Abstract
The effects of the most potent aryl hydrocarbon receptor (AhR) agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on bile acid (BA) homeostasis was examined in male and female wild-type and AhR-null mice shortly after 4-day exposure, rather than at a later time when secondary non-AhR dependent effects are more likely to occur. TCDD had similar effects on BA homeostasis in male and female mice. TCDD decreased the concentration of total- (Σ) BAs in liver by approximately 50% (all major BA categories except for the non-6,12-OH BAs), without decreasing the expression of the rate limiting BA synthetic enzyme (Cyp7a1) or altering the major BA regulatory pathways (FXR) in liver and intestine. Even though the Σ-BAs in liver were markedly decreased, the Σ-BAs excreted into bile were not altered. TCDD decreased the relative amount of 12-OH BAs (TCA, TDCA, CA, DCA) in bile and increased the biliary excretion of TCDCA and its metabolites (TαMCA, TUDCA); this was likely due to the decreased Cyp8b1 (12α-hydroxylase) in liver. The concentration of Σ-BAs in serum was not altered by TCDD, indicating that serum BAs do not reflect BA status in liver. However, proportions of individual BAs in serum reflected the decreased expression of Cyp8b1. All these TCDD-induced changes in BA homeostasis were absent in AhR-null mice. In summary, through the AhR, TCDD markedly decreases BA concentrations in liver and reduces the 12α-hydroxylation of BAs without altering Cyp7a1 and FXR signaling. The TCDD-induced decrease in Σ-BAs in liver did not result in a decrease in biliary excretion or serum concentrations of Σ-BAs.
Keywords: Aryl hydrocarbon receptor, TCDD, bile acids, biliary excretion, taurodeoxycholic acid, Cyp8b1
INTRODUCTION
Efficient biliary excretion is crucial for (1) protecting the organism from harmful xenobiotics (metabolism, and excretion), (2) elimination of excess or toxic endogenous compounds and (3) metabolic/endocrine coordination of liver, intestine, and intestinal microbiome. The primary driving force of the enterohepatic circulation is the synthesis and secretion of bile acids (BAs) into the bile canaliculi by the hepatocytes, and the active re-uptake of BAs by intestine and liver.
Amphiphilic BAs are synthesized from cholesterol through multiple enzymatic steps in the classic (neutral) and alternative (acidic) BA synthetic pathways. The rate-limiting enzyme of the BA synthesis is cholesterol 7α-hydroxylase (CYP7A1), a cytochrome P450 enzyme that converts cholesterol to 7α-hydroxycholesterol in the classic pathway. The alternative pathway (Cyp27a1 and Cyp7b1) has been reported to contribute an average of 9%, 35%, 55% and 50% to the total bile acid synthesis in humans, female mice, male mice and rats, respectively (Vlahcevic et al., 1997; Duane and Javitt, 1999; Schwarz et al., 2001). The sterol 12α-hydroxylase (CYP8B1) enzyme is solely responsible for the biosynthesis of the trihydroxy-BA, cholic acid (CA), in both pathways. Therefore the activity of Cyp8b1 controls the hydrophobicity of the BA pool through the ratio of CA to chenodeoxycholic acid (CDCA) in humans (Gafvels et al., 1999; Vlahcevic et al., 2000). In addition to CA and CDCA in rodents, 6-OH BAs, namely α- and β-muricholic acids (MCA) are formed. After synthesis, the primary BAs are conjugated with glycine or taurine to increase solubility and decrease pKa. Conjugated BAs are actively transported into the biliary canaliculi by the bile salt export protein (Bsep) and Multidrug resistance-associated protein 2 (Mrp2) (Keppler et al., 1999; Stieger et al., 2007). In the intestinal lumen, primary BAs are further metabolized by the intestinal microbiome, producing secondary bile acids such as deoxycholic acid (DCA) and lithocholic acid (LCA). BAs are reabsorbed in the terminal ileum by the apical sodium-dependent bile acid transporter (Asbt) and are effluxed into the portal circulation by organic solute transporter (Ost)α+β (Hagenbuch and Dawson, 2004). In the liver, the Ntcp and Oatp1b2 transporters are responsible for the uptake of conjugated and unconjugated BAs (Ananthanarayanan et al., 1988; Cattori et al., 2000; Csanaky et al., 2011; Slijepcevic et al., 2015).
BA concentrations and composition of the BA pool is regulated primarily by the intestinal FXR (through Fgf15-Fgfr4) and hepatic FXR (through Shp) that regulate BA synthesis and transporters (Goodwin et al., 2000; Inagaki et al., 2005). Adaptive responses in the biliary excretion for elimination of xeno- and endogenous compounds is crucial for survival. Therefore environmental chemicals and endogenous compounds are detected by various nuclear receptors and other transcription factors (e.g. AhR, PXR, CAR, PPARα, Nrf2) that regulate drug metabolism genes and transporters (Aleksunes and Klaassen, 2012). It can be hypothesized that these nuclear receptors have an impact not only on drug metabolism genes but also have effects on BA homeostasis as part of the adaptive response to xenobiotics.
One of these xenosensors is the aryl hydrocarbon receptor (AhR), which is a member of the helix-loop-helix receptors (Burbach et al., 1992). AhR is activated by several exogenous ligands such as dioxin-like compounds, polycyclic aromatic hydrocarbons, plant flavonoids, polyphenols, indoles and endogenous tryptophan metabolites (Murray et al., 2014). Activated AhR translocates to the nucleus where it heterodimerizes with the AhR nuclear translocator (ARNT) (Reyes et al., 1992). The AhR-ARNT complex binds to aryl hydrocarbon response elements (AHREs) and causes changes in the transcription of target genes such as Cyp1a1 (Probst et al., 1993). AhR activation is also essential for normal organ development, regulation of immune response and the endocrine system, and upregulation of drug metabolism enzymes (e.g. Cyp1a1, 1a2, 1b1, Ugt1a1). However continuous activation of AhR receptor leads to weight loss, immunosuppression, hepatic steatosis, and cancers (Nebert, 2017).
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is the most potent exogenous model activator of the AhR, and this receptor is necessary to mediate most of the toxicity of TCDD (Fernandez-Salguero et al., 1996). Chronic TCDD exposure leads to increased lipid accumulation in the liver, causing steatosis, steatohepatitis, and fibrosis (Nault et al., 2016; Nault et al., 2017). TCDD increases hepatic cholesterol and the excess cholesterol enhances BA production, but later Cyp7a1 is repressed (Fletcher et al., 2005; Dere et al., 2011; Kakizuka et al., 2015). TCDD also decreases bile flow in a dose-dependent manner, with a gradually delayed onset reaching the lowest rate between 10 and 20 days after TCDD administration (Yang et al., 1977). BA excretion in rats decreased 10 days after a single dose of 25 μg/kg TCDD (Peterson et al., 1979). Adding CA and dehydro-CA to feed decreases the lethality of TCDD in mice, whereas adding cholestyramine increases the lethality (Manara et al., 1982). However, in rats, adding CA to the lab chow did not decrease the lethality caused by high dose TCDD (Manara et al., 1984). UDCA was also found protective against TCDD-induced testicular injury in mice (Kwon et al., 2004). It was also found that TCDD augments liver damage in bile duct-ligated mice (Ozeki et al., 2011)
Although the previous studies strongly suggest that BA homeostasis is changed by TCDD, none systematically examined the early (direct) effects of the model AhR activator compound TCDD on BA homeostasis. Therefore the present study aimed to examine the short-term (5-day) effects of TCDD on individual bile acids, synthetic enzymes, transporters and regulators in wild-type and AhR-null mice. Because some responses to TCDD have been reported to be different in male and female mice (Lee et al., 2015), studies were done in both male and female mice to reveal possible gender-specific effects of TCDD on bile acid homeostasis. It is also known that TCDD and its analogs can affect the composition of the microbiome (Zhang et al., 2015), therefore, in the present study, TCDD was administered intraperitoneally to decrease the potential direct effects of TCDD on the intestinal microbiome.
MATERIALS AND METHODS
Chemicals
Bile acid standards were purchased from Steraloids, Inc. (Newport, RI) and Sigma-Aldrich (St. Louis, MO). The AhR ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) was a generous gift from Dr. Karl K. Rozman (University of Kansas Medical Center, Kansas City, KS). All other chemicals including corn oil were purchased from Sigma-Aldrich unless otherwise noted.
Animals
Male and female C57BL/6 wild-type (WT) mice were obtained from Charles River Laboratories, Inc. (Wilmington, MA). Mice were acclimated for at least one week in a standard temperature-, light-, and humidity-controlled facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Mice were provided Laboratory Rodent Chow 8604 (Harlan, Madison, WI) and drinking water ad libitum. Studies were approved by the Institutional Animal Care and Use Committee of University of Kansas Medical Center. To use mice of approximately the same weight, male mice were used at 12-15 weeks of age, whereas females were 16–19 weeks of age.
Tissue collection following TCDD treatment
Although TCDD induces the mRNA expression of Cyp1a1 within 24 hrs (Forgacs et al., 2013), the increase in protein of other AhR target Cyps are delayed after 72 hrs of TCDD exposure (Santostefano et al., 1997). Evaluating the short-term effects of TCDD on BA homeostasis also requires a few enterohepatic recirculations of BAs to reach a new steady state before the obvious onset of long-term (wasting) effects of TCDD. Based on these considerations, we used the dosing schedule of our previous study to maximize the short-term AhR activation, also, this dosing schedule makes the results comparable with our other nuclear receptor agonists studies (Petrick and Klaassen, 2007; Lickteig et al., 2016). Corn oil (5 ml/kg) or TCDD (37 μg/kg/5 ml in corn oil) was administered intraperitoneally (i.p.) for four consecutive days to male and female mice (6–8 mice per group). At 24 hours after the last administration of TCDD, mice were anesthetized with 50 mg/kg pentobarbital (Nembutal, Lundbeck Inc, Deerfield, IL), blood was collected from the suborbital veins, and livers and ilea were harvested. Serum samples were separated using Microtainer separating tubes (BD Biosciences, San Jose, CA). Samples were frozen in liquid nitrogen and stored at −80°C until further analysis.
Bile collection following TCDD treatment
To assess the effect of TCDD on biliary excretion of BAs, separate groups of male and female mice were treated with either corn oil vehicle or TCDD as mentioned above. On day 5, mice were anesthetized with a ketamine/midazolam mixture (100 and 5 mg/kg, respectively, i.p.) and the common bile duct of each mouse was cannulated with the shaft of a 30-gauge needle attached to PE-10 tubing. Bile was collected for 40 min in pre-weighed 0.6-ml microcentrifuge tubes that were immersed in ice. The volume of bile samples was determined gravimetrically, taking 1.0 as specific gravity.
RNA extraction
The total RNA of livers and ilea was extracted using RNA-Bee reagent (Tel-Test, Inc., Friendswood, TX), according to the manufacturer’s protocol. RNA concentrations were quantified using a NanoDrop1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE) at a wavelength of 260 nm. RNA integrity was confirmed by agarose gel electrophoresis and ethidium bromide staining of 5 μg of total RNA to visualize intact 18S and 28S bands.
Messenger RNA Quantification
The majority of mRNA of genes in liver and ileum samples were quantified using QuantiGene Plex 2.0 Assay (Affymetrix/Panomics, Inc., Fremont, CA). Individual bead-based oligonucleotide probe sets, specific for each gene examined, were developed by Affymetrix/Panomics, Inc. Genes and reference sequence numbers are available at https://www.thermofisher.com (sets #21330 and #21383). Samples were analyzed using a Bio-Plex 200 System Array reader with Luminex 100 xMAP. Data were acquired using Bio-Plex Data Manager version 5.0 (Bio-Rad, Hercules, CA).
In addition to the bead array, for some mRNAs, reverse transcription quantitative polymerase chain reaction (RT-qPCR) was applied, namely Abca1, Abcg5, Abcg8, Atp8b1, Bcrp, ß-klotho, Ent1, Ibabp, Mate1, Mdr1, Mrp1, Mrp4, Mrp6, Oatp1a4, Oatp2b1, Oat2, Oct1, Ostα, and Ostß as described recently in detail (Lickteig et al., 2016; Renaud et al., 2016). Briefly, total RNA was reverse transcribed with the High Capacity cDNA Reverse Transcription Kit from Applied Biosystems (Foster City, CA). Power SYBR Green Master Mix (Applied Biosystems) was used for qPCR analysis. Fluorescence was quantified with an Applied Biosystems 7300 Real Time PCR System. Differences in gene expression between groups were calculated using the comparative ΔΔCt method. All data were standardized to the internal control ribosomal protein L13A (liver) or glyceraldehyde 3-phosphate dehydrogenase (ileum). Relative mRNA levels were calculated with vehicle controls set at 100% for each gender.
Bile acid analysis in liver, bile, and serum
Sample extraction and quantification of individual BAs by UPLC-MS/MS were performed according to methods described previously (Alnouti et al., 2008; Zhang and Klaassen, 2010).
Statistical Analysis
All statistical analyses were performed with an IBM-SPSS 23.0 computer program (IBM, Armonk, NY). Individual values were log-transformed to obtain normal distribution. The differences between control and TCDD exposed groups were determined by Student t test with significance set at P<0.05. All data are presented as the mean ± S.E.M. Asterisks (*) denote differences between control and TCDD-exposed male or female mice.
Experiments on AhR-null mice
To determine whether AhR is responsible for the TCDD-induced changes in WT mice, the same studies were performed simultaneously with AhR-null mice as mentioned above with WT mice. AhR-null mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and were characterized previously (Schmidt et al., 1996). Mice were backcrossed to (>99% congenic) to Charles River C57BL/6 background. Because TCDD did not significantly alter BA homeostasis in AhR-null mice, the results with AhR-null mice are presented in the supplemental section.
RESULTS
Effects of TCDD on hepatic concentrations and composition of bile acids
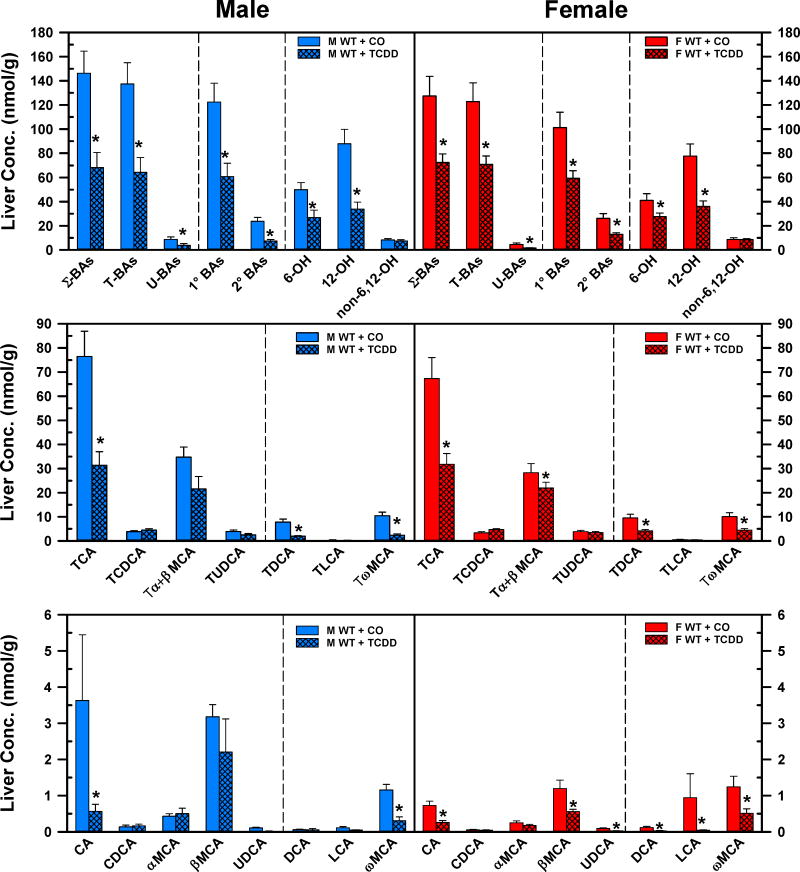
Fig. 1 demonstrates the concentrations of BAs in livers of corn oil- and TCDD-exposed mice. TCDD produced a marked decrease in BA concentrations in the liver. TCDD decreased the concentration of Σ-BAs in both males (−53%) and females (−43%). TCDD diminished the concentrations of T-BAs (M: −53%, F: −42%), U-BAs (M: −50%, F: −64%), 1°BAs (M: −50%, F: −50%), and 2° BAs (M: −69%, F: −50%) in livers of WT male and female mice (Fig. 1. Top panels). TCDD reduced the 12-OH BAs −61% and −54%, and reduced 6-OH BAs −42% and −33% in the livers of male and female mice, respectively. Surprisingly, the non-6,12-OH BAs were not altered by TCDD in either gender. After TCDD exposure, the decrease of hepatic 12-OH BAs was due to the marked decreases in TCA (M: −59%, F: −53%), TDCA (M: −75%, F: −57%), and CA (M: −84%, F: −65%). In addition, in female mice, the decreased concentration of DCA (−76.2%) also contributed to the lower amount of hepatic 12-OH BAs. The decrease in hepatic concentration of the 6-OH BAs was mainly due to the decrease of TωMCA (M: −78%, F: −56%), and ωMCA (M: −74%, F: −59%). In female mice, the concentrations of other 6-OH BAs, namely Tα+βMCA (−22%) and βMCA (−53%) were also decreased in the liver by TCDD. Although the cumulative concentration of non-6,12-OH BAs did not change significantly in male and female mice, the hepatic concentration of UDCA in female mice decreased (−69%) after TCDD exposure.
Fig. 1.
Effect of TCDD on hepatic concentrations of total bile acids (top), individual T-conjugated bile acids (middle), and individual unconjugated bile acids (bottom) in WT male (blue bars) and female mice (red bars). Corn oil (vehicle) or TCDD (37 μg/kg) was administered daily (IP) for 4 days to male and female mice (at least 6 mice per treatment group). On the 5th day livers were harvested and individual BAs were quantified by UPLC-MS/MS. Bars represent the mean ±SE mice per group. Asterisks indicate significant difference (p < 0.05) from the respective value of the WT mice. Primary bile acids (1°BAs), secondary bile acids (2°BAs), 6-hydroxylated bile acids (6-OH), 12α-hydroxylated (12-OH) bile acids, cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), females (F), lithocholic acid (LCA), males (M), muricholic acid (MCA), Non-6, non-12α-hydroxylated bile acids (non-6,12-OH), total bile acids (Σ-BAs), T-conjugated bile acids (T-BAs), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), unconjugated bile acids (U-BAs), ursodeoxycholic acid (UDCA), wild-type mice (WT). Color image is available in the online version of the article.
The relative proportions of the individual BAs in livers of male and female corn oil or TCDD-exposed mice are presented in Fig. 2. The T-conjugated BAs represented over 93% of the BAs in liver, and TCDD treatment did not change the proportion of T-BAs and U-BAs in either male or female mice (Fig. 2. Top pie charts). The proportion of 1° BAs was 84% and 80%, whereas the percentage of 2° BAs was 16% and 20% in male and female control mice, respectively. The fraction of 2° BAs decreased in males (−5%), but not in female mice (−2%) after TCDD treatment (Fig. 2. Middle pie charts). In the livers of control mice, the most abundant bile acids were 12-OH BAs (M: 59%, F: 61%), followed by 6-OH BAs (M: 35%, F: 32%). The smallest fraction was the non-6,12-OH BAs (M: 6%, F: 7%). TCDD treatment almost doubled the non-6,12-OH BAs in both males and females, whereas the percentage of 12-OH BAs were reduced in both males (−9%) and females (−12%). Although TCDD tended to increase the proportion of 6-OH BAs in both genders, it was significant only in female mice (+6%). TCDD increased the relative proportion of TCDCA (M: +4%, F: +4.1%), Tα+βMCA (M: +6.5%, F: +8%), TUDCA (M: +1%, F: +1.7%), TLCA (M: +0.2%), CDCA (M: +0.15%), αMCA (M: +0.4%), whereas TCDD decreased the relative proportion of TCA (M: −5.3% (NS), F: −9.8%) TωMCA (M: −3.6%), CA (M: −1.3% (NS), F: −0.2%), ωMCA (M: −0.5%).
Fig 2.
Effect of TCDD on hepatic composition of individual bile acids in WT male and female mice. Corn oil (vehicle) or TCDD (37 μg/kg) was administered daily (IP) for 4 days to male and female mice (at least 6 mice per treatment group). On the 5th day livers were harvested and the individual BAs were quantified by UPLC-MS/MS. Each section in pie charts and bars was calculated to represent the mean proportion of an individual BA relative to the Σ-BA concentration. Asterisks indicate significant difference (p < 0.05) from the respective value of the WT. Primary bile acids (1°BAs), secondary bile acids (2°BAs), 6-hydroxylated bile acids (6-OH), 12α-hydroxylated (12-OH) bile acids, cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), females (F), lithocholic acid (LCA), males (M), muricholic acid (MCA), Non-6-, non-12α-hydroxylated bile acids (non-6,12-OH), total bile acids (Σ-BAs), T-conjugated bile acids (T-BAs), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), unconjugated bile acids (U-BAs), ursodeoxycholic acid (UDCA), wild-type mice (WT). Color image is available in the online version of the article.
To determine whether the AhR is responsible for the above mentioned TCDD-induced changes, a similar experiment was performed with AhR-null mice. In contrast to WT mice, TCDD did not significantly influence the hepatic concentration of any BA (Suppl. Fig. 1). The results from AhR-null mice are available in the Supplemental Materials.
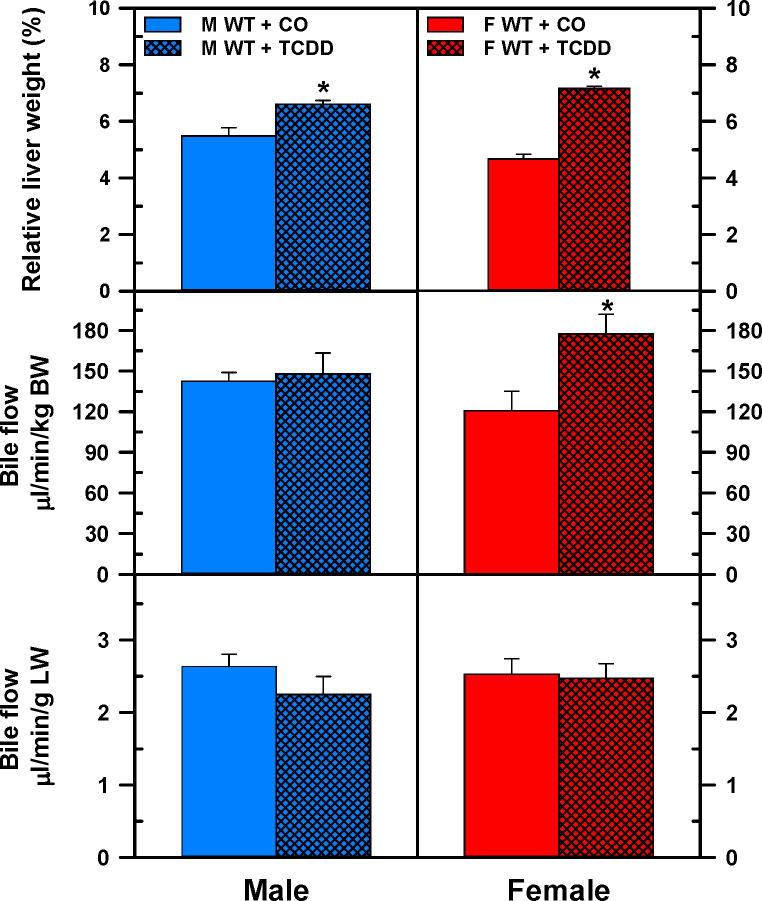
Effects of TCDD on liver weight and bile flow
TCDD increased liver weights of male (+20%) and female (+53%) mice (Fig. 3. Top panels). TCDD did not alter the bile flow calculated either per body weight or per liver weight in male mice (Fig.3. Left middle and bottom left panels), but in females, TCDD increased bile flow by 47% when it was calculated per body weight (Fig. 3. Right middle panel). Nevertheless, there were no significant differences in bile flow between control and TCDD-exposed female mice when calculated per gram liver weight (Fig. 3. Bottom right panel). In AhR-null mice the liver weight was not altered by TCDD. Surprisingly, however, the bile flow per body weight decreased 25% in TCDD-exposed male AhR-null mice, but not in female mice (Suppl. Fig 2.).
Fig 3.
Relative liver weight and bile flow in male and female WT mice. Corn oil (vehicle) or TCDD (37 μg/kg) was administered daily (IP) for 4 days to male and female mice (at least 6 mice per treatment group). On the 5th day, livers and bile were collected. Liver weight is expressed as a percent of body weight (BW) (top). Bile flow rates were normalized to BW (middle) and liver weight (bottom). Bars represent means ± SE of mice per group. Asterisks indicate significant difference (p < 0.05) from the respective value of the WT mice. Females (F), males (M), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), wild-type mice (WT). Color image is available in the online version of the article.
Effects of TCDD on biliary excretion and composition of bile acids
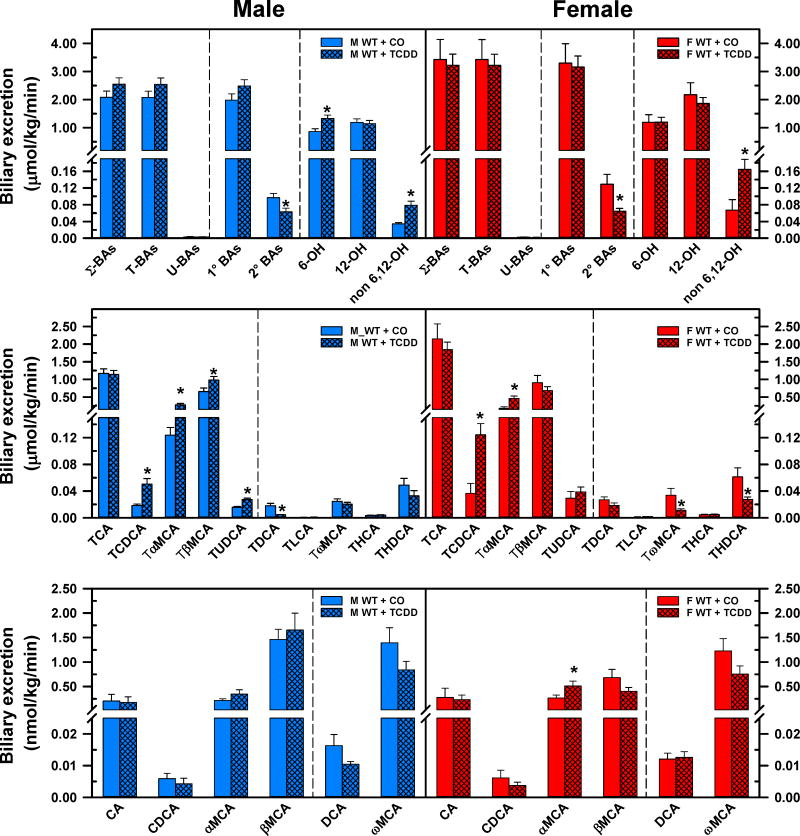
The effects of TCDD on biliary excretion of BAs are shown in Fig. 4. TCDD did not alter the biliary excretion of Σ-BAs, T-BAs, U-BAs, or 1°BAs, but significantly decreased the 2° BAs (M: −34.6%, F: −50.1%), and increased the non-6,12-OH BAs (M: 1.3-fold, F: 1.5-fold). TCDD increased the 6-OH BAs by 54% in male mice, but not in female mice (Fig. 4. Top panels). The enhanced biliary excretion of non-6,12-OH BAs in TCDD-exposed mice was mainly due to the 1.8-fold and 2.4-fold increase in the biliary excretion of TCDCA in male and female mice, respectively. In addition to the enhanced excretion of TCDCA, the increased excretion of its 7β-epimer TUDCA in male mice (+74%), but not in female mice, also contributes to the augmented biliary excretion of non-6,12-OH BAs. In male mice, TCDD increased the biliary excretion of primary 6-OH BAs, TαMCA (1.3-fold) and TβMCA (+50%), but did not change the biliary excretion of secondary 6-OH BAs. In contrast to males, in female mice TCDD increased only the biliary excretion of TαMCA (1.6-fold) and αMCA (+93%), but not the excretion of TβMCA (Fig. 4. Top and middle panels). In addition, TCDD decreased the biliary excretion of secondary 6-OH BAs such as TωMCA (−67%) and THDCA (−55%) in female mice. This remarkable decrease in the biliary excretion of secondary 6-OH BAs after TCDD treatment contributed to the more pronounced decrease of 2°BAs in female than in male mice. In male mice, the only significant decrease in biliary excretion of TDCA (−75%) was responsible for the reduced biliary excretion of 2°BAs after AhR activation. A similar tendency was found in the biliary excretion of DCA after TCDD treatment in male mice, but that change was not statistically significant. Contrary to the increased formation and excretion of CDCA in TCDD-exposed mice, biliary excretion of TLCA was not affected either in male or female mice (Fig 4. Middle panels).
Fig 4.
Effect of TCDD on biliary excretion of total bile acids (top), individual T-conjugated bile acids (middle), and individual unconjugated bile acids (bottom) in WT male (blue bars) and female mice (red bars). Corn oil (vehicle) or TCDD (37 μg/kg) was administered daily (IP) for 4 days to male and female mice (at least 6 mice per treatment group). On the 5th day bile was collected for 40 minutes and the individual BAs were quantified by UPLC-MS/MS. Bars represent the mean ±SE of mice per group. Asterisks indicate significant difference (p < 0.05) from the respective value of the WT. Primary bile acids (1°BAs), secondary bile acids (2°BAs), 6-hydroxylated bile acids (6-OH), 12α-hydroxylated (12-OH) bile acids, Non-6-, non-12α-hydroxylated bile acids (non-6,12-OH), cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), females (F), hyocholic acid (HCA), hyodeoxycholic acid (HDCA), lithocholic acid (LCA), Males (M), muricholic acid (MCA), total bile acids (Σ-BAs), T-conjugated bile acids (T-BAs), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), unconjugated bile acids (U-BAs), ursodeoxycholic acid (UDCA), wild-type mice (WT). Color image is available in the online version of the article.
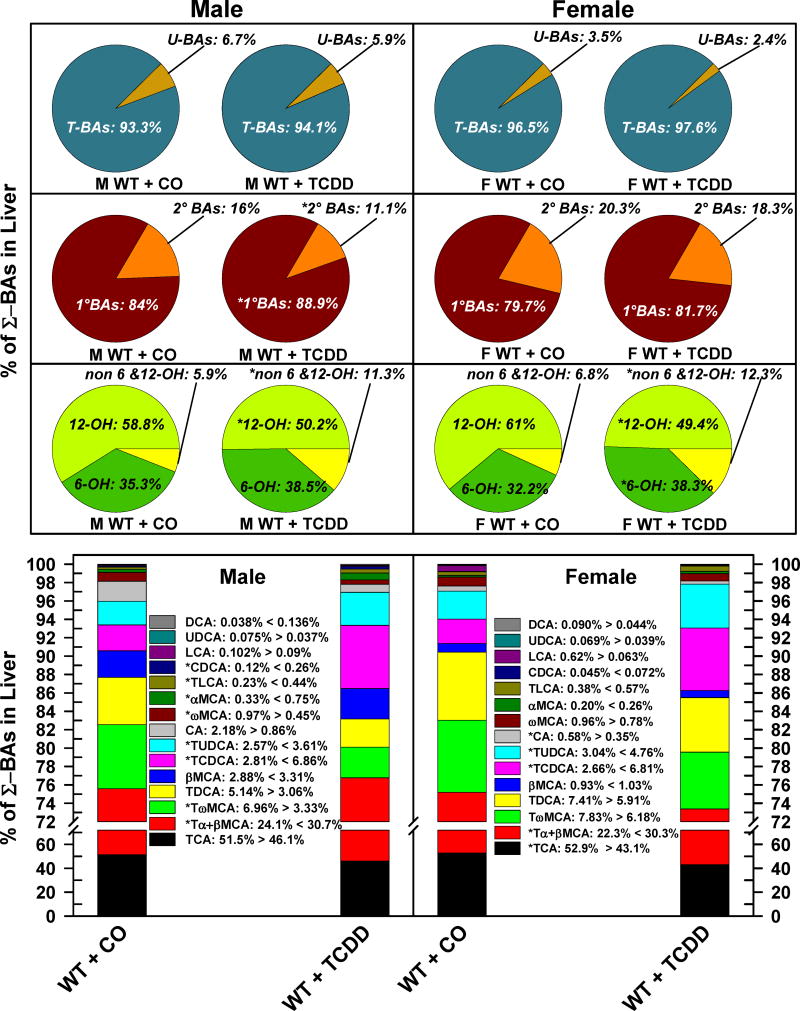
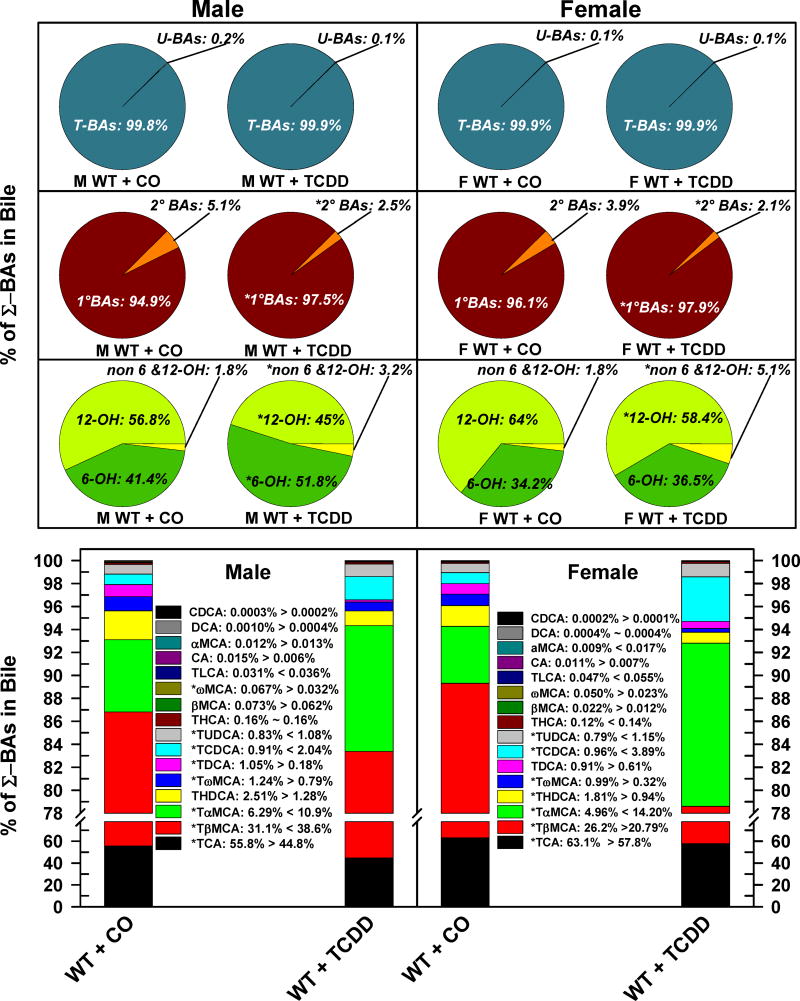
The relative percentage of each BA in bile is depicted in Fig. 5. As expected, both male and female mice excreted almost exclusively T-BAs (M: 99.8%, F: 99.9%) and a minimal amount of unconjugated BAs. TCDD did not alter the relative proportion of T-BAs and U-BAs in either male or female mice (Fig. 5. Top pie charts). However, TCDD decreased the proportion of 2° BAs in both male (5.1% to 2.5%) and female mice (3.9% to 2.1%) (Fig. 5. Middle pie charts). The relative proportion of 12-OH BAs decreased in both males (−11.8%) and females (−5.6%), whereas the relative proportion of biliary non-6,12-OH-BAs almost doubled in male mice, and tripled in female mice. Interestingly, the biliary proportion of 6-OH BAs was relatively lower in corn oil treated female (34%) than male mice (41%). After TCDD treatment, the 6-OH BAs increased by 10.4% in male mice, but it did not change significantly in female mice (Fig. 5. Bottom pie charts). TCDD decreased the relative proportion of TCA and TωMCA in both genders, whereas TDCA decreased in male mice, and THDCA and TβMCA decreased in female mice. TCDD increased the relative proportion of TCDCA, TUDCA, and TαMCA in both genders. Surprisingly, TCDD treatment altered the relative proportion of TβMCA in male and female mice in opposite directions: in males, the relative proportion of TβMCA increased by 7.5%, whereas it decreased 5.3% in females. In AhR-null mice, TCDD treatment did not change the biliary excretion/composition of BAs (Supplemental Fig. 3.).
Fig 5.
Effect of TCDD on biliary composition of individual bile acids in WT male and female mice. Corn oil (vehicle) or TCDD (37 μg/kg) was administered daily (IP) for 4 days to male and female mice (at least 6 mice per treatment group). On the 5th day bile was collected for 40 minutes and the individual BAs were quantified by UPLC-MS/MS. Each section in pie charts and bars represent the mean proportion of an individual BA relative to the Σ-BA concentration. Asterisks indicate significant difference (p < 0.05) from the respective value of the WT mice. Primary bile acids (1°BAs), secondary bile acids (2°BAs), 6-hydroxylated bile acids (6-OH), 12α-hydroxylated (12-OH) bile acids, Non-6-, non-12α-hydroxylated bile acids (non-6,12-OH), cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), females (F), hyocholic acid (HCA), hyodeoxycholic acid (HDCA), lithocholic acid (LCA), Males (M), muricholic acid (MCA), total bile acids (Σ-BAs), T-conjugated bile acids (T-BAs), unconjugated bile acids (U-BAs), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), ursodeoxycholic acid (UDCA), wild-type mice (WT). Color image is available in the online version of the article.
Effects of TCDD on serum concentrations and composition of bile acids
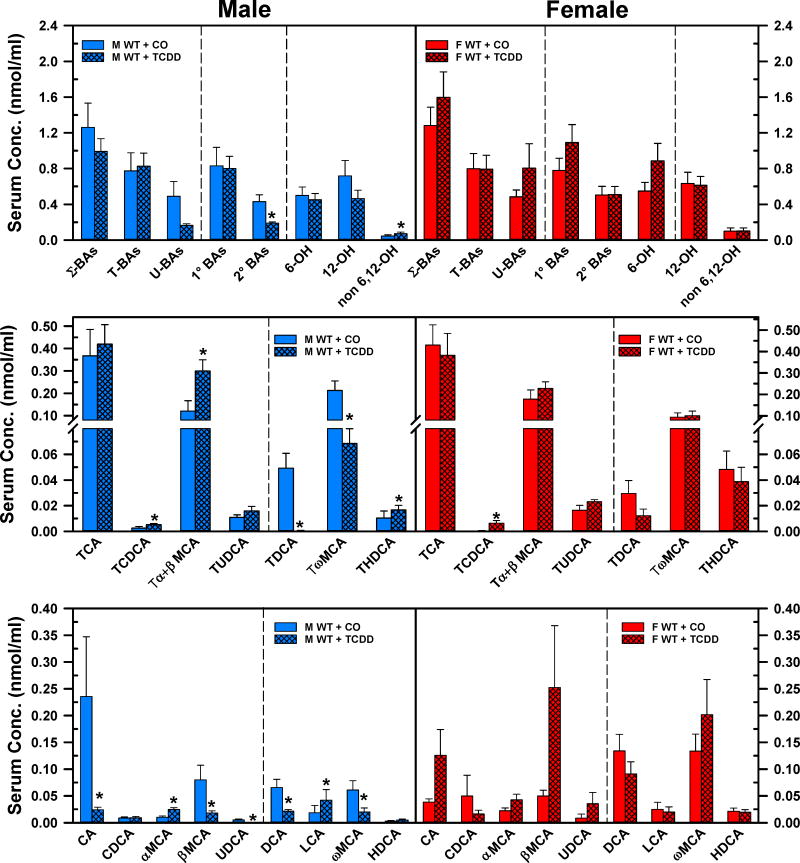
Fig. 6. depicts the concentrations of BAs in serum of corn oil- and TCDD-exposed mice. TCDD did not change the concentrations of Σ-BAs, T-BAs, U-BAs, 1°BAs, 6-OH BAs, or 12-OH BAs in either male or female mice. However, TCDD decreased the 2°BAs (−56%) and increased the non-6,12-OH BAs (+58%) in males, but not in females (Fig. 6. Top panels). In male mice, TCDD increased the serum concentrations of TCDCA (1-fold), Tα+βMCA (1.5-fold), α-MCA (1.4-fold), LCA (1.3-fold), and THDCA (60%), whereas it decreased the serum concentrations of TDCA (−99%), TωMCA (−68%), CA (−90%), βMCA (−77%), UDCA (−98%), DCA (−67%), and ωMCA (−67%) (Fig. 6. Left middle and bottom panels). Surprisingly, in female mice after TCDD treatment, the only statistically significant change was the 12.8-fold increase in the concentration of the non-6,12-OH BA TCDCA (Fig. 6, Right middle panel). It is interesting to note that after TCDD treatment several BAs in sera tended to show opposite changes in female mice compared to male mice. There was a marked tendency for an increase in serum concentrations of CA, βMCA, αMCA, ωMCA in TCDD-exposed female mice, however, because of the large variability, none of these changes were statistically significant.
Fig. 6.
Effect of TCDD on serum concentration of total bile acids (top), individual T-conjugated bile acids (middle), and individual unconjugated bile acids (bottom) in WT male (blue bars) and female mice (red bars). Corn oil (vehicle) or TCDD (37 μg/kg) was administered daily (IP) for 4 days to male and female mice (at least 6 mice per treatment group). On the 5th day blood was collected and the individual BAs were quantified in sera by UPLC-MS/MS. Bars represent the mean ± SE of mice per group. Asterisks indicate significant difference (p < 0.05) from the respective value of the WT mice. Primary bile acids (1°BAs), secondary bile acids (2°BAs), 6-hydroxylated bile acids (6-OH), 12α-hydroxylated (12-OH) bile acids, cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), females (F), hyodeoxycholic acid (HDCA), lithocholic acid (LCA), males (M), muricholic acid (MCA), Non 6-, non-12α-hydroxylated bile acids (non-6,12-OH), total bile acids (Σ-BAs), T-conjugated bile acids (T-BAs), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), unconjugated bile acids (U-BAs), ursodeoxycholic acid (UDCA), wild-type mice (WT). Color image is available in the online version of the article.
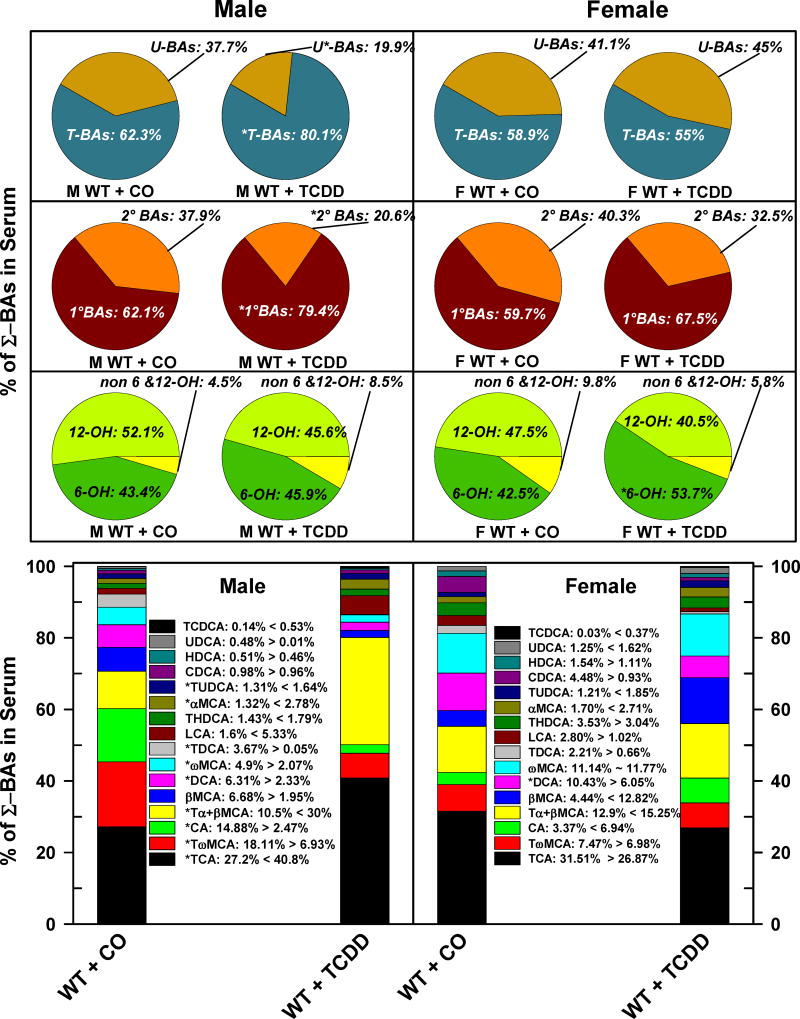
The relative percentage of each BA in serum is depicted in Fig. 7. The relative proportion of U-BAs were 38% and 41% in male and female mice, respectively (Fig. 7. Top panels). TCDD decreased the relative percentage of U-BAs in male mice (−19%), but not in female mice. The relative proportion of 2°BAs was approximately 38% in male mice and 40% in female mice. TCDD decreased the fraction of 2°BAs by 17% in male mice and tended to decrease it in females. In serum, the most abundant BAs are the 12-OH BAs (M: 52%, F: 48%), followed by the 6-OH BAs (M: 43%, F:43%), and non-6,12-OH BAs (M: 4.5%, F: 9.8%). TCDD did not alter the proportions of the major categories of BAs in male mice. Nonetheless, there was a tendency for a decrease in 12-OH BAs, and increase in 6-OH BAs and non-6,12-OH BAs (Fig. 7. Bottom left pie charts). In female mice, TCDD tended to decrease the 12-OH BAs and non-6,12-OH BAs but increased the proportion of 6-OH BAs (to 54%). TCDD treatment of male mice increased the relative proportion of TCA (13.6%), Tα+βMCA (19.4%), TUDCA (0.32%), αMCA (1.46%), LCA (3.73%), whereas it decreased the relative percentage of TDCA (−3.6%), TωMCA (−11.2%), CA (−12.4%), DCA (−4%), and ωMCA (−2.8%) in serum (Fig. 7. Left bar chart). Interestingly, TCDD treatment of female mice produced a decrease only in serum DCA (−4.3%), although TCDD tended to decrease the relative proportion of TCA, CDCA, and LCA, and tended to increase Tα+βMCA, CA, and βMCA. TCDD did not alter the concentrations of BAs in the serum of male and female AhR-null mice, with the exceptions of CDCA (−54%) and αMCA (−62%), which were decreased in the sera of male mice (Suppl. Fig. 4.).
Fig. 7.
Effect of TCDD on serum composition of individual bile acids in WT male and female mice. Corn oil (vehicle) or TCDD (37 μg/kg) was administered daily (IP) for 4 days to male and female mice (at least 6 mice per treatment group). On the 5th day blood was collected and the individual BAs were quantified in sera by UPLC-MS/MS. Each section in pie charts and bars represent the mean proportion of an individual BA relative to the Σ-BA concentration. Asterisks indicate significant difference (p < 0.05) from the respective value of the WT. Primary bile acids (1°BAs), secondary bile acids (2°BAs), 6-hydroxylated bile acids (6-OH), 12α-hydroxylated (12-OH) bile acids, cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), females (F), hyodeoxycholic acid (HDCA), lithocholic acid (LCA), males (M), muricholic acid (MCA), Non-6-, non 12α-hydroxylated bile acids (non-6,12-OH), total bile acids (Σ-BAs), T-conjugated bile acids (T-BAs), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), unconjugated bile acids (U-BAs), ursodeoxycholic acid (UDCA), wild-type mice (WT). Color image is available in the online version of the article.
Effects of TCDD on mRNA expression of major BA synthesizing enzymes and major hepatic regulating factors of BA homeostasis
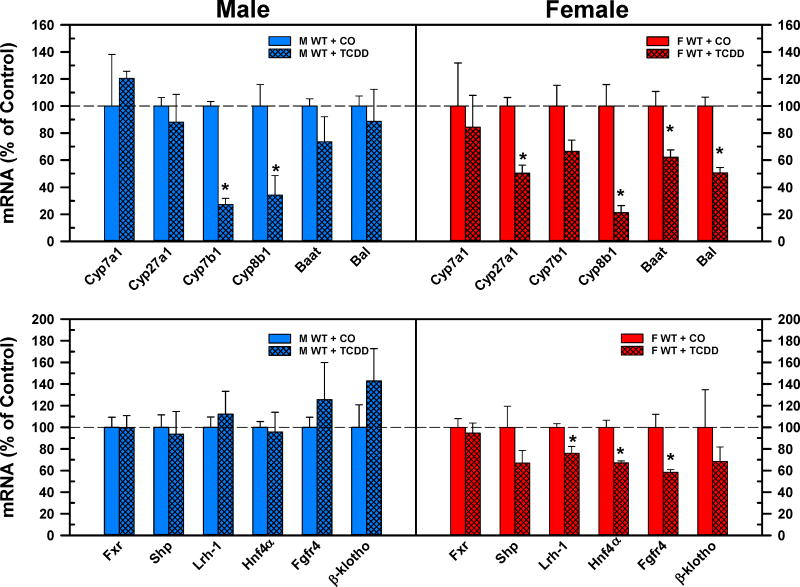
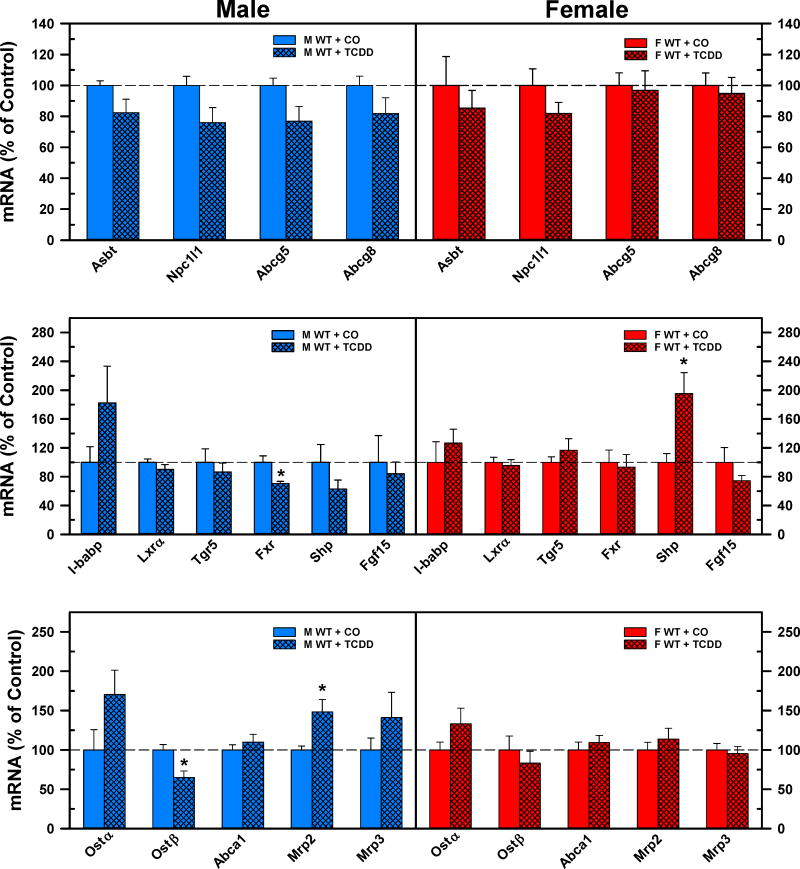
The top panel of Fig. 8 demonstrates the mRNA expression of the major bile acid synthetic enzymes. TCDD did not change the mRNA expression of Cyp7a1, the rate-limiting enzyme in the classical pathway, either in male or female mice. The Cyp27a1 enzyme is responsible for the side chain hydroxylation in both the classical and alternative pathway. TCDD did not alter the gene expression of Cyp27a1 in male mice but decreased it 50% in female mice. Cyp7b1 is responsible for 7α-hydroxylation in the alternative pathway. TCDD significantly decreased the mRNA expression of Cyp7b1 in male mice (−73%), but only tended to decrease it in female mice. The complementary decrease in the mRNA expression of Cyp27a1 and Cyp7b1 indicates the downregulation of the alternative bile acid synthetic pathway in both male and female mice after TCDD injection. TCDD did not change the mRNA expression of the BA conjugation enzymes in male mice but decreased the mRNA expression of Baat (−38%) and Bal (−50%) in female mice. The mRNA expression of sterol 12α-hydroxylase (Cyp8b1) was markedly reduced in both male (−66%) and female (−79%) mice after TCDD administration.
Fig. 8.
Effect of TCDD on mRNA of BA synthesis (top) and regulation (bottom) genes in livers of WT male (blue bars) and female mice (red bars). Corn oil (vehicle) or TCDD (37 μg/kg) was administered daily (IP) for 4 days to male and female mice (at least 6 mice per treatment group). On the 5th day livers were harvested. Total RNA was analyzed by QuantiGene Plex 2.0 Assay, as well as by RT-qPCR. Relative mRNA levels were calculated with vehicle controls set as 100%. Bars represent the relative percentage mRNA expression ± SE of mice per treatment group. Asterisks indicate significant difference (p < 0.05) from the respective value of the WT mice. Bile acid CoA:amino acid N-acyltransferase (Baat), Bile acid CoA ligase (Bal), Cytochrome p450 (Cyp), Farnesoid x receptor (Fxr), females (F), Fibroblast growth factor receptor (Fgfr4), Hepatocyte nuclear factor 4a (Hnf4a), Liver receptor homolog-1 (Lrh-1), males (M), Small heterodimer partner (Shp), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), wild-type mice (WT). Color image is available in the online version of the article.
The bottom panel of Fig. 8. demonstrates the effects of TCDD on the hepatic regulators of BA homeostasis. TCDD did not alter the gene expression of the major hepatic regulators of BA homeostasis. In contrast, TCDD decreased the mRNA expression of Lrh1 (−24%), HNF4α (33%), and Fgfr4 (−42%) in female mice.
TCDD given to AhR-null mice did not cause any significant changes in BA synthetic enzymes in either male or female mice. However, TCDD tended to decrease the mRNA expression of Cyp7a1 and increase Shp (+27%) and β-Klotho (1.5-fold) in male AhR-null mice (Suppl. Fig. 5).
Effects of TCDD on mRNA expression of major hepatic sinusoidal uptake, canalicular and basolateral efflux transporters
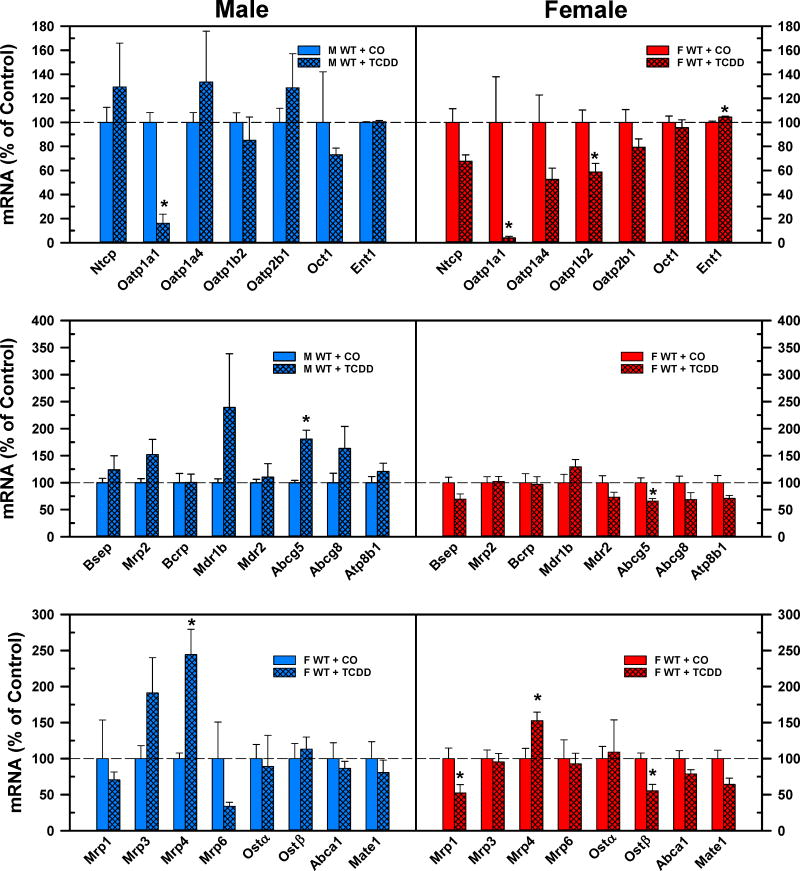
The top panel of Fig. 9 demonstrates the effect of TCDD on basolateral uptake transporters in male and female mice. TCDD markedly decreased the mRNA expression of male predominant Oat1a1 (Cheng et al., 2006) in both male (−84%) and female (−96%) mice. In female mice, in addition to Oatp1a1, the gene expression of the unconjugated BA transporter Oatp1b2 (Csanaky et al., 2011) was also decreased (−41%) by TCDD. In female mice, TCDD slightly increased the mRNA expression of Ent1 (+5%). The middle panel of Fig. 9 shows that TCDD did not alter the mRNA expression of the canalicular efflux transporters, except for Abcg5, which increased in male (+81%) but decreased in female (−34%) mice. In male mice, the mRNA expression of Mdr1b tended to increase, but it was not statistically significant. The bottom panel of Fig. 9 demonstrates the effect of TCDD on the mRNA expression of the basolateral efflux transporters. TCDD increased the mRNA of Mrp4 in male (1.4-fold) and female (+53%) mice. In female mice, TCDD decreased the mRNA expression of Mrp1 (−48%) and Ostβ (−45%).
Fig. 9.
Effect of TCDD on mRNA expression of basolateral uptake (top), canalicular (middle), and basolateral efflux (bottom) transporters in livers of WT male (blue bars) and female mice (red bars). Corn oil (vehicle) or TCDD (37 μg/kg) was administered daily (IP) for 4 days to male and female mice (at least 6 mice per treatment group). On the 5th day livers were harvested. Total RNA was analyzed by QuantiGene Plex 2.0 Assay, as well as by RT-qPCR. Bars represent the relative percentage mRNA expression ± SE of mice per group. Asterisks indicate significant difference (p < 0.05) from the respective value of the WT mice. Breast cancer resistance protein (Bcrp), Bile salt export pump (Bsep), Equilibrative nucleoside transporter (Ent), females (F), males (M), Multidrug and toxin extrusion transporter (Mate), Multidrug resistance protein (Mdr), Multidrug resistance-associated protein (Mrp), Na(+)-taurocholate cotransporting polypeptide (Ntcp), Organic anion transporting polypeptide (Oatp), Organic cation transporter (Oct), Organic solute transporter (Ost), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), wild-type mice (WT). Color image is available in the online version of the article.
In AhR-null mice, after TCDD treatment, the marked decrease in mRNA expression of Oatp1a1 was absent, even more, its gene expression tended to increase in both males and females. In addition, the mRNA expression of Oatp1a4 significantly increased in male AhR-null mice. Similarly to male WT mice, the gene expression of Abcg5 also significantly increased in male AhR-null mice. In female AhR-null mice, there were no significant changes in hepatic transporters, except Mrp6 (+58%). The mRNA expression of Ostα was strongly induced by TCDD in female AhR null mice, but because of high variability it was not significant (Suppl. Fig. 6.).
Effects of TCDD on mRNA expression of major ileal BA apical and basolateral transporters, and regulating factors of BA homeostasis
The top panels of Fig. 10 compare the mRNA expression of apical transporters in the ilea after corn oil or TCDD treatment of WT male and female mice. TCDD did not change any of the quantified apical BA/cholesterol transporters in either male or female mice. TCDD did not affect the mRNA expression of the ileal regulators of BA homeostasis, with the exceptions of a decrease in Fxr (−30%) in male mice, and an increase of Shp (+95%) in female mice (Fig. 10. Middle panels). The bottom panels of Fig. 10 depict changes in basolateral transporters in the ilea after TCDD treatment. TCDD caused significant changes in the mRNA expression of basolateral transporters only in male mice, but not in female mice. In male mice, the gene expression of Ostβ was decreased 35%, whereas the mRNA expression of Mrp2 was increased by 48% following TCDD treatment.
Fig 10.
Effect of TCDD on mRNA expression of BA regulators and transporters in ilea of WT male (blue bars) and female (red bars) mice. Corn oil (vehicle) or TCDD (37 μg/kg) was administered daily (IP) for 4 days to male and female mice (at least 6 mice per treatment group). On the 5th day ilea were harvested. Total RNA was analyzed by QuantiGene Plex 2.0 Assay, as well as by RT-qPCR. Relative mRNA levels were calculated with vehicle controls set as 100%. Bars represent the relative percentage mRNA expression ± SE of mice per group. Asterisks indicate significant difference (p < 0.05) from the respective value of the WT. ATP-binding cassette (Abc), Apical sodium-dependent bile acid transporter (Asbt), Farnesoid X Receptor (Fxr), females (F), Fibroblast growth factor (Fgf), I-babp (ileal bile acid binding protein), Liver x receptor a (Lxra), males (M), Multidrug resistance-associated protein (Mrp), Nieman-Pick c1-like 1 (Npc1l1), Organic solute transporter (Ost), total bile acids (Σ-BAs), Small heterodimer partner (Shp), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), Transmembrane G protein-coupled receptor 5 (Tgr5), wild-type mice (WT). Color image is available in the online version of the article.
In AhR-null mice, TCDD did not change the mRNA expression of any quantified ileal BA transporter or regulatory factor in female mice (Suppl. Fig. 7. Right panels). Surprisingly, TCDD induced the mRNA expression of Fgf15 by 58% and tended to increase gene expression of another intestinal Fxr target gene, Shp. In contrast to WT mice, TCDD did not alter the mRNA expression of Ostβ and tended to increase Mrp2, in AhR-null mice. However, the gene expression of basolateral transporter Abca1 was significantly induced by TCDD in AhR-null male mice (Suppl. Fig. 7. Left panels).
DISCUSSION
TCDD is a potent compound which produces a complex and diverse toxicity. Specific toxicological features of TCDD originate from its chemical structure, which allows binding to the AhR, minimal biotransformation, and accumulation in high fat containing tissues. One of the characteristic properties of TCDD is delayed lethality, which usually occurs between 1 and 6 weeks after a single exposure to TCDD. Before death, animals undergo a significant weight loss, which is known as the wasting syndrome (Pohjanvirta and Tuomisto, 1994). The progressive dose-related loss of body weight in mice starts 4 to 6 days after exposure to TCDD. The serum concentration of the BA precursor cholesterol does not change within 4 days, but decreases by the 8th day, and then increases by 17–21 days after TCDD exposure (Chapman and Schiller, 1985). Persistent activation of the AhR and alteration of the expression of its target genes mediate TCDD toxicity that typically requires weeks to develop, which means activation of the AhR may evoke indirect secondary pathophysiological responses. For instance, the immune signaling genes in liver are induced a week after TCDD administration, following immune cell infiltration (Boverhof et al., 2005). Besides the importance of AhR in the toxicological processes, it is also involved in the regulation of cell cycle, stem cells, immune functions, and the endocrine system (Bock, 2017b; Bock, 2017a)
To assess the short-term direct effects of AhR activation, two factors should be considered: (1) effective induction of AhR target genes such as Cyp1a1, and (2) evaluation before the obvious onset of the wasting syndrome, which is approximately on the 7th day after the first exposure to TCDD (Chapman and Schiller, 1985; Kelling et al., 1985). Based on these considerations, in this study the mice were exposed for 4 consecutive days with 37 μg/kg TCDD i.p. and examined on the 5th day. This exposure induces the AhR target gene Cyp1a1 up to 1200-fold without changing the gene expression of AhR (Petrick and Klaassen, 2007), and without significant changes in body weight.
This short term exposure to TCDD produced the most marked changes in the BA concentrations in the liver, in comparison to the serum and bile. TCDD decreased the BA concentrations by approximately 50% in liver of both male and female mice (Fig 1). In contrast, TCDD did not alter the BA concentrations in livers of AhR-null mice (Suppl. Figs 1.); this indicates that the short term changes in BA homeostasis after TCDD are AhR-dependent. Similarly in rats, TCDD (60 μg/kg) decreased the concentration of BAs in liver 7 days after exposure (Kakizuka et al., 2015). However, after repeated oral exposure to TCDD for one month, the BA concentration in livers of mice increased (Fader et al., 2017). These data call attention to the effects of TCDD during the early stage (within one week after TCDD exposure) compared to the long term effects of TCDD, when secondary mechanisms are likely to contribute to changes in BA homeostasis.
Contrary to the 50% decrease of BAs in the liver (Fig 1), TCDD surprisingly did not change the mRNA expression of the major rate limiting BA synthetic enzyme Cyp7a1, but decreased the gene expression of the alternative pathway enzymes in both male and female WT mice (Fig 8). In contrast, after long-term exposure to TCDD, Cyp7a1 mRNA was decreased by 21–30 days (Lu et al., 2011; Fader et al., 2017). Likewise, there were no changes in the mRNA expression of the major regulators of BA homeostasis in the liver, namely Fxr-Shp, Lrh1, Hnf4α, and Fgfr4-βklotho (Fig 8). Similar to the liver, there were no meaningful changes in the mRNA expression of the regulators of BA homeostasis in the ileum (Fxr, Shp, Fgf15) after TCDD exposure. Taken together, short term i.p. administration of TCDD has minimal impact on the major BA regulatory pathways (intestinal Fxr-Fgf15 and hepatic Fxr-Shp pathways) and the rate limiting classical BA synthetic enzyme (Cyp7a1) in WT mice.
The mechanism by which TCDD lowers BA concentration in the liver is unknown, but surprisingly, the short term activation of constitutive androstane receptor (CAR) has very similar consequences in repressing Cyp8b1 expression and reducing the BA concentration in liver (Lickteig et al., 2016). It is known that TCDD increases the expression of CAR and its downstream genes in mice (Petrick and Klaassen, 2007). Changes in CAR expression may also cause changes in crosstalk between CAR and other nuclear receptors (Xiao et al., 2010). In addition, TCDD in Nrf2-null mice downregulates the Cyp8b1 and the enzymes of alternative pathway more than in WT mice, indicating the potential role of oxidative stress, especially in the long-term regulation of BA homeostasis (Lu et al., 2011). Taken together, short term AhR activation may have complex effects on bile acid synthetic enzymes and transporters without a direct influence on FXR-orchestrated major regulatory pathways. Further research is needed to clarify the role and the relationship of AhR/CAR/Nrf2 in the regulation of BA homeostasis after TCDD exposure. It has to be also noted that changes in mRNA gene expression are not always parallel with translation and function of proteins. The protein levels and enzyme/transporter activities were not quantified in this study due to technical limitations. Mouse specific antibodies and substrates of many isoforms of enzymes, transporters and nuclear receptors are not available. Hopefully in the future, complex quantitative proteomic and functional analyses will be easily available to understand the changes in the abundance and functions of the proteins of BA homeostasis after TCDD exposure.
TCDD produced the most noticeable decrease in 12-OH BAs in liver, namely CA and its taurine conjugate TCA and their 7α-dehydroxy metabolites, DCA and TDCA (Figs 1 and 2). The 12α-hydroxylation of BAs is performed by only one liver-specific enzyme, Cyp8b1 (Gafvels et al., 1999; Vlahcevic et al., 2000). In accordance with the decreased 12-OH BAs, TCDD markedly decreased Cyp8b1 expression in both male and female WT mice (Fig 8), but not in AhR-null mice (Suppl. Fig 5). This finding suggests that Cyp8b1 is regulated by AhR activation, which determines the ratio of the two human 1°BAs, CA and CDCA, and the hydrophobicity of the BA pool. However, in rodents, the dihydroxy CDCA (and UDCA) are further metabolized to trihydroxy 6-OH α- and βMCA. Similarly in Cyp8b1-null mice, α- and βMCA replace the missing 12-OH CA and its metabolites (Li-Hawkins et al., 2002). It is known that rodent specific 6-OH BAs (MCAs) act as FXR antagonists in the intestine, which can decrease the downregulation of Cyp7a1 (through the Fgf15-Fgfr4 pathway) and eventually increase BAs in the body (Sayin et al., 2013). Therefore it can be hypothesized that downregulation of Cyp8b1 may have different consequences in rodents than in humans, because humans do not produce MCAs, which are FXR antagonists, and the hydrophobicity indices of the tri-OH MCAs indicate that TαMCA and TβMCA are even more hydrophilic than TCA itself (Heuman, 1989).
Even though TCDD decreased the Σ-BA concentration in liver, it did not decrease the biliary excretion of BAs (Fig 4.). It is important to note that the biliary excretion of TCA and CA were maintained at the same rate as in control mice, even though TCA and its 12-OH metabolites are less in the livers of TCDD exposed mice (Fig 1). However the biliary excretion of the biologically more active FXR agonist (CDCA) and antagonist (MCAs) increased. The biliary excretion of non-6,12-OH BAs (mainly TCDCA) was markedly enhanced (M: 1.3-fold, F: 1.5-fold) by TCDD, however the portion of these BAs is relatively low in mice. In addition the biliary excretion of 6-OH BA TαMCA in male and female mice (+TβMCA in males) increased. As a consequence of these changes, the fraction of 12-OH BAs (mainly TCA) decreased in bile, whereas the proportion of non-6,12-OH BAs (mainly TCDCA and TUDCA) in both sexes and 6-OH BAs (MCA) increased in males (Fig 5).
TCDD decreased the concentrations of 2°BAs, especially (T)DCA and (T)ωMCA in liver, bile, and serum. 2°BAs are formed in the intestine by the gut microbiota. The 7α/β-dehydroxylation of 1°BAs is mainly processed by Clostridia (Uchida et al., 1999; Wells et al., 2000). CDCA and MCAs inhibit the germination of Clostridium difficile (Sorg and Sonenshein, 2010; Francis et al., 2013). It can be hypothesized that increased biliary excretion of TCDCA and the MCAs into the intestine may inhibit the bacterial 7α/β-dehydroxylation activity of Clostridia, and thus contribute to the reduced formation of 2°BAs. TCDD can alter the intestinal microbiome when given orally (Lefever et al., 2016) but probably has fewer effects when it is given i.p.
The results of the current research indicate again that the concentrations of serum BAs do not reflect changes in BA homeostasis in the liver (Hofmann and Hagey, 2014). TCDD did not alter the total serum BA concentration in WT mice (Fig 6), contrary to the markedly lower BA content of the liver. In female mice, TCDD decreased the U-BAs in serum of male but not in female mice. TCDD exposure decreased Oatp1b2 in female, but not in male mice (Fig 9). Because Oatp1b2 is responsible for the hepatic uptake of U-BAs (Csanaky et al., 2011), this may be the mechanism for the gender difference in serum levels of U-BAs (Fig 6). Similar gender differences in Oatp1b2 expression and U-BAs were observed in aging male and female mice (Fu et al., 2012).
In summary, after i.p. administration of the model AhR activator, TCDD decreased BAs in mouse liver without significant impact on Cyp7a1 and the major BA homeostasis regulator of the hepatic and intestinal FXR pathways. However, AhR activation suppressed the 12α-hydroxylase Cyp8b1. The downregulation of Cyp8b1 decreased the relative abundance of 12-OH BAs and increased the concentration and biliary excretion of TCDCA and its metabolites (mainly TαMCA) without changing bile flow. All of these changes were absent in AhR-null mice after i.p. injection of TCDD.
Supplementary Material
Highlights.
Short term TCDD exposure decreased total bile acids in liver approximately 50%.
TCDD did not alter total bile acid excretion into bile.
TCDD did not alter total bile acid concentrations in serum.
TCDD decreased the percentage of 12-OH bile acids.
TCDD decreased the bile acid 12-hydroxylase (Cyp8b1) in liver.
Acknowledgments
Funding Information
This work was supported by the National Institutes of Health grants ES009649, DK092069 and the Children’s Mercy Startup fund for ILC.
The authors would like to thank all members of our laboratory for technical assistance with blood and tissue collection.
ABBREVIATIONS
- 1°BAs
Primary bile acids
- 2°BAs
Secondary bile acids
- 6-OH
6-hydroxylated
- 12-OH
12α-hydroxylated
- Abc
ATP-binding cassette
- AhR
Aryl hydrocarbon receptor
- Asbt
Apical sodium-dependent bile acid transporter
- BA
Bile acid
- Baat
Bile acid CoA:amino acid N-acyltransferase
- Bal
Bile acid CoA ligase
- Bcrp
Breast cancer resistance protein
- Bsep
Bile salt export pump
- CA
Cholic acid
- CAR
Constitutive Androstane Receptor
- CDCA
Chenodeoxycholic acid
- Cyp
Cytochrome p450
- DCA
Deoxycholic acid
- Ent
Equilibrative nucleoside transporter
- F
Female
- FXR
Farnesoid X Receptor
- Fgfr4
Fibroblast growth factor receptor 4
- Fgf15
Fibroblast growth factor 15
- HDCA
Hyodeoxycholic acid
- Hnf4α
Hepatocyte nuclear factor 4α
- i.p
intraperitoneal
- LCA
Lithocholic acid
- LRH1
Liver receptor homolog 1
- Lxrα
Liver x receptor α
- M
Male
- Mate
Multidrug and toxin extrusion transporter
- MCA
Muricholic acid
- Mdr
Multidrug resistance protein
- Mrp
Multidrug resistance-associated protein
- Ntcp
Na(+)-taurocholate cotransporting polypeptide
- Npc1l1
Nieman-Pick c1-like 1
- non-6,12-OH
Non-6,12-hydroxylated
- NS
not-significant
- Oatp
Organic anion transporting polypeptide
- Oct
Organic cation transporter
- Ost
Organic solute transporter
- Σ-BAs
Sum (total) of bile acids
- Shp
Small heterodimer partner
- T-BAs
Taurine-conjugated bile acids
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- Tgr5
Takeda-G-protein-coupled receptor 5
- U-BAs
Unconjugated bile acids
- UDCA
Ursodeoxycholic acid
- UPLC-MS/MS
Ultraperformance Liquid Chromatography–Tandem Mass Spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleksunes LM, Klaassen CD. Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARalpha-, and Nrf2-null mice. Drug Metab Dispos. 2012;40:1366–1379. doi: 10.1124/dmd.112.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnouti Y, Csanaky IL, Klaassen CD. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;873:209–217. doi: 10.1016/j.jchromb.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthanarayanan M, von Dippe P, Levy D. Identification of the hepatocyte Na+-dependent bile acid transport protein using monoclonal antibodies. J Biol Chem. 1988;263:8338–8343. [PubMed] [Google Scholar]
- Bock KW. From dioxin toxicity to putative physiologic functions of the human Ah receptor in homeostasis of stem/progenitor cells. Biochem Pharmacol. 2017a;123:1–7. doi: 10.1016/j.bcp.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Bock KW. Human and rodent aryl hydrocarbon receptor (AHR): from mediator of dioxin toxicity to physiologic AHR functions and therapeutic options. Biol Chem. 2017b;398:455–464. doi: 10.1515/hsz-2016-0303. [DOI] [PubMed] [Google Scholar]
- Boverhof DR, Burgoon LD, Tashiro C, Chittim B, Harkema JR, Jump DB, Zacharewski TR. Temporal and dose-dependent hepatic gene expression patterns in mice provide new insights into TCDD-Mediated hepatotoxicity. Toxicol Sci. 2005;85:1048–1063. doi: 10.1093/toxsci/kfi162. [DOI] [PubMed] [Google Scholar]
- Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattori V, Hagenbuch B, Hagenbuch N, Stieger B, Ha R, Winterhalter KE, Meier PJ. Identification of organic anion transporting polypeptide 4 (Oatp4) as a major full-length isoform of the liver-specific transporter-1 (rlst-1) in rat liver. FEBS letters. 2000;474:242–245. doi: 10.1016/s0014-5793(00)01596-9. [DOI] [PubMed] [Google Scholar]
- Chapman DE, Schiller CM. Dose-related effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in C57BL/6J and DBA/2J mice. Toxicol Appl Pharmacol. 1985;78:147–157. doi: 10.1016/0041-008x(85)90314-x. [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher J, Lu H, Klaassen CD. Endocrine regulation of gender-divergent mouse organic anion-transporting polypeptide (Oatp) expression. Mol Pharmacol. 2006;70:1291–1297. doi: 10.1124/mol.106.025122. [DOI] [PubMed] [Google Scholar]
- Csanaky IL, Lu H, Zhang Y, Ogura K, Choudhuri S, Klaassen CD. Organic anion-transporting polypeptide 1b2 (Oatp1b2) is important for the hepatic uptake of unconjugated bile acids: Studies in Oatp1b2-null mice. Hepatology. 2011;53:272–281. doi: 10.1002/hep.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Lo R, Celius T, Matthews J, Zacharewski TR. Integration of genome-wide computation DRE search, AhR ChIP-chip and gene expression analyses of TCDD-elicited responses in the mouse liver. BMC Genomics. 2011;12:365. doi: 10.1186/1471-2164-12-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duane WC, Javitt NB. 27-hydroxycholesterol: production rates in normal human subjects. J Lipid Res. 1999;40:1194–1199. [PubMed] [Google Scholar]
- Fader KA, Nault R, Zhang C, Kumagai K, Harkema JR, Zacharewski TR. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-elicited effects on bile acid homeostasis: Alterations in biosynthesis, enterohepatic circulation, and microbial metabolism. Sci Rep. 2017;7:5921. doi: 10.1038/s41598-017-05656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140:173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- Fletcher N, Wahlstrom D, Lundberg R, Nilsson CB, Nilsson KC, Stockling K, Hellmold H, Hakansson H. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters the mRNA expression of critical genes associated with cholesterol metabolism, bile acid biosynthesis, and bile transport in rat liver: a microarray study. Toxicol Appl Pharmacol. 2005;207:1–24. doi: 10.1016/j.taap.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Forgacs AL, Dere E, Angrish MM, Zacharewski TR. Comparative analysis of temporal and dose-dependent TCDD-elicited gene expression in human, mouse, and rat primary hepatocytes. Toxicol Sci. 2013;133:54–66. doi: 10.1093/toxsci/kft028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MB, Allen CA, Sorg JA. Muricholic acids inhibit Clostridium difficile spore germination and growth. PLoS One. 2013;8:e73653. doi: 10.1371/journal.pone.0073653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu ZD, Csanaky IL, Klaassen CD. Gender-divergent profile of bile acid homeostasis during aging of mice. PLoS One. 2012;7:e32551. doi: 10.1371/journal.pone.0032551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafvels M, Olin M, Chowdhary BP, Raudsepp T, Andersson U, Persson B, Jansson M, Bjorkhem I, Eggertsen G. Structure and chromosomal assignment of the sterol 12alpha-hydroxylase gene (CYP8B1) in human and mouse: eukaryotic cytochrome P-450 gene devoid of introns. Genomics. 1999;56:184–196. doi: 10.1006/geno.1998.5606. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Dawson P. The sodium bile salt cotransport family SLC10. Pflugers Arch. 2004;447:566–570. doi: 10.1007/s00424-003-1130-z. [DOI] [PubMed] [Google Scholar]
- Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719–730. [PubMed] [Google Scholar]
- Hofmann AF, Hagey LR. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res. 2014;55:1553–1595. doi: 10.1194/jlr.R049437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Kakizuka S, Takeda T, Komiya Y, Koba A, Uchi H, Yamamoto M, Furue M, Ishii Y, Yamada H. Dioxin-Produced Alteration in the Profiles of Fecal and Urinary Metabolomes: A Change in Bile Acids and Its Relevance to Toxicity. Biological & pharmaceutical bulletin. 2015;38:1484–1495. doi: 10.1248/bpb.b15-00235. [DOI] [PubMed] [Google Scholar]
- Kelling CK, Christian BJ, Inhorn SL, Peterson RE. Hypophagia-induced weight loss in mice, rats, and guinea pigs treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam Appl Toxicol. 1985;5:700–712. doi: 10.1016/0272-0590(85)90194-0. [DOI] [PubMed] [Google Scholar]
- Keppler D, Cui Y, Konig J, Leier I, Nies A. Export pumps for anionic conjugates encoded by MRP genes. Advances in enzyme regulation. 1999;39:237–246. doi: 10.1016/s0065-2571(98)00015-6. [DOI] [PubMed] [Google Scholar]
- Kwon YI, Yeon JD, Oh SM, Chung KH. Protective effects of ursodeoxycholic acid against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced testicular damage in mice. Toxicol Appl Pharmacol. 2004;194:239–247. doi: 10.1016/j.taap.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Lee J, Prokopec SD, Watson JD, Sun RX, Pohjanvirta R, Boutros PC. Male and female mice show significant differences in hepatic transcriptomic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. BMC Genomics. 2015;16:625. doi: 10.1186/s12864-015-1840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefever DE, Xu J, Chen Y, Huang G, Tamas N, Guo TL. TCDD modulation of gut microbiome correlated with liver and immune toxicity in streptozotocin (STZ)-induced hyperglycemic mice. Toxicol Appl Pharmacol. 2016;304:48–58. doi: 10.1016/j.taap.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Hawkins J, Gafvels M, Olin M, Lund EG, Andersson U, Schuster G, Bjorkhem I, Russell DW, Eggertsen G. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. The Journal of clinical investigation. 2002;110:1191–1200. doi: 10.1172/JCI16309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickteig AJ, Csanaky IL, Pratt-Hyatt M, Klaassen CD. Activation of Constitutive Androstane Receptor (CAR) in Mice Results in Maintained Biliary Excretion of Bile Acids Despite a Marked Decrease of Bile Acids in Liver. Toxicol Sci. 2016;151:403–418. doi: 10.1093/toxsci/kfw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Cui W, Klaassen CD. Nrf2 protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced oxidative injury and steatohepatitis. Toxicol Appl Pharmacol. 2011;256:122–135. doi: 10.1016/j.taap.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manara L, Coccia P, Croci T. Persistent tissue levels of TCDD in the mouse and their reduction as related to prevention of toxicity. Drug metabolism reviews. 1982;13:423–446. doi: 10.3109/03602538209029988. [DOI] [PubMed] [Google Scholar]
- Manara L, Coccia P, Croci T. Prevention of TCDD toxicity in laboratory rodents by addition of charcoal or cholic acids to chow. Food Chem Toxicol. 1984;22:815–818. doi: 10.1016/0278-6915(84)90120-0. [DOI] [PubMed] [Google Scholar]
- Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 2014;14:801–814. doi: 10.1038/nrc3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nault R, Fader KA, Ammendolia DA, Dornbos P, Potter D, Sharratt B, Kumagai K, Harkema JR, Lunt SY, Matthews J, Zacharewski T. Dose-Dependent Metabolic Reprogramming and Differential Gene Expression in TCDD-Elicited Hepatic Fibrosis. Toxicol Sci. 2016;154:253–266. doi: 10.1093/toxsci/kfw163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nault R, Fader KA, Lydic TA, Zacharewski TR. Lipidomic Evaluation of Aryl Hydrocarbon Receptor-Mediated Hepatic Steatosis in Male and Female Mice Elicited by 2,3,7,8-Tetrachlorodibenzo-p-dioxin. Chem Res Toxicol. 2017;30:1060–1075. doi: 10.1021/acs.chemrestox.6b00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW. Aryl hydrocarbon receptor (AHR): “pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog Lipid Res. 2017;67:38–57. doi: 10.1016/j.plipres.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki J, Uno S, Ogura M, Choi M, Maeda T, Sakurai K, Matsuo S, Amano S, Nebert DW, Makishima M. Aryl hydrocarbon receptor ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin enhances liver damage in bile duct-ligated mice. Toxicology. 2011;280:10–17. doi: 10.1016/j.tox.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RE, Madhukar BV, Yang KH, Matsumura F. Depression of adenosine triphosphatase activities in isolated liver surface membranes of 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated rats: correlation with effects on ouabain biliary excretion and bile flow. J Pharmacol Exp Ther. 1979;210:275–282. [PubMed] [Google Scholar]
- Petrick JS, Klaassen CD. Importance of hepatic induction of constitutive androstane receptor and other transcription factors that regulate xenobiotic metabolism and transport. Drug Metab Dispos. 2007;35:1806–1815. doi: 10.1124/dmd.107.015974. [DOI] [PubMed] [Google Scholar]
- Pohjanvirta R, Tuomisto J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals: effects, mechanisms, and animal models. Pharmacol Rev. 1994;46:483–549. [PubMed] [Google Scholar]
- Probst MR, Reisz-Porszasz S, Agbunag RV, Ong MS, Hankinson O. Role of the aryl hydrocarbon receptor nuclear translocator protein in aryl hydrocarbon (dioxin) receptor action. Mol Pharmacol. 1993;44:511–518. [PubMed] [Google Scholar]
- Renaud HJ, Klaassen CD, Csanaky IL. Calorie Restriction Increases P-Glycoprotein and Decreases Intestinal Absorption of Digoxin in Mice. Drug Metab Dispos. 2016;44:366–369. doi: 10.1124/dmd.115.064766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- Santostefano MJ, Ross DG, Savas U, Jefcoate CR, Birnbaum LS. Differential time-course and dose-response relationships of TCDD-induced CYP1B1, CYP1A1, and CYP1A2 proteins in rats. Biochem Biophys Res Commun. 1997;233:20–24. doi: 10.1006/bbrc.1997.6389. [DOI] [PubMed] [Google Scholar]
- Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M, Russell DW, Dietschy JM, Turley SD. Alternate pathways of bile acid synthesis in the cholesterol 7alpha-hydroxylase knockout mouse are not upregulated by either cholesterol or cholestyramine feeding. J Lipid Res. 2001;42:1594–1603. [PubMed] [Google Scholar]
- Slijepcevic D, Kaufman C, Wichers CG, Gilglioni EH, Lempp FA, Duijst S, de Waart DR, Elferink RP, Mier W, Stieger B, Beuers U, Urban S, van de Graaf SF. Impaired uptake of conjugated bile acids and hepatitis b virus pres1-binding in na(+) -taurocholate cotransporting polypeptide knockout mice. Hepatology. 2015;62:207–219. doi: 10.1002/hep.27694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg JA, Sonenshein AL. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. Journal of bacteriology. 2010;192:4983–4990. doi: 10.1128/JB.00610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger B, Meier Y, Meier PJ. The bile salt export pump. Pflugers Arch. 2007;453:611–620. doi: 10.1007/s00424-006-0152-8. [DOI] [PubMed] [Google Scholar]
- Uchida K, Satoh T, Narushima S, Itoh K, Takase H, Kuruma K, Nakao H, Yamaga N, Yamada K. Transformation of bile acids and sterols by clostridia (fusiform bacteria) in Wistar rats. Lipids. 1999;34:269–273. doi: 10.1007/s11745-999-0363-y. [DOI] [PubMed] [Google Scholar]
- Vlahcevic ZR, Eggertsen G, Bjorkhem I, Hylemon PB, Redford K, Pandak WM. Regulation of sterol 12alpha-hydroxylase and cholic acid biosynthesis in the rat. Gastroenterology. 2000;118:599–607. doi: 10.1016/s0016-5085(00)70267-8. [DOI] [PubMed] [Google Scholar]
- Vlahcevic ZR, Stravitz RT, Heuman DM, Hylemon PB, Pandak WM. Quantitative estimations of the contribution of different bile acid pathways to total bile acid synthesis in the rat. Gastroenterology. 1997;113:1949–1957. doi: 10.1016/s0016-5085(97)70015-5. [DOI] [PubMed] [Google Scholar]
- Wells JE, Berr F, Thomas LA, Dowling RH, Hylemon PB. Isolation and characterization of cholic acid 7alpha-dehydroxylating fecal bacteria from cholesterol gallstone patients. J Hepatol. 2000;32:4–10. doi: 10.1016/s0168-8278(00)80183-x. [DOI] [PubMed] [Google Scholar]
- Xiao L, Xie X, Zhai Y. Functional crosstalk of CAR-LXR and ROR-LXR in drug metabolism and lipid metabolism. Advanced drug delivery reviews. 2010;62:1316–1321. doi: 10.1016/j.addr.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Yang KH, Croft WA, Peterson RE. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on plasma disappearance and biliary excretion of foreign compounds in rats. Toxicol Appl Pharmacol. 1977;40:485–496. doi: 10.1016/0041-008x(77)90075-8. [DOI] [PubMed] [Google Scholar]
- Zhang L, Nichols RG, Correll J, Murray IA, Tanaka N, Smith PB, Hubbard TD, Sebastian A, Albert I, Hatzakis E, Gonzalez FJ, Perdew GH, Patterson AD. Persistent Organic Pollutants Modify Gut Microbiota-Host Metabolic Homeostasis in Mice Through Aryl Hydrocarbon Receptor Activation. Environ Health Perspect. 2015;123:679–688. doi: 10.1289/ehp.1409055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Klaassen CD. Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. J Lipid Res. 2010;51:3230–3242. doi: 10.1194/jlr.M007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.