Abstract
Introduction
There is concern that surgical quality initially declines during the learning phase of robotic surgery. At our institution, we used a multi-surgeon programmatic approach to the introduction of robotic surgery. The purpose of this study was to evaluate outcomes of patients treated during the first year of our program.
Methods
This is a historical cohort of all radical prostatectomy patients during a one-year period. Baseline, perioperative, and long-term followup data were prospectively and retrospectively collected. Treatment failure was a composite of any postoperative radiation, androgen-deprivation, or prostate-specific antigen (PSA) ≥0.2.
Results
During the study period, 225 radical prostatectomy procedures were performed (104 robotic and 121 open). Baseline characteristics were similar between groups (p>0.05). All patients were continent and 74% were potent prior to surgery. Mean estimated blood loss (280 cc vs. 760 cc; p<0.001) and blood transfusion (0% vs. 8.3%; p=0.002) was lower in the robotic cohort. Non-transfusion complications were similar between groups (13% vs. 12%; p=0.7). Mean hospital stay was shorter in the robotic cohort (1.4 vs. 2.5 days). There was no difference in overall positive margin rate (38% vs. 43%; p=0.4) or treatment failure at a median followup of 3.5 years (p=0.4). Robotically treated patients were more often continent (89% vs. 77%; p=0.02) and potent (48% vs. 32%; p=0.02).
Conclusions
Using an inclusive multi-surgeon approach, robotic pros-tatectomy was introduced safely at a Canadian academic institution.
Introduction
Robotic assisted laparoscopic radical prostatectomy (RALRP) has gained popularity for the surgical treatment of prostate cancer. Advantages of the robotic approach include better surgeon ergonomics, improved visualization, and easier instrumentation compared to pure laparoscopy. RALRP may result in less blood loss, fewer blood transfusions, less time in hospital, and quicker recovery with equivalent cancer outcomes and long-term quality of life compared to an open surgical approach.1–6 Disadvantages of robotic prostatectomy is the higher cost per case, and potentially the learning curve associated with adoption of a new surgical procedure.
Hospitals may offer only open or robotic surgery while others offer both based on surgeon preference and resource availability. Over the last decade, the use of RALRP has increased in many jurisdictions. In the U.S., a robotic approach increased from approximately 1% of patients in 2001 to 70% by 2012.7,8 In contrast to the rapid uptake of robotic surgery in the U.S., uptake in Canada has been much slower. The proportion of cases being performed robotically in Canada was 19% in 2012–2013.9 Presumably, the cost of robotic surgery will decrease over time and more centres in Canada will develop a robotic surgery program and offer this surgical option to prostate cancer patients.
There is no standard method to optimally implement a robotic surgery program. Some centres may limit the number of surgeons who perform robotic surgery, while others may adopt a more inclusive, multi-surgeon approach. At our institution, the robotic surgery program was introduced using a multi-surgeon approach. We believed that inclusion of multiple surgeons would allow for a more rapid program expansion and we hoped there would not be a negative impact on patient outcomes. In this study, we report the outcomes of patients receiving robotic surgery during the first year of implementation of our multi-surgeon robotic surgery program compared to patients over the same time period treated with open prostatectomy.
Methods
Clinical setting
Institutional ethics review board approval was obtained. This was a historical cohort of prospectively collected data for all patients who underwent radical prostatectomy (open or robotic) from October 31, 2011 to October 31, 2012 at our institution. The surgeons performing RALRP included one with robotic surgery fellowship training (RB), two experienced in laparoscopic prostatectomy (BB, JW), and one experienced in open prostatectomy but with no laparoscopic experience (CM). All surgeons performing open prostatec-tomy had oncology fellowship training (RB, IC, CM) or were highly experienced with this procedure (FD, RG, JM, JO, and JW). No criteria were used to select patients for RALRP or open prostatectomy. The choice of which procedure was performed was based on who operated on the patient and resource availability. Since the wait times were generally longer for RALRP, higher-risk patients may have more frequently received open prostatectomy to reduce their wait time to surgery.
All surgeons performing RALRP attended a training session at an Intuitive Surgical training facility prior to the first case. The robotic-trained urologist mentored the other surgeons until both the mentor and the mentee were confident that routine mentoring was no longer required. A common anesthesia pathway was used, but no specific anesthesiol-ogy training or anesthesiologist was required. The nursing team leader (RW) received training at an Intuitive Surgical training facility and he subsequently trained the remainder of the nursing team. All patients followed a common perioperative care pathway. All surgeons followed similar surgical steps using standardized instruments and sutures. All RALRPs were performed using the da Vinci surgical robot system (Intuitive Surgical, Sunnyvale, CA, U.S.) and a trans-peritoneal approach.
Baseline characteristics
Preoperative characteristics, including patient age, body mass index (BMI), preoperative prostate-specific antigen (PSA), prostate volume, patient American Society of Anesthesiologists (ASA) class, PSA density, prostate volume, preoperative erectile function, digital rectal exam (DRE) result, biopsy Gleason score, number of positive cores, and tumour grade, were abstracted from the medical record.
Surgery characteristics
Intraoperative parameters, such as the estimated blood loss (EBL), number of nodes removed, nerve-sparing technique, operative time, intraoperative complications, and positive margin status, were prospectively recorded in a database.
Postoperative outcomes
Postoperative complications until discharge were collected from the medical record. Postoperative complications were classified by their severity based on the Clavien-Dindo scale.10 The Clavien-Dindo classification system is used to grade the severity of adverse events that occur as a result of surgical procedures. More details on this classification system can be found online (http://www.surgicalcomplication.info/index-2.html). As part of the clinical pathway, all robotic patients had pelvic drain fluid analyzed for creatinine irrespective of symptoms and output volumes. Open prostatectomy patients did not have routine drain creatinine analyzed unless the drain output was high. Patients were typically seen at least three, six, nine, 12, 18, 24, and 36 months postoperatively and oncological and functional outcomes were recorded at each visit. Biochemical failure was defined as PSA ≥0.2. If postoperative radiation or androgen deprivation was initiated, the date of therapy commencement was recorded. A composite outcome of primary treatment failure was defined as one or more of: a postoperative PSA ≥0.2, postoperative radiation, or postoperative androgen deprivation.
Data analysis
Baseline characteristics and outcomes of patients receiving open and robotic surgery were summarized and compared using descriptive statistics. Treatment failure-free survival was calculated from surgery to any of the composite endpoint events. Patients were censored at last followup or death. The Kaplan-Meier method was used to estimate survival, and a log-rank test was used to test for differences in the treatment failure-free survival between groups. Univariable and multivariable cox proportional hazard regression was used to estimate hazard ratios of cancer recurrence associated with the surgical approach, preoperative PSA levels, pathological Gleason score, and pathological tumour stage. A p value <0.05 was considered significant. No adjustment was made for multiple testing and all analyses were performed using SAS (SAS Institute Inc., Cary, NC, U.S.). To encourage participation of all surgeons, it was decided a priori that no analysis would be performed comparing results between individual surgeons.
Results
Patient characteristics
A total of 225 patients underwent radical prostatectomy during the study period; 104 were performed robotically and 121 were performed using an open approach. The number of cases for each surgeon in the robotic groups was 36, 34, 30, and 4. The number of cases for each surgeon in the open group was 30, 29, 28, 12, 10, 8, 3, and 1. The patients in the robotic cohort were slightly younger and more frequently had a family history of prostate cancer (Table 1). Fewer robotically treated patients received a preoperative magnetic resonance imaging (MRI) and more received a nerve-sparing procedure (p<0.001). The mean preoperative PSA in the entire cohort was 8.3±6.2 ng/ml and mean prostate volume was 34.5±20.3 cc. The biopsy Gleason score was 6 in 52 (22.3%), 7 in 135 (57.9%), and ≥8 in 38 (19.8%) patients. Incomplete followup was similar between groups, with 20 (16.5%) patients lost to followup in the open group and 14 (13.5%) in the robotic group.
Table 1.
Patient demographics and disease characteristics
| Variable | Open (n=121) | Robotic (n=104) | p |
|---|---|---|---|
| Age (years), mean ± SD | 62.9±6.7 | 60.8±6.5 | 0.02 |
| BMI ± SD | 28.6±3.9 | 28.0±3.6 | 0.29 |
| ASA class (%) | 0.26 | ||
| 1 | 4 (3.3) | 6 (5.8) | |
| 2 | 78 (65) | 74 (71.8) | |
| 3 | 36 (30) | 23 (22.3) | |
| 4 | 2 (1.7) | 0 (0) | |
| Preoperative PSA (ng/mL), mean ± SD | 8.58±6.73 | 7.89±5.63 | 0.33 |
| PSA density ± SD | 0.28±0.27 | 0.26±0.19 | 0.75 |
| Family history of prostate cancer (%) | 21 (17.4) | 47 (45.9) | <0.0001 |
| Prostate volume (mL), mean ± SD | 36.7±24.9 | 32.0±13.1 | 0.33 |
| Neoadjuvant androgen deprivation (%) | 8 (6.7) | 6 (5.8) | 0.80 |
| Preoperative MRI (%) | 52 (43%) | 14 (13%) | <0.0001 |
| Nerve-sparing technique (%) | <0.0001 | ||
| Non | 34 (30.4) | 18 (17.3) | |
| Unilateral | 30 (26.8) | 11 (10.6) | |
| Bilateral | 48 (42.9) | 75 (61.0) | |
| Preoperative erectile function (%) | 0.66 | ||
| Inadequate for penetration | 32 (28.1) | 23 (22.8) | |
| Adequate for penetration | 76 (66.7) | 73 (72.3) | |
| Adequate with PED5 inhibitor | 6 (5.4) | 5 (4.9) | |
| DRE (%) | 0.24 | ||
| Normal | 90 (74.4) | 70 (67.3) | |
| Abnormal | 31 (25.6) | 34 (32.7) | |
| Biopsy Gleason score (%) | 0.45 | ||
| 6 | 27 (22.3) | 25 (24.0) | |
| 7 | 70 (57.9) | 65 (63.0) | |
| ≥8 | 24 (19.8) | 14 (13.5) | |
| Positive cores, mean ± SD | 4.3±2.57 | 4.3±2.3 | 0.66 |
| Prostatectomy tumour volume (%) | 15.7 (15.7) | 12.4 (10.1) | 0.49 |
| Length of followup (months), mean ± SD | 33.9±11.1 | 34.0±9.5 | 0.38 |
| Lost to followup (%) | 20 (16.5) | 14 (13.5) |
ASA: American society of anesthesiologists; BMI: body mass index; DRE: digital rectal exam; MRI: magnetic resonance imaging; PED5: phosphodiesterase type-5; PSA: prostate-specific antigen; SD: standard deviation.
Surgical parameters
Surgical outcomes are presented in Table 2. The operating room time was similar between groups (p=0.10). The blood loss was 278.7 ±220.1 ml in the robotic group compared to 756.8 ±451.8 ml in the open group (p<0.0001). No patients in the robotic group required a blood transfusion compared to 10 (8.3%) patients in the open group (p=0.002). Among the 10 patients receiving a transfusion in the open cohort, an average of 2.1±1.0 units of blood was transfused (median 2, range 1–3). The number of lymph nodes dissected was similar between groups (p=0.19). Other than blood transfusion, there were no intraoperative complications in either cohort and no patients were converted from a robotic approach to open. The risk of a non-transfusion-related postoperative complication was 12.4% overall, 11.6% in the open group and 13.5% in the robotic group. No patients had a Grade 4 or 5 complication. Five (5%) robotic patients had a Grade 3 complication compared to nine (7%) open patients. Eight patients had anastomotic leak, six (6%) in the robotic group compared to two (2%). Six were treated conservatively with no intervention, and two were treated with prophylactic antibiotics, both of whom were in the robotic group. Two patients in the open group and two patients in the robotic group were diagnosed with a postoperative urinary tract infection. No patient in the robotic group experienced a bladder neck contracture compared to six patients (5%) in the open group. Other documented complications included hiatal hernia exacerbation, incision seromas, lymphoceles, meatal stenosis, and wound infections. The mean hospital stay was 1.4±0.7 days in the robotic group compared to 2.5±0.9 days in the open group (p<0.0001).
Table 2.
Operative characteristics and adverse events
| Open (n=121) | Robotic (n=104) | p | |
|---|---|---|---|
| OR time, minutes ± SD | 270.82±65.9 | 282.7±63.2 | 0.10 |
| Transfusion (%) | 10 (8.3) | 0 (0) | 0.002 |
| Estimated blood loss, mL ± SD | 756.8±451.8 | 278.7± 220.1 | <0.0001 |
| Length of stay, mean days ± SD | 2.5±0.9 | 1.4±0.7 | <0.0001 |
| Lymph nodes dissected ± SD | 7.4±6.2 | 6.04±4.5 | 0.19 |
| Total postoperative complications (%) | 14 (11.6) | 14 (13.5) | 0.67 |
| Grade I | 2 (1.6) | 4 (3.8) | |
| Grade II | 3 (2.5) | 5 (4.8) | |
| Grade III | 9 (7.4) | 5 (4.8) | |
| Specific postoperative complication (%) | |||
| Anastomotic leak | 2 (1.7) | 6 (5.8) | 0.04 |
| Grade I | 2 (1.7) | 4 (3.8) | |
| Grade II | 0 (0) | 2 (1.9) | |
| Bladder neck contracture | 6 (5.0) | 0 (0) | |
| Grade I | 0 (0) | 0 (0) | |
| Grade III | 6 (4.1) | 0(0) | |
| Hernia | 0 (0) | 3 (2.9) | |
| Grade III | 0 (0) | 3 (2.9) | |
| Incision seroma | 0 (0) | 1 (1.0) | |
| Grade II | 0 (0) | 1 (1.0) | |
| Lymphocele | 2 (1.7) | 2 (1.9) | |
| Grade III | 2 (1.7) | 2 (1.9) | |
| Meatal stenosis | 1 (0.8) | 0 (0) | |
| Grade III | 1 (0.8) | 0 (0) | |
| Urinary tract infection | 2 (1.7) | 2 (1.9) | |
| Grade II | 2 (1.7) | 2 (1.9) | |
| Wound infection | 1 (0.8) | 0 (0) | |
| Grade II | 1 (0.8) | 0 (0) |
OR: operating room; SD: standard deviation.
Oncological outcomes
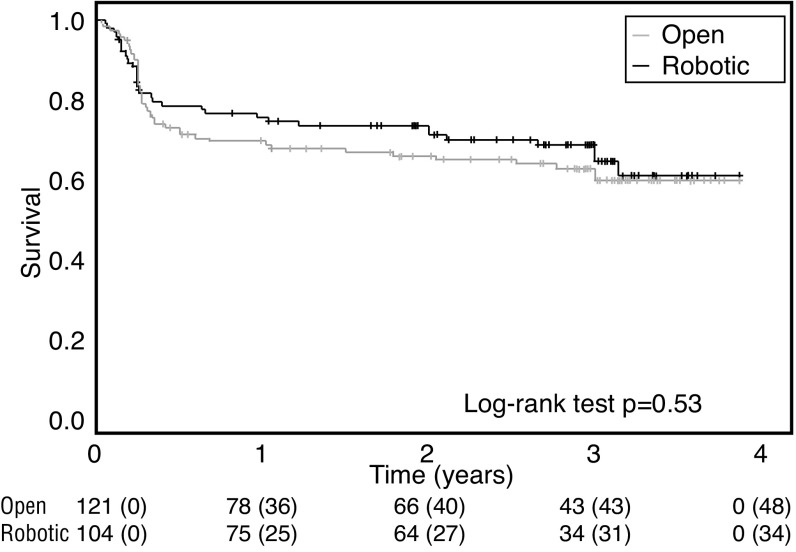
Oncological outcomes are presented in Table 3. The pros-tatectomy pathology specimen showed Gleason score 3+3, 3+4, 4+3 and ≥8 to be comparable between groups (p=0.81). The risk of a positive surgical margin was similar between groups (p=0.4). No significant difference was observed when patients were stratified by pT2 or pT3 tumours. The risk of cancer recurrence (p=0.48), the number of patients having a 36-month PSA ≥0.2 (p=0.97), and the number of patients requiring postoperative hormonal (p=0.35) or radiation (p=0.62) therapy was comparable between groups. The overall three-year treatment failure-free survival using the composite endpoint was 62.0%, with no statistical difference between the two cohorts (p=0.53) (Fig. 1). Preoperative PSA, prostatectomy tumour stage, and prostatectomy Gleason score were significantly associated with an increased risk of cancer recurrence (Table 4). The surgical approach was not predictive of cancer recurrence.
Table 3.
Oncological outcomes
| Open (n=121) | Robotic (n=104) | p | |
|---|---|---|---|
| Treatment failure (%) | 45 (37.2) | 34 (32.7) | 0.48 |
| PSA ≥0.2 (%) | 6 (5) | 5 (5) | 0.97 |
| Postoperative androgen-deprivation (%) | 24 (20.2) | 16 (15.4) | 0.35 |
| Postoperative pelvic radiation (%) | 38 (31.9) | 30 (28.9) | 0.62 |
| Prostatectomy Gleason score (%) | 0.81 | ||
| 3+3 | 9 (7.4) | 11 (10.7) | |
| 3+4 | 67 (55.4) | 54 (52.4) | |
| 4+3 | 31 (25.6) | 28 (27.2) | |
| ≥8 | 14 (11.6) | 10(9.7) | |
| Prostatectomy tumour stage (%) | 0.03 | ||
| T2 | 56 (46.3) | 64 (61.5) | |
| T3 | 64 (52.9) | 40 (38.5) | |
| T4 | 1 (0.8) | 0 (0) | |
| Positive margin rate (%) | |||
| Overall | 52 (43.0) | 39 (38.0) | 0.40 |
| T2 tumours only | 23 (41.1) | 19 (29.7) | 0.19 |
| T3 tumours only | 28 (43.8) | 20 (50.0) | 0.53 |
PSA: prostate-specific antigen.
Fig. 1.
Kaplan-Meier curve for primary treatment failure-free survival stratified by surgical technique.
Table 4.
Univariable and multivariable analysis of disease characteristics with cancer recurrence following radical prostatectomy
| Variable | Univariable | Multivariable | |||
|---|---|---|---|---|---|
|
| |||||
| Comparison | Hazard ratio | p | Hazard ratio | p | |
| Surgical approach | Open vs. robotic | 1.2 (0.74–1.8) | 0.53 | 0.8 (0.5–1.3) | 0.44 |
| Preoperative PSA (per ng/ml) | N/A | 1.1 (1.0–1.1) | <0.0001 | 1.1 (1.0–1.1) | <0.0001 |
| Gleason score | 3+3 vs. 3+4 | Not estimable | Not estimable | ||
| 4+3 vs. 3+4 | 3.6 (2.1–5.9) | <0.0001 | 2.7 (1.6–4.6) | 0.0004 | |
| ≥8 vs. 3+4 | 7.8 (4.3–14.2) | <0.0001 | 5.5 (2.9–10.4) | <0.0001 | |
| Pathological stage | T3 vs. T2 | 5.6 (3.3–9.6) | <0.0001 | 3.0 (1.7–5.3) | 0.0002 |
PSA: prostate-specific antigen.
Functional outcomes
Preoperative erectile function was slightly higher in the robotic cohort (77% vs. 72% able to achieve penetration). At last followup, more patients had erectile function adequate for penetration in the robotic group (48% vs. 32%; p=0.02) (Table 5). When stratified by nerve-sparing technique, of the patients who underwent a bilateral nerve-sparing procedure, a higher proportion of patients in the robotic group had adequate postoperative erectile function (p=0.04) (Table 6). At last followup, almost 90% of patients in the robotic group were fully continent compared to 77.4% of patients in the open group (p=0.02). When stratified by nerve-sparing technique, similar associations were observed (Table 7).
Table 5.
Long-term functional outcomes
| Open (n=121) | Robotic (n=104) | p | |
|---|---|---|---|
| Postoperative erectile function, n (%) | 0.02 | ||
| Adequate for penetration with or without PDE5 inhibitors | 36 (31.9) | 49 (47.6) | |
| Not adequate for penetration | 77 (68.1) | 54 (52.4) | |
| Postoperative continence, n (%) | 0.02 | ||
| Continent (no pads) | 89 (77.4) | 92 (89.3) | |
| Incontinent (1 or more pads) | 26 (22.6) | 11 (10.7) |
PED5: phosphodiesterase type-5.
Table 6.
Patient postoperative erectile function with or without PDE5 inhibitors stratified by nerve-sparing technique
| Nerve-sparing technique | Approach | Postoperative erectile function | |
|---|---|---|---|
|
| |||
| Normal function, n (%) | No function, n (%) | ||
| None | Open | 1 (3.2) | 30 (96.8) |
| Robotic | 0 (0) | 18 (100) | |
| Unilateral | Open | 12 (41.4) | 17 (58.6) |
| Robotic | 2 (20) | 8 (80) | |
| Bilateral* | Open | 19 (43.2) | 25 (56.8) |
| Robotic | 47 (62.7) | 28 (37.3) | |
p<0.05.
PED5: phosphodiesterase type-5.
Table 7.
Patient postoperative urinary continence stratified by nerve-sparing technique
| Nerve-sparing technique | Approach | Postoperative urinary continence | |
|---|---|---|---|
|
| |||
| Fully continent, n (%) | Incontinent, n (%) | ||
| None | Open | 22 (71) | 9 (29) |
| Robotic | 15 (83.3) | 3 (16.7) | |
| Unilateral | Open | 23 (79.3) | 6 (20.7) |
| Robotic | 9 (90) | 1 (10) | |
| Bilateral | Open | 37 (80.4) | 9 (19.6) |
| Robotic | 68 (90.7) | 7 (9.3) | |
Discussion
At our institution, we adopted a programmatic approach and assembled a core team of surgeons, nurses, administrators, and anesthetists who were dedicated to the initiation of the program and who consistently attended planning meetings, training sessions, and actual walk-through surgical rehearsals prior to the first case being done. All surgeons completed a standardized set of robotic surgical simulation programs on the da Vinci simulator and we included multiple surgeons with a consistent surgical team with the aim of mitigating some of the potential concerns with the learning phase of new technology.11
The present study reports three-year followup data on 225 patients who had prostatectomy performed during the first year of our robotic surgery program, 104 of which were performed robotically. We had permissive inclusion criteria, as is reflected in the similar baseline characteristics. Reassuringly, we did not observe worse cancer outcomes in RALRP patients compared to those treated by experienced surgeons using an open approach. Furthermore, the benefits of the laparoscopic approach were consistent with other series. RALRP patients had lower blood loss,3,4,6 fewer transfusions,6,12,13 and shorter length of hospital stay.11,12 In the current study, there were no intraoperative complications or conversions to an open approach. The overall postoperative complication rate in our study was 12.4%, and the rate was comparable between the two groups. Some studies have shown a decrease in complications associated with RALRP,14,15 others have simply shown equivalence to open cases;16,17 however, some evidence suggests the complications experienced with RALRP are less severe,12 consistent with our findings. Five percent of patients in the open group experienced a bladder neck contracture, while none of the patients in the robotic group experienced such a complication. The higher risk of anastomotic leak in the robotic cohort is of questionable clinical significance, and may be a reflection of detection bias since all RALRP patients had drain fluid creatinine analyzed.
While we believe our inclusive-surgeon approach facilitated good patient outcomes, we do not know if outcomes would have been better using a more restrictive-surgeon approach. To our knowledge, and not surprisingly, there are no randomized trials or observational studies comparing robotic implementation strategies. At least one published study using a single-surgeon approach has shown a potential decrease in patient outcomes during the learning phase, but it is unclear if this would have been improved or worsened using a multi-surgeon approach.18 Similarly, while there are small series of multi-surgeon approaches with favourable safety outcomes, it is unclear if this would have been altered with a single-surgeon series.19 Certainly, having a dedicated anesthesia and nursing team are important to facilitate the safe and efficient implementation of a robotic surgery program,20 and the team benefits by the larger number of cases that are possible by including multiple surgeons. Lastly, a surgeon mentor trained in robotic surgery is critical to program success.21,22
This is an observational study and associations we observed may be due to confounding or bias. The selection between treatments was based primarily on the preference of the surgeon evaluating the patient or the resources available. Another limitation was the inconsistent use of validated patient function instruments. It is also worth noting that this is a single-centre study and generalizability of our findings has not yet been established.
Conclusion
Using an inclusive multi-surgeon approach with a robotically trained mentor, RALRP was introduced safely at a Canadian academic institution. Short-term and long-term patient outcomes during the first year of our robotic surgery program were equivalent or better than patients treated with open surgery during the same time period. By including multiple surgeons, a high procedure volume was achieved and this allowed for a rapid attainment of experience for the anesthesia and dedicated nursing teams. We believe our approach should be considered by other institutions initiating a robotic surgery program.
Footnotes
See related commentary on page 44
Competing interests: Dr. Morash has been an advisor for Abbvie, Astellas, Ferring, Janssen, and Sanofi; and has participated in clinical trials supported by Abbvie. Dr. Lavallée has been an advisor for Ferring and Sanofi; and has received a grant from Sanofi. Dr. Cagiannos has been an advisor for Abbvie and Ferring; and has received speaker honoraria from Abbvie, Acerus, and Ferring. The remaining authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Yaxley JW, Coughlin GD, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy vs. open radical retropubic prostatectomy: Early outcomes from a randomized controlled phase 3 study. Lancet. 2016;388:1057–66. doi: 10.1016/S0140-6736(16)30592-X. https://doi.org/10.1016/S0140-6736(16)30592-X. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Agency for Drugs and Technologies in Health. Robot-assisted Surgery versus Open Surgery and Laparoscopic Surgery: Clinical Effectiveness and Economic Analyses. 2011. [Accessed Jan. 18, 2018]. Available at https://www.cadth.ca/robot-assisted-surgery-versus-open-surgery-and-laparoscopic-surgery-clinical-effectiveness-and. [PubMed]
- 3.Ahlering TE, Woo D, Eichel L, et al. Robot-assisted vs. open radical prostatectomy: A comparison of one surgeon’s outcomes. Urology. 2004;63:819–22. doi: 10.1016/j.urology.2004.01.038. https://doi.org/10.1016/j.urology.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 4.Farnham SB, Webster TM, Herrell SD, et al. Intraoperative blood loss and transfusion requirements for robotic-assisted radical prostatectomy vs. radical retropubic prostatectomy. Urology. 2006;67:360–3. doi: 10.1016/j.urology.2005.08.029. https://doi.org/10.1016/j.urology.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 5.Patel VR, Sivaraman A. Current status of robot-assisted radical prostatectomy: Progress is inevitable. Oncology. 2012;26:616–9. [PubMed] [Google Scholar]
- 6.Tewari A, Srivasatava A, Menon M, et al. A prospective comparison of radical retropubic and robot-assisted prostatectomy: Experience in one institution. BJU Int. 2003;92:205–10. doi: 10.1046/j.1464-410x.2003.04311.x. https://doi.org/10.1046/j.1464-410X.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 7.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs. open radical prostatec-tomy. JAMA. 2009;302:1557–64. doi: 10.1001/jama.2009.1451. https://doi.org/10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 8.Lowrance WT, Eastham JA, Savage C, et al. Contemporary open and robotic radical prostatectomy practice patterns among urologists in the United States. J Urol. 2012;187:2087–92. doi: 10.1016/j.juro.2012.01.061. https://doi.org/10.1016/j.juro.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canadian Institute for Health Information (CIHI) Analysis in Brief The Delivery of Radical Prostatectomy to Treat Men With Prostate Cancer. 2014. [Accessed Jan. 18, 2018]. Available at https://secure.cihi.ca/free_products/ProstateSurgeryinCanada_EN_web.pdf.
- 10.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. https://doi.org/10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steers WD, LeBeau S, Cardella J, et al. Establishing a robotics program. Urol Clin North Am. 2004;31:773–80. doi: 10.1016/j.ucl.2004.06.004. https://doi.org/10.1016/j.ucl.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Carlsson S, Nilsson AE, Schumacher MC, et al. Surgery-related complications in 1253 robot-assisted and 485 open retropubic radical prostatectomies at the Karolinska University Hospital, Sweden. Urology. 2010;75:1092–7. doi: 10.1016/j.urology.2009.09.075. https://doi.org/10.1016/j.urology.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 13.Punnen S, Meng MV, Cooperberg MR, et al. How does robot-assisted radical prostatectomy (RARP) compare with open surgery in men with high-risk prostate cancer? BJU Int. 2013;112:E314–20. doi: 10.1111/j.1464-410X.2012.11493.x. https://doi.org/10.1111/j.1464-410X.2012.11493.x. [DOI] [PubMed] [Google Scholar]
- 14.Trinh QD, Sammon J, Sun M, et al. Perioperative outcomes of robot-assisted radical prostatectomy compared with open radical prostatectomy: Results from the nationwide inpatient sample. Eur Urol. 2012;61:679–85. doi: 10.1016/j.eururo.2011.12.027. https://doi.org/10.1016/j.eururo.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Tewari A, Sooriakumaran P, Bloch DA, et al. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol. 2012;62:1–15. doi: 10.1016/j.eururo.2012.02.029. https://doi.org/10.1016/j.eururo.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 16.Ficarra V, Novara G, Fracalanza S, et al. A prospective, non-randomized trial comparing robot-assisted laparoscopic and retropubic radical prostatectomy in one European institution. BJU Int. 2009;104:534–9. doi: 10.1111/j.1464-410X.2009.08419.x. https://doi.org/10.1111/j.1464-410X.2009.08419.x. [DOI] [PubMed] [Google Scholar]
- 17.Barton MK. No cost or safety advantage to robot-assisted radical prostatectomy compared with open-procedure surgery for patients with prostate cancer. CA Cancer J Clin. 2014;64:293–4. doi: 10.3322/caac.21241. https://doi.org/10.3322/caac.21241. [DOI] [PubMed] [Google Scholar]
- 18.Povolotskaya N, Woolas R, Brinkmann D. Implementation of a robotic surgical program in gyneco-logical oncology and comparison with prior laparoscopic series. Int J Surg Oncol. 2015;2015:814315. doi: 10.1155/2015/814315. https://doi.org/10.1155/2015/814315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon EO, Bautista TC, Blumberg JM, et al. Rapid implementation of a robot-assisted prostatec-tomy program in a large health maintenance organization setting. J Endourol. 2010;24:461–5. doi: 10.1089/end.2009.0212. https://doi.org/10.1089/end.2009.0212. [DOI] [PubMed] [Google Scholar]
- 20.Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2017 doi: 10.1097/SPV.0000000000000413. [Epub ahead of print]. https://doi.org/10.1097/SPV.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 21.Luthringer T, Aleksic I, Caire A, et al. Developing a successful robotics program. Curr Opin Urol. 2012;22:40–6. doi: 10.1097/MOU.0b013e32834d5455. https://doi.org/10.1097/MOU.0b013e32834d5455. [DOI] [PubMed] [Google Scholar]
- 22.Zorn KC, et al. Training, credentialing, proctoring, and medicolegal risks of robotic urological surgery: Recommendations of the society of urologic robotic surgeons. J Urol. 2009;182:1126–32. doi: 10.1016/j.juro.2009.05.042. https://doi.org/10.1016/j.juro.2009.05.042. [DOI] [PubMed] [Google Scholar]