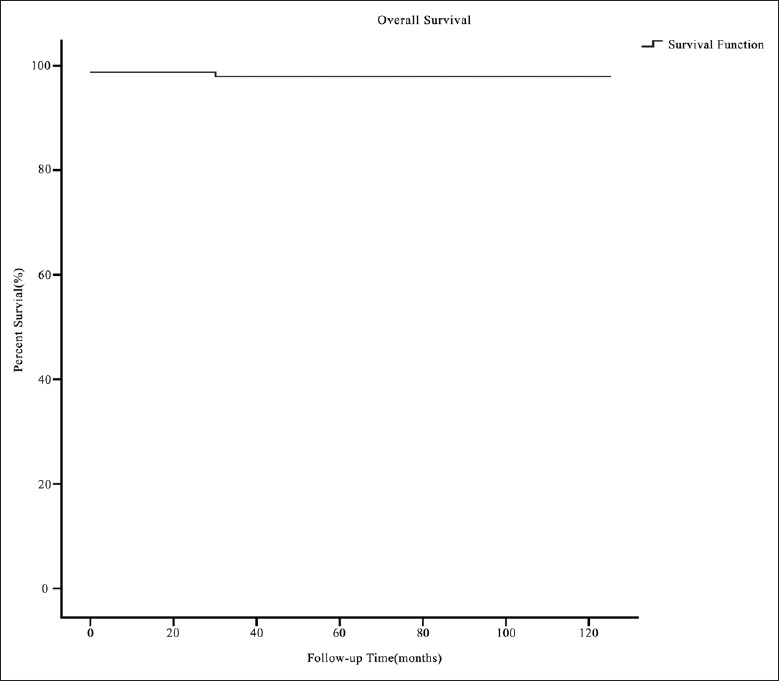
The survival curve of the Ebstein's anomaly patients with tricuspid valvuloplasty or tricuspid valve replacement.
Tricuspid valvuloplasty versus tricuspid valve replacement
The overall mortality rate in the TVP group (1.0%) was lower than that in the TVR group (8.7%); however, this difference was not statistically significant (Fisher's exact test, P = 0.053). The reoperation ratio was 1.0% in the TVP group and 0.0% in the TVR group (Fisher's exact test, P = 1.000). The rate of rehospitalization for arrhythmia was lower in the TVP group (6.5%) than in the TVR group (9.5%); however, this difference was not statistically significant (Fisher's exact test, P = 0.641). During the follow-up period, the rate of NYHA class ≥ III in the TVP group was lower than that in the TVR group (Fisher's exact test, P = 0.510). The rate of recurrent TR in the TVP group (16.6%) was higher than that in the TVR group (4.8%); however, this difference was not statistically significant (Fisher's exact test, P = 0.212; Table 3).
Tricuspid valvuloplasty versus tricuspid valve replacement (propensity score matching)
There were significant differences in the rate of NYHA ≥ III and in Carpentier type between the TVP and TVR groups. When the rate of NYHA ≥ III and Carpentier type were taken into account, propensity score matching was performed in a 1:1 manner. Then, 22 patients were extracted from each group.
Before matching, more patients in the TVR group than in the TVP group had NYHA class ≥ III (χ2 = 4.264, P = 0.039), the cardiothoracic ratio of the TVR group was larger than that of the TVP group (t = 0.044, P = 0.044), and there was a significant difference in Carpentier type between the TVR and TVP groups (Fisher's exact test, P = 0.001) [Table 2]. After propensity score matching, no significant differences in the rate of NYHA ≥ III (Fisher's Exact Test, P = 1.000), the cardiothoracic ratio (t = −1.439, P = 0.158), or the Carpentier type (Fisher's exact test, P = 0.068) were observed [Table 2]. The operative and postoperative data after propensity score matching are presented in Table 3.
The early mortality rate was 0.0% in the TVP group and 9.1% in the TVR group (Fisher's exact test, P = 0.488). The intubation time and length of ICU stay in the TVR group were longer than those in the TVP group (t = −3.727, P = 0.001 and t = −2.245, P = 0.030, respectively). During the perioperative period, the rates of secondary thoracotomy, urgent thoracotomy in the ICU, third-degree atrioventricular block, and need for CRRT in the TVR group were higher than those in the TVP group; however, these differences were not statistically significant. No complications requiring mechanical support occurred in the two groups.
The overall mortality rate was 0.0% in the TVP group and 9.1% in the TVR group (Fisher's exact test, P = 0.488). During follow-up period, no reoperations were performed in the two groups. The rehospitalization rate was same between the two groups (9.1%). The rate of recurrent TR in the TVP group (18.2%) was higher than that in the TVR group (5.0%) (Fisher's exact test, P = 0.346). The rate of NYHA class ≥ III in the TVP group (0.0%) was lower than that in the TVR group (5.0%); however, this difference was not statistically significant.
Arrhythmias
Seven (3.1%) patients with Type B WPW syndrome had a one-stage surgery for arrhythmia and Ebstein's anomaly. The midterm outcomes were optimal, and all patients were free from recurrence [Table 4]. However, the other patients developed new-onset arrhythmias after operation. Specifically, seven patients developed atrial fibrillation (two patients underwent successful radiofrequency ablation, and the others were treated with drugs), and one patient developed paroxysmal ventricular tachycardia (she was treated with drugs, and the arrhythmia disappeared 3 months after operation).
Table 4.
Outcome of surgical ablation and surgical division in seven patients
| Number | Age (years | Current operations (SA1, SD2) | Rhythm | Postoperative medication | NYHA class preoperatively/postoperatively | ||
|---|---|---|---|---|---|---|---|
| Before operation | After operation | During follow-up/follow-up time (months) | |||||
| 1 | 21 | 2 | WPW | NSR, CRBB | NSR, CRBB/3 | None | II/I |
| 2 | 8 | 2 | WPW | NSR, CRBB | NSR, CRBB/84 | None | II/I |
| 3 | 10 | 1 | WPW | NSR | NSR/4 | None | II/II |
| 4 | 13 | 1 | WPW | NSR, CRBB | NSR, CRBB/41 | None | II/I |
| 5 | 44 | 2 | WPW | NSR | NSR/51 | None | III/I |
| 6 | 11 | 2 | WPW | NSR | NSR/10 | None | II/I |
| 7 | 40 | 2 | WPW | NSR | NSR/8 | None | II/I |
SA: Surgical ablation; SD: Surgical division; WPW: Wolff-Parkinson-White; NSR: Normal sinus rhythm; CRBB: Complete right branch block; NYHA: New York Heart Association.
In the early postoperative period, third-degree atrioventricular block occurred in seven patients (TVP 4 vs. TVR 3; P = 0.025), five of whom were treated with permanent pacemakers. Two patients refused pacemaker placement; however, they were asymptomatic during the follow-up period. In addition, one patient was implanted with a permanent pacemaker because of sinus bradycardia.
Left ventricular outflow tract obstruction
A total of six patients in our cohort experienced episodes of LVOTO during corrective surgery for Ebstein's anomaly [Table 5]. Severe LVOTO was observed in three patients, whose left ventricle outflow tract gradient >50 mmHg (1 mmHg = 0.133 kPa). There were no early or late deaths. Four patients were given conservative treatment, including supplementing blood volume, increasing blood pressure, and stopping using inotropes, during corrective surgery, three of these patients had uneventful recoveries. However, one patient had a difficult recovery; this patient's echocardiogram showed that the middle part of the ventricular septum still protruded into the left ventricular during the follow-up period. Conservative treatment was unsuccessful in two patients with transverse plication. These patients subsequently underwent surgery, in which the plication stitches of the ventricular septum were removed, and good results were obtained.
Table 5.
Characteristics of the six patients with LVOTO
| Number | ARVP (T, L) | LVOT gradient (mmHg) | SAM (Y, N) | LVOT (Y, N) | Intraoperative/postoperative treatment | NYHA class (pre/post) | ||
|---|---|---|---|---|---|---|---|---|
| During operation | Discharge | Follow-up results/time (months) | ||||||
| 1 | T | 100 | Y | Y | N | N/116 | Surgical treatment | II/I |
| 2 | T | 100 | Y | Y | N | N/114 | Surgical treatment | III/II |
| 3 | L | >30 | Y | Y | N | N/6 | Conservative treatment | II/I |
| 4 | L | >30 | Y | Y | N | N/92 | Conservative treatment | II/II |
| 5 | T | 32 | Y | Y | N | N/73 | Conservative treatment | II/II |
| 6 | L | 140 | Y | Y | N | N/51 | Conservative treatment | III/I |
1 mmHg = 0.133 kPa. ARVP: Atrialized right ventricle plication; T: Transverse; L: Longitudinal; LVOTO: Left ventricle outflow tract obstruction; SAM: Systolic anterior motion; Y: Yes; N: No; LVOT: Left ventricle outflow tract; NYHA: New York Heart Association.
DISCUSSION
Wilhelm Ebstein's scholarly description of the TV abnormality bearing his name was published in 1866.[9] Ebstein's anomaly is a rare congenital heart disease. Although the disease has general characteristics, each affected patient has different pathologic and hemodynamic characteristics, resulting in different natural histories. Patients with mild forms of Ebstein's anomaly can be symptom free throughout their lives, but patients with severe forms can die in utero. As there are no randomized trials of patients with Ebstein's anomaly, the current international guidelines are restricted by a lack of definitive evidence.[10,11] In our study, 224 children and adults participated in midterm follow-up. Except for the Mayo Clinic, no cardiac center has reported on a larger number of Ebstein's anomaly patients. Therefore, the outcome data from our center may supplement those used for the treatment of Ebstein's anomaly.
Survival
Surgical outcomes differ markedly between neonates and nonneonates. In a study involving 80 neonates, the in-hospital mortality rate was 14%, and the overall estimated 15-year survival rate was 67%.[12] Another cardiac center reported that the in-hospital mortality rate was as high as 31%.[13] At our center, in a population with a mean operative age of 31.2 ± 15.7 years, the early mortality rate was 1.3%, and the overall survival rate was 97.9% at 5 and 10 years. The early and overall mortality rates were fairly low, perhaps owing to the older age of the sample. Other studies with nonneonates had similar results. In a study including 48 patients with a median operative age of 5.6 years, the in-hospital mortality rate was 4.2%, and the overall mortality rate was 8.3%.[14] In a study of a Mayo Clinic population with a mean operative age of 24 years, the early mortality rate was 6%, and the 10-year survival rate was 85%.[3]
There were four deaths at our center. Three patients died due to heart failure in the early postoperative stage, and one patient died due to cardiac arrest of an unknown cause during the follow-up period. Based on our findings and those of other reports, we concluded that the main cause of death was heart failure.[15,16,17]
Tricuspid valvuloplasty versus tricuspid valve replacement
Whether TVP or TVR is the better treatment for Ebstein's anomaly remains controversial. Brown et al.[3] reported that 62.5% of 539 patients underwent TVR and that both TVP and TVR were associated with good long-term survival. They argued that one must resist the temptation to conclude that valve repair is inherently superior to valve replacement and that TVR can eliminate the volume overload affecting the right heart more effectively than TVP. However, when TVR is performed, the following complications may occur: thrombotic events, bleeding incidents, bioprosthesis degeneration, and mechanical valve dysfunction. We thought that the goal of the operation should be to improve the NYHA class and mortality outcomes and decrease the rate of complication. In our study, the length of ICU stay and intubation time were longer in the TVP group than in the TVR group, and the rates of urgent thoracotomy in the ICU, third-degree atrioventricular block, and need for CRRT in the TVP group were lower than those in the TVR group. These results proved that recovery proceeded more smoothly in the TVP group than in the TVR group and explain why the early mortality rate in the TVP group was lower than that in the TVR group. The overall mortality rate in the TVP group was lower than that in the TVR group. In addition, the rates of NYHA class ≥ III and mortality were lower in the TVP group than in the TVR group after propensity score matching, despite that there were no significant differences in these rates between the two groups. Given the findings mentioned above, the outcomes of the TVP group were superior to those of the TVR group. Malhotra et al.[18] reported that 57 nonneonatal patients underwent Ebstein's anomaly repairs and that optimal results were obtained. They argued that avoiding prosthetic TVR was a major guiding force in their philosophy regarding the surgical management of Ebstein's anomaly.
Arrhythmias
Downward displacement of the septal leaflet of the TV is associated with discontinuity between the central fibrous body and the septal atrioventricular ring, which are joined by direct muscular connections, thus creating a potential substrate for accessory atrioventricular connections and pre-excitation.[19] These atrioventricular accessory conduction pathways are located at the true tricuspid annulus, which corresponds to the location of the atrialized right ventricle. Khositseth et al.[20] found that all of the accessory conduction pathways were on the right side. The following two approaches were used to eliminate the accessory conduction pathways with the special pathological characteristics: radiofrequency catheter ablation, and surgical ablation or division.[21,22] The outcomes of surgical ablation or division may be superior to those of radiofrequency catheter ablation.[23] In our study, the optimal outcomes of the one-step surgery proved the effectiveness of surgical ablation or division. Furthermore, as most of the accessory conduction pathways were on the right side, for Ebstein's anomaly patients with Type B WPW syndrome, one-stage surgery could be performed without intraoperative electrophysiologic mapping. However, the number of patients in whom this procedure was performed was small; our findings need to be verified by more studies.
Not only TVR but also TVP might lead to third-degree atrioventricular block. Luu et al.[17] reported that 5 (10%) patients were treated with a permanent pacemaker, and Legius et al.[23] reported that 10% of patients needed treatment with a permanent pacemaker. In our cohort, the rate of pacemaker placement in the TVP group was significantly lower than that in the TVR group, which indicated that the conduction bundle was more vulnerable in the TVR group.
Left ventricular outflow tract obstruction
Although leftward displacement of the ventricular septum may be common, to our knowledge, there are no reports on the occurrence of LVOTO as a complication during corrective surgery for Ebstein's anomaly. Hirata et al.[24] reported a case of Ebstein's anomaly complicated by LVOTO secondary to a deformed basal septum attributable to an atrialized right ventricle, and Waterhouse[25] described a case of dynamic LVOTO due to severe TR. Although the atrialized right ventricle was also large in our patients, no LVOTO occurred before operation. As good results were obtained after the plication stitches of ventricular septum were removed in Patients 1 and 2, we speculated that the LVOTO was caused by the transverse plication stitches of the ventricular septum in the two patients. The transverse plication stitches of the ventricular septum might change the shape of the ventricular septum to some extent and thus cause LVOTO. Patient 6, whose atrialized right ventricle was longitudinally folded, also experienced LVOTO, which could be attributed to the shortening of the long axis of the right ventricle. We speculated that the shortening of the longitudinal axis of the right ventricle might lead to changes in the shape of the ventricular septum, causing it to protrude into the left ventricular outflow tract. Patient 6 had a difficult recovery because the LVOTO was not treated surgically, which indicated that intraoperative intervention for the atrialized right ventricle is necessary in patients with severe LVOTO.
Limitations
Due to its retrospective nature, we did not have follow-up on RV function, and last, novel echocardiographic techniques were not used to assess RV function due to their unavailability. Some parts of the data collection were retrospective, and hence, missing values were inevitable. The number of the TVR group is relatively small, which may cause bias. As the number of patients with the complication of LVOTO or Type B WPW is small, longer follow-up and expanded size are needed.
In conclusion, Ebstein's anomaly patients treated with TVP or TVR can experience optimal outcomes with midterm follow-up. However, as the outcome of the TVP group was superior to that of the TVR group, TVP should be the first-choice treatment when available. Optimal outcomes can be obtained in patients with Type B WPW syndrome who undergo a one-stage operation. The occurrence of severe LVOTO during corrective surgery for Ebstein's anomaly might be related to improper operation of the atrialized right ventricle.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Attenhofer Jost CH, Connolly HM, Dearani JA, Edwards WD, Danielson GK. Ebstein's anomaly. Circulation. 2007;115:277–85. doi: 10.1161/CIRCULATIONAHA.106.619338. doi: 10.1161/CIRCULATIONAHA.106.619338. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–900. doi: 10.1016/s0735-1097(02)01886-7. doi: 10.1016/S0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 3.Brown ML, Dearani JA, Danielson GK, Cetta F, Connolly HM, Warnes CA, et al. The outcomes of operations for 539 patients with Ebstein anomaly. J Thorac Cardiovasc Surg. 2008;135:1120–36. doi: 10.1016/j.jtcvs.2008.02.034. 1136.e1-7. doi: 101016/JJTCVS200802034. [DOI] [PubMed] [Google Scholar]
- 4.Oh JK, Holmes DR, Jr , Hayes DL, Porter CB, Danielson GK. Cardiac arrhythmias in patients with surgical repair of Ebstein's anomaly. J Am Coll Cardiol. 1985;6:1351–7. doi: 10.1016/s0735-1097(85)80224-2. doi: 10.1016/S0735-1097(85)80224-2. [DOI] [PubMed] [Google Scholar]
- 5.Olson TM, Porter CB. Electrocardiographic and electrophysiologic findings in Ebstein's anomaly.Pathophysiology, diagnosis, and management. Prog Pediatr Cardiol. 1993;2:38–50. doi:10.1016/0002-9149(82)90048-0. [Google Scholar]
- 6.Mair DD. Ebstein's anomaly: Natural history and management. J Am Coll Cardiol. 1992;19:1047–8. doi: 10.1016/0735-1097(92)90292-u. doi: 10.1016/0735-1097(92)90292-U. [DOI] [PubMed] [Google Scholar]
- 7.Carpentier A, Chauvaud S, Macé L, Relland J, Mihaileanu S, Marino JP, et al. A new reconstructive operation for Ebstein's anomaly of the tricuspid valve. J Thorac Cardiovasc Surg. 1988;96:92–101. [PubMed] [Google Scholar]
- 8.Sealy WC, Wallace AJ, Ramming KP, Gallagher JJ, Svenson RH. An improved operation for the definitive treatment of the Wolff-Parkinson-White syndrome. Ann Thorac Surg. 1974;17:107–13. doi: 10.1016/s0003-4975(10)65617-2. [DOI] [PubMed] [Google Scholar]
- 9.Mazurak M, Kusa J. The two anomalies of Wilhelm Ebstein. Tex Heart Inst J. 2017;44:198–201. doi: 10.14503/THIJ-16-6063. doi: 10.14503/THIJ-16-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, et al. ESC guidelines for the management of grown-up congenital heart disease (New version 2010) Eur Heart J. 2010;31:2915–57. doi: 10.1093/eurheartj/ehq249. doi: 10.1093/eurheartj/ehq249. [DOI] [PubMed] [Google Scholar]
- 11.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults with Congenital Heart Disease). Developed in Collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e143–263. doi: 10.1016/j.jacc.2008.10.001. doi: 10.1016/j.jacc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Luxford JC, Arora N, Ayer JG, Verrall CE, Cole AD, Orr Y, et al. Neonatal Ebstein anomaly: A 30-year institutional review. Semin Thorac Cardiovasc Surg. 2017;29:206–12. doi: 10.1053/j.semtcvs.2017.01.012. doi: 10.1053/j.semtcvs.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Reemtsen BL, Fagan BT, Wells WJ, Starnes VA. Current surgical therapy for Ebstein anomaly in neonates. J Thorac Cardiovasc Surg. 2006;132:1285–90. doi: 10.1016/j.jtcvs.2006.08.044. doi: 10.1016/j.jtcvs.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 14.Kim MS, Lim HG, Kim WH, Lee JR, Kim YJ. Long-term results after surgical treatment of Ebstein's anomaly: A 30-year experience. Korean Circ J. 2016;46:706–13. doi: 10.4070/kcj.2016.46.5.706. doi: 10.4070/kcj.2016.46.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarris GE, Giannopoulos NM, Tsoutsinos AJ, Chatzis AK, Kirvassilis G, Brawn WJ, et al. Results of surgery for Ebstein anomaly: A multicenter study from the European Congenital Heart Surgeons Association. J Thorac Cardiovasc Surg. 2006;132:50–7. doi: 10.1016/j.jtcvs.2005.10.062. doi: 10.1016/j.jtcvs.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 16.Badiu CC, Schreiber C, Hörer J, Ruzicka DJ, Wottke M, Cleuziou J, et al. Early timing of surgical intervention in patients with Ebstein's anomaly predicts superior long-term outcome. Eur J Cardiothorac Surg. 2010;37:186–92. doi: 10.1016/j.ejcts.2009.06.052. doi: 10.1016/j.ejcts.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 17.Luu Q, Choudhary P, Jackson D, Canniffe C, McGuire M, Chard R, et al. Ebstein's anomaly in those surviving to adult life – A single centre experience. Heart Lung Circ. 2015;24:996–1001. doi: 10.1016/j.hlc.2015.03.016. doi: 10.1016/j.hlc.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra SP, Petrossian E, Reddy VM, Qiu M, Maeda K, Suleman S, et al. Selective right ventricular unloading and novel technical concepts in Ebstein's anomaly. Ann Thorac Surg. 2009;88:1975–81. doi: 10.1016/j.athoracsur.2009.07.019. doi: 10.1016/j.athoracsur.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Frescura C, Angelini A, Daliento L, Thiene G. Morphological aspects of Ebstein's anomaly in adults. Thorac Cardiovasc Surg. 2000;48:203–8. doi: 10.1055/s-2000-6893. doi: 10.1055/s-2000-6893. [DOI] [PubMed] [Google Scholar]
- 20.Khositseth A, Danielson GK, Dearani JA, Munger TM, Porter CJ. Supraventricular tachyarrhythmias in Ebstein anomaly: Management and outcome. J Thorac Cardiovasc Surg. 2004;128:826–33. doi: 10.1016/j.jtcvs.2004.02.012. doi: 10.1016/j.jtcvs.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Orczykowski M, Derejko P, Bodalski R, Urbanek P, Zakrzewska-Koperska J, Sierpiński R, et al. Radiofrequency catheter ablation of accessory pathways in patients with Ebstein's anomaly: At 8 years of follow-up. Cardiol J. 2017;24:1–8. doi: 10.5603/CJ.a2016.0111. doi: 10.5603/CJ.a2016.0111. [DOI] [PubMed] [Google Scholar]
- 22.Bockeria L, Golukhova E, Dadasheva M, Revishvili A, Levant A, Bazaev V, et al. Advantages and disadvantages of one-stage and two-stage surgery for arrhythmias and Ebstein's anomaly. Eur J Cardiothorac Surg. 2005;28:536–40. doi: 10.1016/j.ejcts.2005.04.047. doi: 10.1016/j.ejcts.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 23.Legius B, Van De Bruaene A, Van Deyk K, Gewillig M, Troost E, Meyns B, et al. Behavior of Ebstein's anomaly: Single-center experience and midterm follow-up. Cardiology. 2010;117:90–5. doi: 10.1159/000318041. doi: 10.1159/000318041. [DOI] [PubMed] [Google Scholar]
- 24.Hirata K, Yagi N, Kubota S, Wake M, Tengan T. Case of Ebstein anomaly complicated by left ventricular outflow tract obstruction secondary to deformed basal septum attributable to atrialized right ventricle. Circulation. 2016;133:e33–7. doi: 10.1161/CIRCULATIONAHA.115.016208. doi: 10.1161/CIRCULATIONAHA.115.016208. [DOI] [PubMed] [Google Scholar]
- 25.Waterhouse DF, Murphy TM, McCreery CJ, O'Hanlon R. An unusual cause of dynamic left ventricular outflow obstruction: An unusual case of dynamic LVOTO. Int J Cardiol. 2015;197:282–3. doi: 10.1016/j.ijcard.2015.06.084. doi: 10.1016/j.ijcard.2015.06.084. [DOI] [PubMed] [Google Scholar]


