Abstract
Adolescent brothers were diagnosed with testicular germ cell tumors within the same month. Both were found to have multiple renal cysts on pre-treatment imaging done for staging. The proband, his brother, and their mother, were all found to have a novel splice variant in intron 8 of the PKD1 gene by clinical exome sequencing. This is the second family ever reported with both FTGCT and ADPKD, and the first described association of FTGCT with a splice variant in PKD1. We suggest this novel variant in PKD1 may convey increased risk for FTGCT in addition to causing ADPKD.
Keywords: PKD1, familial testicular germ cell tumor, exome sequencing
Introduction
Familial testicular germ cell tumors (FTGCT) have been well-described and thought to be secondary to the combined effect of multiple, common, low-penetrance risk alleles. Several single nucleotide polymorphisms (SNPs) have been implicated as risk alleles. Autosomal dominant polycystic kidney disease (ADPKD) is well known to be caused by pathogenic variants in PKD1 and PKD2 mutations. PKD1 encodes the protein polycystin-1 thought to play a role in cell growth, proliferation, migration, and cell-to-cell interactions in ciliated epithelia including the testes. To date, only one report of FTGCT and a PKD1 mutation has been described. We describe two brothers with FTGCT and ADPKD likely due to a PKD1 mutation identified using clinical exome sequencing (CES).
Case Report
The proband, a 17 year old male, presented with right testicular enlargement and frank hematuria. A testicular ultrasound was performed, revealing a testicular mass. Pathology from a right orchiectomy confirmed the diagnosis of testicular germ cell tumor (embryonal carcinoma 60%, teratoma 35%, yolk sac tumor 5%). A CT of the abdomen and pelvis was done as part of his staging workup and incidentally found bilateral multicystic kidneys.
Approximately two weeks later, his 15 year old brother, noticed left testicular enlargement. He had a testicular ultrasound and a left orchiectomy which confirmed a testicular germ cell tumor (embryonal carcinoma 30%, teratoma 70%). He also had a CT of the abdomen and pelvis as part of his staging workup and was found to have bilateral multi-cystic kidneys like his brother.
Both brothers were treated with two cycles of bleomycin, etoposide, and cisplatin (BEP), which was tolerated well in each case. Because of the presence of testicular germ cell tumors and polycystic kidney disease in both brothers, the family was determined to be candidates for CES in order to determine a possible genetic link for these findings.
Methods
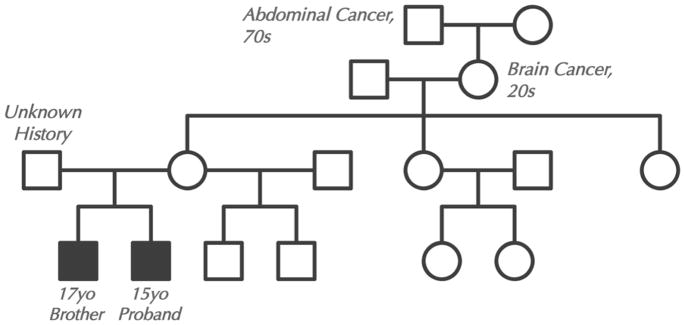
Family history with a four-generation pedigree was obtained (Figure 1). Blood samples were collected after informed consent on the proband, his brother, and their mother. The father was not available for testing and his medical history was unknown. CES was performed in a CLIA approved clinical lab at the UCLA Clinical Genomics Center using the standard protocol. Agilent SureSelect Human All Exon 50 Mb XT kit was used for exome capture and Illumina HiSeq 2500 was used for sequencing. To confirm the results obtained by CES, a peripheral blood sample from the brother was sent to Prevention Genetics. DNA was extracted using a 5 Prime ArchivePure DNA Blood Kit. PCR was used to amplify the implicated exons and flanking non-coding sequence. Sequencing was done using the ABI Big Dye Terminator v.3.0 kit. Products were resolved by electrophoresis on an ABI 3730xl capillary sequencer. Sequencing was performed in both the forward and reverse directions.
Figure 1.
Four generation pedigree.
Results
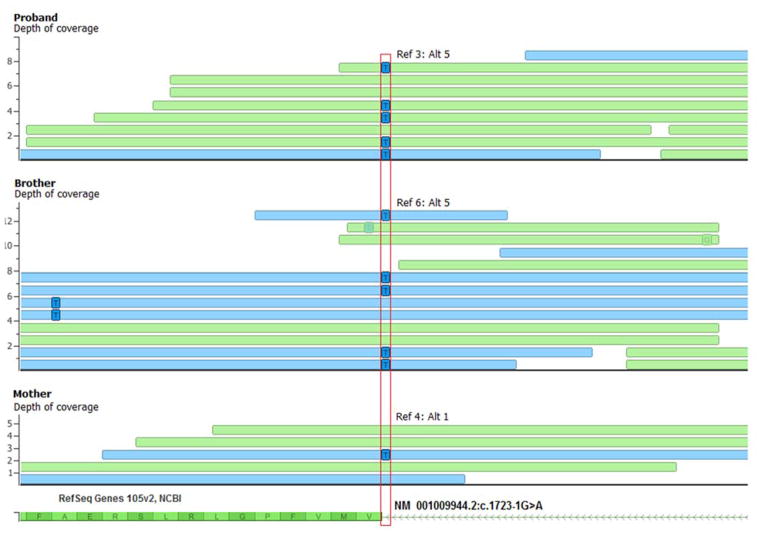
CES identified, in both brothers, a maternally inherited NM_001009944.2:c.1723-1G>A variant predicted to disrupt the canonical splice acceptor site in intron 8 of the PKD1 gene (Figure 2). Due to its disruption of the splice acceptor site, it is expected to be a pathogenic variant. Variants in the PKD1 gene are already known to be associated with autosomal dominant polycystic kidney disease (ADPKD). However, this variant is novel, as it has not been previously observed in any context, including the ExAC database of over 63,000 human exomes. No additional variants in cancer predisposition genes were identified through CES. Interestingly, a survey of PKD1 mutations in COSMIC (Catalogue of Somatic Mutations in Cancer) reveals overabundance of specific missense and truncating variants in a number of tumors, including testicular germ cell tumors, suggesting a potential role for somatic PKD1 variants in oncogenesis.
Figure 2.
Exome sequencing reads (blue: forward reads; green: reverse reads) around the PKD1 variant c.1723-1G>A (red vertical box) are plotted for the proband, brother, and mother with only the alternate allele shown in squares within each read. Y-axis is depth-of-coverage and number of reads supporting the reference allele (Ref: C) and alternate allele (Alt: T) is noted for each case.
Discussion
The finding of both FTGCT and ADPKD in this family is unusual, as this has only been reported in one other family to date with several members affected with ADPKD, and in which the proband and his father also had testicular seminoma. In that family, they noted a possible linkage of testicular seminoma to PKD1, but a specific mutation was not identified [2]. We report a family with two brothers affected with both FTGCT and ADPKD, in which we identified a maternally inherited PKD1 mutation.
ADPKD is known to be associated with heterozygous mutations in the PKD1 gene, located on chromosome 16p13.3. This accounts for 80–85% of cases. This leads to a defective polycystin 1 protein causing alteration in cell signaling pathways. This results in increased cell growth and fluid accumulation, causing the progressive development of multiple cysts and often leading to end stage renal disease. Frameshift, missense, nonsense, splicing, insertion, and rearrangement mutations have all been identified [10]. Specifically, a variant in the same splice acceptor we identified, c.1723-2A>C, has been previously reported as a cause of ADPKD, supporting this newly identified variant is also pathogenic.
FTGCT has been determined to be due to the combination of several genetic mutations as opposed to a mutation in one high-penetrance allele as originally hypothesized. Using genome-wide association study (GWAS) analysis, over 25 SNPs involving pathways such as KIT/KITLG signaling, alternative pathways of male germ cell development and differentiation, telomerase function, microtubule assembly and DNA damage repair have been identified in both familial and sporadic testicular germ cell tumors [8]. Together, a number of these mutations increase the relative risk of being affected with TGCT. PKD1, until now, has not been described as one of these involved genes.
Based on the findings in this family, not only does the novel variant found contribute to ADPKD, but it likely also increases the risk of FTGCT in the male members of the family. As abdominal CT imaging is a standard part of the staging workup for patients with FTGCT, polycystic kidney disease should be identified if present. Alternatively, in those patients with ADPKD, if they are found to have the identified PKD1 c.1723-1G>A variant, an increased risk for FTGCT should be considered.
Acknowledgments
Support was provided by the David Geffen School of Medicine, UCLA Children’s Discovery and Innovation Institute, UCLA Jonsson Comprehensive Cancer Center, and UCLA Clinical Genomics Center.
Abbreviations Key
- ADPKD
Autosomal Dominant Polycystic Kidney Disease
- FTGCT
Familial Testicular Germ Cell Tumor
- CES
Clinical Exome Sequencing
Footnotes
Conflict of Interest Statement
No conflicts of interest to disclose.
References
- 1.Greene MH, Mai PL, Loud JT, et al. Familial testicular germ cell tumors (FTGCT) – overview of a multidisciplinary etiologic study. Andrology. 2014 Oct 9; doi: 10.1111/andr.294. [DOI] [PubMed] [Google Scholar]
- 2.Teh BT, Linblad K, Nord B, et al. Familial testicular cancer: lack of evidence for trinucleotide repeat expansions and association with PKD1 in one family. J Med Genet. 1999 Apr;36(4):348–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Jehani RM, Povey S, Delhanty JD, et al. Loss of heterozygosity on chromosome arms 5q, 11p, 13q, and 16p in human testicular germ cell tumors. Genes Chromosomes Cancer. 1995 Aug;13(4):249–56. doi: 10.1002/gcc.2870130404. [DOI] [PubMed] [Google Scholar]
- 4.Exome Aggregation Consortium. The Broad Institute; Retrieved January 2015 from http://exac.broadinstitute.org. [Google Scholar]
- 5.The European Polycystic Kidney Disease Consortium. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell. 1994;77(6):881–894. doi: 10.1016/0092-8674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 6.Audrezet MP, Cornec-Le Gall E, Chen JM, et al. Autosomal dominant polycystic kidney disease: comprehensive mutation analysis of PKD1 and PKD2 in 700 unrelated patients. Hum Mutat. 2012;33(8):1239–1250. doi: 10.1002/humu.22103. [DOI] [PubMed] [Google Scholar]
- 7.Ruark E, Seal S, McDonald H, et al. Identification of nine new susceptibility loci for testicular cancer, including variants near DAZL and PRDM14. Nat Genet. 2013 Jun;45(6):686–9. doi: 10.1038/ng.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litchfield K, Shipley J, Turnbull C. Common variants identified in genome-wide association studies of testicular germ cell tumour: an update, biological insights and clinical application. Andrology. 2015 Jan;3(1):34–46. doi: 10.1111/andr.304. [DOI] [PubMed] [Google Scholar]
- 9.Simms RJ. Autosomal dominant polycystic kidney disease. BMJ. 2016 Feb 11;352:j679. doi: 10.1136/bmj.i679. [DOI] [PubMed] [Google Scholar]
- 10.Audrezet MP, Cobiere C, Lebbah S, et al. Comprehensive PKD1 and PKD2 Mutation Analysis in Prenatal Autosomal Dominant Polycystic Kidney Disease. J Am Soc Nephrol. 2015 Jul 9; doi: 10.1681/ASN.2014101051. pil: ASN.2014101051. [DOI] [PMC free article] [PubMed] [Google Scholar]