Abstract
Background
Myosin phosphatase target subunit 1 (MYPT1) serves as a subgroup of myosin phosphatases, and is frequently low-expressed in human cancers. However, little is known about the effects of MYPT1 in gastric cancer (GC).
Material/Methods
In our study, MYPT1 expression was detected by quantitative real-time reverse transcription PCR (qRT-PCR) in GC tissues, different advanced pathological stages of GC tissues, and preoperative and postoperative patients. Kaplan-Meier analysis was used to measure the overall survival of GC patients. MYPT1 expression was analyzed by qRT-PCR and Western blot assays in GES-1 cells and GC cells. Cell proliferation, cycle, and migration and invasion abilities were detected by CCK-8, flow cytometry, and Transwell assays. E-cadherin, TIMP-2, MMP-2, MMP-9 RhoA, and p-RhoA expressions were assessed by qRT-PCR and Western blot assays in treated SNU-5 cells.
Results
Our results indicated that MYPT1 was down-regulated in GC tissues and cells, and is related to clinical stages and overall survival of GC. Functional research demonstrated that overexpression of MYPT1 can inhibit cell proliferation, cell cycle progression, and migration and invasion of GC cells. Many studies on mechanisms reported that overexpression of MYPT1 dramatically improved the expression levels of cell cycle-related genes (Cyclin D1 and c-myc), significantly increased epithelial marker (E-cadherin) expression, and decreased invasion-associated genes (TIMP-2 and MMP-2) expressions in SNU-5 cells. In addition, we found that MYPT1 suppressed RhoA phosphorylation.
Conclusions
We verified that MYPT1 inhibits GC cell proliferation and metastasis by regulating RhoA phosphorylation.
MeSH Keywords: Myosin-Light-Chain Phosphatase, rhoA GTP-Binding Protein, Stomach Neoplasms, Transcellular Cell Migration
Background
Gastric cancer (GC) is one of the most common malignant tumors, with the third highest mortality rate of cancer in the world [1,2]. In China, the morbidity and mortality of GC ranks high on the list of malignant tumors, and there are more than 400 000 new-onset cases each year [3,4]. At present, diagnosing and treating GC are becoming quite advanced [5–7]. However, there are no symptoms, or only mild symptoms, in the early stages of GC [8,9]. Patients lacking early diagnostic biomarkers are often diagnosed late and cannot be treated at the optimal time [9]. The main treatments for GC patients are surgical resection and chemoradiation [10,11]. However, the 5-year survival rate of GC is only 40% because of frequent recurrence [12,13]. At present, the lack of diagnostic biomarkers, prognostic indicators, and effective therapeutic targets seriously limits effective treatment of GC. Therefore, it is important for the mechanism researches of the development of GC. Recently, accumulating evidence has shown that the alteration of MYPT1 expression is involved in tumorigenesis [14–16].
The myosin phosphatase-targeting protein (MYPT) family mainly includes MYPT1, MYPT2, MYPT3, MBS85, and TIMAP, which serve as targeted and regulated subunits and can confirm the substrate-specificity of the type 1 phosphatase subunit (PP1cδ) [17]. MYPT1, MYPT2, and MBS85 genes contain c-terminal leucine zipper domain, which can regulate the depolymerization and the protein interaction [18]. The MYPT family also can be regulated by phosphorylation activated by different protein kinases. For example, MYPT1, MYPT2, and MBS85 phosphorylated by Rho kinase finally can inhibit phosphatase activity in the process of smooth muscle contraction and Ca2+ sensitivity [19].
MYPT1 is mainly involved in the RhoA/ROCK signaling pathway, and RhoA/ROCK kinase can regulate MYPT1, MYPT2, and MBS85 expression through phosphorylation, while the phosphorylation sites of MYPT1 induced by ROCK are mainly Thr696 and Thr853 [20,21]. The roles and mechanisms of MYPT1 in smooth muscle cell contraction or non-myocyte movement are: ROCK1/2 and ZIPK inhibit MYPT1 activity through promoting the phosphorylation of the T696 and T853 sites in MYPT1; CPI-17 and PH-1 can directly inhibit MYPT1, release Ca2+, activate myosin through myosin light chain kinase (MLCK), and then induce smooth muscle cell contraction or non-smooth muscle cell migration [22–24]. In addition, PKA and PKG can promote phosphorylation of Ser668, Ser692, Ser695, and Ser852 sites in MYPT1 to activate its activity. Telokin can directly activate MYPT1, MYPT1 activation can inhibit myosin light streptokinase, and then relax smooth muscle cells [25,26].
The MYPT family play important roles in the development of diseases such as cancer, hypertension, and Parkinson’s disease [27–29]. Studies have indicated that MYPT1 plays vital roles in the development and progression of cancers, such as cell cycle, migration, and invasion [16,30–33]. The compounds of MYPT1 and protein phosphatase 1 (PP1) can dephosphorylate the receptor interacting protein, and inhibit the activation of proteins [34,35]. MYPT1 can reduce the vasodilatation reaction mediated by nitrogen oxide through changing the generation of MYPT1 LZ+ [36]. MYPT1 has many different binding sites and subcellular interactions, and resistance or activation of MYPT1 expression may be a therapeutic target for tumors [37]. In this study, we demonstrated the expression level of MYPT1 in GC tissues and cells, and the relationship with clinical stages and overall survival of GC. We also verified the roles of MYPT1 in GC cell proliferation, cell cycle progression, migration, and invasion. Furthermore, we proved the mechanisms of MYPT1 in GC, such as the regulation of MYPT1 on cell cycle-related genes (Cyclin D1 and c-myc) expressions, the regulation of MYPT1 on invasion-associated genes (E-cadherin, TIMP-2, MMP-2, and MMP-9), and the regulation of MYPT1 on RhoA phosphorylation. We found that MYPT1 may be a novel therapeutic target for the treatment of GC.
Material and Methods
Clinical samples
Gastric cancer tissue samples and adjacent normal tissues (5 cm away from the tumor) from 68 GC patients were collected from Tongde Hospital of Zhejiang Province from February 2011 to June 2016. Informed consent was obtained from all GC patients, and ethics approval was granted by the Ethics Committee of Tongde Hospital of Zhejiang Province. All tissues were immediately put in liquid nitrogen after removal from GC patients, and stored at −80°C until used.
Peripheral blood from 43 GC patients was extracted before and 14 d after surgery. We collected 5 ml of blood and allowed it to clot at room temperature for 30 min to 2 h. Serum was isolated from all blood samples using a two-step centrifugation protocol (2000 g for 10 min at 4°C, 12 000 g for 10 min at 4°C). After separation, serum samples were added into RNase DNase-free tubes and stored at −80°C.
Cell culture
Gastric epithelial cell (GES-1) and GC cell lines (SNU-5, MKN-45, BGC-823, SGC-7901, AGS, and HGC-27) were purchased from the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). All cells were inoculated into a sterile culture bottle, and incubated in RPMI-1640 medium (HyClone, Cat. No. SH30027) with 10% fetal bovine serum (FBS, Cat. 10082-147, Gibco/Life Technologies, Norwalk, CT), 100 U/mL penicillin and 100 U/mL streptomycin (Invitrogen, Carlsbad, CA, Cat. No. 15140-12) at 37°C and 5% CO2 saturation. The culture medium was replaced every 2–3 d. Cells were digested with 0.25% trypsin ion when cell fusion reached to 80%–90%, and were inoculated to culture bottles (25 cm2) with 5×105 cells.
Plasmid construct and transfection
To construct the pcDNA3.1-MYPT1 expression vector, the complete sequence of human MYPT1 gene was synthesized and inserted into a pcDNA3.1 (+) vector (GenePharma, Shanghai, China). SNU-5 cells (5×105 cells) at the logarithmic phase were inoculated in 6-well plates and incubated for 12 h. Then, cells were transfected with pcDNA3.1 and pcDNA3.1-MYPT1 plasmid using Lipofectamine 2000 reagent (Invitrogen, cat. no. 11668-019) and Opti-MEM reduced serum medium (Life Technologies), according to the manufacturer’s instructions, when the cell fusion rate reached to 80%.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from the treated SNU-5 cells by using TRIzol reagent (Invitrogen).
The RNA concentration was quantitated by using the OD value. Then total RNA (1 μg) was used to synthesize complementary DNA (cDNA) using the high-capacity cDNA reverse transcription Kit (cat. no. 4368814) in a total volume of 20 μl according to manufacturer’s instructions. RT-qPCR reactions were performed using SYBR Green (Applied Biosystems, cat #4368577) in a total volume of 20 μl according to the manufacturer’s instructions. The reaction process was as follows: 95°C for 10 min, 95°C for 15 s for 40 cycles, and 60°C for 60 s. The results were measured on a ABI 7500 real-time PCR instrument (Applied Biosystems, Carlsbad, CA). The relative mRNA expression levels were analyzed using 2−ΔΔCt method [38]. The primers were as follows:
MYPT1: 5′-GAGCCTCCGGTGGTGAAG-3′ (forward),
5′-GGCAGTGAGTCCGTCCAC-3′ (reverse);
Cyclin D1: 5′-CTTCCTGTCCTACTACCGCC-3′ (forward),
5′-CTCCTCCTCTTCCTCCTCCT-3′ (reverse);
c-myc: 5′-AGCATACATCCTGTCCGTCC-3′ (forward), 5′-CAAGAGTTCCGTAGCTGTTCA-3′ (reverse);
E-cadherin: 5′-ACT GAT TTT CCC ACG GAC CT-3′ (forward),
5′-CTC CTC GCT TTC CAT GTG TG-3′ (reverse);
TIMP-2: 5′-TTCAAAGGGCCTGAGAAGGA-3′ (forward),
5′-TCAGGCTCTTCTTCTGGGTG-3′ (reverse);
MMP-2: 5′-GATACCCCTTTGACGGTAAGGA-3′ (forward),
5′-CCTTCTCCCAAGGTCCATAGC-3′ (reverse);
MMP-9: 5′-TTCAGGGAGACGCCCATTTC-3′ (forward), 5′-AAACCGAGTTGGAACCACGA-3′ (reverse);
GAPDH: 5′-GCC ATC ACA GCA ACA CAG AA-3′ (forward),
5′-GCC ATA CCA GTA AGC TTG CC-3′ (reverse).
Western blot assay
Total protein was extracted from the treated SNU-5 cells using RIPA lysis buffer (100–200 μL lysate, cat# 89901; Thermo Scientific, Rockford, IL, USA). Protein was quantified using a BCA protein quantification kit (23225, Pierce, Rockford, IL, USA). Then, 30 μg protein was separated using 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE, 150V, 90 min), and transferred onto a polyvinylidene difluoride (PVDF, Millipore, Billerica, MA) membrane (100 V, 90 min). The PVDF membrane was blocked with 5% non-fat dried milk in TBST solution for 1 h at room temperature, and incubated with primary antibodies at 4°C overnight. The next day, the PVDF membrane was washed 3 times with TBST solution (10 min/time), and incubated with secondary antibody (cat. number LK2003L, Sungene Biotech Co., Ltd, China) for 1 h at room temperature. The protein was detected using an enhanced chemiluminescence (ECL) substrate kit (Thermo scientific Pierce) and ImageJ software (NIH, Bethesda, Maryland, USA). The gray values of objective proteins and internal reference were analyzed by Quantity One V4.6.2 software (Bio-Rad, USA). The primary antibodies were anti-GAPDH antibody (Dilution 1: 2000; Abcam, ab8245), anti-Bax antibody (Dilution 1: 1000; Abcam, ab32503), anti-MYPT1 antibody (Dilution 1: 1000; BD Biosciences, Cat 612164), anti-Cyclin D1 antibody (Dilution 1: 1000; Cell Signaling Technology, cat. 2922), anti-c-myc antibody (Dilution 1: 1500; Abcam, ab32072), anti-E-cadherin antibody (Dilution 1: 1000; Cell Signaling Technology, Cat# 3195S), anti-TIMP-2 antibody (Dilution 1: 1000; Abcam, cat. no. ab180630), anti-MMP-2 antibody (Dilution 1: 1000; Cat. No. PF023, Millipore, Darmstadt, Germany), anti-MMP-9 antibody (Dilution 1: 50; Santa Cruz, cat # sc-21733), anti-p-RhoA antibody (Dilution 1: 1000; Abcam, ab41435), anti-RhoA antibody (Dilution 1: 2000; Abcam, cat. no. ab187027).
Proliferation assay
SNU-5 cells (5×104 cells/mL) in the logarithmic phase were seeded into 96-well plates in 200 μL of medium and transfected with pcDNA3.1 and pcDNA3.1-MYPT1 for 48 h at 37°C with 5% CO2. To detect cell proliferation, 10 μL of Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) was added to each well. After 3 h, the absorbance was detected at 450 nm using a Thermo Multiskan Ex plate reader (Thermo Fisher, United Kingdom).
Flow cytometric analysis
The SNU-5 cells (6×107 cells) at the logarithmic phase were seeded into 6-well plates and were transfected with pcDNA3.1 and pcDNA3.1-MYPT1 for 48 h in an incubator (37°C, 5% CO2). Cells were collected and treated with pre-cooled 70% ethanol (1 ml), and then the treated cells were treated with 500 μL phosphate-buffered saline (PBS, cat#AM9625) containing propidium iodide (PI. Cat. P4170, Sigma-Aldrich, USA, 50 μg/mL), RNase A (100 μg/mL, Cat# 109169), and 0.2%Triton X-100 (Sigma-Aldrich, cat. no. T8787) in the dark for 30 min. Finally, the results were obtained by using a flow cytometer, and cell cycle analysis was performed by FlowJo software (Tree Star, Ashland, OR).
Transwell assay
Cell migration and invasion were confirmed by detecting the ability of cells to move to another place. The cells at the logarithmic phase were digested, washed with PBS twice, and suspended in RPMI-1640 medium without FBS, and the concentration was adjusted to 2×105 cells/ml. Cells suspensions (100 μL) were added to the polycarbonate membrane of the upper chamber with (for the invasion assay) or without (for the migration assay) Matrigel (BD Bioscience, San Diego, CA).
The bottom chamber was filled with complete medium (500 μL). The cells were incubated at 37°C for 24 h. Cells on the bottom of the coated Transwell chamber were washed twice, fixed with 4% paraformaldehyde (Cat#P6148) for 30 min, and stained with 0.1% crystal violet (no. C3886-100G0; Sigma-Aldrich, St. Louis, MO) for 15 min at room temperature. After drying, the number of migrated or invaded cells was analyzed from 5 randomly selected fields under a microscope at a magnification of ×100.
Statistical analysis
All the experimental results are presented as mean ±SD of at least 3 independent experiments. The data were analyzed by using IBM SPSS Version 20 with the t test. Statistical significance was defined as P<0.05.
Results
MYPT1 was down-regulated in GC
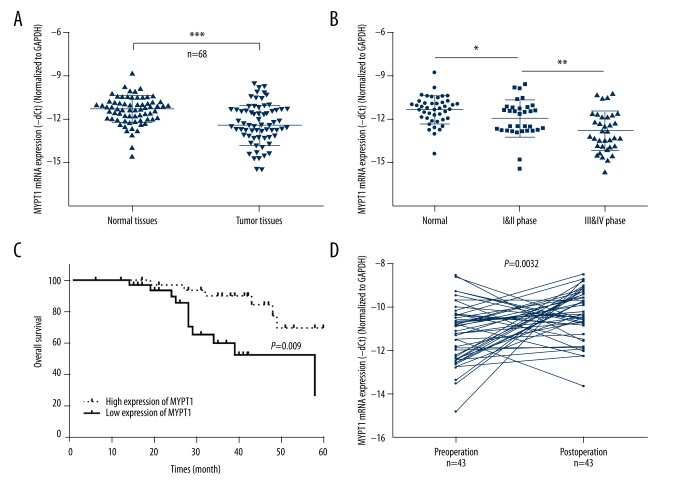
To explore MYPT1 expression levels in GC, we used qRT-PCR assay to measure its expression in 68 pairs of GC (tumor tissues) and adjacent non-cancerous tissues (normal tissues). The results indicated that the mRNA expression level of MYPT1 was significantly decreased in GC tissues compared with normal tissues (P<0.001, Figure 1A). Furthermore, we found that MYPT1 expression was significantly decreased in phase I and II (N1 and N2, n=33) compared with normal tissues (N0, n=43) (P<0.05); and was significantly down-regulated in phase III and IV (N3 and N4, n=35) compared with phase I and II (N1 and N2, n=33) (P<0.01, Figure 1B).
Figure 1.
Relative MYPT1 expression in GC and the relationship with overall survival of GC. (A) The mRNA expression level of MYPT1 was detected by qRT-PCR assay in GC tissues and corresponding non-tumor tissues (n=68, *** P<0.001). (B) MYPT1 expression was measured by qRT-PCR assay in different advanced pathological stages normal (N0, n=43), I & II phase (N1 and N2, n=33), III and IV phase (N3 and N4, n=35), * P<0.05, ** P<0.01. (C) Based on MYPT1 expression in GC tissues, the overall survival of GC patients was calculated by Kaplan-Meier analysis (P=0.009). (D) MYPT1 expression was analyzed by qRT-PCR assay in preoperative and postoperative patient serum (n=43, P=0.0032).
MYPT1 expression was related to overall survival of GC
To further explore the correlation between MYPT1 expression and GC survival, Kaplan-Meier analysis was used to assess the overall survival of GC patients according to MYPT1 expression. The results revealed that GC patients with high MYPT1 expression had a dramatically longer survival time compared with those with low MYPT1 expression (P=0.009, Figure 1C). In addition, we found that MYPT1 expression was higher in postoperative patients than in preoperative patients (P=0.0032, Figure 1D). Therefore, MYPT1 expression was associated with survival and prognosis.
Overexpression of MYPT1 inhibits GC cell proliferation
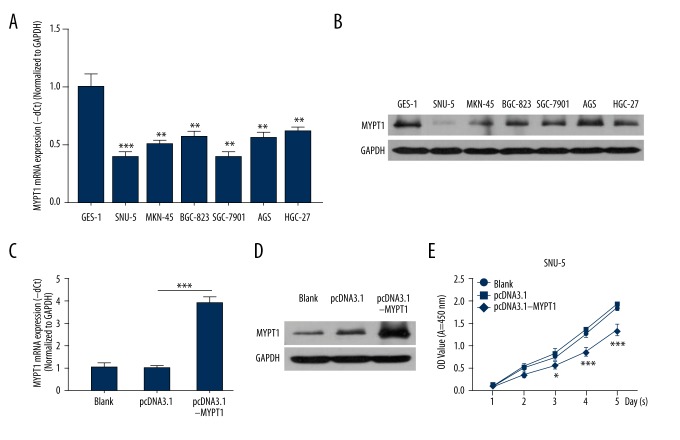
According to the above observations, we then demonstrated the function and mechanism of MYPT1 in GC. Firstly, MYPT1 expression was detected in gastric epithelial cell (GES-1) and GC cell lines (SNU-5, MKN-45, BGC-823, SGC-7901, AGS, and HGC-27). The results revealed that MYPT1 expression was markedly inhibited in 6 GC cell lines compared with the GES-1 cells (P<0.01, P<0.001, Figure 2A, 2B). We also found that MYPT1 expression was lower in SNU-5 cells than in other GC cell lines, and we chose to use SNU-5 cells in subsequent experiments. SNU-5 cells were treated with PBS (Blank), pcDNA3.1, and pcDNA3.1-MYPT1 for 48 h, respectively. MYPT1 expression was analyzed in treated SNU-5 cells, and the results showed that MYPT1 was highly expressed in the pcDNA3.1-MYPT1 group compared with the pcDNA3.1 group (P<0.001, Figure 2C, 2D). In addition, we proved that overexpression of MYPT1 significantly inhibited the proliferation ability of SNU-5 cells (P<0.05, P<0.001, Figure 2E).
Figure 2.
Overexpression of MYPT1 inhibits GC cell proliferation. MYPT1 expression was analyzed by qRT-PCR (A) and Western blot (B) assays in gastric epithelial cell (GES-1) and GC cell lines (SNU-5, MKN-45, BGC-823, SGC-7901, AGS, and HGC-27), ** P<0.01, *** P<0.001 vs. GES-1 group. (C) SNU-5 cells were treated with PBS (Blank), pcDNA3.1, pcDNA3.1-MYPT1 for 48 h, respectively. MYPT1 mRNA expression level was evaluated by qRT-PCR assay (*** P<0.001). (D) MYPT1 protein expression level was measured by Western blot assay in treated SNU-5 cells. (E) CCK-8 assay was performed to detect SNU-5 cell proliferation (* P<0.05, *** P<0.001).
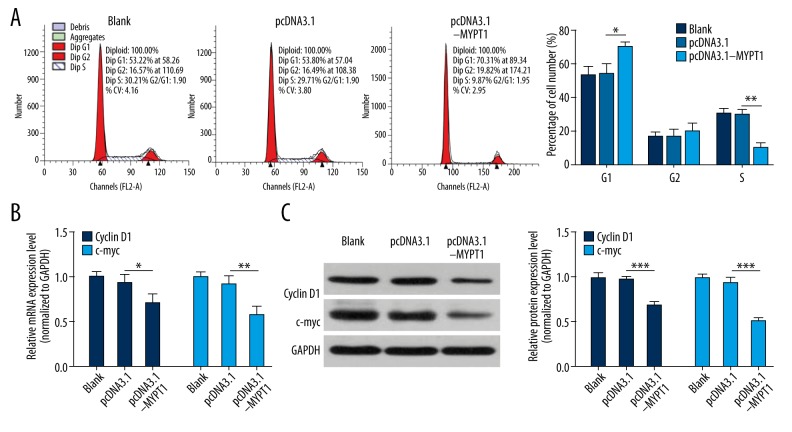
Overexpression of MYPT1 induces SNU-5 cell cycle arrest in G1 phase
Cell cycle is connected with cell proliferation. Therefore, we further assessed the cell cycle distributions using flow cytometry. SNU-5 cells transfected with pcDNA3.1-MYPT1 showed significant G1 arrest and S phase reduction compared with the pcDNA3.1 group (P<0.05, P<0.01, Figure 3A). In addition, we analyzed cell cycle-related genes (Cyclin D1 and c-myc) expressions using qRT-PCR and Western blot assays, and found that overexpression of MYPT1 dramatically improved the expression levels of Cyclin D1 and c-myc in SNU-5 cells (P<0.05, P<0.01, P<0.001, Figure 3B, 3C).
Figure 3.
Overexpression of MYPT1 induces SNU-5 cell cycle arrest in G1 phase. (A) The cell cycle was analyzed flow cytometry in treated SNU-5 cells, and the values of G1, S, and G2 were shown in the bar graphs (* P<0.05, ** P<0.01). (B) qRT-PCR and (C) Western blot assays were performed to analyze the mRNA and protein expression levels of Cyclin D1 and c-myc in treated SNU-5 cells (* P<0.05, ** P<0.01, *** P<0.001).
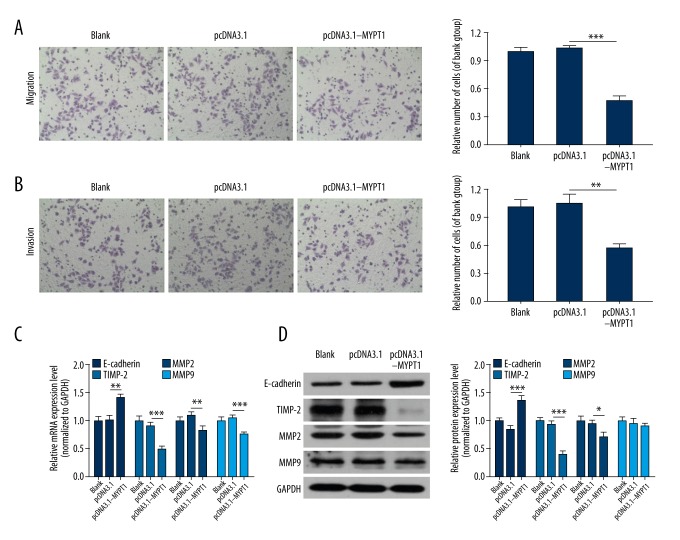
Overexpression of MYPT1 inhibits SNU-5 cell migration and invasion
Because we found that overexpression of MYPT1 inhibited GC cell proliferation and induced GC cell cycle arrest, we further assessed the effect of MYPT1 on migration and invasion, and the data showed that overexpression of MYPT1 markedly inhibited the migration and invasion capacities of SNU-5 cells (P<0.01, P<0.001, Figure 4A, 4B). We also analyzed the influences of MYPT1 on metastasis-associated genes (E-cadherin, TIMP-2, MMP-2, and MMP-9) expressions. As shown in Figure 4C and 4D, overexpression of MYPT1 remarkably increased E-cadherin expression and decreased TIMP-2 and MMP-2 expressions (P<0.05, P<0.01, P<0.001).
Figure 4.
Overexpression of MYPT1 inhibits SNU-5 cell migration and invasion. The migration (A) and invasion (B) abilities of the treated SNU-5 cells were measured by Transwell assays (** P<0.01, *** P<0.001). (C) qRT-PCR and (D) Western blot assays were performed to detect the mRNA and protein expression levels of E-cadherin, TIMP-2, MMP-2 and MMP-9 in treated SNU-5 cells (* P<0.05, ** P<0.01, *** P<0.001).
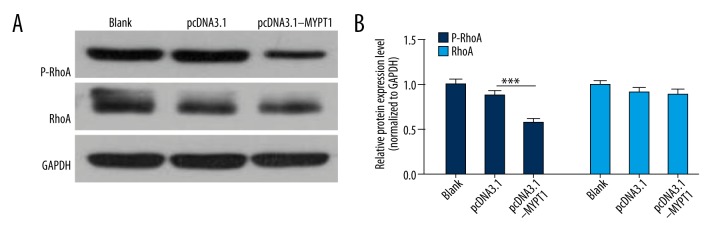
Overexpression of MYPT1 suppresses RhoA phosphorylation
Studied have shown that small GTPases, which are oncogenic genes, have important effects in the tumorigenic process [39,40]. RhoA was a major member of the Rho family of small GTPases-Ras-like proteins, which is involved in proliferation, differentiation, migration, and invasion of cancers [41–45]. Therefore, we analyzed the effect of MYPT1 on RhoA expression, and found that p-RhoA expression was obviously down-regulated in the pcDNA3.1-MYPT1 group compared with the pcDNA3.1 group (P<0.001, Figure 5).
Figure 5.
Overexpression of MYPT1 suppresses RhoA phosphorylation. RhoA and p-RhoA expressions were detected by Western blot assay in treated SNU-5 cells, and the relative expression levels were counted from 3 independent experiments (*** P<0.001).
Discussion
GC is one of the most frequently diagnosed cancers and is the second leading cause of cancer-related death worldwide [46,47]. China has the highest incidence of GC and the highest rates of GC mortality [48]. Although several strategies have been proposed for GC screening, most patients are diagnosed at advanced stage, with dismal outcome [49]. Although several molecularly targeted drugs have been developed, most advanced GC has a poor prognosis [50], and a new appropriate site for targeted therapy needs to be found. At present, MYPT1 has been established as having a key role in regulating various biological and pathological processes in a great number of human diseases [14,51].
ROCK is a serine/threonine kinase that can participate in the regulation of cell adhesion, movement, proliferation, differentiation, and apoptosis. Myoglobin phosphatase (MLCP) and myosin light chain (MLC) are the 2 main substrates of ROCK [52]. MLCP is composed of catalytic domain, MLC-binding subunit, and non-catalytic subunits, and the action sites are located in the MYPT-1 binding subunit of MLCP [53]. Studies have indicated that myosin phosphatase-RhoA interacting protein (M-RIP) can combine Rho A/ROCK and MLCP. MYPT-1 is phosphorylated by ROCK, which causes MLCP inactivation; however, MYPT-1 phosphorylation can lead to MYPT-1 dissociation, which can block the dephosphorylation effect of MLCP on MLC. Both of them can increase the content of phosphorylated MLC, and finally result in enhanced contraction of smooth muscle [54]. In addition, ROCK can phosphorylate MLCP and directly phosphorylate MLC to increase the content of phosphorylated MLC [55].
In our study, we found that MYPT1 was significantly decreased in GC tissues, corresponding to the TNM stage of GC. We also found that GC patients with high MYPT1 expression had a longer survival time, and MYPT1 expression was higher in postoperative patients than in preoperative patients. In addition, the results indicated that overexpression of MYPT1 can suppress RhoA phosphorylation. In functional experiments, we have demonstrated that MYPT1 inhibits GC cell proliferation, migration, and invasion, and induces GC cell cycle arrest.
Previous studies have demonstrated that MYPT1 and CPI-17 can regulate basal LC20 phosphorylation in gastric fundus, murine gastric antrum, and proximal colon smooth muscles [56]; MYPT1 phosphorylation can regulate mammalian mitotic progression [57]; MYPT1 can affect contractility and microtubule acetylation to regulate matrix assembly and integrin adhesions [58]; MYPT1 degradation can promote the development of tolerance to nitric oxide in porcine pulmonary artery [59]; MYPT1 can affect vascular smooth muscle function and maintain blood pressure balance [60]; and phosphorylation of CPI-17 and MYPT1 can induce Ca2+ sensitization in intestinal smooth muscle [61]. In addition, a study showed that MYPT1 can promote the cycle progression of cancer cells [15]. In the present study, we found that MYPT1 expression is related to the development of GC; therefore, we suggest that MYPT1 might be a potential biomarker and therapeutic strategy for GC.
C-myc is an important regulation factor of the cell cycle, which is located in chromosome 8. C-myc can regulate many downstream genes, and then regulate the progression of cell cycle and apoptosis [62,63]. In the past few decades, it was found that C-myc participates in the tumorigenesis of many malignant tumors [64]. In addition, C-myc is strictly regulated in normal cells and is out of control in tumor cells [65,66]. Cyclin Ds are the positive regulatory factors of the cell cycle, and can make cells access the S phase. A study proved that cyclin D1, which is a representative Cyclin D, has the most direct relationship with tumors. Overexpression of cyclin D1 can lead to G1 phase decrease and division speed acceleration [67]. In our study, we demonstrated that MYPT1 dramatically increased Cyclin D1 and c-myc expression, and our data indicate that MYPT1 inhibits GC cell proliferation and induces GC cell cycle arrest by Cyclin D1 and c-myc.
Matrix metalloproteinase (MMP) is secreted by connective tissue and is part of the extracellular matrix degradation Zn2+-dependent protease family [68]. Studies showed that MMPs, such as MMP-2 and MMP-9, play major roles in the physiological and pathological processes of embryonic development, cell migration, angiogenesis, wound healing, atherosclerosis, malignant tumor infiltration, and metastasis [69–71]. Tissue inhibitor of metalloproteinases (TIMPs) is a set of low molecular weight glycoproteins that are widely distributed in tissues and fluids that can be produced and secreted by fibroblasts, epithelial cells, and endothelial cells. In addition, TIMPs are multifunctional proteins which can inhibit the activity of MMPs [72]. TIMPs participate in extracellular matrix remodeling and various pathological processes, such as tumor invasion, diffusion metastasis, and tissue fibrosis [73,74]. Previous research has shown that E-cadherin plays an important role in cellular adhesion in tumors, and its deletion was associated with tumor metastasis [75]. In our study, we found that MYPT1 increased E-cadherin expression and decreased TIMP-2 and MMP-2 expressions. The results indicate that MYPT1 inhibits GC cell migration and invasion by regulating E-cadherin, TIMP-2, and MMP-2.
Conclusions
In summary, MYPT1 expression is associated with TNM stage, survival time, and prognosis of GC patients. In addition, MYPT1 inhibits GC cell proliferation, migration, and invasion via activating RhoA phosphorylation. This study identifies MYPT1 as a novel prognostic marker and candidate drug target for GC.
Footnotes
Source of support: This work was supported by Zhejiang Provincial Science and Technology Agency on public welfare technology application research plan’s social development project (Grant number 2017C33130)
Conflict of interests
None.
References
- 1.Wadhwa R, Song S, Lee JS, et al. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10(11):643–55. doi: 10.1038/nrclinonc.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jou E, Rajdev L. Current and emerging therapies in unresectable and recurrent gastric cancer. World J Gastroenterol. 2016;22(20):4812–23. doi: 10.3748/wjg.v22.i20.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo X, Guo L, Ji J, et al. miRNA-331-3p directly targets E2F1 and induces growth arrest in human gastric cancer. Biochem Biophys Res Commun. 2010;398(1):1–6. doi: 10.1016/j.bbrc.2010.05.082. [DOI] [PubMed] [Google Scholar]
- 4.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12(1):17–20. doi: 10.3748/wjg.v12.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma H, Tian Y, Yu X. Targeting smoothened sensitizes gastric cancer to chemotherapy in experimental models. Med Sci Monit. 2018;24:1493–500. doi: 10.12659/MSM.903012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahin AG, Aydin C, Unver M, Pehlivanoglu K. Predictive value of preoperative neutrophil lymphocyte ratio in determining the stage of gastric tumor. Med Sci Monit. 2018;24:1973–79. doi: 10.12659/MSM.900681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao HH, Wang BJ, Wu Y, Huang Q. High expression of angiogenic factor with G-patch and FHA domain1 (AGGF1) predicts poor prognosis in gastric cancer. Med Sci Monit. 2018;24:1286–94. doi: 10.12659/MSM.903248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bairamov RB, Abdullaeva RT. [The impact of early gastric cancer diagnosis on indices of survival in patients after radical surgical intervention]. Klin Khir. 2013;(6):18–21. in Russian. [PubMed] [Google Scholar]
- 9.Pasechnikov V, Chukov S, Fedorov E, et al. Gastric cancer: Prevention, screening and early diagnosis. World J Gastroenterol. 2014;20(38):13842–62. doi: 10.3748/wjg.v20.i38.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foo M, Leong T. Adjuvant therapy for gastric cancer: current and future directions. World J Gastroenterol. 2014;20(38):13718–27. doi: 10.3748/wjg.v20.i38.13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Min C, Bangalore S, Jhawar S, et al. Chemoradiation therapy versus chemotherapy alone for gastric cancer after R0 surgical resection: A meta-analysis of randomized trials. Oncology. 2014;86(2):79–85. doi: 10.1159/000354641. [DOI] [PubMed] [Google Scholar]
- 12.Huang D, Wang H, Liu R, et al. miRNA27a is a biomarker for predicting chemosensitivity and prognosis in metastatic or recurrent gastric cancer. J Cell Biochem. 2014;115(3):549–56. doi: 10.1002/jcb.24689. [DOI] [PubMed] [Google Scholar]
- 13.Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet. 2016;388(10060):2654–64. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 14.Chirino YI, Garcia-Cuellar CM, Garcia-Garcia C, et al. Airborne particulate matter in vitro exposure induces cytoskeleton remodeling through activation of the ROCK-MYPT1-MLC pathway in A549 epithelial lung cells. Toxicol Lett. 2017;272:29–37. doi: 10.1016/j.toxlet.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Cho HS, Suzuki T, Dohmae N, et al. Demethylation of RB regulator MYPT1 by histone demethylase LSD1 promotes cell cycle progression in cancer cells. Cancer Res. 2011;71(3):655–60. doi: 10.1158/0008-5472.CAN-10-2446. [DOI] [PubMed] [Google Scholar]
- 16.Lin ZY, Chen G, Zhang YQ, et al. MicroRNA-30d promotes angiogenesis and tumor growth via MYPT1/c-JUN/VEGFA pathway and predicts aggressive outcome in prostate cancer. Mol Cancer. 2017;16(1):48. doi: 10.1186/s12943-017-0615-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Tanaka J, Ito M, Feng J, et al. Interaction of myosin phosphatase target subunit 1 with the catalytic subunit of type 1 protein phosphatase. Biochemistry. 1998;37(47):16697–703. doi: 10.1021/bi980782x. [DOI] [PubMed] [Google Scholar]
- 18.Tan I, Ng CH, Lim L, Leung T. Phosphorylation of a novel myosin binding subunit of protein phosphatase 1 reveals a conserved mechanism in the regulation of actin cytoskeleton. J Biol Chem. 2001;276(24):21209–16. doi: 10.1074/jbc.M102615200. [DOI] [PubMed] [Google Scholar]
- 19.Muranyi A, Derkach D, Erdodi F, et al. Phosphorylation of Thr695 and Thr850 on the myosin phosphatase target subunit: Inhibitory effects and occurrence in A7r5 cells. FEBS Lett. 2005;579(29):6611–15. doi: 10.1016/j.febslet.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 20.Khasnis M, Nakatomi A, Gumpper K, Eto M. Reconstituted human myosin light chain phosphatase reveals distinct roles of two inhibitory phosphorylation sites of the regulatory subunit, MYPT1. Biochemistry. 2014;53(16):2701–9. doi: 10.1021/bi5001728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khromov A, Choudhury N, Stevenson AS, et al. Phosphorylation-dependent autoinhibition of myosin light chain phosphatase accounts for Ca2+ sensitization force of smooth muscle contraction. J Biol Chem. 2009;284(32):21569–79. doi: 10.1074/jbc.M109.019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudson CA, Heesom KJ, Lopez Bernal A. Phasic contractions of isolated human myometrium are associated with Rho-kinase (ROCK)-dependent phosphorylation of myosin phosphatase-targeting subunit (MYPT1) Mol Hum Reprod. 2012;18(5):265–79. doi: 10.1093/molehr/gar078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh DK, Sarkar J, Raghavan A, et al. Hypoxia modulates the expression of leucine zipper-positive MYPT1 and its interaction with protein kinase G and Rho kinases in pulmonary arterial smooth muscle cells. Pulm Circ. 2011;1(4):487–98. doi: 10.4103/2045-8932.93548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu G, Xu M, Xu K, Hu Y. Benidipine protects kidney through inhibiting ROCK1 activity and reducing the epithelium-mesenchymal transdifferentiation in type 1 diabetic rats. J Diabet Res. 2013;2013:174526. doi: 10.1155/2013/174526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler T, Paul J, Europe-Finner N, et al. Role of serine-threonine phosphoprotein phosphatases in smooth muscle contractility. Am j Physiol Cell Physiol. 2013;304(6):C485–504. doi: 10.1152/ajpcell.00161.2012. [DOI] [PubMed] [Google Scholar]
- 26.Filter JJ, Williams BC, Eto M, et al. Unfair competition governs the interaction of pCPI-17 with myosin phosphatase (PP1-MYPT1) Elife. 2017;6 doi: 10.7554/eLife.24665. pii: e24665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan R, Xu X, Chen M, et al. Advances in the studies of roles of Rho/Rho-kinase in diseases and the development of its inhibitors. Eur J Med Chem. 2013;70:613–22. doi: 10.1016/j.ejmech.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 28.Grassie ME, Moffat LD, Walsh MP, MacDonald JA. The myosin phosphatase targeting protein (MYPT) family: A regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1delta. Arch Biochem Biophys. 2011;510(2):147–59. doi: 10.1016/j.abb.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Scotto-Lavino E, Garcia-Diaz M, Du G, Frohman MA. Basis for the isoform-specific interaction of myosin phosphatase subunits protein phosphatase 1c beta and myosin phosphatase targeting subunit 1. J Biol Chem. 2010;285(9):6419–24. doi: 10.1074/jbc.M109.074773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkinson S, Paterson HF, Marshall CJ. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol. 2005;7(3):255–61. doi: 10.1038/ncb1230. [DOI] [PubMed] [Google Scholar]
- 31.Patel RA, Forinash KD, Pireddu R, et al. RKI-1447 is a potent inhibitor of the Rho-associated ROCK kinases with anti-invasive and antitumor activities in breast cancer. Cancer Res. 2012;72(19):5025–34. doi: 10.1158/0008-5472.CAN-12-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X, Zhang Y, Wang S, Shi W. Effect of fasudil on growth, adhesion, invasion, and migration of 95D lung carcinoma cells in vitro. Can J Physiol Pharmacol. 2010;88(9):874–79. doi: 10.1139/y10-047. [DOI] [PubMed] [Google Scholar]
- 33.Yang X, Zheng F, Zhang S, Lu J. Loss of RhoA expression prevents proliferation and metastasis of SPCA1 lung cancer cells in vitro. Biomed Pharmacother. 2015;69:361–66. doi: 10.1016/j.biopha.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Pinheiro AS, Marsh JA, Forman-Kay JD, Peti W. Structural signature of the MYPT1-PP1 interaction. J Am Chem Soc. 2011;133(1):73–80. doi: 10.1021/ja107810r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geetha T, Langlais P, Caruso M, Yi Z. Protein phosphatase 1 regulatory subunit 12A and catalytic subunit delta, new members in the phosphatidylinositide 3 kinase insulin-signaling pathway. J Endocrinol. 2012;214(3):437–43. doi: 10.1530/JOE-12-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuen SL, Ogut O, Brozovich FV. Differential phosphorylation of LZ+/LZ− MYPT1 isoforms regulates MLC phosphatase activity. Arch Biochem Biophys. 2014;562:37–42. doi: 10.1016/j.abb.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Given AM, Ogut O, Brozovich FV. MYPT1 mutants demonstrate the importance of aa 888–928 for the interaction with PKGIalpha. Am J Physiol Cell Physiol. 2007;292(1):C432–39. doi: 10.1152/ajpcell.00175.2006. [DOI] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Chang EH, Gonda MA, Ellis RW, et al. Human genome contains four genes homologous to transforming genes of Harvey and Kirsten murine sarcoma viruses. Proc Natl Acad Sci USA. 1982;79(16):4848–52. doi: 10.1073/pnas.79.16.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeFeo D, Gonda MA, Young HA, et al. Analysis of two divergent rat genomic clones homologous to the transforming gene of Harvey murine sarcoma virus. Proc Natl Acad Sci USA. 1981;78(6):3328–32. doi: 10.1073/pnas.78.6.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen S, Wang J, Gou WF, et al. The involvement of RhoA and Wnt-5a in the tumorigenesis and progression of ovarian epithelial carcinoma. Int J Mol Sci. 2013;14(12):24187–99. doi: 10.3390/ijms141224187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gou L, Wang W, Tong A, et al. Proteomic identification of RhoA as a potential biomarker for proliferation and metastasis in hepatocellular carcinoma. J Mol Med. 2011;89(8):817–27. doi: 10.1007/s00109-011-0753-3. [DOI] [PubMed] [Google Scholar]
- 43.Ito H, Morishita R, Tabata H, Nagata K. Roles of Rho small GTPases in the tangentially migrating neurons. Histol Histopathol. 2014;29(7):871–79. doi: 10.14670/HH-29.871. [DOI] [PubMed] [Google Scholar]
- 44.Katayama K, Imai F, Campbell K, et al. RhoA and Cdc42 are required in pre-migratory progenitors of the medial ganglionic eminence ventricular zone for proper cortical interneuron migration. Development. 2013;140(15):3139–45. doi: 10.1242/dev.092585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S, Konstantinidis DG, Yang JQ, et al. Gene targeting RhoA reveals its essential role in coordinating mitochondrial function and thymocyte development. J Immunol. 2014;193(12):5973–82. doi: 10.4049/jimmunol.1400839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamashima C. Current issues and future perspectives of gastric cancer screening. World J Gastroenterol. 2014;20(38):13767–74. doi: 10.3748/wjg.v20.i38.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20(16):4483–90. doi: 10.3748/wjg.v20.i16.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 49.Zagouri F, Papadimitriou CA, Dimopoulos MA, Pectasides D. Molecularly targeted therapies in unresectable-metastatic gastric cancer: A systematic review. Cancer Treat Rev. 2011;37(8):599–610. doi: 10.1016/j.ctrv.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Arkenau HT. Gastric cancer in the era of molecularly targeted agents: Current drug development strategies. J Cancer Res Clin Oncol. 2009;135(7):855–66. doi: 10.1007/s00432-009-0583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang X, Di J, Zhang Y, et al. The Rho-kinase inhibitor inhibits proliferation and metastasis of small cell lung cancer. Biomed Pharmacother. 2012;66(3):221–27. doi: 10.1016/j.biopha.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 52.Kimura K, Ito M, Amano M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273(5272):245–48. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 53.Furuyama T, Komori K, Shimokawa H, et al. Long-term inhibition of Rho kinase suppresses intimal thickening in autologous vein grafts in rabbits. J Vasc Surg. 2006;43(6):1249–56. doi: 10.1016/j.jvs.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 54.Feng J, Ito M, Ichikawa K, et al. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274(52):37385–90. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- 55.Shin HM, Je HD, Gallant C, et al. Differential association and localization of myosin phosphatase subunits during agonist-induced signal transduction in smooth muscle. Circ Res. 2002;90(5):546–53. doi: 10.1161/01.res.0000012822.23273.ec. [DOI] [PubMed] [Google Scholar]
- 56.Bhetwal BP, An CL, Fisher SA, Perrino BA. Regulation of basal LC20 phosphorylation by MYPT1 and CPI-17 in murine gastric antrum, gastric fundus, and proximal colon smooth muscles. Neurogastroenterol Motil. 2011;23(10):e425–36. doi: 10.1111/j.1365-2982.2011.01769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiyoda T, Sugiyama N, Shimizu T, et al. LATS1/WARTS phosphorylates MYPT1 to counteract PLK1 and regulate mammalian mitotic progression. J Cell Biol. 2012;197(5):625–41. doi: 10.1083/jcb.201110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joo EE, Yamada KM. MYPT1 regulates contractility and microtubule acetylation to modulate integrin adhesions and matrix assembly. Nat Commun. 2014;5:3510. doi: 10.1038/ncomms4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma H, He Q, Dou D, et al. Increased degradation of MYPT1 contributes to the development of tolerance to nitric oxide in porcine pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2010;299(1):L117–23. doi: 10.1152/ajplung.00340.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiao YN, He WQ, Chen CP, et al. Myosin phosphatase target subunit 1 (MYPT1) regulates the contraction and relaxation of vascular smooth muscle and maintains blood pressure. J Biol Chem. 2014;289(32):22512–23. doi: 10.1074/jbc.M113.525444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mori D, Hori M, Murata T, et al. Synchronous phosphorylation of CPI-17 and MYPT1 is essential for inducing Ca(2+) sensitization in intestinal smooth muscle. Neurogastroenterol Motil. 2011;23(12):1111–22. doi: 10.1111/j.1365-2982.2011.01799.x. [DOI] [PubMed] [Google Scholar]
- 62.Kaptein JS, Lin CK, Wang CL, et al. Anti-IgM-mediated regulation of c-myc and its possible relationship to apoptosis. J Biol Chem. 1996;271(31):18875–84. doi: 10.1074/jbc.271.31.18875. [DOI] [PubMed] [Google Scholar]
- 63.Song A, Ye J, Zhang K, et al. Lentiviral vector-mediated siRNA knockdown of c-MYC: Cell growth inhibition and cell cycle arrest at G2/M phase in Jijoye cells. Biochem Genet. 2013;51(7–8):603–17. doi: 10.1007/s10528-013-9590-0. [DOI] [PubMed] [Google Scholar]
- 64.Dang CV. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med. 2013;3(8) doi: 10.1101/cshperspect.a014217. pii: a014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miyawaki Y, Kawachi H, Ooi A, et al. Genomic copy-number alterations of MYC and FHIT genes are associated with survival in esophageal squamous-cell carcinoma. Cancer science. 2012;103(8):1558–66. doi: 10.1111/j.1349-7006.2012.02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsiatis AC, Herceg ME, Keedy VL, et al. Prognostic significance of c-Myc expression in soft tissue leiomyosarcoma. Modern Pathol. 2009;22(11):1432–38. doi: 10.1038/modpathol.2009.113. [DOI] [PubMed] [Google Scholar]
- 67.Elkady AI, Abuzinadah OA, Baeshen NA, Rahmy TR. Differential control of growth, apoptotic activity, and gene expression in human breast cancer cells by extracts derived from medicinal herbs Zingiber officinale. J Biomed Biotechnol. 2012;2012:614356. doi: 10.1155/2012/614356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Yadav L, Puri N, Rastogi V, et al. Matrix metalloproteinases and cancer – roles in threat and therapy. Asian Pac J Cancer Prev. 2014;15(3):1085–91. doi: 10.7314/apjcp.2014.15.3.1085. [DOI] [PubMed] [Google Scholar]
- 69.Di Carlo A. Matrix metalloproteinase-2 and -9 in the sera and in the urine of human oncocytoma and renal cell carcinoma. Oncol Rep. 2012;28(3):1051–56. doi: 10.3892/or.2012.1864. [DOI] [PubMed] [Google Scholar]
- 70.Fic P, Zakrocka I, Kurzepa J, Stepulak A. [Matrix metalloproteinases and atherosclerosis]. Postepy Hig Med Dosw. 2011;65:16–27. doi: 10.5604/17322693.931536. in Polish. [DOI] [PubMed] [Google Scholar]
- 71.Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol. 2012;302(11):F1351–61. doi: 10.1152/ajprenal.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015;44–46:247–54. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 73.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3(12) doi: 10.1101/cshperspect.a005058. pii: a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beavon IR. The E-cadherin-catenin complex in tumour metastasis: structure, function and regulation. Eur J Cancer. 2000;36(13 Spec):1607–20. doi: 10.1016/s0959-8049(00)00158-1. [DOI] [PubMed] [Google Scholar]