Abstract
Objectives
We compared nicotine concentrations in one brand of refill fluids that were purchased in 4 countries and labeled 0 mg of nicotine/mL. We then identified counterfeit e-cigarette products from these countries.
Methods
Overall, 125 e-cigarette refill fluids were purchased in Nigeria, the United States (US), England, and China. Nicotine concentrations were measured using high performance liquid chromatography and compared to labeled concentrations. Refill fluids were examined to identify physical differences and grouped into authentic and counterfeit products.
Results
Whereas nicotine was in 51.7% (15/29) of the Nigerian, 3.7% (1/27) of the Chinese and 1.6% (1/61) of the American refill fluids (range = 0.4 – 20.4 mg/mL), 8 British products did not contain nicotine. Products from China, the US, and Nigeria with trace amounts of nicotine (0.4 to 0.6 mg/mL) were authentic; however, all products from Nigeria with more than 3.7 mg/mL were counterfeit.
Conclusions
We introduce 2 novel issues in the e-cigarette industry, the production of counterfeit refill fluids under a brandjacked label and inclusion of nicotine in 81.3% of the counterfeit products labeled 0 mg/mL. This study emphasizes the need for better control and monitoring of nicotine containing products and sales outlets.
Keywords: electronic cigarettes, counterfeit tobacco-related products, nicotine, addiction, toxicity, public health, tobacco regulation
Nicotine is readily available in electronic cigarette (e-cigarette) refill fluids that are sold worldwide. Nicotine concentrations in these products range from 0 to over 100 mg/mL, and product concentration labels are often inaccurate.1–3 Even do-it-yourself flavoring products used to create these fluids sometimes contain nicotine.4 The widespread distribution and use of nicotine-containing e-cigarette products presents a new public health problem that could increase nicotine addiction, cause poisoning, and lead to other unwanted health effects.5–12
In this study we evaluated e-cigarette refill fluids produced by one manufacturer and sold worldwide. Specifically, we quantified nicotine in products labeled 0 mg/mL, evaluated products to determine authenticity, and identified counterfeit zero nicotine refill fluids that contained nicotine.
METHODS
Sample Collection and Assessment
Between March 2015 and May 2016, 125 of LiQua e-cigarette refill fluids (Ritchy Group Limited) were purchased in Nigeria (29 refill fluids, 7 flavors, purchased over-the-counter in an Abuja department store and at an online store in Lagos), the United States (61 refill fluids, 50 flavors, purchased over the Internet from Kansas and California), England (8 refill fluids, in 8 flavors, purchased over the Internet from Northamptonshire), and China (27 refill fluids, 25 flavors, purchased over the Internet from Xiamen and Guangdong). These countries were chosen to represent different: (1) global regions; (2) levels of economic development; and (3) levels of consumer product regulation and quality. Labeled nicotine concentration for all 125 products was “0 mg/mL,” which was interpreted as zero nicotine. Ritchy Group Limited is a Russian company with production plants in China and Italy and contact centers in Moscow, Kansas, the Czech Republic, and China that distributes products to over 85 countries (www.ritchy.com). Ritchy was chosen because of its broad global distribution of refill fluids, thereby enabling comparison of products purchased in the 4 countries. When possible, products with the same flavors were purchased in multiple countries.
Each product was assigned an inventory number, photographed, and stored at 4°C. All products were received sealed and undamaged, and were analyzed within one month of receipt. All products came in individual boxes, except those from Guangdong (China). Coloration of each fluid was compared visually.
Nicotine Concentration Quantification
We used high-performance liquid chromatography (HPLC) to quantify nicotine in each sample using a method described previously.3 The limit of quantification for nicotine was 10 μg/ml with a limit of detection of 50 ng/ml.
Authentication of E-cigarette Refill Fluids
We defined counterfeit products as ones not manufactured by Ritchy but sold under the Ritchy label. We used the Quick Response (QR) barcode, European Article Number (EAN) barcode, and guidelines from consumer websites to determine if refill fluids were authentic or counterfeit.13,14 We examined products for the presence of QR codes as recommended by personnel at Ritchy. QR codes on refill fluids have 5 sets of 4-digit numbers printed on white stickers located on the bottom or the caps of refill fluid boxes or bottles. These codes were either inputted or scanned into the verification section (www.ritchy.com/check) on the Ritchy website, which recognizes numbers that belong to authentic Ritchy products and distinguishes ones not generated by Ritchy.
We also accessed the globally used 13-digit EAN barcode, which identifies items for sale at retail establishments, for authentication. This barcode consists of: (1) the GS1 prefix which identifies the country where the product was manufactured or the member organization to which the manufacturer is registered; (2) the unique manufacturer’s identification code assigned by the GS1 office; (3) the item or product code which is selected by the manufacturer; and (4) the check digit which proves that the manufacturer has thoroughly inspected the item. EAN barcodes appeared on stickers pasted or printed directly on each refill fluid box or bottle and were scanned using ICONIT software. The user is directed to an Internet site that: (1) identifies the product as a Ritchy product; (2) fails to identify the product; or (3) identifies an incorrect Ritchy product indicating the barcode had been hijacked. A second line of EAN identification was performed using a government supported online database (www.gepir.gs1.org) that provides information on the company, products, and illegal EAN numbers.
In addition, we used guidelines from e-cigarette websites and forums to identify counterfeit LiQua products.13,14 These criteria included the quality of printing on boxes and bottles (which is inferior on counterfeit products), the appearance of identical product images on the Ritchy website, and the packaging of the product in a box at the time of receipt, which is characteristic of authentic Ritchy products. “Product Name on Database” was not available for the LiQua Q and LiQua HP products and some premium LiQua flavors.
RESULTS
Nicotine Concentrations in Zero Nicotine Products
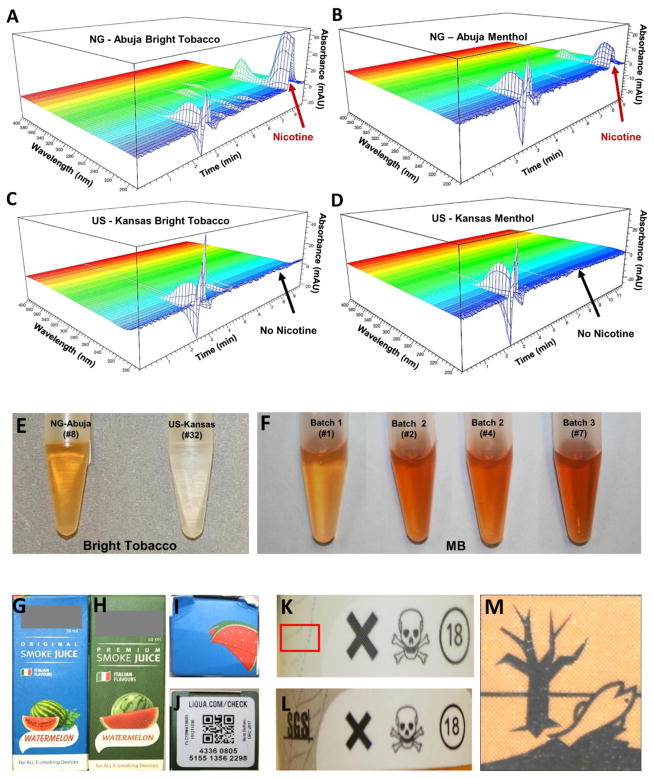
Nicotine was quantified in 125 LiQua e-cigarette refill fluids labeled 0 mg (Table 1, Figures 1A–D, and Supplementary Table 1). No nicotine was found in 108 samples (Table 1 and Supplementary Table 1). Figures 1A and 1B show Nigerian products that contained nicotine peaks as indicated by the red arrow at 8 min in Bright Tobacco flavor (A) and Menthol flavor (B). Figures C and D show that the same flavors purchased in the United States (US) contained no nicotine, as indicated by the black arrows. Samples of Two-Apples from the US, China, and Lagos contained trace amounts of nicotine (range = 0.4 to 0.6 mg/mL), probably due to contamination or carry over during manufacturing. In contrast, all LiQua Bright Tobacco, MB, and Menthol flavors purchased in Abuja (N = 13), contained 3.7 – 20.4 mg/mL of nicotine (Table 1). Nicotine concentrations varied within the same flavor purchased at separate times, eg, the first set of MB fluids contained 20.4 mg/mL of nicotine (product #1, Table 1), whereas the second (products #2 – #4) and third (products #5 – #7) sets contained 12.3 and 14.6 mg/mL, respectively.
Table 1.
Counterfeit and Suspected Counterfeit E-cigarette Refill Fluidsa
| # | Cob | Flavor | [Q] (mg/mL)c | Colorationd | QRe | EANf | Mfr. Nameg | Product Name on Database |
|---|---|---|---|---|---|---|---|---|
| 1 | NG-A | MB | 20.4 ± 0.3 | Coral | NC | IC | RGHK | Variety (0mg) |
| 2 | NG-A | MB | 12.3 ± 0.2 | Orange | NC | IC | RGHK | Variety (0mg) |
| 3 | NG-A | MB | 12.4 ± 0.2 | Orange | NC | IC | RGHK | Variety (0mg) |
| 4 | NG-A | MB | 12.3 ± 0.1 | Coral | NC | IC | RGHK | Variety (0mg) |
| 5 | NG-A | MB | 14.9 ± 0.4 | Deep Orange | NC | IC | RGHK | Variety (0mg) |
| 6 | NG-A | MB | 15.5 ± 0.4 | Deep Orange | NC | IC | RGHK | Variety (0mg) |
| 7 | NG-A | MB | 13.6 ± 0.6 | Deep Orange | NC | IC | RGHK | Variety (0mg) |
| 8 | NG-A | Bright Tob. | 13.6 ± 0.2 | Coral | NC | IC | RGHK | Energy Drink (18mg) |
| 9 | NG-A | Bright Tob. | 12.9 ± 0.5 | Light Orange | NC | IC | RGHK | Energy Drink (18mg) |
| 10 | NG-A | Menthol | 9.2 ± 0.0 | Clear (translucent) | NC | IC | RC: 13 | Illegal Number |
| 11 | NG-A | Menthol | 3.7 ± 0.0 | Clear | NC | IC | RC: 13 | Illegal Number |
| 12 | NG-A | Menthol | 4.2 ± 0.1 | Clear (translucent) | NC | IC | RC: 13 | Illegal Number |
| 13 | NG-A | Menthol | 4.1 ± 0.0 | Clear (translucent) | NC | IC | RC: 13 | Illegal Number |
| 14 | NG-A | Watermelon | ND | Clear | NC | IC | SLHK | No record found |
| 15 | NG-A | Watermelon | ND | Clear | NC | IC | SLHK | No record found |
| 16 | NG-A | Watermelon | ND | Clear | NC | IC | SLHK | No record found |
| 17 | CN-G | Two Apples | 0.4 ± 0.0 | Yellow | NB | NB | N/A | N/A |
| 18 | CN-G | Cola | ND | Clear | NB | NB | N/A | N/A |
| 29 | CN-G | Peach | ND | Clear | NB | NB | N/A | N/A |
| 20 | CN-G | Licorice | ND | Clear w/YT | NB | NB | N/A | N/A |
| 21 | CN-G | Brownie | ND | Clear | NB | NB | N/A | N/A |
| 22 | CN-G | Berry Mix | ND | Clear | NB | NB | N/A | N/A |
| 23 | CN-G | Cheesecake | ND | Clear | NB | NB | N/A | N/A |
| 24 | CN-G | Ry4 Tob. | ND | Pale yellow | NB | NB | N/A | N/A |
| 25 | CN-G | Bright Tob. | ND | Pale yellow | NB | NB | N/A | N/A |
| 26 | CN-G | Virginia Tob. | ND | Clear w/YT | NB | NB | N/A | N/A |
| 27 | CN-G | Traditional Tob. | ND | Pale yellow | NB | NB | N/A | N/A |
| 28 | CN-G | Mild Kretek Tob. | ND | Clear w/YT | NB | NB | N/A | N/A |
| 29 | CN-G | Red Oriental Tob. | ND | Clear w/YT | NB | NB | N/A | N/A |
| 30 | CN-G | Golden Oriental Tob. | ND | Clear w/YT | NB | NB | N/A | N/A |
| 31 | CN-G | American Blend Tob. | ND | Clear | NB | NB | N/A | N/A |
| 32 | CN-G | Goldenrod Oriental Tob. | ND | Clear w/YT | NB | NB | N/A | N/A |
| 33 | CN-G | Vermillion Oriental Tob. | ND | Yellow-Orange | NB | NB | N/A | N/A |
Note.
#1 – 16 were verified to be counterfeit using all criteria. Packaging for #17–33 was not available and these were suspected of being counterfeit. Supplementary Table 1 contains all authentic products
Co =Country and location of product purchase (NG-A = Nigeria, Abuja; CN-G = China, Guangdong)
[Q] = Quantified nicotine concentration (± standard deviation) using HPLC (ND = Not Detected)
Coloration = Color of the refill fluids (YT = yellow tint;)
QR = Availability and verification of manufacturer’s Quick Response Code (C = Correct code = Verified; NC/NB = No Code/No Box = Unverified)
EAN = Availability and verification of company and product information using the European Article Number barcode (IC = Incorrect; NB = None)
Mfr. Name = Name of manufacturer to which product EAN barcode is linked; RGHK = Ritchy Group Ltd HK; SLHK = Spoilt Ltd HK; RC:13 = Illegal/None; N/A = Not Available
Figure 1.
Comparison of 0 mg E-cigarette Refill Fluids and Identification of Counterfeit Products
Note.
(A–D) Three-dimensional HPLC chromatograms showing presence or absence of nicotine in e-cigarette products labeled 0 mg of nicotine. X axis = time (minutes), Y-axis = absorbance (mAU), and Z-axis = wavelength (nm).
(E–F) These are the color variations between identical refill fluids for Bright Tobacco Nigeria versus USA (E) and MB flavors (F).
(G–J) Differences in packaging between Watermelon from Nigeria (G) without a QR code (I) and USA (H) with a QR code for authentication (J).
(K –L) Warning labels and certification logos on Bright Tobacco refill fluid boxes purchased in the USA (K) without the SGS logo (red box) and in Nigeria (L) with the SGS logo.
The ecotoxic symbol (M) was present on only the counterfeit LiQua refill fluids.
Physical Properties of E-cigarette Refill Fluids
Within LiQua flavor groups, color varied by country, eg, Bright Tobacco purchased in Abuja was coral to light orange, but clear in other countries (Table 1, Supplementary Table 1, and Figure 1E). The color of LiQua MB flavors purchased in Abuja at separate times varied from coral to orange (Figure 1F). This color variation in counterfeit products is suggestive of inconsistencies during manufacture. Watermelon-flavored products purchased in Abuja were clear and identical to those purchased in the US (Table 1; Supplementary Table 1).
Labeling on Abuja products was fuzzy and of inferior quality compared to products from other countries which were of superior quality. Watermelon- flavored fluids from Abuja were in blue boxes without a QR code for authentication (Figures 1G and 1I), but the Kansas sample was in a green box with a QR code (Figures 1H and 1J) and was identical to the image on the Ritchy website. Bright Tobacco labels from Abuja were printed on a tan background, but labels from other countries were on white backgrounds that were identical to images on the Ritchy website. The MB flavor had no semblance to product images on www.ritchy.com but existed only on websites discussing “Fake LiQua e-juices.” Samples from Guangdong, China were not received with boxes; therefore, the semblance and quality of packaging could not be evaluated.
All products from Abuja had identical lot/batch numbers unlike products from other countries, which had different lot/batch numbers for each sample. Only the “variety pack of ten,” purchased from Santa Clara (California) and Xiamen had the same production lot/batch numbers on the fluids as well as on the variety pack box.
Identification of Counterfeit Products
We examined refill fluids to determine if they were counterfeit (Table 1) or authentic (Supplementary Table 1) using the QR code, EAN barcode, and differences in physical properties of the products. Using QR codes, we verified that products from the US (except for one), England, Lagos, and China (Xiamen) were authentic. Products from Abuja had no QR codes on their boxes and products from Guangdong (China) were received without boxes, and therefore, their authenticity could not be verified (Table 1).
We obtained additional information on counterfeit products using the EAN barcode (Table 1). Counterfeit Nigerian products were registered to: (1) Ritchy Group LTD but were linked to the incorrect product, eg, the 10 ml Bright Tobacco code identified it as a 30 ml Energy Drink; (2) Spoilt LTD, a different company, identified by the barcode as an “illegal number” (eg, watermelon); or (3) no company, meaning matching documents were unavailable (eg, menthol flavors) and it could not be verified (Table 1). All flavors from other locations had barcodes and were identical to flavors found on www.ritchy.com.
Labeling and Warning Symbols
All boxes had a skull and crossbones, over 18, and X (harmful) symbols (Figures 1K and 1L); however, only the counterfeit samples had the Société Générale de Surveillance (SGS) insignia and the ecotoxic symbol (Figures 1L and 1M). SGS is a worldwide organization that inspects, verifies, tests, and certifies that imported goods have been checked and meet quality control standards (www.sgs.com). Similar health warnings were reported on the bottles or boxes of all refill fluids.15 Only LiQua HP flavors stated that a user should “contact a poison center or seek medical assistance if you feel ill after use.”
Association between Nicotine and Counterfeit Refill Fluids
We used the above criteria to determine that 16/125 refill fluids labeled 0 mg were counterfeit products sold under a brandjacked label. About 81% (13/16) of the counterfeit products contained nicotine (3.7 – 20.4 mg/mL). The 3 counterfeit flavors with nicotine were MB, Menthol, and Bright Tobacco. Approximately 19% (3/16) of watermelon flavored LiQua, purchased in Abuja, were also counterfeit but did not contain nicotine.
DISCUSSION
We introduce novel issues in tobacco control and global health – the production of counterfeit e-cigarette refill fluids and the inclusion of nicotine in counterfeit products labeled 0 mg. The identification of nicotine in e-cigarette products that should be nicotine-free is a health concern for several reasons. First, zero-nicotine users with access to counterfeit products could develop an unwanted addiction that may be difficult to break. Secondly, a growing number of pregnant women use nicotine-free refill fluids16 and could unwittingly expose their fetuses/newborns to a neuroteratogen. 5 Thirdly, refill fluids containing nicotine have caused numerous poisonings, often in children;11,17 this potential danger is not apparent from the mislabeled counterfeit products. Finally, some ecigarette users gradually decrease nicotine use with e-cigarettes.18 If these users purchase counterfeit products containing nicotine, they may be unsuccessful in weaning themselves off nicotine.
Refill fluid users can identify counterfeit products using the criteria presented in this paper. Counterfeit fluids purchased in Abuja were ₦500.00 NGN in contrast to authentic products purchased from recommended LiQua distributors in Lagos for ₦1500.00 NGN. Although counterfeit products with nicotine were only purchased in Abuja, these products are readily distributable to other countries, and we had no difficulty bringing them into the USA. In addition, the counterfeit products varied in color within flavors, suggesting inconsistencies in their manufacture.
Unlike earlier generations,19 the authentic products in this study were generally labeled with safety warnings and reasonably accurate nicotine concentrations. LiQua Q flavors purchased in California carried the Proposition 65 warning stating the product contains substances that may cause cancer or produce reproductive/developmental problems. 20 However, only LiQua HP flavors contained warnings such as not recommended for non-smokers, contact with skin maybe toxic, keep out of reach of children and pets, and contact a poison center if you feel ill after use. The SGS logo implies products have undergone supervision and quality control from acquisition of raw materials through manufacturing to final production and distribution. Users of refill fluids should be skeptical of this logo as it appeared only on counterfeit products.
Counterfeit products have been problematic in the conventional tobacco cigarette industry.21 Our study demonstrates that the problem of counterfeit products extends to the e-cigarette retail market. However, because our study is limited to products from one company and 4 countries, future studies are needed to determine the breadth of counterfeit e-cigarette sales.
Conclusions
This is the first report that counterfeit e-cigarette products with inaccurate nicotine labeling and invalid quality control certification logos are being produced under a brandjacked label. Users of these products would be exposed to nicotine without their knowledge, which could lead to unwanted nicotine-induced health effects, as recently summarized by the US Surgeon General.12 In addition, the counterfeit products varied in color within flavors, suggesting inconsistencies in their manufacture. These data will be useful in establishing regulatory policies for e-cigarettes.
IMPLICATIONS FOR TOBACCO REGULATION
We introduce a new issue in the emerging e-cigarette industry, the inclusion of nicotine in counterfeit products labeled 0 mg/mL. Nicotine also has been reported in some DIY e-cigarette flavor products that should be nicotine-free.4 Mislabeled counterfeit and DIY e-cigarette products containing nicotine are a public health concern that could be addressed by agencies involved in the regulation of tobacco products. These findings emphasize the need for education of e-cigarette users to the existence of zero-nicotine products that contain nicotine and for identification and confiscation of counterfeit products.
Supplementary Material
Acknowledgments
Grant number R21DA037365 to PT from the National Institutes of Health (NIH) and the FDA Center for Tobacco Products supported this research. EO was supported in part by California Institute for Regenerative Medicine Bridges award grant number TB01-0777 and BD was supported by a National Science Foundation Predoctoral Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the FDA, or other granting agencies. The authors thank Rachel Behar for her helpful comments on the manuscript and Paul Gerald Iyaji and Baba Onotu for making the Nigeria products available for analysis. Use of trade names is only for information purpose and does not imply endorsement.
Footnotes
Conflict of Interest Statement
No conflicts of interest are reported by any of the authors.
Contributor Information
Esther E. Omaiye, Graduate Student, Environmental Toxicology Graduate Program, University of California, Riverside, CA.
Iliana Cordova, Undergraduate Student, Department of Cell Biology and Neuroscience, University of California, Riverside, CA.
Barbara Davis, Graduate Student, Department of Cell Biology and Neuroscience, University of California, Riverside, CA.
Prue Talbot, Professor, Department of Cell Biology and Neuroscience, University of California Riverside, CA.
References
- 1.Trehy ML, Ye W, Hadwiger ME, et al. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. Journal of Liquid Chromatography & Related Technologies. 2011;34(14):1442–1458. [Google Scholar]
- 2.Cameron JM, Howell DN, White JR, et al. Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tob Control. 2013;23(1):77–78. doi: 10.1136/tobaccocontrol-2012-050604. [DOI] [PubMed] [Google Scholar]
- 3.Davis B, Dang M, Kim J, Talbot P. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob Res. 2014;17(2):134–141. doi: 10.1093/ntr/ntu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis B, Razo A, Nothnagel E, et al. Unexpected nicotine in do-it-yourself electronic cigarette flavourings. Tob Control. 2015;25(e1):e67–e68. doi: 10.1136/tobaccocontrol-2015-052468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122(2):125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49(1):57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantrell FL. Adverse effects of e-cigarette exposures. J Community Health. 2013;39(3):614–616. doi: 10.1007/s10900-013-9807-5. [DOI] [PubMed] [Google Scholar]
- 8.Kim JW, Baum CR. Liquid nicotine toxicity. Pediatr Emerg Care. 2015;31(7):517–521. doi: 10.1097/PEC.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 9.Winer S. [Accessed January 27, 2017];Police investigating toddler’s death from nicotine overdose. Available at: http://timesofisrael.com/police-investigating-toddler-death-from-nicotine-overdose.
- 10.Mohney G. [Accessed January 27, 2017];First child’s death from liquid nicotine as “Vaping” grows. Available at: http://abcnews.go.com/Health/childs-death-liquid-nicotine-reported-vapinggains-popularity/story?id=27563788.
- 11.Hua M, Talbot P. Potential health effects of electronic cigarettes: a systematic review of case reports. Prev Med Rep. 2016;4:169–178. doi: 10.1016/j.pmedr.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services (DHHS), Office of the Surgeon General. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: DHHS; 2016. [PubMed] [Google Scholar]
- 13. [Accessed January 27, 2017];How to Spot Fake LiQua Juice. Available at: https://plainvape.wordpress.com/2013/11/10/how-to-spot-fakeliqua-juice/
- 14. [Accessed January 27, 2017];I doubt this LiQua e-liquid is Original: FastTech Counterfeit Electronic Cigarette Products with Mislabeled Nicotine Concentrations 354 forums. doi: 10.18001/TRS.3.3.10. Available at: https://www.fasttech.com/forums/1297106/t/1127136/i-doubt-this-liqua-e-liquidis-original. [DOI] [PMC free article] [PubMed]
- 15.Kim S, Goniewicz M, Yu S, et al. Variations in label information and nicotine levels in electronic cigarette refill liquids in South Korea: regulation challenges. Intl J Environ Res Public Health. 2015;12(5):4859–4868. doi: 10.3390/ijerph120504859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mark KS, Farquhar B, Chisolm MS, et al. Knowledge, attitudes, and practice of electronic cigarette use among pregnant women. J Addict Med. 2015;9(4):266–272. doi: 10.1097/ADM.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 17.American Association of Poison Control Centers. [Accessed January 27, 2017];E-cigarettes and liquid nicotine data. Available at: https://aapcc.s3.amazonaws.com/files/library/E-cig__Nicotine_Web_Data_through_12.2016.pdf.
- 18.McQueen A, Tower S, Sumner W. Interviews with “Vapers”: implications for future research with electronic cigarettes. Nicotine Tob Res. 2011;13(9):860–867. doi: 10.1093/ntr/ntr088. [DOI] [PubMed] [Google Scholar]
- 19.Trtchounian A, Talbot P. Electronic nicotine delivery systems: is there a need for regulation? Tob Control. 2010;20(1):47–52. doi: 10.1136/tc.2010.037259. [DOI] [PubMed] [Google Scholar]
- 20.Office of Environmental Health Hazard Assessment. State of California Environmental Protection Agency Office of Environmental Health Hazard Assessment Safe Drinking Water and Toxic Enforcement Act of 1986. [Accessed January 27, 2017];Chemicals Known to the State to Cause Cancer or Reproductive Toxicity. 2016 Available at: http://oehha.ca.gov/media/downloads/proposition-65//p65single10212016.pdf.
- 21.National Research Council and Institute of Medicine. Understanding the U.S. Illicit Tobacco Market: Characteristics, Policy Context, and Lessons from International Experiences. Washington, DC: The National Academies Press; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.