Abstract
Introduction
Due to their immunostimulatory properties TLR ligands are used prophylactically to protect against a variety of viral and bacterial pathogens in mammals. Knowledge of the molecular and functional aspects of TLRs is essential for a better understanding of the immune system and resistance to diseases in birds. For that reason, this study attempted to determine the impact of TLR21 stimulation by its synthetic ligand (CpG ODN, class B) on the chicken immune system.
Material and Methods
Sixty embryonated chicken eggs were randomly allocated into three groups (control and two experimental groups). On day 18 of embryonic development, chickens in one experimental group were administered in ovo a low dose of CpG ODN and the birds of the second experimental group were given a high dose of the ligand. Spleens were collected at 1, 2, 5, and 10 days post-hatching (dph) for analysis of IFN-α, IFN-β, IFN-γ, IL-6, and IL-10 expression using qRT-PCR.
Results
Significant differences were observed in mRNA expression levels of all the measured cytokines associated with the modulation and regulation of the immune response at different time points.
Conclusion
The obtained data clearly demonstrate that immune response induction takes place after in ovo administration of class B CpG ODN, and that the ligand has the ability to induce cytokine responses in neonatal chicken spleen.
Keywords: chicken, chTLR21, CpG ODN, immunomodulation, cytokines
Introduction
The immune system of birds is considered an important model in immunology. The basic principles of the avian immune system are very similar to their mammalian counterparts. Comprehension of the immune system’s mechanisms is essential for a better understanding of resistance to diseases in birds.
Recent findings have clearly demonstrated that the components of the innate immune system may also have at least some of the features of the adaptive immune system (16). This integration and mutual infiltration give the immune response flexibility and resistance, and thus provide protection against various pathogens. Through the stimulation of the innate immune system with various compounds, cellular activation and cytokine production can be obtained, protecting chickens against viral pathogens.
Immune cells involved in the mechanisms of the innate immune response have the ability to distinguish self-antigens from foreign ones due to their specialised receptors. These receptors include highly conserved ligand-binding proteins, namely toll-like receptors (TLRs), which are present in several species including mice, humans, and chickens (6). TLRs, belonging to the pattern recognition receptors (PRR), are responsible for recognising the conserved structural motifs derived from a variety of microorganisms, known as pathogen-associated molecular patterns (PAMPs) (26). Binding the suitable ligand to the TLR triggers a signalling cascade that leads to the activation of many adaptor proteins, transcription factors, and consequently the production of proinflammatory cytokines, chemokines, and defensive molecules. Most vertebrates encode from 10 to 13 different TLRs. TLRs 1−10 have been identified in humans, while TLRs 1−13 have been identified in mice. Recent studies on TLR expression in chickens confirmed their evolutionarily conserved nature and identified 10 different TLR genes (18). Six of these (TLR 2a, 2b, 3, 4, 5, and 7) are clear orthologues of TLRs found in mammals (4). Chicken TLR21 (chTLR21) does not have a mammalian orthologue; however, it is sensitive and responsive to unmethylated cytosine triphosphate deoxynucleotide-phosphodiester-guanine triphosphate deoxynucleotide (CpG) DNA similarly to the mammalian TLR9 ligand. Synthetic oligodeoxynucleotides containing unmethylated CpG motifs (CpG ODN) are similar to CpG DNA from microorganisms and are able to cause series of immunostimulatory effects in vertebrates. In experimental research this phenomenon is used to increase the resistance of the host through the stimulation of signal transduction from TLRs by synthetic analogues of their natural ligands (33).
In vitro studies showed induction of interleukin-1b (IL-1b), interleukin-6 (IL-6), interferons (IFNs), and nitric oxide (NO) in chicken peripheral blood mononuclear cells (PBMC) and macrophages treated with CpG ODN (9). Although the immune response mediated via TLR has been fairly well studied in vitro, there are few studies regarding chickens’ response to CpG in vivo. Immunomodulation with the use of CpG ODN was shown in 1-day-old and adult birds (1, 18). However, its mechanism has not yet been well characterised in neonatal birds. Moreover, it is unknown how the response kinetics changes after in ovo administration of CpG in post-hatch chicks and how long these changes last.
Due to their immunostimulatory properties, TLR ligands are used prophylactically to protect against a variety of viral and bacterial pathogens in mammals (29, 32). In ovo delivery of CpG ODN induced protection against E. coli and Salmonella typhimurium in chickens after hatching (11, 38). In addition, CpG ODN application in one-day-old chicks significantly reduced mortality and degree of internal organs colonisation of birds experimentally infected with Salmonella (14, 37). Furthermore, it has been shown that TLR stimulation with their ligands inhibits the replication of certain viruses, e.g. avian influenza virus (AIV), Marek’s disease virus (MDV), and infectious bronchitis virus (IBV) (8, 30, 37). It has also been proven that in ovo use of CpG ODN stimulated the primary immune response against Salmonella enteritidis, and also reduced intestinal colonisation by these bacteria (25). However, the mechanism of immune modulation and CpG DNA activity in the antiviral response has not been fully explained.
The aim of the present study was to determine the impact of toll-like receptor TLR21 stimulation by in ovo administration of its synthetic ligand (CpG ODN, class B) on selected parameters (IL-6, IL-10, IFN-α, IFN-β, and IFN-γ) of the post-hatching chicken immune system. Since class B CpG-ODN can induce B-cell proliferation, the spleen was chosen as the target organ for analysis of cytokine expression in neonatal chickens.
Material and Methods
Animals
Specific-pathogen-free (SPF) embryonic eggs were purchased from Valo BioMedia (Germany) and hatched chicks were maintained in cages at biosafety level 2 animal facility with feed and water ad libitum.
TLR ligand
Synthetic class B CpG ODN (5’-TCGTCGTTGTCGTTTTGTCGTT-3’) with phosphorothioate backbone was purchased from TIB Molbiol (Germany). It was selected for the study because of its proven safety in ovo (11). The ligand was dissolved in sterile phosphate-buffered saline (PBS, pH 7.4) to its working concentrations.
Experimental design
The embryonated eggs were randomly allocated into three groups. On day 18, the chicken embryos were administered in ovo CpG ODN (directly into the yolk sac): 10 µg in 200 µL PBS/egg for group 1 and 50 μg CpG ODN in 200 μL PBS/egg for group 2. The third group of chicken eggs was administered 200 μL of PBS as above. Eggs from all groups were left to hatch. At 1, 2, 5, and 10 days post-hatching (dph), five chicks from each group were euthanised and the spleen was immediately collected for RNA isolation.
RNA extraction
The received organ samples were homogenised in PBS (10% w/v) with antibiotics (2,000 U/mL of penicillin and 2 mg/mL of streptomycin). Total RNA was isolated from the homogenised spleens using RNeasy Mini kit (Qiagen, Germany) according to the manufacturer’s protocol. Total RNA was eluted in 50 μL of RNase-free water and stored at –70°C until used. The concentration and quality of the obtained RNA was assessed by the ratio of the optical densities at 260 nm and 280 nm wavelengths using a NanoDrop 2000 spectrophotometer (Thermo Scientific, USA).
Quantitative RT-PCR
Expression of cytokine genes was assessed by real-time quantitative reverse transcription PCR (qRT-PCR) using a QuantiTect Probe RT-PCR Kit (Qiagen) with primers and probes previously described (10, 17, 22, 28, 29). Primers and probes were synthesised by Genomed (Poland) and their specific sequences are shown in Table 1. All PCR reactions were performed using the Applied Biosystems 7500 system (Applied Biosystems, USA) with the following cycle profile: one cycle of 50°C for 30 min and one cycle of 95°C for 15 min, followed by 95°C for 15 sec and 60°C for 60 sec for 40 cycles. The expression of the housekeeping gene (β-actin) was used for normalisation of data. The assays were performed in duplicate including no-template controls. The median and mean values were calculated and the expression levels of IL-6, IL-10, IFN-α, IFN-β, and IFN-γ were described as 2−ΔΔCt.
Table 1.
Primers and probes used in the study
| Target gene | Sequence (5’-3’) | Reference |
|---|---|---|
| IL-6 | F: GCTCGCCGGCTTCGA | Kaiser et al. (17) |
| R: GGTAGGTCTGAAAGGCGAACAG | ||
| Probe: AGGAGAAATGCCTGACGAAGCTCTCCA | ||
| IL-10 | F: CATGCTGCTGGGCCTGAA | Kumar et al. (22) |
| R: CGTCTCCTTGATCTGCTTGATG | ||
| Probe: CGACGATGCGGCGCTGTCA | ||
| IFN-α | F: GACAGCCAACGCCAAAGC | Eldaghayes et al. (10) |
| R: GTCGCTGCTGTCCAAGCATT | ||
| Probe: CTCAACCGGATCCACCGCTACACC | ||
| IFN-β | F: CCTCCAACACCTCTTCAACATG | Peroval et al. (28) |
| R: TGGCGTGCGGTCAAT | ||
| Probe: TTAGCAGCCCACACACTCCAAAACACTG | ||
| IFN-γ | F: GTGAAGAAGGTGAAAGATATCATGGA | Kaiser et al. (17) |
| R: GCTTTGCGCTGGATTCTCA | ||
| Probe: TGGCCAAGCTCCCGATGAACGA | ||
| β-actin | F: CATCCTCACCCTGAAGTACC | Vora et al. (39) |
| R: GCTCATTGTAGAAGGTGTGG | ||
| Probe: CACGGCATCGTCACCAACTG | ||
Statistical analysis
Statistical analysis of the obtained results was performed using Statistica 6.0. Arithmetic means, standard deviations (SD), and minimum and maximum values were calculated for the studied groups. Non-parametric tests were used to analyse the results. The Kruskal-Wallis test was used in order to compare the level of expression values (RQ) among all of the studied groups, while the Mann-Whitney U test compared two independent groups. Comparisons were considered significant at P ≤ 0.05.
Results
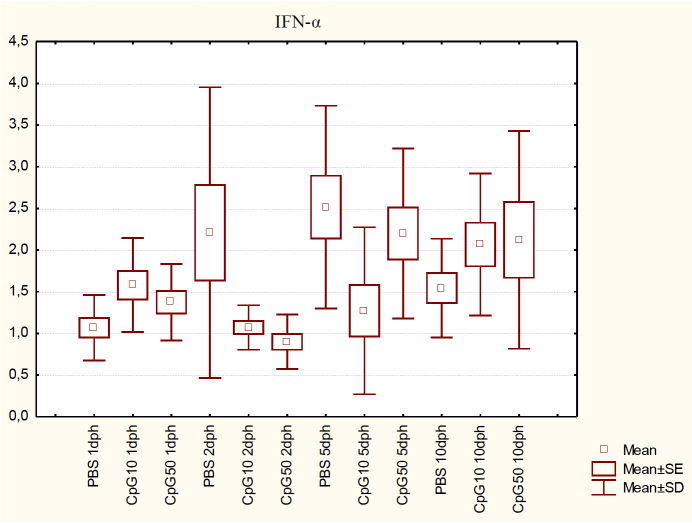
The kinetics of IFNα expression in the spleens of CpG10 and CpG50 ODN-administered birds compared to control birds are shown in Fig. 1. IFNα transcripts were up-regulated on 1 dph for both doses of ligand, but only the increase in the CpG10 group was statistically significant (P < 0.05). On 2 and 5 dph, transcript levels in both groups were down-regulated compared to the PBS-treated group, but only the low dose of ligand on 5 dph significantly inhibited IFNα expression (P < 0.05). In contrast, on 10 dph the obtained results did not differ significantly between the PBS control group and the CpG-stimulated groups (P > 0.05).
Fig. 1.
IFN-α expression in the spleen of chicks at 1, 2, 5, and 10 dph after in ovo CpG ODN treatment
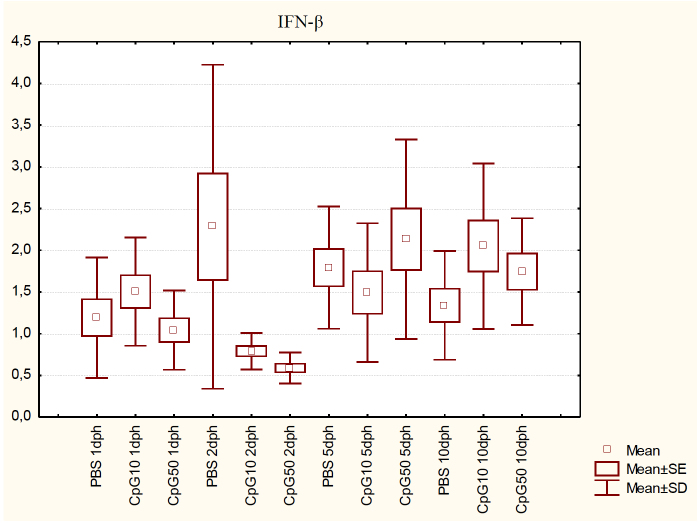
The IFN-β mRNA expression level after CpG administration was not affected significantly on 1 dph, whereas CpG treatment resulted in a significant (P < 0.05) decrease in IFN-β expression in the CpG50 group on 2 dph when compared with the control group. No significant differences between gene expression levels on 5 dph were noted, whereas on 10 dph IFN-β expression increased in both treated groups; however, only the lower dose of ligand resulted in a statistically significant difference (Fig. 2).
Fig. 2.
IFN-β expression in the spleen of chicks at 1, 2, 5, and 10 dph after in ovo CpG ODN treatment
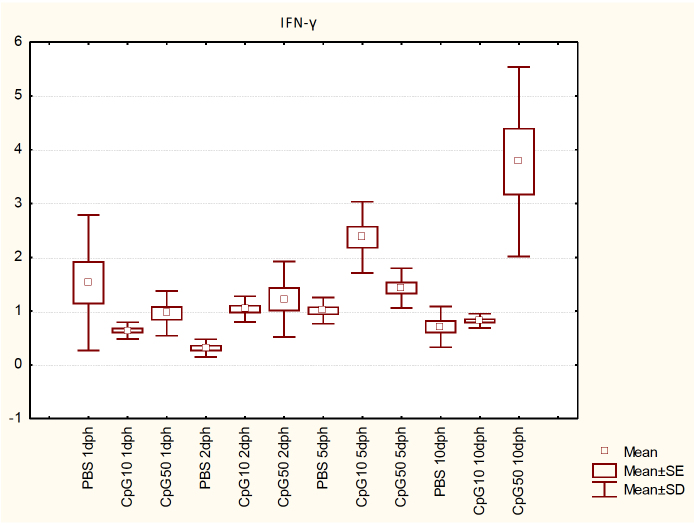
IFN-γ transcript levels in both CpG-stimulated groups were not statistically different from the PBS treated control group on 1 dph. On 2, 5, and 10 dph, IFN-γ expression was increased in CpG-treated chicks compared with the control group (P < 0.05). Both doses of ligands raised IFN-γ expression to a threefold higher level on 2 dph, while on 5 dph the lower dose influenced its production more strongly, resulting in an almost 2.5 times greater expression in comparison with the PBS-treated group (P < 0.05). The higher dose resulted in a nearly 1.5-fold increase (P < 0.05). The highest impact was noted on day 10 in the CpG50 group, where the expression increased more than 5-fold, whereas the increase in the CpG10 group was not significant (Fig. 3).
Fig. 3.
IFN-γ expression in the spleen of chicks at 1, 2, 5, and 10 dph after in ovo CpG ODN treatment
The transcriptional responses of relevant inflammatory interleukins (IL-6 and IL-10), which are suggestive of TLR stimulation, were also examined.
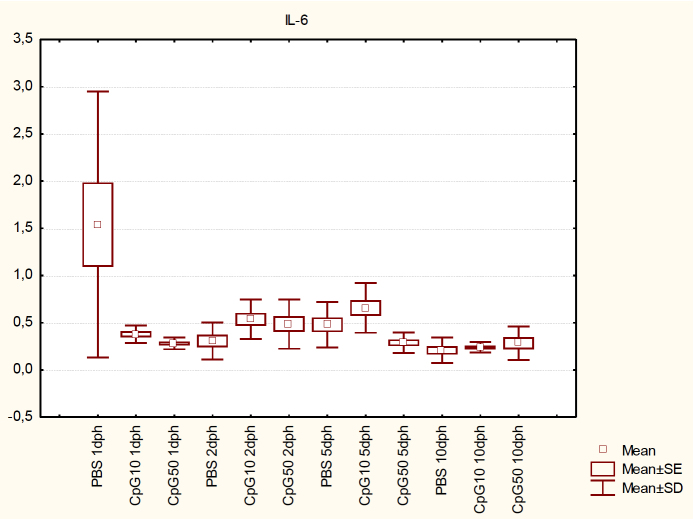
Both doses of CpG treatment significantly (P < 0.05) decreased IL-6 mRNA level on 1 dph. IL-6 expression in both groups was about 5 times lower than in the control group. On 2 dph a significant increase (P < 0.05) was found in the CpG10 group, while the higher dose of ligand did not lead to any significant differences. On day 5, in contrast, IL-6 expression decreased significantly after administration of the higher dose of CpG (P < 0.05). Finally, there were no differences between the treated groups and the control group regarding IL-6 expression on 10 dph (P < 0.05) (Fig. 4).
Fig. 4.
IL-6 expression in the spleen of chicks at 1, 2, 5, and 10 dph after in ovo CpG ODN treatment
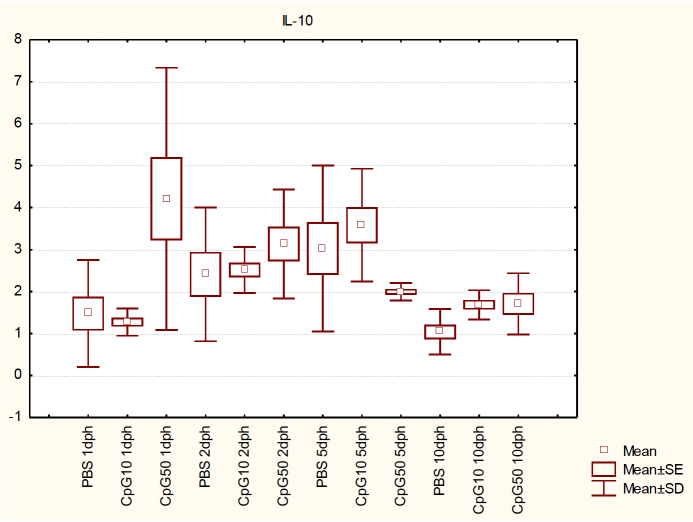
Lastly, IL-10 mRNA expression was significantly up-regulated by 3 times on 1 dph in the spleen from the high-dose-CpG–treated group (P < 0.05) compared to the PBS-treated group. Interestingly, the lower dose of CpG did not cause any significant differences (P > 0.05). On 2 and 5 dph, no differences between the CpG and control groups were observed. However, on 10 dph transcript levels in both CpG-treated groups increased significantly compared to the control group (P < 0.05). The results are presented in Fig. 5.
Fig. 5.
IL-10 expression in the spleen of chicks at 1, 2, 5, and 10 dph after in ovo CpG ODN treatment
Discussion
Knowledge of the molecular and functional aspects of TLRs is essential for a better understanding of the immune system and resistance to diseases in birds. In addition, it provides valuable information on the evolution of the immune system of vertebrates. TLR signalling in chickens has several unique properties, both in terms of specificity of TLR ligand complex formation and signalling pathway activation. Bearing in mind the current extremely rapid expansion of the poultry industry and simultaneous reduction in the use of medications, there is a great need to develop new strategies for prevention and control of diseases that affect profitability. This also includes the search for new drugs and immunomodulatory agents to provide alternatives to the ones currently in use.
In our study we have chosen the TLR21 receptor, as its stimulation with the suitable ligand can modulate the immune system of chickens through production of type I and II interferons (5).
Type I interferons (IFN-α and IFN-β) have anti-viral activity and increase MHC class I molecule expression. They are also able to activate both macrophages and natural killer (NK) cells. It is an essential element involved in protecting against multiple viral pathogens. They not only enhance the expression of the interferon-inducible genes, but also inhibit the transcription and translation of the virus and promote apoptosis in infected cells (30). Type II interferon (IFN-γ) is a pleiotropic cytokine that participates in most stages of the inflammatory and immune responses. Its antiviral activity is enhanced in combination with type I interferons (31). Type II IFNs associated with TH1 response also exhibit antiviral properties, partly through NK and CD8+ T cell activation. These cells show antiviral activity through targeted release of the cytotoxic granules towards infected cells, thereby promoting apoptosis. Therefore, CpG ODN, which induces a TH1 response in chickens, proved to be effective in preventing infection with various viral pathogens, including avian influenza and infectious bronchitis viruses (3, 8, 27). IL-6, as the proinflammatory cytokine, plays an important role in both the innate and adaptive immune systems and induces inflammatory responses during the disease. It promotes antibody production by B cells and switches monocyte differentiation from dendritic cells to macrophages (19). IL-10 is an anti-inflammatory cytokine with regulatory functions (16). It down-regulates activated dendritic cells and macrophages and, moreover, suppresses Th1 and Th2 responses (20).
Our data clearly demonstrate that immune response induction after CpG ODN in ovo administration in post-hatching chicken spleen has some similarities with the incidences observed and presented previously. CpG ODN treatment in mammals was shown to rapidly up-regulate IFN-α, IFN-γ, IL-1β, IL-6, IL-12, and IL-18 mRNAs (19, 21). Up-regulation of type I IFNs occurs during early stages of viral infection of the cells, as well as in plasmacytoid dendritic cells stimulated by TLR ligands (9). Class B CpG ODN, as used in our study, is a potent stimulator for B cells, and thus preferentially induces cytokines other than IFNs of type 1 (2). However, previous studies showed a slight increase in IFN-α mRNA expression in mammals and chickens after administration of class B CpG ODN (20, 21). Furthermore, this type of alterations in the expression of type I IFNs may affect the antiviral activity of IFN-γ. We also noted slight alterations of IFN-α and IFN-β mRNA. These changes were ambiguous (an initial increase, then a decrease and again an increase on 10 dph). These puzzling differences may arise from the kinetics of type I IFN expression on different days post-hatching in the control group or the different times when expression was measured. In any case, our results confirm the above-mentioned reports that class B CpG ODN rather weakly induces type I IFN.
Generally, a significant increase in IFN-γ mRNA expression at almost all time points for both doses of CpG ODN was observed. Such up-regulation of IFN-γ mRNA level corresponds with the previously reported expression of this cytokine in the spleen and bursal cells of one-day-old chicks treated with CpG ODN 2007 (27). Similarly, chicken PBMCs and macrophages stimulated with different CpG ODNs in vitro have shown a significant induction of IFN-γ mRNAs (12). These changes of IFN-γ in the spleen may result from the strong TLR-mediated B cell response. Interestingly, suggested by Sekellick et al. (31), a correlation may exist between a drastic increase in IFN-γ and changes in IFN-α and IFN-β expression on 10 dph.
IL-6 is a proinflammatory cytokine secreted by the immune cells after stimulation and pathogen infections (24). Again, as in the case of IFN-β, changes observed in IL-6 expression were ambiguous, being different for each dose. Our results are not consistent with previous publications. The absence of rapid induction of IL-6 mRNA expression in neonatal chickens in our study differs from the significant induction of this cytokine in chicken embryos and other animal species as described in the literature (20, 21). Furthermore, an increase in IL-6 mRNA expression was observed in in vitro studies in chicken splenocytes in response to CpG ODN (15). This suppression of IL-6 gene expression in our experiment on 1 dph may suggest an anti-inflammatory role of CpG ODN 2007. In the above mentioned studies, the expression of IL-6 was measured over periods from a few to a dozen hours after ligand administration. In our study, to check the length of changes in IL-6 expression, it was measured at long-term intervals in neonatal birds after in ovo CpG ODN application. In addition, these results may be the consequence of differences in constitutive expression of the TLR21 between the chickens used in the study. Our previous data (unpublished) showed that TLR21 expression in 3-week-old SPF chicks was significantly higher than in broilers. Therefore, TLR21 stimulation with its ligand can also have a different course and trigger different effects. Moreover, it was also observed that the expression of some of the immune response genes varied significantly between different chicken organs during viral infection (35). A similar mechanism could exist during TLR stimulation.
Previous studies suggest that in mammals, expression of IL-10 down-regulates CpG ODN-induced inflammation by its influence on the expression of other cytokines, e.g. IL-12 (40). We also observed an increase in IL-10 mRNA expression in response to CpG ODN in ovo administration. This is in accordance with previous reports that showed a significant increase in the expression of IL-10 mRNA in chickens at early time points after administration of CpG-ODN (36). Our results are also in agreement with previous in vitro studies that implied IL-10 production by B cells, dendritic cells, and thrombocytes in response to CpG ODN (23, 34). Looking at the cytokine profile and earlier reports it seems that IL-10 expression may undergo major changes a few hours post-administration of CpG ODN.
In conclusion, we have shown that in ovo administration of class B CpG ODN has the ability to induce cytokine responses in neonatal chicken spleen. This study is the first step in defining the antiviral role of CpG ODN administrated via in ovo route. In addition, we confirmed the safety of CpG ODN in ovo use to enhance neonatal immunity without inducing disease pathology. Its administration on embryonic day 18 had some impact for at least 10 days after hatching. However, further studies should be performed to characterise the expression of other immune response genes. There is a need to correlate the significant changes in cytokine expression observed in our study with any real protection of chickens after the challenge. It is important to examine the effect of combined administration of CpG ODN and other TLR ligands, as it was reported that simultaneous chicken monocyte treatment with CpG ODN and poly I:C (TLR3 ligand) caused a synergistic effect in up-regulation of IFN-γ and IL-10 in comparison with the treatment using individual ligands (13).
Footnotes
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: This work was funded by the KNOW (Leading National Research Centre) Scientific Consortium "Healthy Animal - Safe Food", the Polish Ministry of Science and Higher Education resolution No. 05-1/KNOW2/2015.
Animal Rights Statement: All procedures requiring the use of embryonated eggs and hatched chicks were approved by the Second Local Ethics Committee of Animal Experimentation in Lublin (University of Life Sciences in Lublin, Poland) in accordance with the European and national regulations on animal experiments and welfare.
References
- 1.Albigier B., Dahlberg S., Henriques-Normark B., Normark S.. Role of the innate immune system in host defence against bacterial infections: focus on the Toll-like receptors. J Intern Med. 2007;261:511–528. doi: 10.1111/j.1365-2796.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 2.Asselin-Paturel C., Trinchieri G.. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202:461–465. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barjesteh N., Shojadoost B., Brisbin J.T., Emam M., Hodgins D.C., Nagy É., Sharif S.. Reduction of avian influenza virus shedding by administration of Toll-like receptor ligands to chickens. Vaccine. 2015;33:4843–4849. doi: 10.1016/j.vaccine.2015.07.070. [DOI] [PubMed] [Google Scholar]
- 4.Brownlie R., Allan B.. Avian toll-like receptors. Cell Tissue Res. 2011;343:121–130. doi: 10.1007/s00441-010-1026-0. [DOI] [PubMed] [Google Scholar]
- 5.Callison S.A., Hilt D.A., Boynton T.O., Sample B.F., Robison R., Swayne D.E., Jackwood M.W.. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J Virol Methods. 2006;138:60–65. doi: 10.1016/j.jviromet.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavanagh D., Gelb J.J. Saif Y.M. Diseases of poultry. Blackwell Publishing; Ames, Iowa: 2008. Infectious bronchitis; pp. 117–136. [Google Scholar]
- 7.Chrząstek K., Wieliczko A.. Characterization of oligodeoxy-nucleotides containing CpG motifs, their effects on immune cells, and their potential for use in human and veterinary medicine. Med Weter. 2010;66:242–245. [Google Scholar]
- 8.Dar A., Potter A., Tikoo S., Gerdts V., Lai K., Babiuk L.A., Mutwiri G.. CpG oligodeoxynucleotides activate innate immune response that suppresses infectious bronchitis virus replication in chicken embryos. Avian Dis. 2009;53:261–267. doi: 10.1637/8560-121808-Reg.1. [DOI] [PubMed] [Google Scholar]
- 9.Doly J., Civas A., Navarro S., Uze G.. Type I interferons: expression and signalization. Cell Mol Life Sci. 1998;54:1109–1121. doi: 10.1007/s000180050240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eldaghayes I., Rothwell L., Williams A., Withers D., Balu S., Davison F., Kaiser P.. Infectious bursal disease virus: strains that differ in virulence differentially modulate the innate immune response to infection in the chicken bursa. Viral Immunol. 2006;19:83–91. doi: 10.1089/vim.2006.19.83. [DOI] [PubMed] [Google Scholar]
- 11.Gomis S., Babiuk L., Allan B., Willson P., Waters E., Ambrose N., Hecker R., Potter A.. Protection of neonatal chicks against a lethal challenge of Escherichia coli using DNA containing cytosine-phosphodiesterguanine motifs. Avian Dis. 2004;48:813–822. doi: 10.1637/7194-041204R. [DOI] [PubMed] [Google Scholar]
- 12.He H., Crippen T.L., Farnell M.B., Kogut M.H.. Identification of CpG oligodeoxynucleotide motifs that stimulate nitric oxide and cytokine production in avian macrophage and peripheral blood mononuclear cells. Dev Comp Immunol. 2003;27:621–627. doi: 10.1016/s0145-305x(03)00013-2. [DOI] [PubMed] [Google Scholar]
- 13.He H., Genovese K.J., Swaggerty C.L., Mackinnon K.M., Kogut M.H.. Co-stimulation with TLR3 and TLR21 ligands synergistically up-regulates Th1-cytokine IFN-γ and regulatory cytokine IL-10 expression in chicken monocytes. Dev Comp Immunol. 2012;36:756–760. doi: 10.1016/j.dci.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 14.He H., Genovese K.J., Swaggerty C.L., Nisbet D.J., Kogut M.H.. In vivo priming heterophil innate immune functions and increasing resistance to Salmonella Enteritidis infection in neonatal chickens by immune stimulatory CpG oligodeoxynucleotides. Vet Immunol Immunopathol. 2007;117:275–283. doi: 10.1016/j.vetimm.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins K., Lowenthal J.W., Kimpton W., Bean G.D.. The in vitro and in ovo responses of chickens to TLR9 subfamily ligands. Dev Comp Immunol. 2009;33:660–667. doi: 10.1016/j.dci.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser P.. The long view: a bright past, a brighter future? Forty years of chicken immunology pre- and post-genome. Avian Pathol. 2012;41:511–518. doi: 10.1080/03079457.2012.735359. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser P., Rothwell L., Galyov E.E., Barrow P.A., Burnside J., Wigley P.. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis, and Salmonella gallinarum. Microbiology. 2000;146:3217–3226. doi: 10.1099/00221287-146-12-3217. [DOI] [PubMed] [Google Scholar]
- 18.Keestra A.M., de Zoete M.R., Bouwman L.I., van Putten J.P.M.. Chicken TLR21 is an innate CpG DNA receptor distinct from mammalian TLR9. J Immunol. 2010;185:460–467. doi: 10.4049/jimmunol.0901921. [DOI] [PubMed] [Google Scholar]
- 19.Klinman D.M., Yi A.K., Beaucage S.L., Conover J., Krieg A.M.. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci U.S.A. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieg A.M., Efler S.M., Wittpoth M., Al Adhami M.J., Davis H.L.. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J Immunother. 2004;27:460–471. doi: 10.1097/00002371-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Krieg A.M.. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S., Buza J.J., Burgess S.C.. Genotype-dependent tumor regression in Marek’s disease mediated at the level of tumor immunity. Cancer Microenviron. 2009;2:23–31. doi: 10.1007/s12307-008-0018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenert P., Brummel R., Field E.H., Ashman R.F.. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol. 2005;25:29–40. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- 24.Li C.K., Seth R., Gray T., Bayston R., Mahida Y.R., Wakelin D.. Production of proinflammatory cytokines and inflammatory mediators in human intestinal epithelial cells after invasion by Trichinella spiralis. Infect Immun. 1998;66:2200–2206. doi: 10.1128/iai.66.5.2200-2206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackinnon K., He H., Swaggerty C., McReynolds J., Genovese K., Duke S., Nerren J., Kogut M.. In ovo treatment with CpG oligodeoxynucleotides decreases colonization of Salmonella Enteriditis in broiler chickens. Vet Immunol Immunopathol. 2009;127:371–375. doi: 10.1016/j.vetimm.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Medzhitov R.. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 27.Patel B.A., Gomis S., Dar A., Willson P.J., Babiuk L.A., Potter A., Mutwiri G., Tikoo S.K.. Oligodeoxynucleotides containing CpG motifs (CpG-ODN) predominantly induce Th1-type immune response in neonatal chicks. Dev Comp Immunol. 2008;32:1041–1049. doi: 10.1016/j.dci.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Peroval M.Y., Boyd A.C., Young J.R., Smith A.L.. A critical role for MAPK signalling pathways in the transcriptional regulation of Toll like receptors. PLoS ONE 2013. 8:e51243. doi: 10.1371/journal.pone.0051243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulendran B., Kumar P., Cutler C.W., Mohamadzadeh M., Van Dyke T., Banchereau J.. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167:5067–5076. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samuel C.E.. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sekellick M.J., Lowenthal J.W., O'Neil T.E., Marcus P.I.. Chicken interferon types I and II enhance synergistically the antiviral state and nitric oxide secretion. J Interferon Cytokine Res. 1998;18:407–414. doi: 10.1089/jir.1998.18.407. [DOI] [PubMed] [Google Scholar]
- 32.Shinya K., Ito M., Makino A., Tanaka M., Miyake K., Eisfeld A.J., Kawaoka Y.. The TLR4-TRIF pathway protects against H5N1 influenza virus infection. J Virol. 2012;86:19–24. doi: 10.1128/JVI.06168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St Paul M., Brisbin J.T., Abdul-Careem M.F., Sharif S.. Immunostimulatory properties of Toll-like receptor ligands in chickens. Vet Immunol Immunopathol. 2013;152:191–199. doi: 10.1016/j.vetimm.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 34.St Paul M., Mallick A.I., Haq K., Orouji S., Abdul-Careem M.F., Sharif S.. In vivo administration of ligands for chicken toll-like receptors 4 and 21 induces the expression of immune system genes in the spleen. Vet Immunol Immunopathol. 2011;144:228–237. doi: 10.1016/j.vetimm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 35.St Paul M., Mallick A.I., Read L.R., Villanueva A.I., Parvizi P., Abdul-Careem M.F., Nagy E., Sharif S.. Prophylactic treatment with Toll-like receptor ligands enhances host immunity to avian influenza virus in chickens. Vaccine. 2012;30:4524–4531. doi: 10.1016/j.vaccine.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 36.St Paul M., Paolucci S., Barjesteh N., Wood R.D., Schat K.A., Sharif S.. Characterization of chicken thrombocyte responses to Toll-like receptor ligands. PLoS One 2012. 7:e43381. doi: 10.1371/journal.pone.0043381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szczubełek P., Szeleszczuk P.. Synthetic oligodeoxynucleotides consisting of unmethylated cytosine-guanosine dinucleotides as immunostimulators for poultry. Med Weter. 2009;65:823–826. [Google Scholar]
- 38.Taghavi A., Allan B., Mutwiri G., Van Kessel A., Willson P., Babiuk L., Potter A., Gomis S.. Protection of neonatal broiler chicks against Salmonella Typhimurium septicemia by DNA containing CpG motifs. Avian Dis. 2008;52:398–406. doi: 10.1637/8196-121907-Reg. [DOI] [PubMed] [Google Scholar]
- 39.Vora P., Youdim A., Thomas L.S., Fukata M., Tesfay S.Y., Lukasek K., Michelsen K.S., Wada A., Hirayama T., Arditi M., Abreu M.T.. Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol. 2004;173:5398–5405. doi: 10.4049/jimmunol.173.9.5398. [DOI] [PubMed] [Google Scholar]
- 40.Yi A.K., Yoon J.G., Yeo S.J., Hong S.C., English B.K., Krieg A.M.. Role of mitogen-activated protein kinases in CpG DNA-mediated IL-10 and IL-12 production: central role of extracellular signalregulated kinase in the negative feedback loop of the CpG DNA-mediated Th1 response. J Immunol. 2002;168:4711–4720. doi: 10.4049/jimmunol.168.9.4711. [DOI] [PubMed] [Google Scholar]