Abstract
Carbapenemase-producing Klebsiella pneumoniae is globally recognized as one of the greatest threats to public health, and combination therapy may be the chemotherapeutic option. In the present study, we aimed to evaluate the antibacterial effects of colistin/fosfomycin combination against carbapenemase-producing K. pneumoniae. The antibacterial effects were determined in a one-compartment in vitro pharmacokinetic model over a period of 24 h. The initial inoculum was 108 CFU/mL. Low, medium, and high Cmax values of colistin at 0.5, 2, and 5 mg/L as well as Cmax of fosfomycin at 100 mg/L were simulated in the model. Doses of both colistin and fosfomycin were given every 8 h until 24 h. For the colistin- and fosfomycin-susceptible isolate KP47, three combination regimens showed greater killing effect compared with colistin monotherapy. The greatest killing effect was observed in combination regimen containing 5 mg/L colistin. For colistin-heteroresistant and fosfomycin-susceptible isolate KP79, combination regimen containing low dose colistin (0.5 mg/L) showed no synergistic or additive effects. However, combination regimens containing 2 and 5 mg/L colistin maintained the bactericidal effect until 24 h compared with colistin monotherapy. For colistin-heteroresistant and fosfomycin-resistant isolates KP42 and KP11, bactericidal activity was barely enhanced by combination regimens. Moreover, combination regimen containing 5 mg/L colistin could only prevent the emergence of colistin-resistant subpopulation in colistin and fosfomycin-susceptible isolate. It is necessary to know the resistant patterns of the K. pneumoniae before using combination of colistin and fosfomycin in clinical practice.
1. Introduction
The emergence of carbapenem-resistant Enterobacteriaceae (CRE), particularly carbapenemase-producing Klebsiella pneumoniae, has greatly increased over the past decade and become a significant public health concern as carbapenem is one of the few antibiotics to treat severe infections caused by these pathogens [1]. A recent survey from 36 countries in Europe about carbapenemase-producing Enterobacteriaceae, including more than 400 hospitals, has shown that 850 (37%) of 2,301 K. pneumoniae samples are carbapenemase producers [2]. However, the discovery of new antibiotics cannot catch up with growth of antimicrobial resistance [3]. Prevalence of resistance coupled with scarcity of novel antimicrobials forces us to reevaluate some “old but efficacious” antibiotics. Colistin is one of these old drugs.
As a polypeptide antibiotic, colistin belongs to the polymyxin family. Colistin disrupts the outer cell membrane of the Gram-negative bacilli by binding to the lipid A component of the lipopolysaccharide, causing leakage of cytoplasmic contents and bacterial cell death [4]. An alarming increase in the rate of resistance to most available antimicrobials and a shortage of new antimicrobial agents have forced researchers to reevaluate the use of colistin, despite its potential risk of nephrotoxicity and neurotoxicity [5]. Colistin has been recently applied against the widespread multidrug-resistant (MDR) Gram-negative pathogens, including K. pneumoniae. Unfortunately, colistin resistance has also been reported in K. pneumoniae [6]. Heteroresistance observed in colistin monotherapy is considered to be a major cause of the rapid emergence of colistin resistance [7–10], which may be mitigated by combination therapy. In this case, the combination therapy becomes an option. Fosfomycin is another old broad-spectrum antibiotic that inhibits bacterial cell wall synthesis. Due to its excellent clinical efficacy and tolerability, fosfomycin has been successfully used in the treatment of uncomplicated urinary tract infections and pyelonephritis in females. From an in vitro standpoint, fosfomycin generally has high activity against ESBL- and carbapenemase-producing Enterobacteriaceae [11]. A study has showed that 78% of 107 carbapenem-nonsusceptible Enterobacteriaceae isolates remained susceptible to fosfomycin [12]. Our previous in vitro studies have confirmed that combination of colistin and fosfomycin shows synergy against carbapenem-resistant Pseudomonas aeruginosa isolates [13].
In the present study, we aimed to evaluate the antibacterial effects of colistin/fosfomycin combination against carbapenemase-producing K. pneumoniae with varying susceptibility to fosfomycin. An in vitro pharmacokinetic/pharmacodynamic (PK/PD) model was applied to simulate PKs of clinically achievable colistin and fosfomycin at routine doses in patients. The risk of emergence of secondary resistance was investigated by studies of population analysis profiles (PAPs).
2. Materials and Methods
2.1. Strains
Four clinical carbapenem-resistant K. pneumoniae isolates (KP47, KP79, KP11, and KP42) were selected according to different susceptibilities to colistin and fosfomycin. KP11 was collected from the Navy General Hospital; KP42 and KP47 were obtained from Beijing Hospital; and KP79 was isolated from the PLA General Hospital.
Minimum inhibitory concentration (MIC) of colistin was tested via broth (cation adjusted Mueller-Hinton) microdilution method, while MIC of fosfomycin was determined via agar (Mueller-Hinton agar with 25 μg/mL glucose-6-phosphate, G6P) dilution method. CLSI epidemiological cutoff values of colistin and EUCAST breakpoint of fosfomycin for Enterobacteriaceae were employed. If the MIC of colistin against an isolate was ≤2 mg/L, while its subpopulations were able to survive on the plates containing colistin higher than 2 mg/L, such isolate would be defined as heteroresistance to colistin [14]. A biochemical test (Carba NP test II) was used to identify different types of carbapenemase production in K. pneumoniae (classes A, B, and D).
2.2. Antibiotics and Adjuvants
Colistin sulfate (Lot. WXBB6005V ≥ 15,000 U/mg) and G6P (Lot. 423A0313, 98–100%) were obtained from Sigma-Aldrich. Fosfomycin (Lot. 0350-200001, 65.1%) was purchased from the National Institutes for the Food and Drug Control (Beijing, China). Colistin sulfate was applied in this study because colistin methanesulfonate (CMS) is a prodrug that turns into its active form when administrated in vivo [15], although CMS is commonly used in clinical practice.
2.3. K. pneumoniae Genotyping
PCR was used to amplify the β-lactamase-associated genes. Supplementary Table S1 lists 15 sets of primers for β-lactamases of Ambler class A (SME, IMI, VEB, KPC, SHV, GES, and CTX), Ambler class B (NDM, VIM, and IMP), Ambler class C (AmpC), and Ambler class D (OXA-48, OXA-10, OXA-2, and OXA-1) [16]. Amplification procedure is applied as described in previous study with minor revision and suitable for 15 β-lactamase-associated genes listed above [17]. After an initial denaturation step at 94°C for 4 min, amplifications were carried out with 36 cycles at a melting temperature of 94°C for 1 min, an annealing temperature of 49°C for 1 min, and an extension temperature of 70°C for 5 min, followed by a final elongation step at 72°C for 10 min.
2.4. In Vitro PK/PD Model
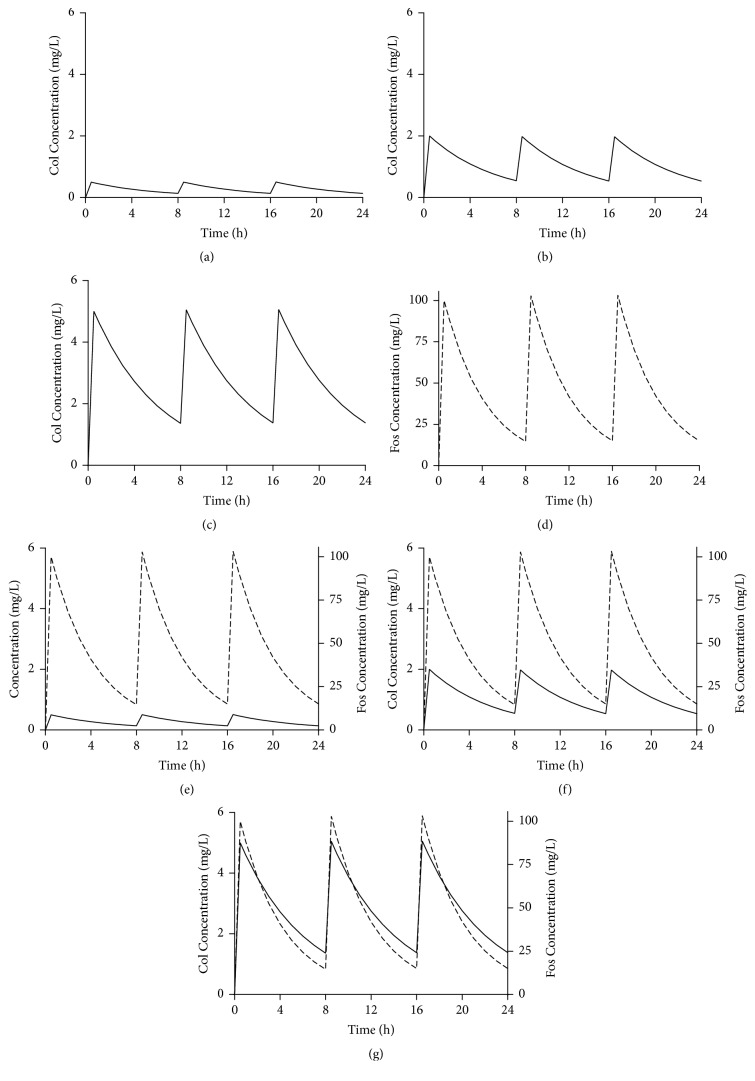
A one-compartment in vitro PK/PD model (PASS-402W System, Japan) was employed to evaluate the bactericidal effects of colistin alone or in combination with fosfomycin over a period of 24 h. The apparatus consisted of one central chamber with a capacity of 200 mL that was connected to a reservoir containing CAMHB broth. The temperature of broth within the system was maintained at 37°C. Fresh broth was pumped into the central chamber through a peristaltic pump at a defined flow rate. There was mixing to ensure distribution. Low, medium, and high Cmax values of colistin at 0.5, 2, and 5 mg/L as well as Cmax of fosfomycin at 100 mg/L were simulated in this PK/PD model. Simulated half-life for colistin and fosfomycin was 4 h and 2.7 h, respectively, as suggested in previously study [18]. Colistin concentrations were modified from the PK profiles in previously published report of in vitro PK/PD study, which maintained a constant concentration of colistin at 0.5, 2, or 5 mg/L [19]. Fosfomycin regimen was also simulated according to the PK parameters achieved in clinic [20]. At the beginning of the experiments, stock solutions of colistin and fosfomycin were injected into the model to achieve the target maximum concentration, and bolus injections were given every 8 h till 24 h. In addition, 25 mg/L of G6P was maintained in the MHB medium throughout the entire experiments. Figure 1 illustrates the targeted steady-state concentration-time profiles of colistin alone or in combination with fosfomycin. In this PK/PD model, synergy is defined as a decrease of ⩾2 log10 in the number of CFU/mL between the combination and its most active component; additivity is defined as a decrease of 1.0 to <2 log10 in the number of CFU/mL between the combination and its most active component [21].
Figure 1.
Targeted steady-state concentration-time profiles for colistin (Col, every 8 h) alone or in combination with fosfomycin (Fos, every 8 h). (a) Col 0.5 mg/L, (b) Col 2 mg/L, (c) Col 5 mg/L, (d) Fos 100 mg/L, (e) Col 0.5 mg/L plus Fos 100 mg/L, (f) Col 2 mg/L plus Fos 100 mg/L, and (g) Col 5 mg/L plus Fos 100 mg/L.
2.5. Bactericidal Effects and Emergence of Colistin Resistance
Briefly, 1 mL of serial samples was drawn from the central chamber at defined time points listed in Table 2 for viable colony count and real-time PAPs. Viable colony count and PAPs were conducted right after sampling. Subsequently, 50 μL of each sample with serial dilution was spirally plated on agar plates. Viable colony count was conducted after 24 h incubation at 35°C.
Table 2.
Colistin and fosfomycin dosage regimens, PK/PD index values, and sampling times in the in vitro PK/PD model.
| Treatment regimen | PK/PD index value | Sampling times (h) for microbiological measurements | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fAUC/MIC | fCmax/MIC | fT > MIC | |||||||||||
| Kp47 | Kp79 | Kp42 | Kp11 | Kp47 | Kp79 | Kp42 | Kp11 | Kp47 | Kp79 | Kp42 | Kp11 | ||
| Colistin monotherapy, target concentration C max /C min (mg/L) | 0, 1, 2, 4, 6, 8, 10, 12, 14, 16, 20, 24 |
||||||||||||
| 0.5/0.14 | 11.38 | 5.69 | 22.76 | 45.52 | 1 | 0.5 | 2 | 4 | 0 | 0 | 100 | 100 | |
| 2/0.54 | 44.92 | 22.46 | 89.84 | 179.68 | 4 | 2 | 8 | 16 | 100 | 18.75 | 100 | 100 | |
| 5/1.36 | 113.82 | 56.91 | 227.64 | 455.28 | 10 | 5 | 20 | 40 | 100 | 100 | 100 | 100 | |
|
| |||||||||||||
| Fosfomycin monotherapy, target concentration C max /C min (mg/L) | 0, 1, 2, 4, 6, 8, 10, 12, 14, 16, 20, 24 |
||||||||||||
| 100/14.58 | 837.26 | 52.33 | <3.27 | <3.27 | 100 | 6.25 | <0.39 | <0.39 | 100 | 100 | 0 | 0 | |
|
| |||||||||||||
| Combination therapy | 0, 1, 2, 4, 6, 8, 10, 12, 14, 16, 20, 24 |
||||||||||||
3. Results
3.1. Characteristics of K. pneumoniae Isolates
All four strains were confirmed as producers of class A carbapenemases by Carba NP test. Table 1 shows the results of MICs and β-lactamase genotyping. The four strains were all carbapenem-resistant with MICs of imipenem and meropenem ≥ 128 mg/L. MIC of colistin against four strains were ≤1 mg/L. However, except for KP47, the other three strains were colistin-heteroresistant because their subpopulations could grow on the nutrient agar with colistin concentration > 2 mg/L. KP47 and KP79 were susceptible to fosfomycin with the MICs of 1 and 16 mg/L, respectively. KP11 and KP42 were resistant to fosfomycin with the MIC > 256 mg/L.
Table 1.
MICs for K. pneumoniae isolates used in this study.
| Isolate | MIC (mg/L) | β-Lactamase genotyping | CarbaNP test | MLST | Colistin-heteroresistanta | |
|---|---|---|---|---|---|---|
| Colistin | Fosfomycin | |||||
| KP47 | 0.5 | 1 | KPC, SHV, OXA-2, OXA-10 | Ambler A | ST11 | No |
| KP79 | 1 | 16 | KPC, CTX, SHV, OXA-1, OXA-2, OXA-10 | Ambler A | ST37 | Yes |
| KP42 | 0.25 | >256 | KPC, CTX, SHV, OXA-10 | Ambler A | ST11 | Yes |
| KP11 | 0.125 | >256 | KPC, CTX, SHV, OXA-2, OXA-10 | Ambler A | ST11 | Yes |
aHeteroresistance to colistin was defined as the existence, in an isolate for which the colistin MIC was ≤2 mg/L, of subpopulations able to grow in the presence of >2 mg/L colistin.
3.2. Antibacterial Effects
3.2.1. Colistin Monotherapy
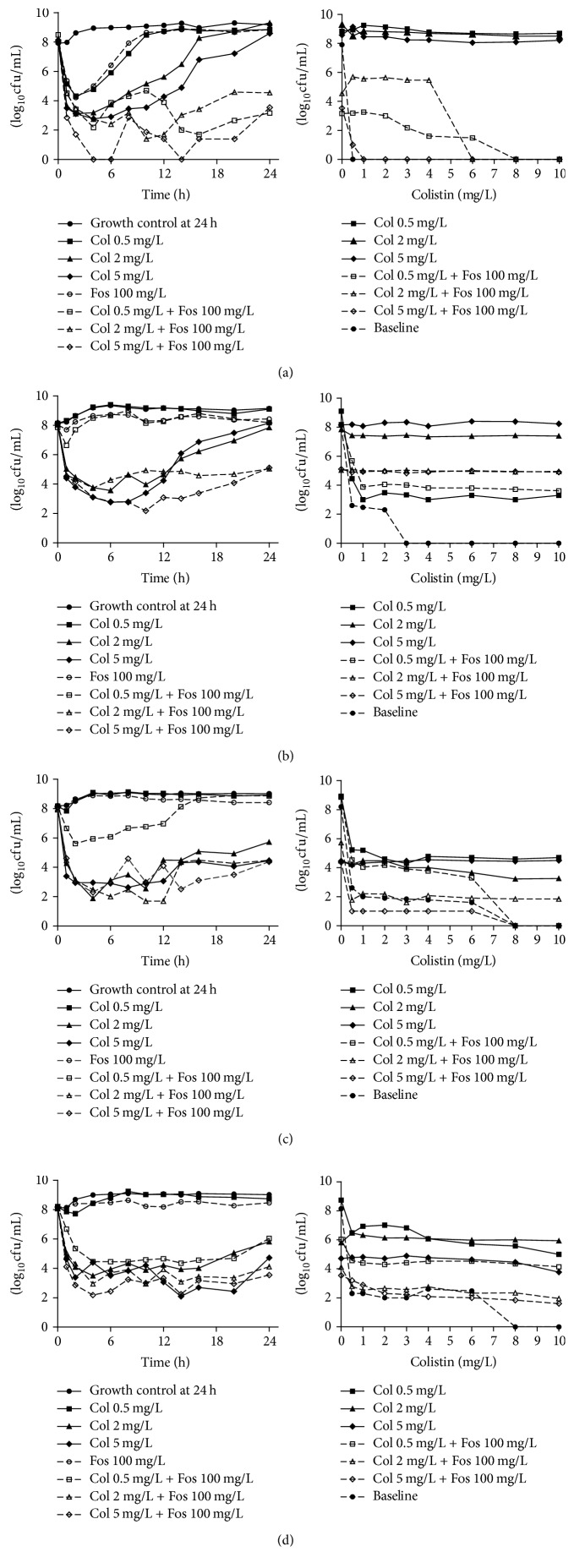
Colistin monotherapy with low, medium, and high Cmax showed different bacterial killing effects against colistin-susceptible and colistin-heteroresistant K. pneumoniae strains. For the colistin-susceptible isolate KP47, colistin alone produced rapid initial (0–4 h) killing effect to 3 log10 CFU/mL or below with all three concentrations of colistin. The regrowth of bacteria to the level of the control group occurred at 10 h, 16 h, and 24 h with colistin monotherapy at 0.5, 2, and 5 mg/L, respectively (Figure 2(a), Table 3). For the colistin-heteroresistant isolates KP79, KP42, and KP11, 0.5 mg/L colistin alone essentially produced no bacterial killing effect against any isolate (Figures 2(b), 2(c), and 2(d), Table 3). Colistin at 2 and 5 mg/L caused a reduction about 4 log10 CFU/mL against KP79 till 10 h, and both regrowth to the level of the control group occurred at 24 h (Figure 2(b), Table 3). For KP42 and KP11, which were heteroresistant to colistin and resistant to fosfomycin, colistin monotherapy at 2 and 5 mg/L achieved greatest bactericidal effect by 10 h (4-5 log10 CFU/mL), and the effect remained to 24 h (2-3 log10 CFU/mL) compared with the control (Figures 2(c) and 2(d), Table 3). In general, greater bactericidal effects were observed in colistin regimen with higher targeted Cmax/MIC. Cmax/MIC of colistin at 5 mg/L against KP42 and KP11, which were higher than other regimen (Table 2), exerted consistent bactericidal effect throughout the 24 h (Table 3).
Figure 2.
(Left) time-kill curves with various clinically relevant dosage regimens of colistin (Col) and fosfomycin (Fos) alone and in combination at an inoculum of 108 CFU/mL. (Right) PAPs at baseline and after 24 h of exposure to colistin monotherapy, colistin-fosfomycin combination therapy, or neither antibiotic (growth control). Fosfomycin monotherapy regimens were not included in colistin-PAP examination. (a) KP47 (colistin-susceptible, fosfomycin-susceptible); (b) KP79 (colistin-heteroresistant, fosfomycin-susceptible); (c) KP42 (colistin-heteroresistant, fosfomycin-resistant); (d) KP11 (colistin-heteroresistant, fosfomycin-resistant).
Table 3.
Log changes at 4, 8, 12, 16, 20, and 24 h at an inoculum of 108 CFU/mL with colistin and/or fosfomycin against K. pneumoniae.
| Isolate | Time (h) |
log change [log10(CFUt) − log10(CFU0)] | ||||||
|---|---|---|---|---|---|---|---|---|
| Col 0.5 mg/L | Col 2 mg/L | Col 5 mg/L | Fos 100 mg/L | Col 0.5 mg/L + Fos 100 mg/L |
Col 2 mg/L + Fos 100 mg/L |
Col 5 mg/L + Fos 100 mg/L |
||
| Kp47 | 4 | −3.23 | −5.00 | −5.24 | −3.00 | −6.30 | −5.30 | −8.13 |
| 8 | −0.80 | −3.63 | −4.57 | −0.08 | −4.17 | −4.87 | −5.27 | |
| 12 | 0.73 | −2.58 | −3.75 | 0.70 | −4.60 | −6.34 | −6.73 | |
| 16 | 0.74 | 0.08 | −1.23 | 0.87 | −6.78 | −4.62 | −8.13 | |
| 20 | 0.78 | 0.52 | −0.81 | 0.66 | −5.83 | −3.46 | −6.73 | |
| 24 | 0.83 | 1.11 | 0.56 | 0.84 | −5.32 | −3.50 | −4.59 | |
|
| ||||||||
| Kp79 | 4 | 1.10 | −4.29 | −5.07 | 0.60 | 0.63 | −4.32 | −4.97 |
| 8 | 1.16 | −3.42 | −5.38 | 0.66 | 1.16 | −3.43 | −5.33 | |
| 12 | 1.03 | −3.40 | −3.94 | 0.27 | 0.41 | −3.19 | −5.01 | |
| 16 | 0.85 | −1.83 | −1.30 | 0.55 | 0.94 | −3.46 | −4.71 | |
| 20 | 0.66 | −1.10 | −0.69 | 0.31 | 0.58 | −3.37 | −4.01 | |
| 24 | 0.97 | −0.21 | −0.01 | 0.38 | 0.32 | −3.00 | −2.97 | |
|
| ||||||||
| Kp42 | 4 | 0.93 | −6.14 | −5.20 | 0.79 | −2.20 | −5.44 | −5.80 |
| 8 | 0.97 | −4.53 | −5.53 | 0.78 | −1.47 | −5.44 | −3.51 | |
| 12 | 0.87 | −3.52 | −5.10 | 0.50 | −1.18 | −6.22 | −4 | |
| 16 | 0.81 | −2.94 | −3.80 | 0.47 | 0.58 | −3.43 | −4.98 | |
| 20 | 0.69 | −3.08 | −4.10 | 0.31 | 0.75 | −3.65 | −4.60 | |
| 24 | 0.76 | −2.29 | −3.70 | 0.32 | 0.71 | −3.44 | −3.72 | |
|
| ||||||||
| Kp11 | 4 | 0.26 | −4.58 | −3.79 | 0.24 | −3.76 | −5.15 | −5.99 |
| 8 | 1.07 | −3.73 | −4.35 | 0.46 | −3.77 | −4.17 | −4.92 | |
| 12 | 0.87 | −3.85 | −5.12 | 0.01 | −3.57 | −4.16 | −4.83 | |
| 16 | 0.70 | −4.06 | −5.48 | 0.36 | −3.66 | −4.65 | − 5 | |
| 20 | 0.67 | −3.02 | −5.74 | 0.10 | −3.54 | −4.74 | −5.21 | |
| 24 | 0.56 | −2.26 | −3.46 | 0.29 | −2.18 | −3.97 | −4.62 | |
The italic font indicates activity (a reduction of ≥1 log10CFU/mL below the initial inoculum); bold font indicates synergy (a decrease of ≥2 log10 in the number of CFU/mL between the combination and its most active component); bold italic font indicates additivity (a decrease of 1.0 to <2 log10 in the number of CFU/mL between the combination and its most active component).
3.2.2. Fosfomycin Monotherapy
Fosfomycin monotherapy was conducted at a Cmax of 100 mg/L. Except for colistin-susceptible isolate KP47, a maximum reduction around 3 log10 CFU/mL was observed at 2 h, and then it started to regrowth. Fosfomycin showed little bacterial killing effect against three colistin-heteroresistant isolates no matter they were resistant or susceptible to fosfomycin.
3.2.3. Combination Therapy
Enhanced bacterial killing effect was particularly evident for the colistin- and fosfomycin-susceptible isolate KP47 (Figure 2(a) and Table 3). A combination therapy of fosfomycin (at 100 mg/L Cmax) and colistin (at 0.5 mg/L Cmax) produced a 3-log10 higher killing effect at 4 h posttreatment compared with the monotherapy. Even at 24 h, combination of fosfomycin and colistin still showed a greater killing effect compared with colistin monotherapy. Combination of fosfomycin and 2 mg/L colistin produced more than 4 log10 greater killing effect compared with colistin monotherapy at 10–24 h. The greatest killing effect was observed in combination regimen containing 5 mg/L colistin. Bacterial killing below the limit of detection sustained from 2 h to 20 h compared with colistin alone. Of the 18 cases from 4–24 h (three combinations across six time points), 15 cases showed synergistic effect, and one case exhibited additive effect (Table 3).
For colistin-heteroresistant and fosfomycin-susceptible isolate KP79, combination of low dose colistin (0.5 mg/L) and fosfomycin showed no synergistic or additive effects compared with colistin alone (Figure 2(b) and Table 3). Moreover, combination of 2 or 5 mg/L colistin and fosfomycin exhibited similar bactericidal effect with colistin monotherapy before 12 h. However, regrowth started after 12 h in colistin monotherapy and almost reached the original inoculum at 24 h, while the combination therapy maintained the bactericidal effect until 24 h. In addition, five cases of synergistic effect and two cases of additive effect were observed in the eight cases from 12–24 h (two combinations across four time points, Table 3).
For colistin-heteroresistant and fosfomycin-resistant isolates KP42 and KP11, enhanced activity was only noticeable in the combination of low dose colistin (0.5 mg/L) and fosfomycin. This combination yielded additional bactericidal effect of more than 2 log10 before 12 h against KP42. After 12 h, its regrowth had no significant difference with low dose colistin alone. For KP11, combination of 0.5 mg/L colistin and fosfomycin sustained a 3-4 log10 CFU lower through 24 h compared with colistin alone. There was no enhancement of bactericidal activity in combination containing 2 or 5 mg/L colistin, showing similar efficacy compared with colistin alone, except that combination resulted in greater activity with additive (predominantly) or synergistic effect at 3-4 time points.
3.3. Emergence of Colistin Resistance
For KP47, the colistin-susceptible strain, colistin monotherapy with Cmax at 0.5, 2, or 5 mg/L at inoculum of 108 CFU/mL could increase the growth of colistin-resistant subpopulations at 24 h. These subpopulations could grow well on the plates containing 10 mg/L colistin (Figure 2(a)). Combinations of low or medium dose of colistin and fosfomycin did not prevent the emergence of colistin-resistant isolates against KP47. The subpopulation could still grow in the presence of 4 or 6 mg/L colistin. However, combination containing 5 mg/L colistin could completely inhibit the emergence of colistin-resistant subpopulations. Not even a single colony could be detected on plates with the concentration of colistin ≥ 1 mg/L by 24 h (Figure 2(a)). However, for colistin-heteroresistant isolates (KP79, KP42, and KP11), no matter they were fosfomycin-susceptible or fosfomycin-resistant, all combinations of colistin and fosfomycin at inoculum of 108 CFU/mL did not eliminate the colistin-resistant subpopulations.
4. Discussion
Colistin has been rekindled as the last resort therapeutic regimen due to the increasing prevalence of MDR Gram-negative pathogens worldwide. However, colistin-resistant species, such as Enterobacteriaceae, Pseudomonas spp., and Acinetobacter spp., have also been reported since colistin is more and more used in the treatment of MDR pathogen-caused infections. It has been reported that the occurrence of colistin resistance may be due to suboptimal use of colistin, such as suboptimal dose or prolonged monotherapy [22, 23]. Even for those pathogens that are initially susceptible to colistin, regrowth can be observed after the colistin monotherapy [21, 24]. In our study, colistin monotherapy induced the colistin resistance against the initially colistin-susceptible isolate KP47 within 24 h. Combination therapy has become an alternative to improve effectiveness and prevent antimicrobial resistance due to the toxicity limiting dose escalation of colistin.
In the present study, we evaluated the antimicrobial effects of colistin/fosfomycin combination against carbapenemase-producing K. pneumoniae in terms of their synergistic activity and ability of preventing emergence of resistant subpopulations. The concentrations of colistin and fosfomycin were selected based on clinically achievable serum free drug concentrations at commonly recommended dosages. The package insert of CMS in the USA recommends that dose of intravenous injection ranges from 2.5 mg/kg to 5 mg/kg per day, which can be divided into two to four equal doses. Cmax of formed colistin can reach 5 mg/L at steady state [25]. Generally speaking, total daily intravenous fosfomycin doses in patients range from 12 to 16 g per day, which can be divided into two-four equal doses. High doses of fosfomycin (up to 24 g) can also be given to patients with central nervous system infections or other severe infections, which will lead to very high maximal plasma concentrations of more than 300 mg/L [26].
Several studies have evaluated the in vitro and in vivo synergism of colistin/fosfomycin combination against Gram-negative bacteria. Wei et al. [27] have found that colistin in combination with fosfomycin shows synergistic effect against 50% of extensively drug-resistant (XDR) A. baumannii. Another clinical trial of 94 patients infected with carbapenem-resistant A. baumannii has demonstrated that those receiving colistin combination regimens show a significantly more favorable microbiological response and lower mortality compared with those receiving colistin monotherapy [28]. For carbapenem-resistant Pseudomonas aeruginosa, combination of colistin and fosfomycin exerts synergistic or partial synergistic effect against 21.84% or 27.59% of 87 isolates, while antagonism is not observed [13]. Combination of colistin and fosfomycin can prevent regrowth of extended-spectrum β-lactamase-producing (ESBL) Escherichia coli at a subinhibitory concentration. This combination also demonstrates the highest cure rate (67%) in a mice model of foreign-body infection, showing a much better effect compared with colistin or fosfomycin alone [29].
Previous study has also reported that colistin in combination with fosfomycin shows synergistic effect against Klebsiella pneumoniae carbapenemase- (KPC-) producing or metallo-β-lactamase- (MBL-) producing K. pneumoniae. In a time-kill study, Souli et al. have investigated the bactericidal effect of colistin/fosfomycin combination against 17 clinical K. pneumoniae isolates carrying blaKPC-2. This combination shows bactericidal activity against 11 (64.7%) and synergistic effect against two (11.8%) of the 17 isolates [30]. Tängdén et al. [31] have found that combination of colistin and fosfomycin exhibits synergistic and bactericidal effect against three of four MBL-producing K. pneumoniae strains (one VIM-KP and two NDM-KP). All of the four strains are susceptible to colistin, while two VIM-KP strains are fosfomycin-susceptible and two NDM-KP strains are fosfomycin-resistant. A clinical research has been performed in 11 ICUs including 48 cases. KPC-2-producing CRKP is isolated from 41 patients (85.4%), as either the single pathogen (23 of 48 cases; 47.9%) or mixed with other pathogens (18 of 48 cases; 37.5%). Colistin or tigecycline is the most common agent administered with fosfomycin. The results have shown that 60% patients with CRKP infections have the satisfactory clinical and microbiological outcomes [32]. Albur et al. [18] have applied a PK/PD model to evaluate the bactericidal effect of combination of colistin (Cmax 3 mg/L) and fosfomycin (Cmax 250 mg/L) against six NDM-1-producing Enterobacteriaceae isolates, including two colistin-susceptible K. pneumoniae. They have found that the combination regimen can increase bactericidal effect and reduce emergence of colistin resistance compared with each monotherapy. Moreover, the antibacterial effects of this combination can last for a longer time and are notable for both fosfomycin-susceptible and fosfomycin-resistant isolates. A recent study in 2017 has evaluated the bactericidal effect of colistin/fosfomycin combination against two KPC-producing K. pneumoniae stains, one of which is susceptible to both colistin and fosfomycin, while the other one is resistant to colistin and susceptible to fosfomycin. In vitro time-kill experiments have revealed that only highest concentrations of colistin (4 mg/L) and fosfomycin (512 mg/L) can achieve synergism in colistin-resistant KPC strains, while synergy is observed for all colistin/fosfomycin combinations against the double-susceptible KPC strain. They have only applied double-susceptible KPC strain in the subsequent PK/PD study. The results have shown that colistin or fosfomycin monotherapy results in rapid proliferation of resistant subpopulations. However, combination of colistin and fosfomycin leads to a rapid reduction of the double-susceptible KPC strain in 6 h and complete suppression of resistant subpopulations until 120 h [33]. Our results on the colistin-susceptible K. pneumoniae isolate were consistent with these reports. The antibacterial effects were enhanced after colistin was used in combination with fosfomycin against KP47, which is susceptible to both colistin and fosfomycin. The highest dose combination (Cmax of 5 mg/L colistin and 100 mg/L fosfomycin) could completely prevent the emergence of colistin-resistant subpopulations. Because the concentration of fosfomycin applied in our study is lower than the above 2 studies (100 mg/L versus 250 and 300 mg/L), we speculated that if higher concentration of fosfomycin was included, more obvious synergism might be observed even in those colistin-heteroresistant strains.
However, the antimicrobial efficacy of the combination of colistin and fosfomycin was not obvious against the colistin-heteroresistant K. pneumoniae, even with the highest dose regimen, although the combination regimen could delay the regrowth of bacteria to a certain extent. Previous PK/PD research on combination of colistin (0.5 or 2 mg/L) and doripenem (Cmax of 2.5 or 25 mg/L over 8 h) at constant concentrations against K. pneumoniae has shown that this combination can both enhance the bactericidal effect and prevent the occurrence of the colistin-resistant colonies at an inoculum of either 106 or 108 CFU/mL [21]. In contrast to their study, constant concentration of colistin was not applied in our study, which might explain the difference between these two studies. However, in general, the combination of colistin and fosfomycin was less effective compared with doripenem in the case of colistin-heteroresistant K. pneumoniae.
There are three limitations in the present study. Colistin and fosfomycin concentrations in CAMHB broth were only measured before experiment. The results showed that the actual concentrations were close to the predicted concentrations with variability of less than 5%. However, the actual concentration of either colistin or fosfomycin was not measured during the real experiments. The second limitation is that the bactericidal effect of combination was only examined up to 24 h. Therefore, we have no idea about resistance and regrowth after 24 h. The third is that only 4 K. pneumoniae strains were applied in the present study, so the representativeness of the results needs to be further verified in more K. pneumoniae isolates and other species.
In conclusion, our study showed that combination of colistin and fosfomycin at clinically achievable concentrations could increase the bactericidal effects against colistin-susceptible K. pneumoniae and prevent the emergence of colistin-resistant subpopulations. However, this synergism and resistance preventing ability was barely observed in colistin-heteroresistant K. pneumoniae, especially in those fosfomycin-resistant isolates. Considering that colistin heteroresistance and fosfomycin resistance are not rare, it is necessary to know the resistant patterns of the K. pneumoniae when using combination of colistin and fosfomycin in clinical practice.
Conflicts of Interest
There are no conflicts of interest to be declared. The authors alone are responsible for the content and writing of the paper.
Funding
This research was supported by the National Natural Science Foundation of China (No. 81573472 and No. 81770004) and Clinical Research Support Funding of PLA General Hospital (No. 2016 FC-CXYY-2013).
Supplementary Materials
Primer sets for β-lactamases used in the PCR were listed as Table S1.
References
- 1.Boucher H. W., Talbot G. H., Bradley J. S., et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clinical Infectious Diseases. 2009;48(1):1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Grundmann H., Glasner C., Albiger B., Aanensen DM., Tomlinson CT., Andrasevic AT., et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis.; 2016. [DOI] [PubMed] [Google Scholar]
- 3.Bassetti M., Merelli M., Temperoni C., Astilean A. New antibiotics for bad bugs: Where are we? Annals of Clinical Microbiology and Antimicrobials. 2013;12(1, article no. 22) doi: 10.1186/1476-0711-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dijkmans A. C., Wilms E. B., Kamerling I. M. C., et al. Colistin: Revival of an Old Polymyxin Antibiotic. Therapeutic Drug Monitoring. 2015;37(4):419–427. doi: 10.1097/FTD.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 5.Durante-Mangoni E., Andini R., Signoriello S., et al. Acute kidney injury during colistin therapy: a prospective study in patients with extensively-drug resistant Acinetobacter baumannii infections. Clinical Microbiology and Infection. 2016;22(12):984–989. doi: 10.1016/j.cmi.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Ah Y.-M., Kim A.-J., Lee J.-Y. Colistin resistance in Klebsiella pneumoniae. International Journal of Antimicrobial Agents. 2014;44(1):8–15. doi: 10.1016/j.ijantimicag.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Halaby T., Kucukkose E., Janssen A. B., et al. Genomic characterization of colistin heteroresistance in Klebsiella pneumoniae during a nosocomial outbreak. Antimicrobial Agents and Chemotherapy. 2016;60(11):6837–6843. doi: 10.1128/AAC.01344-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayol A., Nordmann P., Brink A., Poirel L. Heteroresistance to colistin in Klebsiella pneumoniae associated with alterations in the PhoPQ regulatory system. Antimicrobial Agents and Chemotherapy. 2015;59(5):2780–2784. doi: 10.1128/AAC.05055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meletis G., Tzampaz E., Sianou E., Tzavaras I., Sofianou D. Colistin heteroresistance in carbapenemase-producing Klebsiella pneumoniae. Journal of Antimicrobial Chemotherapy. 2011;66(4):946–947. doi: 10.1093/jac/dkr007.dkr007 [DOI] [PubMed] [Google Scholar]
- 10.Silva A., Sousa A. M., Alves D., Lourenço A., Pereira M. O. Heteroresistance to colistin in Klebsiella pneumoniae is triggered by small colony variants sub-populations within biofilms. Pathogens and Disease. 2016;74(5) doi: 10.1093/femspd/ftw036. [DOI] [PubMed] [Google Scholar]
- 11.Reffert J. L., Smith W. J. Fosfomycin for the treatment of resistant gram-negative bacterial infections. Pharmacotherapy. 2014;34(8):845–857. doi: 10.1002/phar.1434. [DOI] [PubMed] [Google Scholar]
- 12.Kaase M., Szabados F., Anders A., Gatermann S. G. Fosfomycin susceptibility in carbapenem-resistant Enterobacteriaceae from Germany. Journal of Clinical Microbiology. 2014;52(6):1893–1897. doi: 10.1128/jcm.03484-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di X., Wang R., Liu B., et al. In vitro activity of fosfomycin in combination with colistin against clinical isolates of carbapenem-resistant Pseudomas aeruginosa. The Journal of Antibiotics. 2015;68(9):551–555. doi: 10.1038/ja.2015.27. [DOI] [PubMed] [Google Scholar]
- 14.Poudyal A., Howden B. P., Bell J. M., et al. In vitro pharmacodynamics of colistin against multidrug-resistant Klebsiella pneumoniae. Journal of Antimicrobial Chemotherapy. 2008;62(6):1311–1318. doi: 10.1093/jac/dkn425. [DOI] [PubMed] [Google Scholar]
- 15.Bergen P. J., Li J., Rayner C. R., Nation R. L. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy. 2006;50(6):1953–1958. doi: 10.1128/AAC.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kazi M., Drego L., Nikam C., et al. Molecular characterization of carbapenem-resistant Enterobacteriaceae at a tertiary care laboratory in Mumbai. European Journal of Clinical Microbiology & Infectious Diseases. 2015;34(3):467–472. doi: 10.1007/s10096-014-2249-x. [DOI] [PubMed] [Google Scholar]
- 17.Gootz T. D., Lescoe M. K., Dib-Hajj F., et al. Genetic organization of transposase regions surrounding blaKPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City Hospital. Antimicrobial Agents and Chemotherapy. 2009;53(5):1998–2004. doi: 10.1128/AAC.01355-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albur M. S., Noel A., Bowker K., MacGowan A. The combination of colistin and fosfomycin is synergistic against NDM-1-producing Enterobacteriaceae in in vitro pharmacokinetic/pharmacodynamic model experiments. International Journal of Antimicrobial Agents. 2015;46(5):560–567. doi: 10.1016/j.ijantimicag.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Lee H. J., Bergen P. J., Bulitta J. B., et al. Synergistic activity of colistin and rifampin combination against multidrug-resistant acinetobacter baumannii in an in vitro pharmacokinetic/ pharmacodynamic model. Antimicrobial Agents and Chemotherapy. 2013;57(8):3738–3745. doi: 10.1128/AAC.00703-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roussos N., Karageorgopoulos D. E., Samonis G., Falagas M. E. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. International Journal of Antimicrobial Agents. 2009;34(6):506–515. doi: 10.1016/j.ijantimicag.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Deris Z. Z., Yu H. H., Davis K. The combination of colistin and doripenem is synergistic against Klebsiella pneumoniae at multiple inocula and suppresses colistin resistance in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrobial Agents and Chemotherapy. 2012;56:5103–5112. doi: 10.1128/AAC.01064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J., Coulthard K., Milne R., et al. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. Journal of Antimicrobial Chemotherapy. 2003;52(6):987–992. doi: 10.1093/jac/dkg468. [DOI] [PubMed] [Google Scholar]
- 23.Li J., Rayner C. R., Nation R. L., et al. Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration [4] Antimicrobial Agents and Chemotherapy. 2005;49(11):4814–4815. doi: 10.1128/AAC.49.11.4814-4815.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin K.-H., Chuang Y.-C., Lee S.-H., Yu W.-L. In Vitro Synergistic Antimicrobial Effect of Imipenem and Colistin Against an Isolate of Multidrug-resistant Enterobacter cloacae. Journal of Microbiology, Immunology and Infection. 2010;43(4):317–322. doi: 10.1016/S1684-1182(10)60049-7. [DOI] [PubMed] [Google Scholar]
- 25.Biswas S., Brunel J. M., Dubus J. C., Reynaud-Gaubert M., Rolain J. M. Colistin: an update on the antibiotic of the 21st century. Expert Review of Anti-infective Therapy. 2012;10(8):917–934. doi: 10.1586/eri.12.78. [DOI] [PubMed] [Google Scholar]
- 26.Falagas M. E., Vouloumanou E. K., Samonis G., Vardakasa K. Z. Fosfomycin. Clinical Microbiology Reviews. 2016;29(2):321–347. doi: 10.1128/CMR.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei W., Yang H., Liu Y., Ye Y., Li J. In vitro synergy of colistin combinations against extensively drug-resistant Acinetobacter baumannii producing OXA-23 carbapenemase. Journal of Chemotherapy. 2016;28(3):159–163. doi: 10.1179/1973947815Y.0000000030. [DOI] [PubMed] [Google Scholar]
- 28.Sirijatuphat R., Thamlikitkul V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrobial Agents and Chemotherapy. 2014;58(9):5598–5601. doi: 10.1128/AAC.02435-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corvec S., Tafin U. F., Betrisey B., Borens O., Trampuz A. Activities of fosfomycin, tigecycline, colistin, and gentamicin against extended-spectrum-β-lactamase-producing Escherichia coli in a foreign-body infection model. Antimicrobial Agents and Chemotherapy. 2013;57(3):1421–1427. doi: 10.1128/aac.01718-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Souli M., Galani I., Boukovalas S., et al. In vitro interactions of antimicrobial combinations with fosfomycin against KPC-2-producing Klebsiella pneumoniae and protection of resistance development. Antimicrobial Agents and Chemotherapy. 2011;55(5):2395–2397. doi: 10.1128/AAC.01086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tängdén T., Hickman R. A., Forsberg P., Lagerbäck P., Giske C. G., Cars O. Evaluation of double- and triple-antibiotic combinations for VIM- and NDM-producing Klebsiella pneumoniae by in vitro time-kill experiments. Antimicrobial Agents and Chemotherapy. 2014;58(3):1757–1762. doi: 10.1128/AAC.00741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pontikis K., Karaiskos I., Bastani S., et al. Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. International Journal of Antimicrobial Agents. 2014;43(1):52–59. doi: 10.1016/j.ijantimicag.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Zhao M., Bulman Z. P., Lenhard J. R., et al. Pharmacodynamics of colistin and fosfomycin: A 'treasure trove' combination combats KPC-producing Klebsiella pneumoniae. Journal of Antimicrobial Chemotherapy. 2017;72(7):1985–1990. doi: 10.1093/jac/dkx070.dkx070 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sets for β-lactamases used in the PCR were listed as Table S1.