Abstract
Objective
To detect changes in finger photoplethysmography after administration of epidural anaesthesia as a surrogate method for evaluating autonomic nervous system activity.
Methods
We included a total of 46 patients scheduled for elective surgical procedures under lumbar epidural anaesthesia. A Biopac SS4LA pulse plethysmograph transducer was used for photoplethysmography recording, and the device was placed on the first toe of the right leg. The first standard lead of the electrocardiogram was simultaneously measured with the finger photoplethysmography. First measurement was done before the administration of epidural anaesthesia, and second measurement was done 25 minutes post administration of epidural anaesthesia.
Results
The area under the curve of the finger photoplethysmography statistically significantly increased 25 minutes after administration of epidural anaesthesia compared with the first measurement (p=0.0001). The amplitude of the finger photoplethysmography as well as the pulse transit time also statistically significantly increased after administration of epidural anaesthesia.
Conclusion
The area under the curve reflects the changes in sympathetic activity after epidural anaesthesia below the block level. It can be used for the detection of the degree of sympathetic block and, respectively, for epidural block success. Future prospects include detection of sympathetic block cessation as an indicator for discharge from the awakening room and beginning of patient verticalisation.
Keywords: Finger photoplethysmography, epidural anaesthesia, area under the curve, sympathetic blockade
Introduction
Several quantitative measurements can be performed to assess sympathectomy-induced vasodilation following regional anaesthesia. Many parameters derived from the photoplethysmographic waveform have been used to assess sympathectomy levels following regional anaesthesia (1–4). The area under the curve represents the whole surface area under the finger photoplethysmography waveform. The finger photoplethysmography amplitude represents the maximum height of the finger photoplethysmography waveform, and the pulse transit time represents the time it takes for the pulse pressure waveform to propagate through the arterial tree length. Our primary endpoint was to investigate the changes in the area under the curve of the finger photoplethysmography following lumbar epidural anaesthesia. Our secondary endpoint was to investigate the changes in the finger photoplethysmography amplitude and the pulse transit time changes. We hypothesised that the area under the curve as well as the amplitude and the pulse transit time of the finger photoplethysmography significantly increased after lumbar epidural administration with 0.5% bupivacaine.
Methods
After obtaining ethical approval from the Ethical Committee of the University Clinical Hospital Centre Zagreb, we initiated patient recruitment. Written informed consent was obtained from all patients included in the study. A total of 46 patients were scheduled for elective unilateral or bilateral inguinal hernia repair under lumbar epidural anaesthesia. All the patients were classified as either ASA 1 or ASA 2. Other inclusion criteria were age between 18 and 45 years, palpable pulses of the arteria tibialis posterior and arteria dorsalis pedis, normal nail growth and transparency and no external pathological alterations of the blood vessels of the lower extremities. Normal finger photoplethysmography waveform was observed before the administration of epidural anaesthesia in all patients. We excluded from the research patients with accompanying cardiovascular diseases (e.g. hypertension and Raynaud’s syndrome), patients taking preoperative cardiac therapy (particularly, vasoactive drugs) and obese patients (BMI>30). After overnight fasting, all patients received 7.5 mg oral midazolam 60 minutes before anaesthesia administration. Standard monitoring consisting of non-invasive blood pressure cuff, pulse oximeter and electrocardiogram were used to monitor patients throughout the procedure. An epidural space was identified in the L3/L4 interspace, in the sitting position of the patient by loss of resistance technique, using an 18-G Tuohy needle. An epidural catheter was placed 4–5 cm into the epidural space. Aspiration test was performed, and a test dose of 3 mL 0.5% bupivacaine was given. For epidural anaesthesia, a 1 mL/segment of 0.5% bupivacaine+0.1 mL of 0.5% bupivacaine for every 5 cm above a 150 cm height was applied to the patient in the lying position. The study was conducted in the operating theatre with a controlled ambient temperature of 24°C, and except the operating field, right foot and the head, patients were covered with a thin cotton blanket to avoid loss of body heat as much as possible. Body temperature monitoring was not utilised during the procedure. The pin-prick test was performed 20 minutes after the administration of epidural anaesthesia to test the targeted sensory T10 block height. Potential hypotension due to sympathectomy was defined as a drop in systolic blood pressure below 90 mmHg and was treated with boluses of 5–10 mg ephedrine. No patients received intravenous sedation throughout the procedure.
Biopac SS4LA pulse plethysmograph transducer, which uses a source of infra-red light and a photodetector (emitter/detector wavelength=860±900 nm), was used for photoplethysmography recording. The photoplethysmographic device was placed on the first toe of the right leg. Baseline measurement was performed after the patients received prehydration with 500 mL normal saline and before initiating the administration of epidural anaesthesia. The second measurement was performed 25 minutes after the administration of epidural anaesthesia. The first standard lead of the electrocardiogram was simultaneously measured with the photoplethysmographic signal. Besides prehydration, which was given to compensate for expected blood pressure decrease due to sympathectomy, 4 mL kg−1 h−1 for the first 10 kg of body mass+2 mL kg−1 h−1 for the next 10 kg of body mass+1 mL kg−1 h−1 for each kg above 20 kg of body mass were administered throughout the procedure to meet the physiologic fluid requirements. Also, an additional 2 mL kg−1 h−1 normal saline was administered to each patient throughout the rest of the procedure to compensate for evaporative fluid losses through the surgical field.
Every single measurement was performed in a sequence of 180 seconds and stored as a separate file. The software enables re-run of the stored files as a continuous signal and detailed analysis of every measured and stored photoplethysmographic signal. After collecting all the data, we started the measurement analysis. In each single measurement, a sequence that includes 50 photoplethysmographic pulsations with relatively low fluctuations was chosen for analysis. Photoplethysmographic waveform is simultaneously shown on the screen display with the first standard lead of the electrocardiogram (Figure 1).
Figure 1.
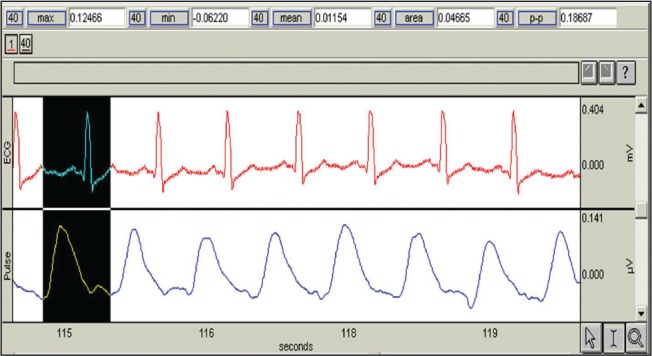
Operating display of the finger photoplethysmography signal
Besides the area under the curve of the finger photoplethysmography, the amplitude of finger photoplethysmography as well as the pulse transit time were also measured. The amplitude represents the maximum height of the photoplethysmographic waveform, and the pulse transit time was calculated by drawing a straight line from the middle of the R wave of the electrocardiogram to the beginning of the photoplethysmographic wave. The three parameters measured at the baseline and 25 minutes after the administration of epidural anaesthesia were compared. The sample size has been calculated according to a pilot study that included 10 patients, for the area under the curve and amplitude. The mean and standard deviation were calculated, and type I error along with the study power were set as 0.01 and 99%, respectively. The pulse transit time sample size fit in the calculated sample size based on the two above mentioned variables. Differences between the two measurements of the single parameters of the same examinees were tested using nonparametric tests for dependent samples (Wilcoxon matched-pairs test). P<0.05 was chosen as the statistically significant limit. Statistical analysis was performed using the programme Statistica 6.
Results
After excluding 6 patients from the research because of the exclusion criteria mentioned in the methods section, statistical analysis was performed in 40 patients. Age, body weight and body height of the patients are shown in Table 1. The area under the curve of the finger photoplethysmography statistically significantly increased 25 minutes after the administration of epidural anaesthesia compared with the baseline measurement. The amplitude of the finger photoplethysmography as well as the pulse transit time also statistically significantly increased after the administration of epidural anaesthesia. Results are shown in Table 2.
Table 1.
Demographic data of the patients
| Age (years) | 41 (23–45) |
| Body weight (kg) | 84 (60–102) |
| Body height (cm) | 179 (158–191) |
Data are presented as median (range).
Table 2.
Results
| Before anaesthesia n=40 |
During lumbar epidural anaesthesia n=40 |
p* | |
|---|---|---|---|
| Area under the curve (μVsec) | 0.042 (0.010–0.1244) | 0.2461 (0.0566–0.5490) | 0.0001 |
| Amplitude (μV) | 0.084 (0.028–0.213) | 0.542 (0.147–0.963) | 0.0001 |
| Pulse transit time (sec) | 0.3000 (0.2350–0.3458) | 0.3050 (0.2667–0.3600) | 0.007 |
Data are presented as median (range).
Wilcoxon’s pair test.
Discussion
The area under the curve of the finger photoplethysmography showed a statistically significant increase 25 minutes after the administration of 0.5% bupivacaine epidural block compared to the baseline value, which we attributed to the effect of the sympathetic block. The waveform of the finger photoplethysmography comprises the ascending and the descending part. The ascending part represents an expansion of the blood vessel wall under the influence of increased pressure, and the descending part represents relaxation of the blood vessel wall between the two heart beats. Both parts of the waveform change with changes in the arterial compliance. The amplitude of the finger photoplethysmography represents the maximum height of the finger photoplethysmography waveform. Similar to the ascending and the descending part of the waveform, amplitude also changes, that is, increases after an increase in the arterial compliance caused by the sympathetic block. Our results also demonstrated that the signal amplitude also increases during lumbar epidural block as the area under the curve of the photoplethysmographic signal increases. The time to peak effect of the epidural block with longer-acting local anaesthetics, such as bupivacaine, is 20–25 minutes (5). In our study, we observed a sensory block at the T10 level in all our patients using a pin-prick test 20 minutes after lumbar epidural administration of 0.5% bupivacaine. The nerve block caused by a local anaesthetic also occurs in a certain order: preganglionic sympathetic nerve fibres are first blocked followed by the nerve fibres responsible for pain, touch and motor function (6–11). With epidural anaesthesia, similar zones of differential sensory and sympathetic blocks are found (12). Therefore, the photoplethysmography derived from the first toe anatomically correlates with the blocked preganglionic sympathetic nerve fibres, because the sympathetic nerve fibres of the first toe arise from the spinal nerves up to the L4 level, which were obviously affected by the epidural block that reached the T10 level.
Besides the area under the curve and the amplitude, we also measured the pulse transit time, which represents the time needed for the pulse to travel from the heart to the peripheral blood vessel. Babchenko (13, 14) claims that the lumbar epidural anaesthesia, administered for operating procedures of the lower abdomen and lower extremities, is accompanied with a decreased sympathetic activity in the lower extremities and toes, which is reflected in a prolonged pulse transit time after the administration of lumbar epidural anaesthesia. From our research, it is evident that the pulse transit time is statistically significantly increased after lumbar epidural anaesthesia (p=0.009, Table 2), which correlates with Babchenko’s observations. Nitzan reports that decrease of the pulse transit time, due to the age of the examinees, is attributed to direct, age-conditioned, structural decreases in arterial compliance and not to the functional effects of age-related blood pressure increase. Nitzan also reports that the pulse transit time parameters are not dependent on diastolic pressure, although measurements are performed at the end of the diastole (15, 16). The average patient age was 41 years, so it can be assumed that among our patients, there were no structural changes of the blood vessel wall that could impact the arterial compliance. Babchenko (13, 14) highlights that arterial compliance increases because of decreased sympathetic activity, which results in higher arterial wall tension and higher pulse pressure speed. The results of our research also confirm the previous findings.
In addition, there were no hypotensive episodes observed in our patients during the first 25 minutes after the administration of the epidural block, hence, no vasoconstrictive agents were used during the period of our photoplethysmography recordings.
Conclusion
A statistically significant increase in the area under the curve of the finger photoplethysmography, as well as the amplitude of the finger photoplethysmography and the pulse transit time 25 minutes after the administration of the lumbar epidural block, is in a tight connexion with the increase in arterial compliance, which results from the decreased tone of the blood vessel muscle wall, as a consequence of the sympathetic block.
The area under the curve of the finger photoplethysmography is a reliable method for detecting changes in sympathetic activity caused by lumbar epidural anaesthesia. It can be used to detect the degree of the sympathetic block and respectively evaluate the success of the epidural sensory block. Future prospects include the detection of the sympathetic block cessation as an indicator for discharge from the awakening room and the beginning of the patient verticalisation.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of University Clinical Hospital Centre Zagreb.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – S.M., M.B.; Design – S.M., M.B.; Supervision – S.M., M.B.; Resources – S.M., M.B., K.R., K.S., M.C.; Materials – S.M., M.B., K.S., M.C.; Data Collection and/or Processing – M.B., K.R., K.S., M.C.; Analysis and/or Interpretation – S.M., K.R.; Literature Search – K.R., K.S., L.M.; Writing Manuscript – S.M., K.R., L.M.; Critical Review – S.M., L.M.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Ginosar Y, Weiniger CF, Meroz Y, Kurz V, Bdolah-Abram T, Babchenko A, et al. Pulse oximeter perfusion index as an early indicator of sympathectomy after epidural anesthesia. Acta Anaesthesiol Scand. 2009;53:1018–26. doi: 10.1111/j.1399-6576.2009.01968.x. https://doi.org/10.1111/j.1399-6576.2009.01968.x. [DOI] [PubMed] [Google Scholar]
- 2.Ginosar Y, Weiniger CF, Kurz V, Babchenko A, Nitzan M, Davidson E. Sympathectomy-mediated vasodilation: a randomized concentration ranging study of epidural bupivacaine. Can J Anaesth. 2009;56:213–21. doi: 10.1007/s12630-008-9036-z. https://doi.org/10.1007/s12630-008-9036-z. [DOI] [PubMed] [Google Scholar]
- 3.Kortekaas MC, Niehof SP, van Velzen MHN, Galvin EM, Huygen FJPM, Stolker RJ. Pulse transit time as a quick predictor of a successful axillary brachial plexus block. Acta Anaesthesiol Scand. 2012;56:1228–33. doi: 10.1111/j.1399-6576.2012.02746.x. https://doi.org/10.1111/j.1399-6576.2012.02746.x. [DOI] [PubMed] [Google Scholar]
- 4.Bergek C, Zdolsek JH, Hahn RG. Non-invasive blood haemoglobin and plethysmographic variability index during brachial plexus block. Br J Anaesth. 2015;114:812–7. doi: 10.1093/bja/aeu484. https://doi.org/10.1093/bja/aeu484. [DOI] [PubMed] [Google Scholar]
- 5.Bernards CM, Hoestetter LS. Epidural and spinal anesthesia. In: Barash PG, Cullen BF, Stoelting RK, Calahan MK, Stock MC, Ortega R, editors. Clinical Anesthesia. Philadelphia: Lippincott Williams and Wilkins; 2013. pp. 905–33. [Google Scholar]
- 6.Panier BM, Avolio AP, Hoeks A, Mancia G, Takazawa K. Methods and devices for measuring compliance in humans. Am J Hypertens. 2002;15:743–53. doi: 10.1016/s0895-7061(02)02962-x. https://doi.org/10.1016/S0895-7061(02)02962-X. [DOI] [PubMed] [Google Scholar]
- 7.Barron SA, Rogowski Z, Kanter Y, Hemli J. DC photoplethysmography in the evaluation of sympathetic vasomotor responses. Clin Physiol. 1993;13:561–72. doi: 10.1111/j.1475-097x.1993.tb00472.x. https://doi.org/10.1111/j.1475-097X.1993.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 8.Beene TK, Eggers GW., Jr Use of the pulse monitor for determining sympathetic block in the arm. Anesthesiology. 1974;40:412–4. doi: 10.1097/00000542-197404000-00023. https://doi.org/10.1097/00000542-197404000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Larsen R. Anästhesie. 5th ed. München Wien Baltimore: Urban&Schwarzenberg; 1995. [Google Scholar]
- 10.Brodner G, Meiβner A, Rolf N, Aken van H. Die thorakale epiduralanästhesie - mehr als eni anästhesieverfahren. Der Anaesthesist. 1997;46:751–62. doi: 10.1007/s001010050465. https://doi.org/10.1007/s001010050465. [DOI] [PubMed] [Google Scholar]
- 11.Kim JM, Arakawa K, Benson KT, Fox DK. Pulse oximetry and circulatory kinetics with pulse volume amplitude measured by photoelectric plethysmographiy. Anaesth Analg. 1986;65:1333–9. https://doi.org/10.1213/00000539-198612000-00015. [PubMed] [Google Scholar]
- 12.Brull SJ, Greene NM. Zones of differential sensory block during extradural anesthesia. Br J Anaesth. 1991;66:651. doi: 10.1093/bja/66.6.651. https://doi.org/10.1093/bja/66.6.651. [DOI] [PubMed] [Google Scholar]
- 13.Babchenko A, Davidson E, Adler D, Ginosar Y, Kurz V, Nitzan M. Increase in puls transit time to the foot after epidural anaesthesia treatment. Med Biol Eng Comput. 2000;38:674–9. doi: 10.1007/BF02344874. https://doi.org/10.1007/BF02344874. [DOI] [PubMed] [Google Scholar]
- 14.Babchenko A, Davidson E, Ginosar Y, Kurtz V, Faib I, Adler D, et al. Photoplethysmographic measurement of changes in total and pulsatile tissue blood volume, following sympathetic blockade. Physiol Meas. 2001;22:389–96. doi: 10.1088/0967-3334/22/2/310. https://doi.org/10.1088/0967-3334/22/2/310. [DOI] [PubMed] [Google Scholar]
- 15.Nitzan M, Khanokh B, Slovik Y. The difference in pulse transit time to the toe and finger measured by photoplethysmography. Physiol Meas. 2002;23:85–93. doi: 10.1088/0967-3334/23/1/308. https://doi.org/10.1088/0967-3334/23/1/308. [DOI] [PubMed] [Google Scholar]
- 16.Nitzan M, Babchenko A, Khanokh B, Landau D. The variability of the photoplethysmographic signal – a potential method for the evaluation of the autonomic nervous system. Physiol Meas. 1998;19:93–102. doi: 10.1088/0967-3334/19/1/008. https://doi.org/10.1088/0967-3334/19/1/008. [DOI] [PubMed] [Google Scholar]


